Abstract
Noonan syndrome and related disorders (Noonan syndrome with multiple lentigines, Costello syndrome, cardiofaciocutaneous syndrome, Noonan syndrome with loose anagen hair, and other related traits) are autosomal dominant traits. Mutations causing these disorders alter proteins relevant for signaling through RAS. Thus, these traits are now collectively called the RASopathies. While the RASopathies have pleiomorphic features, this review will focus on the hypertrophic cardiomyopathy observed in varying percentages of all of these traits. In addition, inherited abnormalities in one pathway gene, RAF1, cause pediatric-onset dilated cardiomyopathy. The pathogeneses for the RASopathy-associated cardiomyopathies are being elucidated, principally using animal models, leading to genotype-specific insights into how signal transduction is perturbed. Based on those findings, small molecule therapies seem possible for RASopathy-associated cardiomyopathies.
Introduction
Hypertrophic cardiomyopathy presenting in infancy and childhood is quite different from the same trait presenting in adolescents and adults. After moving beyond the newborn period where infants of diabetic mothers is the most prevalent cause of hypertrophic cardiomyopathy, there are a wide range of disorders that present in this manner. Among the commonest are Noonan syndrome and several phenotypically related disorders. While sometimes challenging to distinguish from one another clinically and previously categorized as separate disease entities, the elaboration of the molecular causes of these autosomal dominant traits, missense mutations altering genes whose proteins are relevant for RAS-mediated intracellular signaling, has largely reinforced the existing nosology. More importantly, the identification of the causative mutations has enabled the identification of several genotype-phenotype associations, including ones relevant for hypertrophic cardiomyopathy, and allowed for the elaboration of pathogenesis for the cardiac muscle disease, providing insights that suggest these hypertrophic cardiomyopathies may be treatable. Lastly, a gene previously known to cause Noonan syndrome when mutated was recently discovered to have distinct mutations that also cause non-syndromic dilated cardiomyopathy, also with a pathogenesis that suggests therapy. In this review, the disorders of RAS-mediated signaling, the so-called RASopathies, will be discussed with an emphasis on the cardiomyopathies with which they are associated.
RASopathy phenotypes
Noonan syndrome
Noonan syndrome is an autosomal dominant disorder with multisystem involvement including distinctive dysmorphic facial features (most commonly hypertelorism, ptosis, low-set ears, and short webbed neck), short stature, skeletal anomalies (particularly, sternal deformities and cubitus valgus), and intellectual and developmental disabilities [1]. The majority, 80–90%, have cardiovascular involvement that can include a broad range of congenital heart defects, most prevalently valvar pulmonic stenosis, and/or early-onset hypertrophic cardiomyopathy. The prevalence of hypertrophic cardiomyopathy, which is classified as a secondary form of that cardiac muscle disease in the American Heart Association’s system [2], is 20% in Noonan syndrome, of which one-half also have congenital heart defects. The histology of the myocardium in the Noonan syndrome-associated hypertrophic cardiomyopathy is indistinguishable from that observed in sarcomeric hypertrophic cardiomyopathy [3].
The natural history of the hypertrophic cardiomyopathy associated with Noonan syndrome differs in several ways from that delineated for the primary hypertrophic cardiomyopathies caused by mutations altering sarcomeric proteins or other pediatric forms of hypertrophic cardiomyopathy [4, 5]. Noonan syndrome-associated cardiac hypertrophy presents early in life, the median being 5 months, with more than half diagnosed by six months of age. This is far earlier than other pediatric forms of hypertrophic cardiomyopathy, which present at age 8 years on average. At diagnosis, patients with Noonan syndrome and hypertrophic cardiomyopathy are far more likely to have congestive heart failure than other children with hypertrophic cardiomyopathy (24% versus 9%). Those with Noonan syndrome also often present with significant left ventricular outflow obstruction, with an average gradient of 32 mm Hg.
