Abstract
Regeneration and functional recovery of nerves after peripheral nerve injury is the key to peripheral nerve repair. One of the putative therapeutic strategies is to use anti-adhesion polymer films, made of polymeric biomaterials. Recently, a novel biodegradable poly (DL-lactic acid) (PDLLA) film has been prepared using a method of phase transformation with biodegradable polylactic acid polymer as the substrate. This novel, anti-adhesion film has a porous structure, which provides better mechanical properties, better flexibility, more complete diffusion through the polymer of tissue biologic factors like growth factors, and more controllable degradation compared to traditional non-porous films. Little is known, however, about the in vitro and in vivo biocompatibility and cytotoxicity of this type of PDLLA film. Therefore, our aim was to evaluate the biocompatibility and cytotoxicity of this novel PDLLA film using various experimental methods, including a skin irritation test, MTT analysis, and the mouse bone marrow cell micronucleus test, as well as hematology or clinical chemistry measurements in rats after receiving sciatic nerve transection and anastomosis with wrapping of the anastomosis with DLLA films. We demonstrated that exposure to PDLLA film extracts did not generate apparent erythema or edema in rabbit skin and had no effect on the proliferation of Vero cells. Additionally, treatment with PDLLA film extracts did not alter the incidence of micronucleated polychromatic erythrocytes as compared with saline Treated group. Furthermore, implantation of PDLLA film did not alter liver or renal function as measured by serum levels of ALT, AST, TP, A/G, Cr, and BUN, and pathologic examinations showed that implantation of PDLLA film did not cause pathologic changes to the rat liver, kidney, pancreas, or spleen. Taken together, these results suggest that PDLLA films have excellent biocompatibility and no obvious toxicity in vivo, and may be used to prevent nerve adhesion, thereby promoting nerve regeneration.
Keywords: Nerve regeneration, sciatic nerve transection, poly (DL-lactic acid) film, biocompatibility, cytotoxicity
Introduction
Regeneration and functional recovery of nerve fibers after peripheral nerve injury is the key to peripheral nerve repair, which is an important yet unmet medical need which has attracted extensive attention in the development to enhance nerve regeneration. Adhesion of the peripheral nerve to surrounding tissues results in fibrosis of the nerve that contributes to peripheral nerve dysfunction [1]. Therefore, the development of a high performance, anti-adhesion therapy would be of great importance clinically in peripheral nerve repair.
One of the putative therapeutic strategies is to use anti-adhesion polymer films, made of polymeric biomaterials [1-4]. Polymeric biomaterials are used commonly in the diagnosis, treatment, replacement or repair, and regeneration of damaged tissues and organs [5]. Biodegradable biomedical polymers include natural polymers, such as chitosan, chitin, and cellulose, and synthetic polymers, such as polylactic acid (PLA). Polylactic acid (PLA) is linear, aliphatic polyester with good biodegradability [6,7]. PLA can be divided further into poly-L-lactide (PLLA), poly-D-lactic acid (PDLA), and poly-rac-lactide (polylactic-DL-acid, PDLLA), based on molecular isomeric structure [8]. Furthermore, PLAs with different structures and synthetic configurations have a large difference in the mechanical properties and degradation profiles [9,10]. For instance, PLLAs are semi-crystalline polymers, with melting points (Tm) as high as 175-178°C, and degradation times of up to 3-3.5 years. As a result, PLLAs are suitable for orthopedic fixation. In contrast, PDLLA polymer chains are not well structured, so that PDLLA is an amorphous polymer, with a Tm as low as 65°C and a degradation time as rapid as 3-6 months [11-13]. Therefore, PDLLA films seem more suitable for prevention of adhesions in peripheral nerves in vivo during their recovery after trauma.
Biodegradable films should serve their intended anti-adhesive function and the products of their degradation should be biocompatible and non-toxic, without interfering with tissue healing. Currently, commercially available, anti-adhesion polymer films are made mostly of polyester materials, which have poor flexibility, uncontrolled degradation, and lack of permeability that interfere with diffusion of nutrients, growth factors, etc. These properties interfered with the healing process and can cause tissue edema/induration or even necrosis under the films. In addition, PLA may produce toxic solutions in vitro, presumably due to formation of the acidic products of degradation [14]. Additionally, chronic inflammation (presence of macrophages, fibroblasts, giant cells and lymphocytes) was observed in dogs with PLLA used in meniscal reconstruction [15]. Furthermore, subcutaneously implanted, pre-degraded PLLA can elicit fibrous encapsulation, with attraction of macrophages and giant cells in response to the smaller particles [16]. Thus, it is necessary to evaluate the biocompatibility and cytotoxicity of any potential types of biodegradable films before clinical applications.
Recently, by using a method of phase transformation, a novel biodegradable PDLLA film has been prepared composed of a biodegradable polylactic acid polymer [8]. This novel, anti-adhesion film has a porous structure, which provides better mechanical properties, better flexibility, and more controllable degradation compared to traditional, non-porous films. The presence of a porous structure may allow better diffusion of tissue fluids, which is beneficial to the nerve regeneration, on both sides of the film without sacrificing the barrier function. Little is known, however about the in vitro and in vivo biocompatibility and cytotoxicity of this type of PDLLA film. Therefore, in the present study, our aim was to evaluate the biocompatibility and cytotoxicity of this novel PDLLA film using various in vivo and vitro methods, including the skin irritation test, MTT analysis, and the mouse bone marrow cell micronucleus test, as well as measurements of hepatic and renal function as well as histopathologic exam of visceral organs in rats after receiving sciatic nerve transection and anastomosis wrapped with the PDLLA film.
Materials and methods
Animals
All animals were provided by the Animal Center, School of Basic Medicine, Jilin University, China (license No. SCXK (Ji) 2003-001). Animals were housed in a temperature- and humidity-controlled vivarium under a 12 h light/dark cycle. All animals had free access to food and water continuously daily. In the skin irritation tests, we used healthy, adult New Zealand rabbits (n = 6), irrespective of sex, weighting 2.0-3.1 kg. To determine the effects of biodegradable PDLLA films on mouse bone marrow micronucleus, we used healthy mice (n = 50), irrespective of sex, weighing 20±2 g, 7-12 weeks of age. Finally, to examine the in vivo toxicity of biodegradable PDLLA films, Wistar rats (n = 18), irrespective of sex, weighing 200±20 g, for analysis of hematology or clinical analyse and histopathologic examination of visceral organs. All animal experiments were approved by the local Institutional Animal Care and Use Committee. The housing and treatment of the animals followed the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China. All painful operations in animals were performed under an anesthetic with sterile conditions.
