Abstract
The survival rate for bladder cancer is much better when the disease is detected early, so improvements in methodology for early detection would be beneficial. When urine contains neoplastic urothelial cells, it carries biomarkers of the disease. This study aims to develop a test for the detection of urothelial carcinoma in the urine. The sediments from urines of ten patients with carcinoma and ten randomly selected normal controls were tested for cancer biomarkers using high-resolution mass spectroscopy. 212 unique individual proteins were identified. Most of them occurred only once or twice in the entire cohort of cases. When sorting the detected proteins by their subcellular compartments, we were able to develop a test that differentiates between the two sets. When the combination of nuclear and red blood cell proteins was used as the discriminating function, the level of statistical significance was p=0.003, the sensitivity was 90%, the specificity 67% and the area under the Receiver-Operating Characteristic curve (ROC) was 94%. When the lack of any detectible proteins, which includes nuclear proteins, was included as a criterion indicating benign urine, the specificity increased to 80%. This use of cellular compartment localization of the detected proteins in the discriminating function is less restrictive than requiring the presence of specific proteins, and we were able to develop a screening test with this less stringent criterion. This approach can be applied to other tumors, such as breast, lung and colon cancers, where the need for a simple screening test is even greater.
Keywords: Bladder cancer, mass spectroscopy, urine, urothelial carcinoma
Introduction
Urothelial carcinoma is a common disease in the older population, the fourth most commonly diagnosed malignancy in men in the United States, and constitutes approximately 90% of all bladder cancers [1]. As with all malignancies, early detection is the key to effective treatment. When detected early, the 5-year survival rate is approximately 95%; however, 30-50% of tumors are deeply invasive or have lymph node metastases at the time of diagnosis [2], limiting survival. Timely intervention dramatically increases the patient survival rate, and so a search for methods of early detection continues to be a major focus of bladder cancer research.
Urine cytology is the definitive method of screening for urothelial carcinoma. The carcinoma is diagnosed by identifying features of the neoplastic cells stained with the Papanicolaou method. Among other features, the cells have larger nuclei than benign cells, and exhibit a higher nuclear: cytoplasmic ratio (Figure 1). That is, neoplastic cells often have proportionately much less cytoplasm than the transitional cells that normally line the bladder. In addition, though not a diagnostic finding of patients with urothelial carcinoma, red blood cells are frequently present in the urine.
Figure 1.
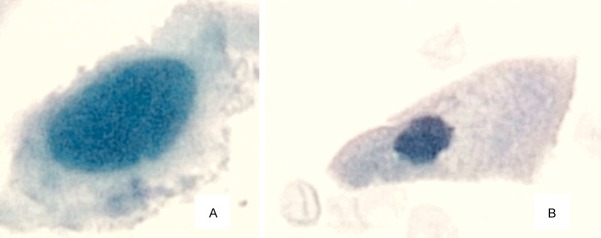
Comparison of a bladder cancer cell and normal bladder lining cell at the same magnification (60x). Panel “A”: A urothelial carcinoma cell exhibits a large nucleus and high nuclear: cytoplasmic ratio compared with Panel “B”: A normal urothelial cell that lines the bladder.
Patients with superficial tumors are generally placed under continued surveillance by routine cystoscopic examinations of the bladder in order to detect new tumor developments as early as possible. Once bladder tumors are identified and removed, surveillance cystoscopy is employed every 3 months for 1 year, then every 6 months for the second year, and yearly thereafter [3]. Since cystoscopy is an invasive procedure, the development of noninvasive urine assays using reliable diagnostic markers would be of benefit to both patients and healthcare providers.
While several tests are designed for early detection of urothelial carcinoma by identification of specific markers, none have particularly high sensitivity and specificity [2]. Many of these tests suffer from high false-positive and/or false negative rates.
Mass spectroscopy has shown promise in detecting biomarkers for carcinoma in several organ systems [4-17]. These tests can be very sensitive since they detect the proteins that are characteristic of the neoplastic cells. Furthermore, the technique is ideal for detecting multiple markers from a single specimen. Commonly, mass spectroscopy is used to detect biomarkers in serum. However, the various proteins in serum are present in vastly different quantities, spanning 10 orders of magnitude, presenting technical difficulties in evaluation of its proteome [18-20].