The presence of hypertrophic cardiomyopathy affects outcomes in Noonan syndrome. Comparing children with and without hypertrophic cardiomyopathy, there is a significant difference in survival. For those without hypertrophic cardiomyopathy, there is nearly complete survival 15 years after diagnosis. For those with hypertrophic cardiomyopathy, survival at that time point is 70%. The primary cause of death for Noonan syndrome-associated hypertrophic cardiomyopathy is congestive heart failure [4–6]. Survival among pediatric patients with hypertrophic cardiomyopathy also differs between those with and without Noonan syndrome. Among those with Noonan syndrome, there is substantial early mortality, with most deaths occurring during infancy. In comparison, the survival curve for other forms of pediatric hypertrophic cardiomyopathy shows a steady, slower mortality rate, such that survival only declines to the level observed in Noonan syndrome 13 years after diagnosis. Longer-term follow up also, however, suggests increased late mortality in Noonan syndrome-associated hypertrophic cardiomyopathy compared to sarcomeric hypertrophic cardiomyopathy [4].
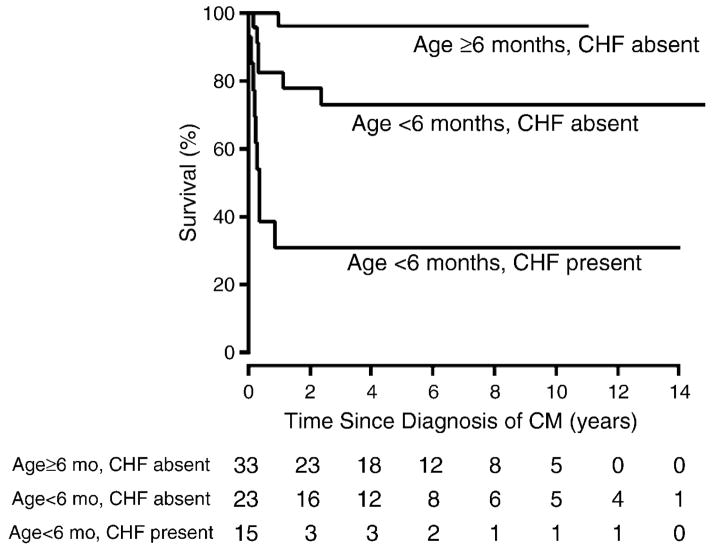
Among individuals with Noonan syndrome and hypertrophic cardiomyopathy, outcomes depend on age of presentation and the presence or absence of congestive heart failure at presentation (Figure 1). Infants with Noonan syndrome presenting before the age of 6 months with congestive heart failure fare poorly, with a 2-year survival of approximately 30%. In comparison, those presenting after 6 months of age and without congestive heart failure enjoy excellent survival of roughly 95% after 2 years. Infants presenting before 6 months of age but without congestive heart failure have an intermediate outlook with 75% survival at 2 years. [5].
Figure 1. Survival by age and congestive heart failure in children with Noonan syndrome and hypertrophic cardiomyopathy.
Estimated survival since diagnosis of hypertrophic cardiomyopathy (CM) in 74 children with Noonan syndrome by age and congestive heart failure (CHF) status at the time of HCM diagnosis. Log-rank P < .001. The size of the risk set is shown below the x-axis. The subgroup of 3 cases with congestive heart failure who were diagnosed at age ≥6 months is not shown (one known to survive 5.5 months post diagnosis, and 2 were not seen after diagnosis). Reprinted with permission from the American Heart Journal [5].
Several additional autosomal dominant disorders with phenotypic overlap with Noonan syndrome have been described subsequently, including Noonan syndrome with multiple lentigines (formerly, LEOPARD syndrome), Costello syndrome, cardiofaciocutaneous syndrome and Noonan syndrome with loose anagen hair (also known as Mazzanti syndrome).