Preparation of extracts from PDLLA biomedical film extracts
Biodegradable PDLLA films (production batch number: 130601F; Changchun SinoBiomaterials, Jilin, China) were used to prepare a solution of PDLLA film extracts (PDLLA/saline = 0.2 g/ml) in accordance with Chinese National Standard GB/T16886.12. All operations were performed under sterile conditions. The PDLLA films were dissolved in saline, and the solution was placed in a water bath shaker (WS2-261-79, Beijing Medical Instrument, Beijing, China) for 72 h at 37°C. The solution was then dispensed into sterile containers and stored at 4°C. For experiments on cell cultures, the solution of PDLLA film extracts was prepared with FBS-containing RPMI-1640 cell culture medium (Gibco, Grand Island, NY, USA), followed by preparation of four concentrations of PDLLA (i.e., 12.5%, 25%, 50%, and 100%). Negative control extracts were prepared using high-density polyethylene with appropriate solvents.
Skin irritation test
The skin irritation test was conducted according to the Chinese National Standard GB/T16886.10-2000. Rabbits were divided randomly into three groups (n = 2/group). Twenty four hours prior to the test, rabbit hair around the dorsal aspect of the back with an area of 5 × 8 cm was removed. Four layers of sterile gauze (2.5 × 2.5 cm) were then used to cover the skin area with a bandage, and the contact area was marked. Saline, dinitrofluorobenzene (a known skin irritant and sensitizer), or PDLLA extracts were applied onto the sterile gauze such that the gauze was fully soaked. The gauze were removed after 24 h, and the skin conditions were observed and recorded after an additional 1, 24 , 48 , and 72 h under natural light or full-spectrum light. Scoring criteria for skin irritation were listed in Table 1. The primary scores of skin irritation of each animal were calculated by subtracting the negative control group (i.e., saline group). A Primary irritation score index (PII) was then calculated using the primary skin irritation score for each animal divided by the total number of animals. Finally, the evaluation of the type of skin reactions was conducted based on criteria list in Table 2.
Table 1.
Skin reaction signs with scoring value
| Reaction | Score |
|---|---|
| Erythema and eschar formation | |
| No erythema | 0 |
| Very slight erythema (barely perceptible) | 1 |
| Well defined erythema | 2 |
| Moderate to severe erythema | 3 |
| Severe erythema (Beet red) to slight eschar formation | 4 |
| Edema formation | |
| No edema | 0 |
| Very slight edema (barely perceptible) | 1 |
| Slight edema (edges of area well defined by definite raising) | 2 |
| Moderate edema (raising approximately 1 mm) | 3 |
| Severe edema (raised more than 1 mm extending beyond the area of exposure) | 4 |
Table 2.
Primary irritation index with irritation reaction
| Average index | Reaction |
|---|---|
| 0-0.4 | Very slight |
| 0.5-1.9 | Slight |
| 2.0-4.9 | Moderate |
| 5.0-8.0 | Severe |
Vero cell culture
Vero cells (School of Basic Medicine, Jilin University, China) were cultured in RPMI-1640 culture medium containing 10% FBS at a temperature of 37°C in an incubator (BB5060, Heraeus, Hanau, Germany) for 24 h until cells reached the end of the logarithmic growth. Vero cells are a cell line derived from monkey renal epithelial cells and have been used often for toxicity studies. Cells were then dispersed with 0.25% trypsin. Cells suspensions (1 × 104 cells/ml) were then prepared after re-suspending cells in culture medium after spinning the dispersed cell medium for 5 min at 1000 rpm.
Cell viability assay
The cell viability assay was conducted in accordance with the Chinese National Standard GB/T16886.5-2003, using non-cancerous Vero cells and the MTT ([3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]) assay [17]. The prepared Vero cell suspension was seeded in the wells of the 96-well plastic cell culture plate (Nest Scientific, USA). To each well, 100 μL of the cell suspension was added. After inoculation, the cells were observed under a phase contrast microscope (Nikon TE300, Japan). After discarding the original culture medium, 100 μL of different concentrations of testing solutions were then added to each well, including PDLLA films extracts, negative control solution (RPMI-1640 medium), or a positive control solution (0.5% phenol solution). Each group consisted of at least 8 wells. The 96-well cell culture plate was then placed in an incubator containing 95% oxygen, 5% carbon dioxide at 37°C. After 24, 48, or 72 h, 20 μl MTT solution was added to each well, followed by additional 5 h incubation. After carefully discarding the solutions in each well, 200 μl DMSO dimethyl sulfoxide was added to each well, and the cell culture plate was shaken for 10 min to form a uniform color. The absorbance was measured at a wavelength of 570 nm with a microplate photometer (BP800, Biohit, Neptune City, USA). The relative growth rate (RGR) was calculated as RGR = (ODexp Mean/ODctl Mean) × 100%. The evaluation of cytotoxicity was in accordance with the ISO2109932-5 standard, using a 5-point grading scale, as shown in Table 3. The RGR value for the negative control group should be no more than grade 1, and the RGR value for the positive control group should be no less than grade 3. Grade 0-1 represents no cytotoxicity, Grade 2 represents mild cytotoxicity, Grade 3-4 represents moderate cytotoxicity, and Grade 5 represents substantial cytotoxicity.
Table 3.
