Abstract
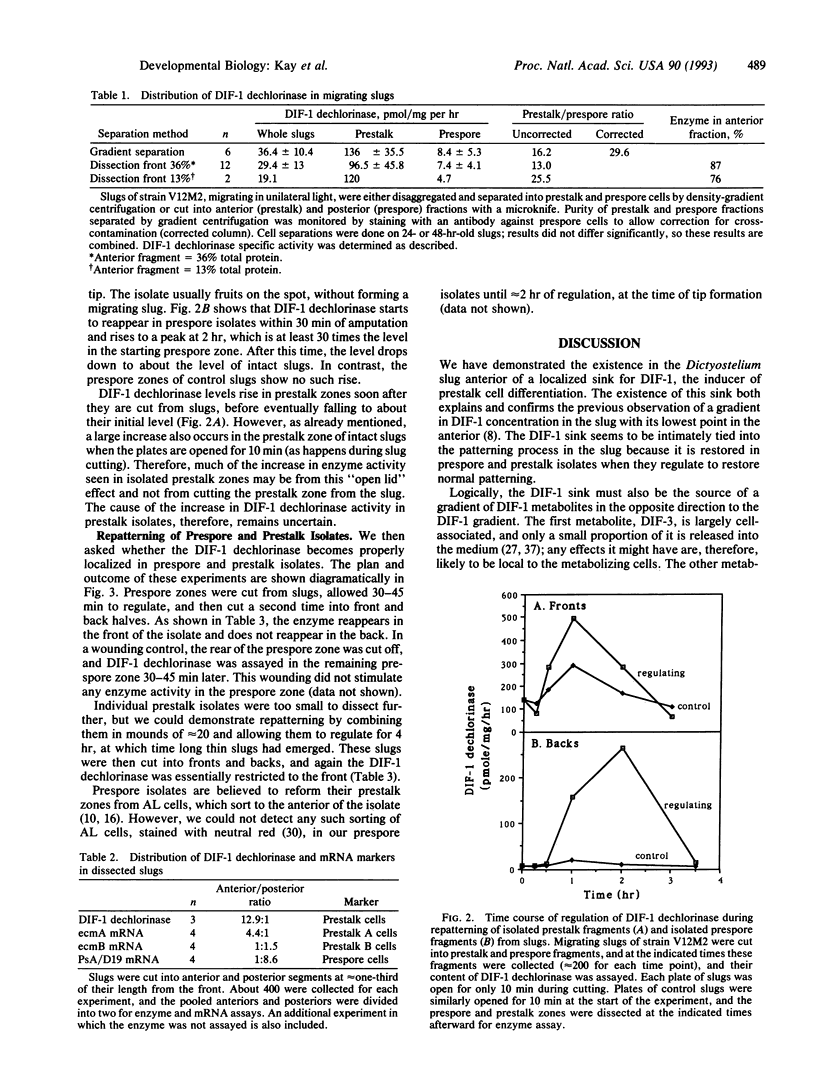
Differentiation-inducing factor 1 [DIF-1; 1-(3,5-dichloro-2,6-dihydroxy-4-methoxyphenyl)-hexan-1-one] induces stalk cell differentiation during Dictyostelium development. It is present as a gradient in the multicellular slug, its lowest concentration being in the anterior. Here we demonstrate the existence of a localized sink for DIF-1, also in the anterior of the slug, which could be responsible for generating the DIF-1 gradient. DIF-1 is metabolized extensively by developing cells, initially by a mono-dechlorination. We used an enzyme assay for DIF-1 dechlorinase to examine its distribution in the slug. DIF-1 dechlorinase activity is 30-fold higher in prestalk cells (largely anterior) compared with prespore cells (posterior) when these are separated from each other on Percoll density gradients. Dissection experiments showed that DIF-1 dechlorinase is 25-fold enriched in the anterior 13% of the slug compared with the rest. These experiments also showed that DIF-1 dechlorinase is more anterior-enriched than the standard prestalk markers, the ecmA and ecmB mRNAs. When cut from a slug, both prestalk and prespore fragments regulate to restore the missing cell type. Prespore fragments rapidly regain (by 30 min) a DIF-1 sink in their anteriors, and prestalk fragments restore a posterior zone with low DIF-1 dechlorinase by 4 hr after cutting. The reappearance of the DIF-1 sink in the anterior of prespore fragments is accomplished without detectable cell sorting and may, therefore, be in response to positional signals. Finally, a localized sink may provide a general way of producing a gradient of a signal substance in a developing embryo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barklis E., Lodish H. F. Regulation of dictyostelium discoideum mRNAs specific for prespore or prestalk cells. Cell. 1983 Apr;32(4):1139–1148. doi: 10.1016/0092-8674(83)90297-0. [DOI] [PubMed] [Google Scholar]
- Berks M., Kay R. R. Combinatorial control of cell differentiation by cAMP and DIF-1 during development of Dictyostelium discoideum. Development. 1990 Nov;110(3):977–984. doi: 10.1242/dev.110.3.977. [DOI] [PubMed] [Google Scholar]
- Brookman J. J., Jermyn K. A., Kay R. R. Nature and distribution of the morphogen DIF in the Dictyostelium slug. Development. 1987 May;100(1):119–124. doi: 10.1242/dev.100.1.119. [DOI] [PubMed] [Google Scholar]
- Devine K. M., Loomis W. F. Molecular characterization of anterior-like cells in Dictyostelium discoideum. Dev Biol. 1985 Feb;107(2):364–372. doi: 10.1016/0012-1606(85)90318-5. [DOI] [PubMed] [Google Scholar]
- Driever W., Nüsslein-Volhard C. A gradient of bicoid protein in Drosophila embryos. Cell. 1988 Jul 1;54(1):83–93. doi: 10.1016/0092-8674(88)90182-1. [DOI] [PubMed] [Google Scholar]
- Durston A. J. Tip formation is regulated by an inhibitory gradient in the dicytostelium discoideum slug. Nature. 1976 Sep 9;263(5573):126–129. doi: 10.1038/263126a0. [DOI] [PubMed] [Google Scholar]