Body fluids, such as urine, that are in direct contact with the neoplastic cells of interest, are more likely to have biomarkers in concentrations that are easily detectable [21]. Characteristic proteins of neoplastic cells are also likely to be present in relatively high concentrations and should be readily detectable, especially if the cells are lysed prior to testing. A mass spectroscopic proteomic analysis of lysed urines may be a promising screening test for urothelial carcinoma.
In this study, the sediments of centrifuged urines of patients with urothelial carcinoma were lysed and examined by mass spectroscopy to identify evidence of carcinoma by a laboratory method with the potential for automation.
Materials and methods
Urine samples
Urine samples that are submitted for cytological analysis are normally saved for two weeks in the transport medium PreservCyt before being discarded. PreservCyt functions by denaturing proteins, and no covalent bonds are formed or broken in the process of preservation. Two week old urine samples were obtained from 10 randomly chosen healthy controls (all males) and 10 patients with urothelial carcinoma (all males). Seven out of the ten cancer patients had high grade carcinoma. The grades of the other three tumors are unknown. Two of the urines from patients with urothelial carcinoma exhibited gross hematuria.
Sample preparation and mass spectroscopy analysis
10 ml of the urine samples were centrifuged at 2000 rpm for 10 minutes, the supernatant was poured off, and 0.5 ml of distilled water was added to the sediment. The samples were then stored at 4°C before processing.
To process, the samples were mixed in an equal volume of the RIPA buffer (Thermo Scientific, cat # 89900) and sonicated for 30 seconds to lyse the cells in the sediment. The protein content was measured. For the samples that had enough detectable protein to be analyzed, the protein concentration was brought to 2 mg/ml with distilled water. The samples were digested with 1:25 (w/w) trypsin (Promega, Madison, WI) overnight at 37 degrees C. The samples were then purified by binding to a ziptip pipette tip (EMD Millipore Corporation, Billerica, MA) and analyzed on the Q-STAR Elite mass spectrometer.
For each typsin digested sample, LC-MS/MS analysis was performed using Eksigent Tempo nanoLC followed with QSTAR Elite mass spectrometer (AB SCIEX). A 60-minute gradient of 2-40% acetonitrile was applied on a C18 reverse phase column (75 µm × 15 cm; buffer A, 2% acetonitrile, 0.1% formic acid; buffer B, 98% acetonitrile, 0.1% formic acid). Using the Smart IDA (Information Dependent Acquisition) features within Analyst® QS 2.0 Software, multiple intelligent MS/MS acquisitions were performed following a second long survey scan.
Protein marker identification and cellular location
Identification of the peptide sequences from the acquired MS/MS data was accomplished using MASCOT search engine version 2.1 (Matrix Science, Boston, MA) with the last updated version of Uniprot/Swiss-Prot protein database [22] merged with random sequences. Methionine oxidation and Asn and Gln deamidation were selected as the only variable modifications. The tolerance for precursor ion and MS/MS fragment mass values was set at 50 ppm and 0.5 Da, respectively. Sequence coverage was automatically calculated within the software. Most of the specimens yielded 20 to 40 identified proteins. Since all of the analyzed specimens yielded at least 20 proteins, the top 20 proteins with strongest matching criteria from each sample were considered as abundant proteins and included in the analysis. To determine the subcellular localization of each protein, the Uniprot/Swiss-Prot database was accessed a second time for each identified protein. Proteins that are localized to both the cytoplasm and the nucleus were designated as pan-cellular proteins.
Statistical analysis
The data from the urines that were positive for cancer were compared with the data from normal urines. A Student’s T-test was manually performed for each pair of cellular compartment data. In addition, sensitivities, specificities and Receiver-Operating Characteristic curves were manually calculated for the proposed tests to differentiate urines containing cancer cells from control urines.
Results
Four (4) of the random control samples had too little protein to perform the analysis, indicating a paucity of cells in the urine. Upon performing the mass spectrometric analysis of the 6 remaining randomly selected urines from patients without carcinoma and 10 urines from patients with urothelial carcinoma, the top 20 proteins with strongest matching criteria from each sample were identified. Therefore, 320 proteins were identified in total. No specific proteins or combinations of specific proteins were identified which could differentiate between cancer and control urines. In fact, most of the identified proteins only appeared once or twice in the entire combined list of proteins. Altogether, 212 unique proteins were identified. To determine the subcellular localization of each protein, the Swiss-Prot database was accessed for each of the 212 proteins [22] a second time. The subcellular compartment for each protein was listed on this website. When comparing cancer containing urines with control urines by the cellular compartments in which the detected proteins reside, differences between the two populations were observed. The number of proteins found in each cellular compartment is listed in Table 1.