Cell relative growth rate (RGR) with cytotoxicity level
| RGR value (%) | Cytotoxicity level |
|---|---|
| ≥100% | 0 |
| 75%-99% | 1 |
| 50%-74% | 2 |
| 25%-49% | 3 |
| 1%-24% | 4 |
| ≤1% | 5 |
Mice bone marrow cell micronucleus test
Mice were divided randomly into five groups (n = 10/group), and two doses of treatment were given intraperitoneally. Mice received the first dose of either saline (154 mM NaCl, 1 ml/kg), PDLLA film extracts (50, 100, or 200 ml/kg), or cyclophosphamide (40 mg/kg), and the second dose of the same treatment 24 h later. The 40 mg/kg dose of cyclophosphamide has been used widely as a positive control in the bone marrow cell micronucleus test [18]. After an additional 6 h, the mice were killed by cervical dislocation. The entire sternum was harvested with excess muscles and blood removed. The sternal bone marrow cells were flushed out with 1 ml of fetal bovine serum (Gibco, USA) into a centrifuge tube. A uniform cell suspension was made by pipet. For the microscopic analysis of micronuclei, a few drops of the cell suspension were placed on a glass slide, fixed, and dried, followed by staining with 10% Giemsa stain (Sigma, USA) for 10-15 min. Cell counting was performed using an inverted microscope (Nikon TE300, Tokyo, Japan). For each mouse, at least 1000 polychromatic erythrocytes (PCEs) were counted, and the micronucleus rate was calculated as follows:
Micronucleus rate (‰) = micronucleated PCEs/total PCEs × 1000
Sciatic nerve transection and anastomosis
To determine the in vivo toxicity of biodegradable PDLLA films, adult Wistar rats (n = 18) were divided randomly into three groups (n = 6/group). Rats were anesthetized by 10% chloral hydrate (0.3 ml/kg; i.p.). After shaving the hair from the mid back to hind limb area, rats were then placed prone on the surgery table with a heating pad underneath to maintain body temperature of approximate 37°C. To allow the exposure of the sciatic nerve, a 3 cm incision was made starting 0.5 cm lateral to the rat midline toward the tibiofemoral articulation, followed by separation of the femoral biceps and gluteus muscles. A microsurgical scissor was used to transect the left side sciatic nerve. The nerve was then repaired by epineural microsutures using 9-0 nylon sutures (Johnson & Johnson medical equipment, Shanghai, China) using binocular loupe magnification. Group 1 received sciatic nerve transection and anastomosis with no special treatment. Group 2 received sciatic nerve transection and anastomosis with artificial nerve conduits (TianXinFu Medical Appliance, Beijing, China). Group 3 received nerve transection and anastomosis with wrapping of the nerve with biodegradable PDLLA film.
Pathology
Blood samples for measurement of hematology or clinical chemistry were collected from the vena cava after 1, 4, or 12 weeks recovery from the nerve transection/reanastomosis while the rat was under isoflurane anesthesia. The following parameters were assessed with a Bayer® Advia 120 hematology analyzer: white blood cell count (WBC), hemoglobin (Hb), hematocrit (HCT), and platelets (PLT). The following serum parameters were assessed with an Olympus® AU640 clinical chemistry analyzer: aspartate aminotransferase activity (AST), alanine aminotransferase activity (ALT), total protein (TP), albumin/globulin ratio (A/G), creatinine (CREA), and urea nitrogen (BUN). Liver, spleen, kidney, and pancreas from the rats were harvested after 1, 4, or 12 weeks recovery after the nerve transection/ reanastomosis (n = 2 rates/time interval) and retained in an appropriate fixative. Microscopic examination of paraffin sections stained with hematoxylin and eosin (H&E) were performed by a veterinary pathologist on all tissues collected from all groups of animals.
Data analysis
All values are presented as mean ± standard deviation (SD) when appropriate. Data were analyzed using one-way analyses of variance (ANOVA) or analyses of variance (ANOVAs) with repeated measures, where appropriate. Significant ANOVA main and interaction effects were further investigated using Tukey post hoc tests, when appropriate. Alpha was set at 0.05.
Results
Effects of PDLLA extracts on skin irritation
After dinitrofluorobenzene treatment, the rabbit skin showed moderate erythema and mild edema. The final primary irritation scores for the dinitrofluorobenzene Treated rabbits were 2.8 and 3.0, with a primary irritation index (PII) of 2.9 (Table 4). According to the standard list in Table 2, this irritation was considered as moderate (Figure 1B). In contrast, saline Treated group had a primary irritation score of zero and a primary irritation index (PII) of zero (Table 4), suggesting saline had no irritation on rabbit skin (Figure 1A). Similarly, all rabbits in the PDLLA extracts Treated group had a primary irritation score of zero and a primary irritation index (PII) of zero (Table 4), suggesting that PDLLA extracts had no irritation on rabbit skin (Figure 1C).
Table 4.
Skin irritation test
| Rabbit No. | Erythema and edema | Score (erythema score, edema score) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 h | 24 h | 48 h | 72 h | Score | |||
| 1 | SALINE | No erythema, no edema | 0 | 0 | 0 | 0 | 0 |
| 2 | SALINE | No erythema, no edema | 0 | 0 | 0 | 0 | 0 |
| 3 | DNFB | Erythema and edema | 2 (1, 1) | 3 (2, 1) | 4 (2, 2) | 2 (1, 1) | 2.8 |
| 4 | DNFB | Erythema and edema | 2 (1, 1) | 3 (2, 1) | 5 (3, 2) | 2 (1, 1) | 3.0 |
| 5 | PDLLA | No erythema, no edema | 0 | 0 | 0 | 0 | 0 |
| 6 | PDLLA | No erythema, no edema | 0 | 0 | 0 | 0 | 0 |
Figure 1.
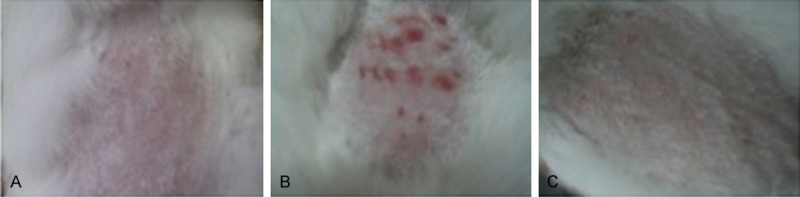
Representative images illustrate the results of skin irritation test. (A) saline-treated group, (B) dinitrofluorobenzene-treated group, and (C) PDLLA film extracts-treated group.