- Early A. E., Williams J. G. A Dictyostelium prespore-specific gene is transcriptionally repressed by DIF in vitro. Development. 1988 Jul;103(3):519–524. doi: 10.1242/dev.103.3.519. [DOI] [PubMed] [Google Scholar]
- Gierer A., Meinhardt H. A theory of biological pattern formation. Kybernetik. 1972 Dec;12(1):30–39. doi: 10.1007/BF00289234. [DOI] [PubMed] [Google Scholar]
- Gregg J. H., Karp G. C. Patterns of cell differentiation revealed by L-[3H]fucose incorporation in Dictyostelium. Exp Cell Res. 1978 Mar 1;112(1):31–46. doi: 10.1016/0014-4827(78)90522-0. [DOI] [PubMed] [Google Scholar]
- Haberstroh L., Firtel R. A. A spatial gradient of expression of a cAMP-regulated prespore cell-type-specific gene in Dictyostelium. Genes Dev. 1990 Apr;4(4):596–612. doi: 10.1101/gad.4.4.596. [DOI] [PubMed] [Google Scholar]
- Hicklin J., Hornbruch A., Wolpert L., Clarke M. Positional information and pattern regulation in hydra: the formation of boundary regions following axial grafts. J Embryol Exp Morphol. 1973 Dec;30(3):701–725. [PubMed] [Google Scholar]
- Insall R., Nayler O., Kay R. R. DIF-1 induces its own breakdown in Dictyostelium. EMBO J. 1992 Aug;11(8):2849–2854. doi: 10.1002/j.1460-2075.1992.tb05352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jermyn K. A., Berks M., Kay R. R., Williams J. G. Two distinct classes of prestalk-enriched mRNA sequences in Dictyostelium discoideum. Development. 1987 Aug;100(4):745–755. doi: 10.1242/dev.100.4.745. [DOI] [PubMed] [Google Scholar]
- Jermyn K. A., Williams J. G. An analysis of culmination in Dictyostelium using prestalk and stalk-specific cell autonomous markers. Development. 1991 Mar;111(3):779–787. doi: 10.1242/dev.111.3.779. [DOI] [PubMed] [Google Scholar]
- Kay R. R., Berks M., Traynor D. Morphogen hunting in Dictyostelium. Development. 1989;107 (Suppl):81–90. doi: 10.1242/dev.107.Supplement.81. [DOI] [PubMed] [Google Scholar]
- Kay R. R. Cell differentiation in monolayers and the investigation of slime mold morphogens. Methods Cell Biol. 1987;28:433–448. doi: 10.1016/s0091-679x(08)61661-1. [DOI] [PubMed] [Google Scholar]
- Kay R. R., Jermyn K. A. A possible morphogen controlling differentiation in Dictyostelium. Nature. 1983 May 19;303(5914):242–244. doi: 10.1038/303242a0. [DOI] [PubMed] [Google Scholar]
- Kay R. R., Taylor G. W., Jermyn K. A., Traynor D. Chlorine-containing compounds produced during Dictyostelium development. Detection by labelling with 36Cl. Biochem J. 1992 Jan 1;281(Pt 1):155–161. doi: 10.1042/bj2810155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopachik W. J., Dhokia B., Kay R. R. Selective induction of stalk-cell-specific proteins in Dictyostelium. Differentiation. 1985;28(3):209–216. doi: 10.1111/j.1432-0436.1985.tb00827.x. [DOI] [PubMed] [Google Scholar]
- Kopachik W., Oohata A., Dhokia B., Brookman J. J., Kay R. R. Dictyostelium mutants lacking DIF, a putative morphogen. Cell. 1983 Jun;33(2):397–403. doi: 10.1016/0092-8674(83)90421-x. [DOI] [PubMed] [Google Scholar]