Noonan syndrome with multiple lentigines
Noonan syndrome with multiple lentigines is the RASopathy that most closely resembles Noonan syndrome but is far rarer (precise prevalence is not known). The distinctive feature of this disorder is the presence of lentigines, small hyperpigmented skin lesions that resemble freckles and typically arise in the thousands on the face, trunk and extremities by age 5 years. Sensorineural deafness is commoner in Noonan syndrome with multiple lentigines compared to Noonan syndrome (~20% versus <5%). Hypertrophic cardiomyopathy is present in ~80% of those with Noonan syndrome with multiple lentigines, the highest rate among the RASopathies [7]. Probably due to its relative rarity, outcome data for hypertrophic cardiomyopathy in Noonan syndrome with multiple lentigines are not currently available. Left ventricular outflow tract obstruction from the hypertrophy is present in about half of those with hypertrophic cardiomyopathy. Rare cases of hypertrophic cardiomyopathy that presents in early infancy and progresses rapidly have been described. Nonetheless, the clinical course of hypertrophic cardiomyopathy in most individuals with Noonan syndrome with multiple lentigines is felt to be relatively benign.
One limitation in these outcome data worth noting is that the information published regarding outcomes for hypertrophic cardiomyopathy in Noonan syndrome were based on clinical diagnoses. Since differentiating Noonan syndrome from Noonan syndrome with multiple lentigines is challenging in infants and toddlers who usually do not exhibit lentigines even if they have the latter disorder, it is conceivable that some proportion of the infants with poor outcomes described in the Noonan syndrome literature actually represent patients with Noonan syndrome with multiple lentigines. As will be discussed later, this is more than a categorization issue, but rather one with important implications regarding the choice of therapies.
Costello syndrome
Costello syndrome is another RASopathy with features overlapping with Noonan syndrome. Distinctive non-cardiac features include coarse features (Fig. 1), papillomata, splayed finger with ulnar deviation and increased prevalence of certain cancers, such as rhabdomyosarcoma and neuroblastoma. The prevalence of hypertrophic cardiomyopathy in Costello syndrome is roughly 65%, notably higher than for Noonan syndrome [8]. Among individuals with Costello syndrome and hypertrophic cardiomyopathy, the pattern is asymmetric in more than 60%. The natural history of Costello syndrome-associated hypertrophic cardiomyopathy is variable but includes severe or progressive disease in 40% of cases and nearly 25% of patients undergoing septal myectomy. The histology of this hypertrophic cardiomyopathy is indistinguishable from sarcomeric hypertrophic cardiomyopathy with myocardial fiber disarray [8, 9].
Cardiofaciocutaneous syndrome
Cardiofaciocutaneous syndrome is a RASopathy distinguishable by the severity of the ectodermal findings (skin and hair) as well as gastrointestinal and neurocognitive impairment [10]. The facial dysmorphia is often striking with macrocephaly, prominent forehead and bi-temporal narrowing
Similar to Noonan syndrome, cardiovascular involvement in cardiofaciocutaneous syndrome is prevalent (~75%) and pulmonary valve stenosis is commonest [10]. Hypertrophic cardiomyopathy is diagnosed in 40% of patients with cardiofaciocutaneous syndrome. Severity varies widely from localized subaortic involvement to severe global hypertrophy with obstruction. Precise outcome data about hypertrophic cardiomyopathy in the context of cardiofaciocutaneous syndrome are not available.
Noonan syndrome with loose anagen hair
Noonan syndrome with loose anagen hair, a RASopathy first described in 2003, is characterized by ectodermal features including darkly pigmented skin with eczema or ichthyosis and loose anagen hair [11]. While a limited number of individuals with Noonan syndrome with loose anagen hair have been described in publications, it appears that a high percentage of affected individuals have cardiovascular involvement [12–15]. Hypertrophic cardiomyopathy prevalence is estimated to be 25%, but its natural history has not been delineated well.
RASopathy genetics
In 2001, Tartaglia and colleagues identified mutations in PTPN11 as a cause of Noonan syndrome in approximately one half of cases [16]. PTPN11 encodes the protein tyrosine phosphatase, SHP2, which has both positive and negative roles in controlling intracellular signaling. It positively controls RAS function and signal flow through the mitogen-activated protein kinase, while negatively modulating the phosphinositide-3-kinase/AKT cascade. The causative mutations, which were all missense defects, altered SHP2 in a manner predicted to result in increased and prolonged phosphatase activity, i.e., gain of function. Biochemical characterization of Noonan syndrome-associated SHP2 mutants confirmed this [17]. Genes subsequently found to also cause Noonan syndrome (KRAS, SOS1, RAF1, BRAF, NRAS, and RIT1) also encode proteins that, when mutated, variably promote signal transduction through RAS and downstream cascades [18]. Specific PTPN11 missense mutations, not observed in Noonan syndrome, were found in 90% of individuals with Noonan syndrome with multiple lentigines [19, 20]. Biochemical studies of the Noonan syndrome with multiple lentigines-associated SHP2 mutants showed perturbations notably different from those for Noonan syndrome [21–23]. Specifically, those mutant SHP2 proteins had reduced catalytic function and specifically perturb the phosphinositide-3-kinase/AKT signaling pathway.