Effects of PDLLA film extract on cell viability
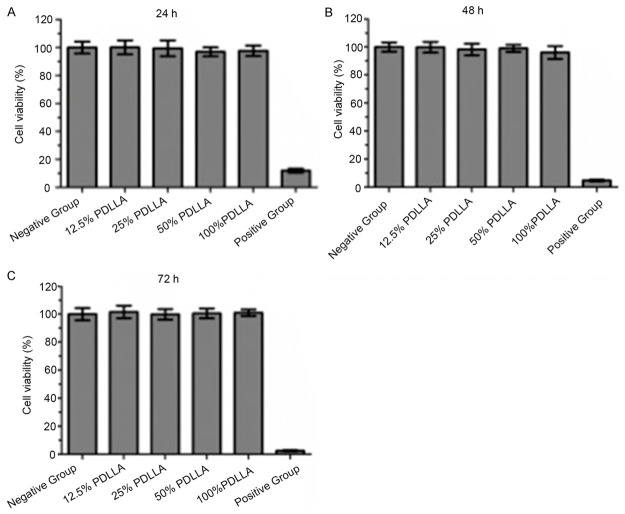
In 0.5% phenol treated group, Vero cells shrank with no apparent cell growth. The RGR value was 12%, 4.7%, and 0% after 24 h, 48 h, and 72 h, respectively (Table 5). According to the criteria listed in Table 3, the level of cytotoxicity was grade 4-5. In contrast, in the Vero cells in RPMI-1640 medium treated group as well as the groups treated with PDLLA films extract, showed adherent growth and normal shape (Table 5) yielding a level of cytotoxicity of grade 0-1. After 24 h, 48 h, or 72 h of incubation, the number of Vero cells increased gradually in both the group treated with RPMI-1640 medium treated group and in all groups treated with PDLLA films extracts (Table 5). There was no significant difference between the groups treated with PDLLA films extracts and RPMI-1640 medium , but there were significant differences between all groups treated with PDLLA films extracts and the 0.5% phenol treated group (p<0.01). These experiments suggest that the PDLLA film extracts were not cytotoxic.
Table 5.
Relative growth rate (RGR%)
| Group | 24 h (%) | 48 h (%) | 72 h (%) |
|---|---|---|---|
| PDLLA | |||
| 100% extract | 97.7 | 96.1 | 100.1 |
| 50% extract | 96.9 | 99.1 | 100.6 |
| 25% extract | 99.5 | 98.2 | 99.9 |
| 12.5% extract | 100.1 | 97.7 | 101.6 |
| Negative control | 100.0 | 100.0 | 100.0 |
| Positive control | 12.0 | 4.7 | 0 |
Effects of PDLLA film extracts on the viability and morphology of Vero cells
After 0.5% phenol treatment, cell viability decreased to approximately 18, 8 and 4% after 24, 48, and 72 h of incubation, respectively (Figure 2). In contrast, treatment with PDLLA film extracts failed to alter Vero cell viability at any of the concentrations tested as with the RPMI-1640 medium treated control group across time (Figure 2). Thus, PDLLA film extracts did not affect Vero cell survival. Furthermore, after 24 or 48 h of incubation with the greatest concentration of PDLLA film extracts, Vero cells showed no abnormal cell morphology, with clear structure, a clearly visible nucleolus, and the clear boundaries between the nucleus and the cytoplasm, similar to RPMI-1640 medium treated control group (Figure 3).
Figure 2.
Cell viability after 24 h (A), 48 h (B), and 72 h (C) incubation. Negative control group represents RPMI-1640 medium treatment. Positive control group represents 0.5% phenol treatment.
Figure 3.
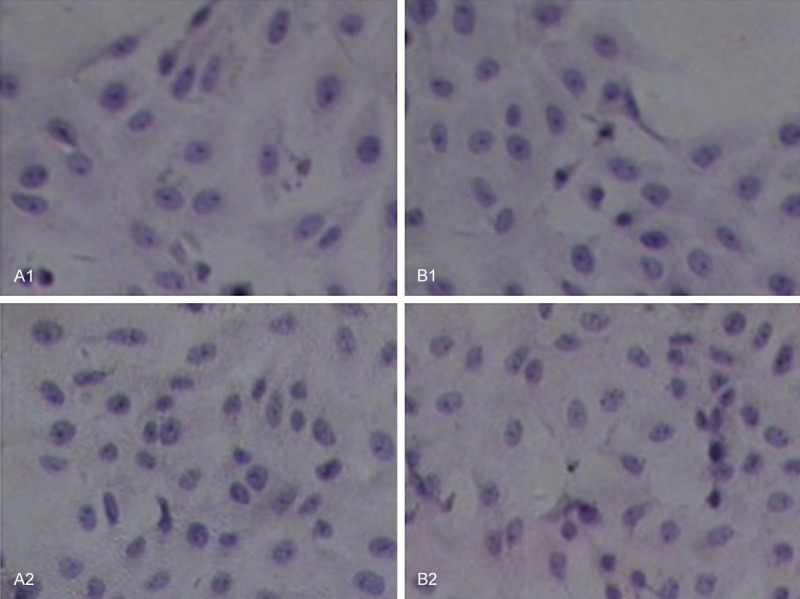
Vero cell morphology under effects of 100% PDLLA film extracts (B1, B2) as compared to RPMI-1640 medium treated control group (A1, A2) after 24 h (A1, B1) and 48 h (A2, B2). In all cultures, the cells showed no abnormal cell morphology, with clear structure, a clearly visible nucleolus, and the clear boundaries between the nucleus and the cytoplasm (magnification × 200).
Effects of PDLLA film extracts on mice bone marrow cell micronucleus
Treatment by cyclophosphamide increased markedly the incidence of micronucleated polychromatic erythrocytes in mice bone marrow (49.7‰; Table 6), which was approximately 20 times greater than in the groups exposed to saline or any dose of PDLLA film extracts groups. Specifically, the incidences of micronucleated polychromatic erythrocytes after the treatment with the low, medium, or high dose of PDLLA were 2.0‰, 2.2‰, and 2.3‰, respectively, which is similar to the incidence in saline treated group (1.9‰; Table 6).
Table 6.
Mouse micronucleus cell
| Group | Mouse (F/M) | PCE Number | Micronucleus cell number | Micronucleus cell percentage (‰) Mean ± SD |
|---|---|---|---|---|
| Saline | 5/5 | 10789 | 20 | 1.9±0.1 |
| PDLLA low dose | 5/5 | 10587 | 21 | 2.0±0.1 |
| PDLLA medium dose | 5/5 | 10237 | 22 | 2.2±0.1 |
| PDLLA high dose | 5/5 | 10564 | 24 | 2.3±0.2 |
| Cyclophosphamide | 5/5 | 10008 | 497 | 49.7±4.0 |
Effects of PDLLA film wrapping on hematology
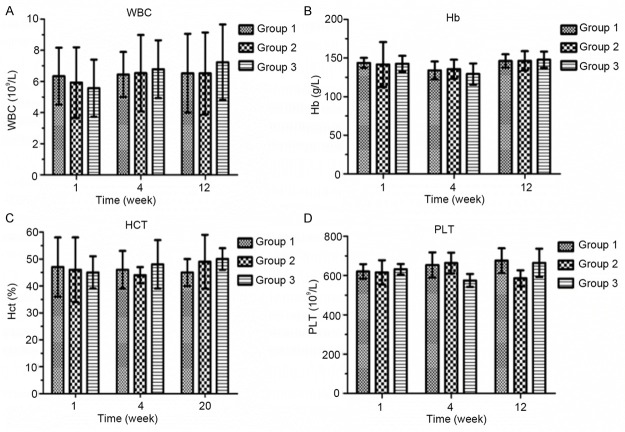
After the sciatic nerve transection and anastomosis, wrapping of the nerve anastomosis with PDLLA film (Group 3) did not alter the amount of WBC, Hb, HCT, or PLT after either 1, 4, or 12 weeks (Figure 4), as compared with direct suturing only (Group 1) or wrapping the nerve anastomosis with artificial nerve conduits wrapping (Group 2).