Table 1.
The cellular distribution of the 20 highest ranked proteins for each of the tested samples
| Compartment | Controls | Cancers | Statistical significance |
|---|---|---|---|
| Cytoplasm | 27 | 49 | n.s. |
| Membrane | 25 | 28 | n.s. |
| Mitochondrion | 0 | 1 | n.s. |
| Nucleus | 10 | 37 | p=0.02 |
| Pan-cellular | 10 | 12 | n.s. |
| rbc | 2 | 11 | n.s.* |
| Secreted | 28 | 35 | n.s. |
| Unknown | 18 | 27 | n.s. |
| Nuclear + rbc | 12 | 48 | p=0.003 |
| Total proteins | 120 | 200 | |
| Total samples | n=6 | n=10 |
5 rbc proteins from 2 cases with gross blood. 6 rbc proteins from one case without gross blood.
The number of proteins that normally reside in the cell nucleus was significantly higher in urines from cancer patients than from controls (p=0.02). In addition, while the number of red blood cell proteins was not significantly different between the two populations, there is a trend toward greater red blood cell proteins in urines with cancer patients (11 proteins) compared with control urines (2 proteins). When the combination of nuclear plus red blood cell proteins is used as the discriminating function, the level of statistical significance increases (p=0.003). Figure 2 shows the combination of the number of nuclear proteins plus red blood cell proteins for each sample.
Figure 2.
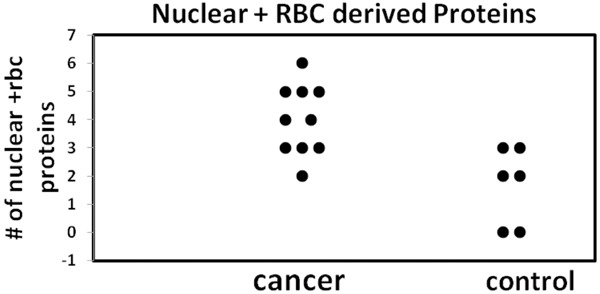
Nuclear and RBC derived proteins from cancer and control cases The sum of nuclear and red blood cell (rbc) derived proteins for each of the samples is shown. The sum (nuclear + rbc proteins) is significantly greater in the sediments of urines from cancer patients than in control patients (p<0.003).
If the test for discriminating between urines from cancer patients and urines from control patients is set at a cut-off of greater than 2 nuclear or red blood cell proteins from the list of the 20 highest ranked proteins in the given sample, 9 out of 10 urines from cancer patients are classified as cancer and 2 out of 6 normal urines are similarly classified. This discriminating function leads to a sensitivity of 90%, a specificity of 67% and an area under the Receiver-Operating Characteristic curve (ROC) of 87% for the test overall (Figure 3).
Figure 3.
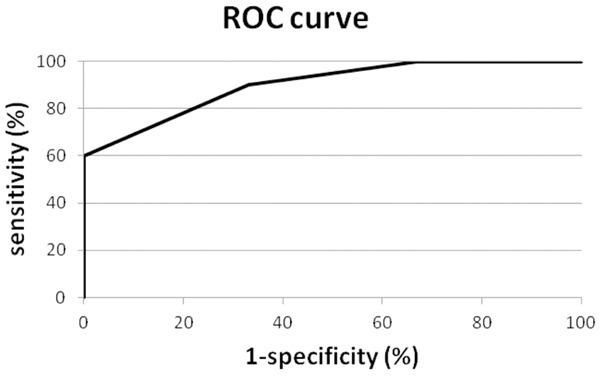
Receiver operating characteristic (ROC) curve for nuclear and RBC derived proteins. The receiver operating characteristic (ROC) curve for the discriminating function defined as the sum of nuclear plus red blood cell derived proteins (Area Under Curve=87%).
As noted above, four of the control samples had too little protein to perform the analysis. This low protein content in the sediment is evidence of an absence of neoplastic cells and corresponding nuclear proteins in the sample. Since neoplastic cells from the tumor are usually shed into the urine of patients with carcinoma, the sediments from urines of cancer patients would, in general, be expected to have a higher cell count and therefore higher protein concentration than controls. So when lack of sufficient protein to perform the analysis is considered evidence of a lack of tumor cells, the number of control urines classified as negative increases to 8. An even higher specificity (80%) and area under ROC curve (94%) is obtained (Figure 4).