Given the striking phenotypic overlap, it is not surprising that genes encoding additional proteins involved in the RAS/mitogen-activated protein kinase signaling pathway were discovered to underlie the other RASopathies. Indeed, HRAS mutations are the sole cause of Costello syndrome [24], KRAS, MEK1, MEK2, and BRAF mutation cause cardiofaciocutaneous syndrome, and a recurrent missense mutation in SHOC2 underlies Noonan syndrome with loose anagen hair. Aside from the Noonan syndrome with multiple lentigines-associated PTPN11 mutations, the other defects for the assorted RASopathies with hypertrophic cardiomyopathy can be generalized as gain-of-function perturbations of signal transduction.
Genotype-phenotype associations
For Noonan syndrome, there are clear differences in the cardiovascular involvement that depend upon the gene mutation. For patients harboring Noonan syndrome-causing PTPN11 or SOS1 mutations, hypertrophic cardiomyopathy prevalence is low [25, 26]. In contrast, individuals with Noonan syndrome due to RAF1 or RIT1 mutations are very likely to develop hypertrophic cardiomyopathy (~85% and 70%, respectively) [27–29]. Because valvar pulmonic stenosis is also prevalent among those harboring RIT1 mutations, the combination of that valve abnormality and hypertrophic cardiomyopathy is frequent among individuals with Noonan syndrome due to RIT1 mutations.
For Costello syndrome, for which all patients have HRAS mutations, a comparison of the two commonest alleles, G12S and G13C, showed no difference in the prevalence of hypertrophic cardiomyopathy (15/33 versus 8/12)[30].
For cardiofaciocutaneous syndrome, two studies contained adequate numbers of individuals with BRAF mutations or MEK defects (either MEK1 or MEK2) to permit a comparison of hypertrophic cardiomyopathy [31, 32]. The prevalence of hypertrophic cardiomyopathy was not significantly different (BRAF 35% and MEK 21%).
For Noonan syndrome with loose anagen hair, nearly all patients have the same SHOC2 mutation so genotype-phenotype comparisons are not relevant.
Pathogenesis of hypertrophic cardiomyopathy in the RASopathies
Noonan syndrome
A mouse bearing the L613V mutation in the Raf1 gene was generated using homologous recombination in embryonic stem cells [33]. This mouse model faithfully recapitulated the major phenotypic features of Noonan syndrome associated with RAF1 mutations including hypertrophic cardiomyopathy, evident as early as two weeks of age. Increased signaling through the RAS/mitogen-activated protein kinase pathway was apparent with increased activation of Mek and Erk in neonatal cardiomyocytes and cardiac fibroblasts with the L613V mutation. Treatment of Raf1L613V/+ mice with a MEK inhibitor from four weeks of age resulted in a reversal of the hypertrophic cardiomyopathy [33].
A mouse bearing the E846K mutation in the Sos1 gene was generated using homologous recombination in embryonic stem cells [34]. This mouse model recapitulated phenotypic features of RASopathies, including aortic valve leaflet thickening that became apparent after age 8.5 months in 40% of the animals. In addition, 20% of the mice exhibited hypertrophic cardiomyopathy with increased signaling through RAS/mitogen-activated protein kinase and also the Rho GTPase Rac. As hypertrophic cardiomyopathy is negatively associated with SOS1 mutations in humans, these latter findings in this mouse model may be less informative.