Figure 4.
Effects of PDLLA wrapping on hematology, including white blood cell count (A; WBC), hemoglobin (B; Hb), hematocrit (C; HCT), and platelets (D; PLT). Group 1 represents direct suturing only after sciatic nerve transection. Group 2 represents wrapping with artificial nerve conduits after sciatic nerve transection and anastomosis. Group 3 represents wrapping with PDLLA film after sciatic nerve transection and anastomosis.
Effects of PDLLA film wrapping on clinical chemistry
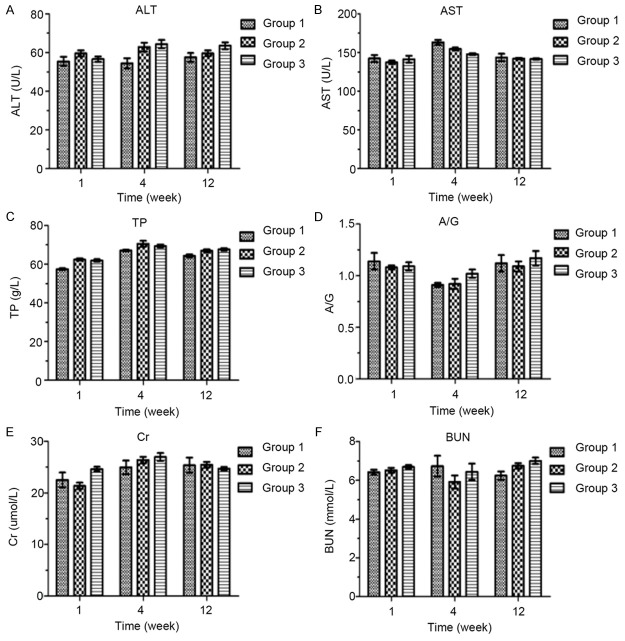
After the sciatic nerve transection and anastomosis, PDLLA film wrapping (Group 3) did not alter the hepatic function (ALT, AST, TP, and A/G) or renal function (Cr, BUN) after either 1, 4, or 12 weeks (Figure 5), as compared with direct suturing only (Group 1) or artificial nerve conduits wrapping (Group 2).
Figure 5.
Effects of wrapping with PDLLA on hepatic and renal functions, which were assessed with an Olympus® AU640 clinical chemistry analyzer: alanine aminotransferase (A; ALT) aspartate aminotransferase (B; AST), total protein (C; TP), albumin/globulin ratio (D; A/G), creatinine (E; Cr), and urea nitrogen (F; BUN). Group 1 represents direct suturing only after sciatic nerve transection. Group 2 represents wrapping with artificial nerve conduits after sciatic nerve transection and anastomosis. Group 3 represents wrapping with PDLLA film after sciatic nerve transection and anastomosis.
Anatomic pathology
Microscopic examination of hematoxylin and eosin stained paraffin sections of liver, spleen, kidney, and pancreas tissues from Wistar rats after 1 week (Figure 6), 4 weeks (Figure 7), or 12 weeks (Figure 8) recovery from the surgery showed that PDLLA film wrapping did not cause any pathologic damage to rat liver, kidneys, pancreas, and spleen.
Figure 6.
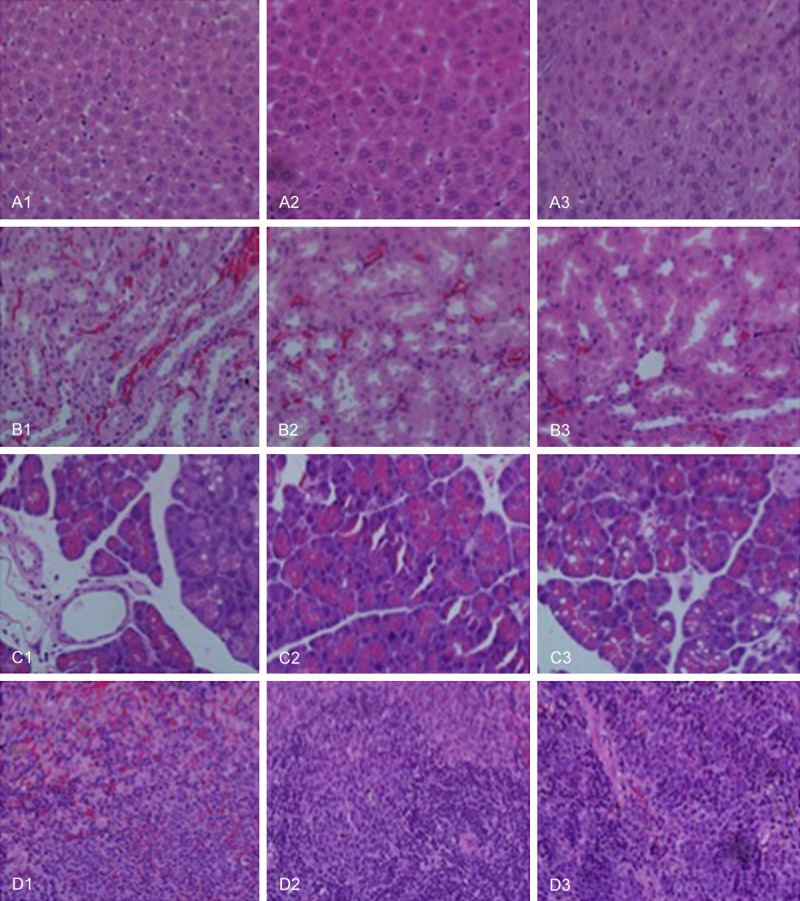
Effects of wrapping with PDLLA on pathologic changes in liver (A), spleen (B), kidney (C), and pancreas (D) after 1 week recovery from sciatic nerve transection and anastomosis. Group 1 represents direct suturing only after sciatic nerve transection. Group 2 represents wrapping with artificial nerve conduits after sciatic nerve transection and anastomosis. Group 3 represents wrapping with PDLLA film after sciatic nerve transection and anastomosis.