Figure 4.
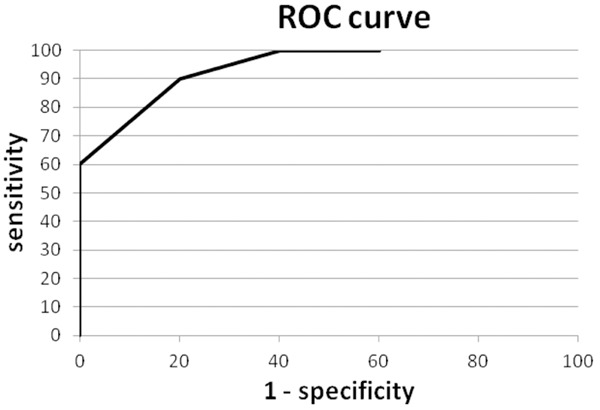
Receiver operating characteristic (ROC) curve for nuclear and RBC derived proteins and sufficient protein. The receiver operating characteristic (ROC) curve for the discriminating function defined as the sum of nuclear plus red blood cell derived proteins with the additional requirement that samples with insufficient protein to perform the analysis are classified as negative (Area Under Curve=94%).
Discussion
Using high-resolution mass spectroscopy, we analyzed 10 urines that were proven to be positive for urothelial carcinoma by cytological examination, and 10 urines that were found to be negative for urothelial carcinoma by cytological examination. Because centrifuging the specimen results in a concentrated quantity of malignant cells in the sediment, we specifically analyzed the sediments from these cases.
We found that the lysed sediments from cancer cases contained significantly more proteins that are localized in the nucleus, compared with control cases (p=0.02). When we include in the analysis proteins that are derived from red blood cells, the difference becomes greater (p=0.003).
Neoplastic urothelial cells contain larger nuclei with little cytoplasm, compared with benign urothelial cells (Figure 1). More nuclear proteins would be expected in the urines of patients with carcinoma when compared with controls. An additional feature of urothelial carcinoma is the presence of blood in the urine. Detection of red blood cell derived proteins is a sensitive test for microscopic hematuria which is often, though not exclusively, found in cancer cases. Therefore, the fact that there are more red blood cell derived proteins in the cancer specimens is not surprising. While two of the malignant specimens had gross hematuria, one of the cases in which red blood cell proteins were detected did not have gross hematuria.
When the criteria for a positive result is detection of three or more of the 20 highest ranked proteins localized either to the nucleus or red blood cell, nine of ten malignant urines are positive by this test, and four of six control urines are negative by this test. This results in 90% sensitivity and 67% specificity for this limited sample. If cases are included where there is a lack of sufficient protein to perform the analysis, there is a corresponding lack of nuclear proteins. The evidence indicates a benign urine, and the specificity of the test increases to 80%.
This proteomic approach successfully distinguishes between urines of cancer patients and urines of controls. Of course only a limited number of cases were analyzed. Because the current study used high resolution mass spectroscopy, it may not be easily streamlined as a high throughput test. Also, each of the 212 proteins was accessed individually on the UniProt/Swiss-Prot site to determine its cellular compartment localization. However, by making use of a simpler instrument such as a time of flight mass spectrometer, and developing a computer application that provides easier access to cellular compartment information for the detected proteins the process may be streamlined for high throughput. Alternatively, programs that predict subcellular localization based on features of the protein such as amino acid sequences could be employed if they have sufficient accuracy [23-25].
The presence of blood in the urine is considered a warning sign of malignancy and, while three of the ten specimens used mass spectroscopic evidence of that feature in the classification, most did not. The majority of the cancer cases were classified as malignant based solely on the presence of nuclear proteins.
When we take into consideration that four of the control cases had insufficient protein in the urine sediment to be analyzed by the method and low total protein content is evidence of low nuclear protein content, a criterion indicative of a negative result, 90% sensitivity and 80% specificity are obtained. Since malignant cells from patients with urothelial carcinoma are often shed into the urine, it is reasonable that, after cell lysis, more protein is obtained from positive than negative urines.
The samples analyzed in this study had been maintained at 4 degrees in the preservative, “Preservcyt” for two weeks prior to the study. As a result, a more limited list of proteins was detected than would likely be obtained from fresh samples. This feature of the study demonstrates the robustness of the technique. Despite storage of the specimens in a preservative for two weeks prior to the study, an effective discriminating function was still obtained. A technique that detects the proteins that are characteristic of neoplastic cells rather than relying on detection of the intact cells by cytology is inherently more sensitive because cellular degradation does not affect the results. Hence fewer cells are required to provide a proteomic profile than are required for detection by cytology.