Noonan syndrome with multiple lentigines
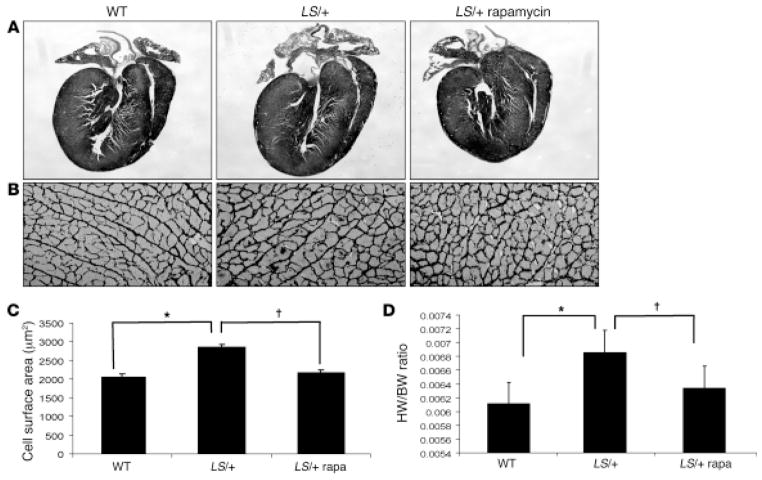
A mouse model of Noonan syndrome with multiple lentigines was generated by introducing the Y279C mutation into the Ptpn11 gene using homologous recombination in embryonic stem cells [35]. The phenotype of the Ptpn11Y279C/+ mice recapitulated several aspects of the Noonan syndrome with multiple lentigines phenotype, particularly hypertrophic cardiomyopathy with postnatal onset. Consistent with prior biochemical analyses of Noonan syndrome with multiple lentigines-associated SHP2 mutant proteins, the Y279C Shp2 in mouse hearts had diminished phosphatase activity. Impaired agonist-induced activation of the mitogen-activated protein kinases Erk1/2 was also observed in cardiomyocytes. In contrast, Akt activation was significantly increased, which was consistent with previous findings obtained in vitro [36], as was its downstream target, the mTor pathway. Treatment of the Ptpn11Y279C/+ mice with rapamycin, an mTor inhibitor, prevented or reversed the hypertrophic cardiomyopathy depending on the timing of the therapy (Figure 2).
Figure 2. Rapamycin normalizes hypertrophic cardiomyopathy in Noonan syndrome with multiple lentigines mice.
(A) Hematoxylin & eosin-stained longitudinal sections of hearts from wild-type (WT) and Noonan syndrome with multiple lentigines (LS/+) mice. Note normalization of hypertrophy in LS/+ hearts after rapamycin treatment (original magnification, ×100). (B) Reticulin stain of paraffin-embedded heart sections from 16-week-old WT and LS/+ mice (original magnification, ×400). (C) Quantification of average area (in μm2) of cardiomyocytes (200–500 cells counted/group) from WT or LS/+ cardiomyocytes isolated from mice that were either vehicle- or rapamycin-treated (2 mg/kg body weight) daily by i.p. injection for 4 weeks, then weekly for 4 weeks. Results are shown as the mean ± standard error of the mean. *P < 0.05, †P < 0.05. (D) Heart weight to body weight ratios of WT and LS/+ mice with vehicle- or rapamycin-treatment, as indicated. *P < 0.05, †P < 0.001. Reprinted with permission from the Journal for Clinical Investigation [35].
Costello syndrome
A mouse model with the HRAS G12V mutation was generated through homologous recombination in embryonic stem cells [37]. The phenotype of the heterozygous mice recapitulates some aspects of Costello syndrome, but the cardiovascular aspects are difficult to interpret. Specifically, these mice exhibited cardiac hypertrophy with fibrosis evident by four months of age, but systemic hypertension was also present. Treatment of the latter with the angiotensin converting enzyme inhibitor captopril partially reversed the cardiac hypertrophy. A conditional Hras allele may be needed to dissect the direct effects on the myocardium from the indirect ones.