Figure 7.
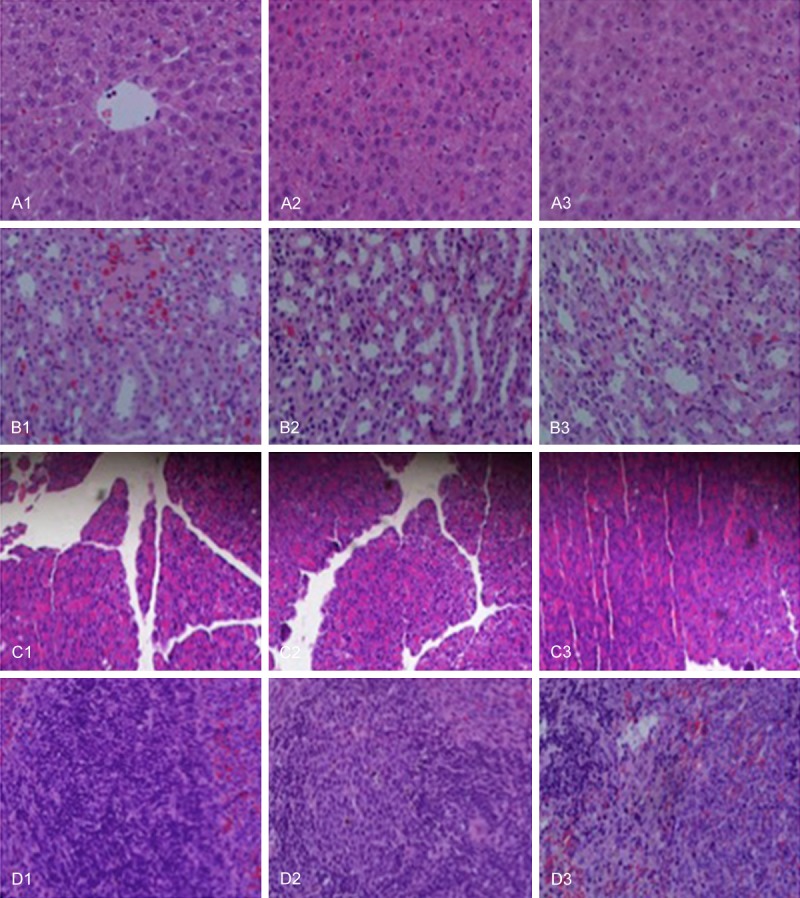
Effects of wrapping with PDLLA on pathologic changes in liver (A), spleen (B), kidney (C), and pancreas (D) after 4 week recovery from sciatic nerve transection and anastomosis. Group 1 represents direct suturing only after sciatic nerve transection. Group 2 represents wrapping with artificial nerve conduits after sciatic nerve transection and anastomosis. Group 3 represents wrapping with PDLLA film after sciatic nerve transection and anastomosis.
Figure 8.
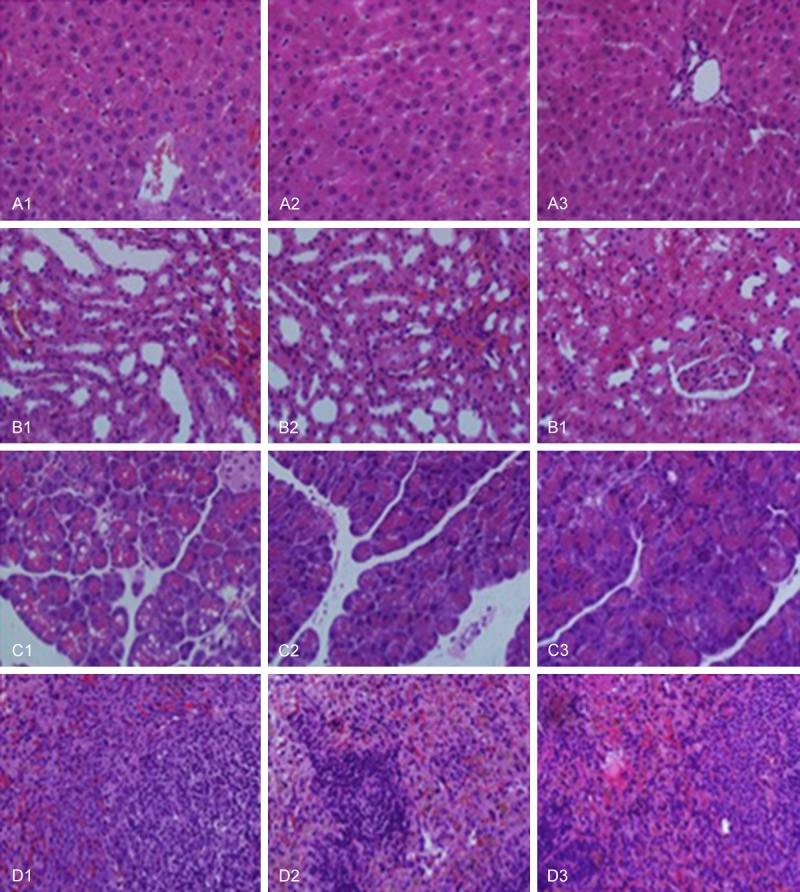
Effects of wrapping with PDLLA on the pathologic changes in liver (A), spleen (B), kidney (C), and pancreas (D) after 12 week recovery from sciatic nerve transection and anastomosis. Group 1 represents direct suturing only after sciatic nerve transection. Group 2 represents wrapping with artificial nerve conduits after sciatic nerve transection and anastomosis. Group 3 represents wrapping with PDLLA film after sciatic nerve transection and anastomosis.
Discussion
In the present study, we evaluated the toxicity of PDLLA films using in vitro cell experiments and in vivo studies in rabbits, rats, and mice. We demonstrated that exposure to PDLLA film extracts did not generate apparent erythema or edema on rabbit skin in a similar pattern as exposure to saline, suggesting that PDLLA films cause no skin irritation. Additionally, using an MTT assay, we demonstrated that 12.5, 25, 50, or 100% of PDLLA film extracts had no effect on the proliferation of Vero cells, suggesting that PDLLA film has no cytotoxicity. Furthermore, to determine the potential genotoxicity of PDLLA film, we conducted a micronucleus test and showed that PDLLA film extracts did not alter the incidence of micronucleated polychromatic erythrocytes suggesting that PDLLA film has no genetic toxicity in mice. Taken together, our results suggested that the biodegradable PDLLA films have minimal biologic toxicity. Future studies may be necessary to further evaluate the long term toxic effects of this biomaterial.