Most screening tests analyze tumor markers in non-concentrated urine. However, many proteins derived from the kidney and plasma are present in the urine, making it difficult to identify proteins derived from the tumor in the unconcentrated urine [23]. Examination of urine sediment where there is a concentrated quantity of malignant cells, essentially eliminates renal and plasma proteins from the analysis. When analyzing tumor markers that are normally intracellular rather than being secreted, analysis of the sediment is preferable to analysis of non-concentrated urine.
Uniquely, the test does not rely on the presence of specific proteins as biomarkers to classify the urine as a malignant sample, instead relying upon the expected location within the cell of the detected proteins, the high nuclear: cytoplasmic ratio of malignant cells, and the association of bladder cancer with hematuria. By relating these features to the compartment localization of the detected proteins, effective criteria for a discriminating function were developed. Studies that examine the cellular compartment of proteins in the proteome of cancer cells have been performed [26-34]; but to our knowledge, none have used their findings to construct a discriminating function as a screening test for cancer.
Use of the cellular compartment localization of the detected proteins in the discriminating function is less restrictive than the stringent requirement of the presence of specific proteins. In this study, the number of unique proteins is 212. So the compartment localization approach reduces the number of variables in the analysis from 212 to 8 (the number of cellular compartments), greatly simplifying the calculations. More generally, the total number of annotated entries in the reference human proteome set is 20,202 [35]. Allowing reduction of the number of possible variables from a very large number down to 8 enables an analysis that otherwise might not be possible.
We show here that this less restrictive attribute can be used in developing a screening test for bladder cancer, but the approach can be applied in developing screening tests for other cancers as well, even when the detection of individual proteins is not helpful. Intact tumor cells from many other cancers are not easily accessible, while proteins leaked from such cells may very well be present in body fluids that are in contact with the cells. In such a case, a proteomic approach could be very beneficial. In general, as in the current study, neoplastic cells from most organ systems have a high nuclear: cytoplasmic ratio, and so remnants of neoplastic cells from most cancers would be expected to supply a relatively large number of nuclear proteins in the appropriate body fluid.
For example, a technique called ductal lavage has been used to identify neoplastic cells in patients with breast cancer, but with only a sensitivity of 23%-77% [36,37]. A technique that does not require observation of intact cells, but relies instead on analysis of the protein content of the sample, may prove more useful. In the bowel, the proteome of fecal samples was studied in mice with a predilection for colon cancer, and the subcellular localization of murine proteins was even determined [38]. However, because sample preparation was different in that protocol, there were not a sufficient number of nuclear proteins to perform a comparison between the number nuclear proteins in cancer prone mice versus control mice. Furthermore, in considering lung cancer, it has been shown that exhaled breath contains many proteins in healthy individuals [39]; it is likely that the proteomic profile would be different for patients with lung cancer.
Another way to categorize cellular proteins is by function. For instance proteins involved in proliferative functions would be expected to be more prevalent in malignant cells, while proteins involved in metabolic or internal cellular structure/anchoring functions would be expected to make up a greater percentage of proteins from benign specimens. Furthermore, in organ-systems that normally secrete an acellular or paucicellular fluid, such as the breast, the presence of secreted proteins may indicate a benign specimen while an increase in intracellular proteins may indicate malignancy. Yet another way to categorize proteins is by the cell of origin of the protein. For instance, if nuclear proteins are increased, it may be important to distinguish between tumor and inflammation in some circumstances, and the cell of origin of the identified proteins would be informative.
In summary, we developed a screening test for bladder cancer without the stringent requirement of identifying specific cancer biomarkers. Using mass spectroscopy, 212 proteins were identified. We were then able to create a test for bladder cancer by examining the cellular compartments in which the detected proteins are usually found. When the combination of nuclear and red blood cell proteins was used as the discriminating function, and the lack of any detectible proteins, including nuclear proteins, was also included as an indication of a normal urine, the sensitivity for the test was 90%, the specificity was 80% and the area under the ROC curve was 94%. Such a characterization of the proteome is reflective of well known cytological differences between malignant and benign cells. The discriminating function includes only knowledge of total protein adequacy and cellular compartment information, and should be amenable to the development of a high throughput test. Furthermore, this approach may be applicable to other tumors, such as breast, lung and colon cancer, where a simple screening test is needed and access to intact tumor cells at an early cancer stage has been problematic.