Dilated cardiomyopathy
Recently, the hypothesis was explored that mutations in RAS/mitogen-activated protein kinase pathway genes might be causative for dilated cardiomyopathy [38]. This was premised on the known roles of RAS pathway signaling in myocardial biology and the overlap between genes causing hypertrophic and dilated cardiomyopathies in general. Nine RAS/mitogen-activated protein kinase genes were screened and missense mutations were found in only one, RAF1, in five of 218 individuals of South Indian ancestry with dilated cardiomyopathy. Subsequent screening of RAF1 in additional cohorts of variable ancestry revealed small numbers of additional mutations (most missense but one frameshift) in South Indian, North Indian and Japanese groups but none in an Italian cohort. No individual with a RAF1 mutation had any extra-cardiac features suggesting a RASopathy syndrome. Limited families were available for analysis but one affected person’s RAF1 variant was documented to have arisen de novo. A review of available clinical information revealed only one difference among those individuals harboring a RAF1 mutation compared to the others- the average age at presentation was younger (12.6 years versus 20 years). In all, 9% of subjects in the dilated cardiomyopathy cohorts presenting in childhood or adolescence had RAF1 mutations.
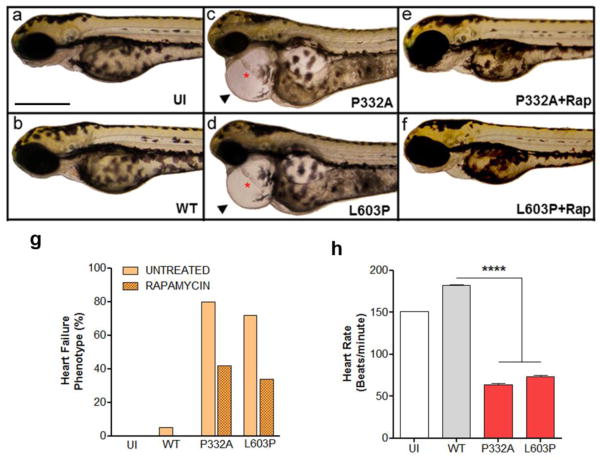
Next, the functional impact of the RAF1 mutations was explored [38]. Through transient expression in human embryonic kidney cells (HEK293), the effects on mitogen-activated protein kinase activation after ligand stimulation was found to be inconsistent among the various mutants and notably different than those associated with RASopathy-associated RAF1 mutants causing hypertrophic cardiomyopathy. Of note, overexpression of the dilated cardiomyopathy-associated RAF1 mutants resulted in consistent activation of AKT and its downstream target tuberin. To examine the effects of the dilated cardiomyopathy-associated RAF1 mutants in vivo, zebrafish models were generated by injecting mRNAs into 1-cell embryos. Overexpression of two RAF1 mutants resulted in a cardiac phenotype with elongation of the atrial and ventricular chambers, marked pericardial edema, and reduced heart rates, while overexpression of wild-type RAF1 did not perturb heart development (Figure 3). As in the HEK293 cells, Erk activation was not altered in the zebrafish models but Akt was hyperactivated. Blocking of Akt hyperactivation using rapamycin partially rescued the cardiac abnormalities.
Figure 3. Dilated cardiomyopathy-associated RAF1 mutants induce heart defects mimicking heart failure phenotype in zebrafish.
Lateral view of zebrafish embryos at 72 hours post fertilization (hpf) that were uninjected (a), injected with WT (b), p.Pro332Ala (c) or p. Leu603Pro (d) RAF1 mRNA. The two representative dilated cardiomyopathy mutants (p.Pro332Ala and p.Leu603Pro) showing string-like cardiac chambers (asterisk) with pericardial edema (arrow). Treatment with rapamycin rescued the heart failure phenotypes in the p.Pro332Ala and p.Leu603Pro RAF1 mRNA-injected embryos (e and f, respectively). Scale bar, 500 μm. g. Percentage of zebrafish embryos at 72 hpf after injection of the indicated RAF1 mRNA exhibiting heart defects with and without rapamycin treatment (n=150 in each group). h. Measurements of mutant heart rate showing severe bradycardia at 72 hpf. Data represent means ± SD of 30 embryos. Reprinted with permission from Nature Genetics [38].