The PDLLA films are designed to prevent nerve adhesion. Therefore, we evaluated the biocompatibility of the PDLLA films in vivo after sciatic nerve transaction in rats. According to literature, we implanted PDLLA films up to 12 weeks [19], to fully evaluate the impact of the chronic exposure of PDLLA biomaterials in the rat model. Our results demonstrated that implantation of PDLLA films between the muscle and sciatic nerve tissue in rats after 1, 4 , or 12 weeks did not increase the white blood cell count, nor change the hemoglobin or platelet count, suggesting that the PDLLA film had no effect on the immune system and the hematopoietic system in rats. Furthermore, PDLLA film implantation did not alter various clinical parameters including ALT, AST, TP, A/G, Cr, and BUN, suggesting PDLLA film has no effect on the hepatic function or renal function. To further confirm the above results, subsequent histopathologic examination showed that PDLLA film implantation did not cause any pathologic damage to rat liver, kidneys, pancreas, or spleen.
In the present study, we evaluated the toxicity of PDLLA films based on the degradation time of PDLLA materials, since the degradation of polymeric biomaterials may affect the tissue in several ways [4,8]. First, the implanted polymeric biomaterials will gradually release various chemical products of degradation, including common additives, impurities, monomers, and oligomers. These chemical components can result in different types of toxic reactions in the tissues by slowly migrating from the interior to the surface and the surrounding tissue, causing inflammation or pathologic changes in the tissues of interest, as well as having systemic reactions. Second, degradation of polymeric biomaterials can produce toxicity effects in human tissues. If the degradation is slow and the product is less toxic, the process of degradation may not cause clinically relevant toxicity in tissues, in contrast, if the degradation is fast and the degradation products are relatively toxic severe acute or chronic inflammation may result.
The physical form of the PDLLA films such as porosity can greatly influence its biocompatibility with tissue in vivo. Usually, biologic nerve grafts made from acellular muscle or collagen are revascularized within the first 4-5 days after implantation by longitudinal ingrowth of vessels from the distal and proximal nerve stump and sprouting of collateral capillaries [20,21]. This process requires diffusion of nutrients, growth factors and other biologically active agents into the area of nerve regeneration. Therefore, permeable anti-adhesive barriers can allow the influx of externally generated wound healing factors and the outward diffusion of waste products. Impermeable barriers may also positively affect nerve regeneration by insulating the area of regeneration, preventing the ingrowth of scar tissue formation and by keeping internally generated growth factors in the local wound area; however, these impermeable barriers may also prevent diffusion of growth factors and nutrients into the injured area. In addition, semi-permeable barriers may also facilitate the formation of a supportive fibrin by allowing inward diffusion of local or systemic healing factors [22]. Importantly, semi-permeable barriers have better effects on nerve regeneration [23-26].
The thickness of the PDLLA films may also contribute to its tissue compatibility. Nerve conduits or barriers with thick walls are usually more rigid leading to impaired handling and difficulty in suturing under the microscope. Furthermore, the rigidity may cause poor in vivo tissue compatibility and provoke local inflammation reactions. It has been reported that regenerated axons were significantly longer in nerve conduits with an average wall thickness of 0.81 mm compared to those in tubes with a thicker wall of 1.1 , 1.28 and 1.44 mm [27]. Less neuroma formatting occurs using thin-walled nerve conduits due to the greater elasticity of thin walls [28], however, very thin walls can cause the nerve conduits to collapse in vivo. For example, amorphous poly (D,L-lactide-ε-caprolactone) tubes with an average wall thickness of 170 μm collapsed 26 weeks postoperatively [29]. Additionally, a substantially portion of the nerve conduits made of electrospun PCL/PLGA fibers with a wall thickness of 150 μm collapsed by four months after surgery [30]. Therefore, it will be necessary in the future to evaluate the effects of various wall thicknesses of the PDLLA films on the biocompatibility.
In summary, we evaluated the in vitro and in vivo biocompatibility and toxicity of PDLLA films. Such polymeric films implanted after the sciatic nerve transection and anastomosis caused no inflammation or tissue damage in various organs in rats. Given the good biocompatibility and little toxicity of PDLLA films in vivo, future studies will be applicable to investigate the effects of PDLLA films on repairing injured nerves.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (No. 3A412T253428).
Disclosure of conflict of interest
None.