Acknowledgements
We would like to thank Ms. Tracey Hall for her assistance in the preparation of specimens prior to processing.
Disclosure of conflict of interest
None.
References
- 1.PDQ Cancer Information Summaries [Internet] Bethesda (MD): National Cancer Institute (US); 2002-. Bladder and Other Urothelial Cancers Screening (PDQ®): Health Professional Version. [Updated 2012 Jan 23] Available from: http://www.ncbi.nlm.nih.gov/books/NBK65774/
- 2.Konety B, Carroll P. Chapter 20. Urothelial Carcinoma: Cancers of the Bladder, Ureter, & Renal Pelvis. In: Tanagho EA, McAninch JW, editors. Smith’s General Urology. 17th ed. New York: McGraw-Hill; 2008. http://www.accesssurgery.com/content.aspx?aID=3128012. Accessed February 2, 2013. [Google Scholar]
- 3.Cooper CS, Joudi FN, Williams RD. Chapter 38. Urology. In: Doherty GM, editor. CURRENT Diagnosis & Treatment: Surgery. 13e. Retrieved February 9, 2013 from http://www.accesssurgery.com/content.aspx?aID=5312459. [Google Scholar]
- 4.Hassanein M, Rahman JS, Chaurand P, Massion PP. Advances in Proteomic Strategies toward the Early Detection of Lung Cancer. Proc Am Thorac Soc. 2011;8:183–188. doi: 10.1513/pats.201012-069MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petricoin EF, Ardekani AM, Hitt BA, Levine PJ, Fusaro VA, Steinberg SM, Mills GB, Simone C, Fishman DA, Kohn EC, Liotta LA. Use of proteomic patterns to identify ovarian cancer. Lancet. 2002;359:572–577. doi: 10.1016/S0140-6736(02)07746-2. [DOI] [PubMed] [Google Scholar]
- 6.Adam BL, Qu Y, Davis JW, Ward MD, Clements MA, Cazares LH, Semmes OJ, Schellhammer PF, Yasui Y, Feng Z, Wright GL Jr. Serum protein fingerprinting coupled with a pattern-matching algorithm distinguishes prostate cancer from benign prostate hyperplasia and healthy men. Cancer Res. 2002;62:3609–3614. [PubMed] [Google Scholar]
- 7.Petricoin EF 3rd, Ornstein DK, Paweletz CP, Ardekani A, Hackett PS, Hitt BA, Velassco A, Trucco C, Wiegand L, Wood K, Simone CB, Levine PJ, Linehan WM, Emmert-Buck MR, Steinberg SM, Kohn EC, Liotta LA. Serum Proteomic Patterns for Detection of Prostate Cancer. J Natl Cancer Inst. 2002;94:1576–1578. doi: 10.1093/jnci/94.20.1576. [DOI] [PubMed] [Google Scholar]
- 8.Vlahou A, Schellhammer PF, Mendrinos S, Patel K, Kondylis FI, Gong L, Nasim S, Wright Jr GL Jr. Development of a Novel Proteomic Approach for the Detection of Transitional Cell Carcinoma of the Bladder in Urine. Am J Pathol. 2001;158:1491–1502. doi: 10.1016/S0002-9440(10)64100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu JK, Chen YD, Zheng S. An integrated approach to the detection of colorectal cancer utilizing proteomics and bioinformatics. World J Gastroenterol. 2004;10:3127–3131. doi: 10.3748/wjg.v10.i21.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sidransky D, Irizarry R, Califano JA, Li X, Ren H, Benoit N, Mao L. Serum Protein MALDI Profiling to Distinguish Upper Aerodigestive Tract Cancer Patients From Control Subjects. J Natl Cancer Inst. 2003;95:1711–1717. doi: 10.1093/jnci/djg099. [DOI] [PubMed] [Google Scholar]
- 11.Coombes KR, Fritsche HA Jr, Clarke C, Chen JN, Baggerly KA, Morris JS, Xiao LC, Hung MC, Kuerer HM. Quality Control and Peak Finding for Proteomics Data Collected from Nipple Aspirate Fluid by Surface-Enhanced Laser Desorption and Ionization. Clin Chem. 2003;49:1615–1623. doi: 10.1373/49.10.1615. [DOI] [PubMed] [Google Scholar]