- Kopachik W. Size regulation in Dictyostelium. J Embryol Exp Morphol. 1982 Apr;68:23–35. [PubMed] [Google Scholar]
- Lokeshwar B. L., Nanjundiah V. Tip regeneration and positional information in the slug of Dictyostelium discoideum. J Embryol Exp Morphol. 1983 Feb;73:151–162. [PubMed] [Google Scholar]
- Morris H. R., Taylor G. W., Masento M. S., Jermyn K. A., Kay R. R. Chemical structure of the morphogen differentiation inducing factor from Dictyostelium discoideum. 1987 Aug 27-Sep 2Nature. 328(6133):811–814. doi: 10.1038/328811a0. [DOI] [PubMed] [Google Scholar]
- Nayler O., Insall R., Kay R. R. Differentiation-inducing-factor dechlorinase, a novel cytosolic dechlorinating enzyme from Dictyostelium discoideum. Eur J Biochem. 1992 Sep 1;208(2):531–536. doi: 10.1111/j.1432-1033.1992.tb17217.x. [DOI] [PubMed] [Google Scholar]
- Ratner D., Borth W. Comparison of differentiating Dictyostelium discoideum cell types separated by an improved method of density gradient centrifugation. Exp Cell Res. 1983 Jan;143(1):1–13. doi: 10.1016/0014-4827(83)90103-9. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Slack J. M. Dorsalization and neural induction: properties of the organizer in Xenopus laevis. J Embryol Exp Morphol. 1983 Dec;78:299–317. [PubMed] [Google Scholar]
- Sternfeld J., David C. N. Fate and regulation of anterior-like cells in Dictyostelium slugs. Dev Biol. 1982 Sep;93(1):111–118. doi: 10.1016/0012-1606(82)90244-5. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI I. IMMUNOCHEMICAL AND IMMUNOHISTOCHEMICAL STUDIES ON THE DEVELOPMENT OF THE CELLULAR SLIME MOLD DICTYOSTELIUM MUCOROIDES. Dev Biol. 1963 Aug;8:1–26. doi: 10.1016/0012-1606(63)90023-x. [DOI] [PubMed] [Google Scholar]
- Takeuchi I., Tasaka M., Oyama M., Yamamoto A., Amagai A. Pattern formation in the development of Dictyostelium discoideum. Prog Clin Biol Res. 1982;85(Pt B):283–294. [PubMed] [Google Scholar]
- Town C. D., Gross J. D., Kay R. R. Cell differentiation without morphogenesis in Dictyostelium discoideum. Nature. 1976 Aug 19;262(5570):717–719. doi: 10.1038/262717a0. [DOI] [PubMed] [Google Scholar]
- Traynor D., Kay R. R. The DIF-1 signaling system in Dictyostelium. Metabolism of the signal. J Biol Chem. 1991 Mar 15;266(8):5291–5297. [PubMed] [Google Scholar]
- Williams J. G., Ceccarelli A., McRobbie S., Mahbubani H., Kay R. R., Early A., Berks M., Jermyn K. A. Direct induction of Dictyostelium prestalk gene expression by DIF provides evidence that DIF is a morphogen. Cell. 1987 Apr 24;49(2):185–192. doi: 10.1016/0092-8674(87)90559-9. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Duffy K. T., Lane D. P., McRobbie S. J., Harwood A. J., Traynor D., Kay R. R., Jermyn K. A. Origins of the prestalk-prespore pattern in Dictyostelium development. Cell. 1989 Dec 22;59(6):1157–1163. doi: 10.1016/0092-8674(89)90771-x. [DOI] [PubMed] [Google Scholar]
- Wolpert L. Positional information and the spatial pattern of cellular differentiation. J Theor Biol. 1969 Oct;25(1):1–47. doi: 10.1016/s0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]