References
- 1.Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int. 2014;2014:698256. doi: 10.1155/2014/698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfister BJ, Gordon T, Loverde JR, Kochar AS, Mackinnon SE, Cullen DK. Biomedical engineering strategies for peripheral nerve repair: surgical applications, state of the art, and future challenges. Crit Rev Biomed Eng. 2011;39:81–124. doi: 10.1615/critrevbiomedeng.v39.i2.20. [DOI] [PubMed] [Google Scholar]
- 3.Konofaos P, Ver Halen JP. Nerve repair by means of tubulization: past, present, future. J Reconstr Microsurg. 2013;29:149–164. doi: 10.1055/s-0032-1333316. [DOI] [PubMed] [Google Scholar]
- 4.Omidi Y, Davaran S. Impacts of Biodegradable Polymers: Towards Biomedical Applications. Handbook of Applied Biopolymer Technology: Synthesis, Degradation and Applications. 2011:388–418. [Google Scholar]
- 5.Hench LL. Bioactive materials: the potential for tissue regeneration. J Biomed Mater Res. 1998;41:511–518. doi: 10.1002/(sici)1097-4636(19980915)41:4<511::aid-jbm1>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 6.Sodergard A, Stolt M. Properties of lactic acid based polymers and their correlation with composition. Progress in Polymer Science. 2002;27:1123–1163. [Google Scholar]
- 7.Garlotta D. A literature review of poly (lactic acid) Journal of Polymers and the Environment. 2001;9:63–84. [Google Scholar]
- 8.Ulery BD, Nair LS, Laurencin CT. Biomedical Applications of Biodegradable Polymers. J Polym Sci B Polym Phys. 2011;49:832–864. doi: 10.1002/polb.22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000;21:2335–2346. doi: 10.1016/s0142-9612(00)00101-0. [DOI] [PubMed] [Google Scholar]
- 10.Sung YK, Song DK. Synthesis and characterization of new biodegradable polymers for biomodeling and biomedical applications. Macromolecular Symposia. 2005;224:239–252. [Google Scholar]
- 11.Zhao J, Liao W, Wang Y, Pan J, Liu F. Preparation and degradation characteristic study of bone repair composite of DL-polylactic acid/hydroxyapatite/decalcifying bone matrix. Chin J Traumatol. 2002;5:369–373. [PubMed] [Google Scholar]
- 12.Xiang Y, Wang YL, Luo YF, Zhang BB, Xin J, Zheng DF. Molecular biocompatibility evaluation of poly(D,L-lactic acid)-modified biomaterials based on long serial analysis of gene expression. Colloids Surf B Biointerfaces. 2011;85:248–261. doi: 10.1016/j.colsurfb.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 13.Ohya Y. Synthesis of nobel poly (lactic acid)-based biodegradable polymers and their application as biomaterials. Kobunshi Ronbunshu. 2002;59:484–498. [Google Scholar]
- 14.Taylor MS, Daniels AU, Andriano KP, Heller J. Six bioabsorbable polymers: in vitro acute toxicity of accumulated degradation products. J Appl Biomater. 1994;5:151–157. doi: 10.1002/jab.770050208. [DOI] [PubMed] [Google Scholar]
- 15.Klompmaker J, Jansen HWB, Veth RPH, Degroot JH, Nijenhuis AJ, Pennings AJ. Porous Polymer Implant for Repair of Meniscal Lesions - a Preliminary-Study in Dogs. Biomaterials. 1991;12:810–816. doi: 10.1016/0142-9612(91)90066-j. [DOI] [PubMed] [Google Scholar]
- 16.Bos RRM, Rozema FR, Boering G, Nijenhuis AJ, Pennings AJ, Verwey AB, Nieuwenhuis P, Jansen HWB, Debruijn WC. Degradation of and Tissue Reaction to Biodegradable Poly (L-Lactide) for Use as Osteosynthesis. Biomaterial-Tissue Interfaces. 1992;10:405–411. doi: 10.1016/0142-9612(91)90128-w. [DOI] [PubMed] [Google Scholar]
- 17.Mosmann T. Rapid Colorimetric Assay for Cellular Growth and Survival - Application to Proliferation and Cyto-Toxicity Assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 18.Krishna G, Petrere J, Anderson J, Theiss J. Use of Cyclophosphamide as a Positive Control in Dominant Lethal and Micronucleus Assays. Mutat Res. 1995;335:331–337. doi: 10.1016/0165-1161(95)00038-0. [DOI] [PubMed] [Google Scholar]
- 19.Ohsumi H, Hirata H, Nagakura T, Tsujii M, Sugimoto T, Miyamoto K, Horiuchi T, Nagao M, Nakashima T, Uchida A. Enhancement of perineurial repair and inhibition of nerve adhesion by viscous injectable pure alginate sol. Plast Reconstr Surg. 2005;116:823–830. doi: 10.1097/01.prs.0000176893.44656.8e. [DOI] [PubMed] [Google Scholar]
- 20.Keilhoff G, Stang F, Wolf G, Fansa H. Biocompatibility of type I/III collagen matrix for peripheral nerve reconstruction. Biomaterials. 2003;24:2779–2787. doi: 10.1016/s0142-9612(03)00084-x. [DOI] [PubMed] [Google Scholar]
- 21.Fansa H, Schneider W, Keilhoff G. Revascularization of tissue-engineered nerve grafts and invasion of macrophages. Tissue Eng. 2001;7:519–524. doi: 10.1089/107632701753213147. [DOI] [PubMed] [Google Scholar]
- 22.Vleggeert-Lankamp CL, de Ruiter GC, Wolfs JF, Pego AP, van den Berg RJ, Feirabend HK, Malessy MJ, Lakke EA. Pores in synthetic nerve conduits are beneficial to regeneration. J Biomed Mater Res A. 2007;80:965–982. doi: 10.1002/jbm.a.30941. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez FJ, Gomez N, Perego G, Navarro X. Highly permeable polylactide-caprolactone nerve guides enhance peripheral nerve regeneration through long gaps. Biomaterials. 1999;20:1489–1500. doi: 10.1016/s0142-9612(99)00055-1. [DOI] [PubMed] [Google Scholar]
- 24.Pego AP, Poot AA, Grijpma DW, Feijen J. Copolymers of trimethylene carbonate and epsilon-caprolactone for porous nerve guides: synthesis and properties. J Biomater Sci Polym Ed. 2001;12:35–53. doi: 10.1163/156856201744434. [DOI] [PubMed] [Google Scholar]
- 25.Uzman BG, Villegas GM. Mouse sciatic nerve regeneration through semipermeable tubes: a quantitative model. J Neurosci Res. 1983;9:325–338. doi: 10.1002/jnr.490090309. [DOI] [PubMed] [Google Scholar]
- 26.Jenq CB, Coggeshall RE. Permeable tubes increase the length of the gap that regenerating axons can span. Brain Res. 1987;408:239–242. doi: 10.1016/0006-8993(87)90379-9. [DOI] [PubMed] [Google Scholar]
- 27.Nicoli Aldini N, Fini M, Rocca M, Giavaresi G, Giardino R. Guided regeneration with resorbable conduits in experimental peripheral nerve injuries. Int Orthop. 2000;24:121–125. doi: 10.1007/s002640000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meek MF, Robinson PH, Stokroos I, Blaauw EH, Kors G, den Dunnen WF. Electronmicroscopical evaluation of short-term nerve regeneration through a thin-walled biodegradable poly (DLLA-epsilon-CL) nerve guide filled with modified denatured muscle tissue. Biomaterials. 2001;22:1177–1185. doi: 10.1016/s0142-9612(00)00340-9. [DOI] [PubMed] [Google Scholar]
- 29.Darren Simon AH, Shanks R. Poly (caprolactone) thin film preparation, morphology, and surface texture. Journal of Applied Polymer Science. 2007;103:8. [Google Scholar]
- 30.Meek MF, Den Dunnen WF, Schakenraad JM, Robinson PH. Long-term evaluation of functional nerve recovery after reconstruction with a thin-walled biodegradable poly (DL-lactide-epsilon-caprolactone) nerve guide, using walking track analysis and electrostimulation tests. Microsurgery. 1999;19:247–253. doi: 10.1002/(sici)1098-2752(1999)19:5<247::aid-micr7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]