- 12.Chapman JR. Mass Spectrometry Ionization Methods and Instrumentation. In: Chapman JR, editor. Methods in Molecular Biology Vol 61: Protein and Peptide Analysis by Mass Spectrometry. Totowa, NJ: Humana Press Inc.; 1996. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch J, Hanson C, Burlingame AL, Matthay MA. Proteomics: current techniques and potential applications to lung disease. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1–23. doi: 10.1152/ajplung.00301.2003. [DOI] [PubMed] [Google Scholar]
- 14.Sorace JM, Zhan M. A data review and re-assessment of ovarian cancer serum proteomic profiling. BMC Bioinformatics. 2003;4:24. doi: 10.1186/1471-2105-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conrads TP, Fusaro VA, Ross S, Johann D, Rajapakse V, Hitt BA, Steinberg SM, Kohn EC, Fishman DA, Whitely G, Barrett JC, Liotta LA, Petricoin EF 3rd, Veenstra TD. High-resolution serum proteomic features for ovarian cancer detection. Endocr Relat Cancer. 2004;11:163–178. doi: 10.1677/erc.0.0110163. [DOI] [PubMed] [Google Scholar]
- 16.Wu JY, Cheng CC, Wang JY, Wu DC, Hsieh JS, Lee SC, Wang WM. Discovery of tumor markers for gastric cancer proteomics. PLoS One. 2014;9:e84158. doi: 10.1371/journal.pone.0084158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu C. The Application of SELDI-TOF-MS in Clinical Diagnosis of Cancers. J Biomed Biotechnol. 2011;2011:245821. doi: 10.1155/2011/245821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1:845–867. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- 19.Anderson L, Hunter CL. Quantitative mass spectrometric multiple reaction monitoring assays for major plasma proteins. Mol Cell Proteomics. 2006;5:573–588. doi: 10.1074/mcp.M500331-MCP200. [DOI] [PubMed] [Google Scholar]
- 20.Hanash S, Taguchi A. The Grand Challenge to Decipher the Cancer Proteome. Nat Rev Cancer. 2010;10:652–660. doi: 10.1038/nrc2918. [DOI] [PubMed] [Google Scholar]
- 21.De Bock M, de Seny D, Meuwis MA, Chapelle JP, Louis E, Malaise M, Merville MP, Fillet M. Challenges for Biomarker Discovery in Body Fluids Using SELDI-TOF-MS. J Biomed Biotechnol. 2010;2010:906082. doi: 10.1155/2010/906082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. http://www.uniprot.org/uniprot/
- 23.Mooney C, Wang YH, Pollastri G. SCLpred: protein subcellular localization prediction by N-to-1 neural networks. Bioinformatics. 2011;27:2812–2819. doi: 10.1093/bioinformatics/btr494. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg T, Hecht M, Hamp T, Karl T, Yachdav G, Ahmed N, Altermann U, Angerer P, Ansorge S, Balasz K, Bernhofer M, Betz A, Cizmadija L, Do KT, Gerke J, Greil R, Joerdens V, Hastreiter M, Hembach K, Herzog M, Kalemanov M, Kluge M, Meier A, Nasir H, Neumaier U, Prade V, Reeb J, Sorokoumov A, Troshani I, Vorberg S, Waldraff S, Zierer J, Nielsen H, Rost B. LocTree3 prediction of localization. Nucleic Acids Res. 2014;42:W350–355. doi: 10.1093/nar/gku396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chou KC, Wu ZC, Xiao X. iLoc-Euk: a multi-label classifier for predicting the subcellular localization of singleplex and multiplex eukaryotic proteins. PLoS One. 2011;6:e18258. doi: 10.1371/journal.pone.0018258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen CL, Lin TS, Tsai Ch, Wu CC, Chung T, Chien KY, We M, Chang YS, Yu JS, Chen YT. Identification of potential bladder cancer markers in urine by abundant-protein depletion coupled with quantitative proteomics. J Proteomics. 2013;85:28–43. doi: 10.1016/j.jprot.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 27.Rieskem C, Borgia B, Schliemann C, Gunthert M, Wunderli-Allenspach H, Giavazzi R, Neri D. Comparative Analysis of the Membrane Proteome of Closely Related Metastatic and Nonmetastatic Tumor Cells. Cancer Res. 2009;69:5406–5414. doi: 10.1158/0008-5472.CAN-08-0999. [DOI] [PubMed] [Google Scholar]
- 28.Karagiannis GS, Pavlou MP, Saraon P, Musrap N, Xie A, Batruch I, Prassas I, Dimitromanolakis A, Petraki C, Diamandis EP. In-depth proteomic delineation of the colorectal cancer exoproteome: Mechanistic insight and identification of potential biomarkers. J Proteomics. 2014;103:121–136. doi: 10.1016/j.jprot.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Patel S, Wetie AG, Darie CC, Clarkson BD. Cancer secretomes and their place in supplementing other hallmarks of cancer. Adv Exp Med Biol. 2014;806:409–442. doi: 10.1007/978-3-319-06068-2_20. [DOI] [PubMed] [Google Scholar]
- 30.Caccia D, Zanetti Domingues L, Miccichè F, De Bortoli M, Carniti C, Mondellini P, Bongarzone I. Secretome compartment is a valuable source of biomarkers for cancer-relevant pathways. J Proteome Res. 2011;10:4196–4207. doi: 10.1021/pr200344n. [DOI] [PubMed] [Google Scholar]
- 31.Raimondo F, Morosi L, Chinello C, Perego R, Bianchi C, Albo G, Ferrero S, Rocco F, Magni F, Pitto M. Protein profiling of microdomains purified from renal cell carcinoma and normal kidney tissue samples. Mol Biosyst. 2012;8:1007–1016. doi: 10.1039/c2mb05372a. [DOI] [PubMed] [Google Scholar]
- 32.Cuomo A, Moretti S, Minucci S, Bonaldi T. SILAC-based proteomic analysis to dissect the “histone modification signature” of human breast cancer cells. Amino Acids. 2011;41:387–399. doi: 10.1007/s00726-010-0668-2. [DOI] [PubMed] [Google Scholar]
- 33.Soldi M, Cuomo A, Bremang M, Bonaldi T. Mass Spectrometry-Based Proteomics for the Analysis of Chromatin Structure and Dynamics. Int J Mol Sci. 2013;14:5402–5431. doi: 10.3390/ijms14035402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leth-Larsen R, Lund R, Hansen HV, Laenkholm AV, Tarin D, Jensen ON, Ditzel HJ. Metastasis-related plasma membrane proteins of human breast cancer cells identified by comparative quantitative mass spectroscopy. Mol Cell Proteomics. 2009;8:1436–1449. doi: 10.1074/mcp.M800061-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. http://web.expasy.org/docs/relnotes/relstat.html.
- 36.Dooley WC, Ljung BM, Veronesi U, Cazzaniga M, Elledge RM, O’Shaugnessy JA, Kuerer Hm, Hung DT, Khan SA, Phillips RF, Ganz PA, Euhus DM, Esserman LJ, Haffty BG, King BL, Kelley MC, Anderson MM, Schmidt PJ, Clark RR, Kass FC, Anderson BO, Troyan SL, Aria RD, Quiring JN, Love SM, Page DL, King EB. Ductal Lavage for Detection of Cellular Atypia in Women at High Risk for Breast Cancer. J Natl Cancer Inst. 2001;93:1624–1632. doi: 10.1093/jnci/93.21.1624. [DOI] [PubMed] [Google Scholar]
- 37.Khan SA, Wiley EL, Rodriguez N, Baird C, Ramakrishnan R, Nayar R, Bryk M, Bethke KB, Staradub VL, Wolfman J, Radmaker A, Ljung BM, Morrow M. Ductal Lavage Findings in Women With Known Breast Cancer Undergoing Mastectomy. J Natl Cancer Inst. 2004;96:1510–1516. doi: 10.1093/jnci/djh283. [DOI] [PubMed] [Google Scholar]
- 38.Ang CS, Rothacker J, Patsiouras H, Burgess AW, Nice EC. Murine fecal proteomics: A model system for the detection of potential biomarkers for colorectal cancer. J chromatogr A. 2010;1217:3330–3340. doi: 10.1016/j.chroma.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Muccilli V, Saletti R, Cunsolo V, Ho J, Gili E, Conte E, Sichili S, Vancheri C, Foti S. Protein profile of exhaled breath condensate determined by high resolution mass spectrometry. J Pharm Biomed Anal. 2015;105:134–149. doi: 10.1016/j.jpba.2014.11.050. [DOI] [PubMed] [Google Scholar]