Taken as a whole, this study showed that there is a non-syndromic RASopathy caused by RAF1 mutations that results in dilated cardiomyopathy that presents in childhood or adolescence. The biochemical profile of this RASopathy, like Noonan syndrome with multiple lentigines, is notable for AKT activation, suggesting possible therapeutic efficacy of using rapamycin or related drugs to block signaling through mTOR.
Therapy development
The cardiomyopathies resulting from mutations altering the RAS-mediated mitogen-activated protein kinase and phosphinositide-3-kinase/AKT pathways provide attractive targets for small molecular therapies. Because there are differing mechanisms for the pathogenesis of the cardiomyopathy, it is apparent that no one drug is likely to be useful for all of the RASopathies.
As documented in the mouse model of PTPN11 mutations causing Noonan syndrome with multiple lentigines, this hypertrophic cardiomyopathy appears to result from increased activation of AKT through mTOR. Several mTOR inhibitors, rapamycin (also known as sirolimus) and related compounds (the so-called rapalogues), are already approved by the Federal Drug Administration. These rapalogues are immunosuppressive and widely used to prevent rejection post transplantation. They have also been employed extensively to prevent coronary re-stenosis after stent placement by embedding the drug on the surface of the stent. The most serious side effects from the rapalogues are interstitial pneumonitis, increased cancer risk, and diabetes.
Tuberous sclerosis is a genetic disorder caused by mutations in TSC1 or TSC2 with increased signaling through AKT. There are ongoing Phase III trials examining the efficacy of the rapalogue, everolimus, for subependymal giant cell astrocyomas and refractory epilepsy and a Phase II trial with everolimus in affected children for neurocognition.
Particularly the last trial is establishing that everolimus can be used safely in children, which provides an opportunity to use that drug or other rapalogues for hypertrophic cardiomyopathy in Noonan syndrome with multiple lentigines. Based on that, Maria Kontarides and colleagues (two of the authors of this review, A.R. and B.D.G. as well as Benjamin Neel) have an active project entitled “Use of rapamycin treatment of hypertrophic cardiomyopathy in patients with LEOPARD syndrome” through the National Center for Advancing Translational Sciences’ Therapeutics for Rare and Neglected Diseases program (http://www.ncats.nih.gov/research/rare-diseases/trnd/projects/ls.html). This project is first examining the use of rapalogues for the existing mouse models of Noonan syndrome with multiple lentigines and, if successful, will hopefully advance to early phase clinical trials in affected individuals.
One can imagine that a similar therapeutic approach might be successful for the dilated cardiomyopathy associated with RAF1 mutations. While the initial studies with the zebrafish model were intriguing, additional pre-clinical information, most likely with a mouse model harboring a disease-related Raf1 knock-in mutation, are needed before undertaking clinical studies for this disorder.
Essentially all of the other RASopathy-associated hypertrophic cardiomyopathies appear to result from gain-of-function mutations that result in increased signaling through ERK, even though both AKT and ERK are hyperactivated by HRAS mutations. Based on the pre-clinical work with the L613V Raf1 mouse, inhibition of MEK may be a tractable approach. Of note, it was shown that a MEK inhibitor could prevent the formation of the hypertrophic cardiomyopathy in that mouse model but no information is available about reversal of that phenotype, which is the more relevant issue clinically. Nonetheless, Novartis is sponsoring a Phase II open-label study examining the safety, pharmacokinetics and tolerability for their MEK inhibitor, MEK162, in adults with Noonan syndrome and hypertrophic cardiomyopathy (ClinicalTrials.gov identifier: NCT01556568). The primary endpoints for this study are the changes in left ventricular mass at 3 and 6 months of therapy as compared to baseline. Of note, another MEK inhibitor, GlaxoSmithKline’s trametinib, received approval from the Food and Drug Administration for the treatment of melanoma with the BRAF V600E mutation. Side effects from this drug include skin problems, stomatitis, lymphedema, hemorrhage and hypertension. There are no data available about the use of trametinib for RASopathy-associated issues including hypertrophic cardiomyopathy.
While use of powerful inhibitors of the central RAS/mitogen-activated protein kinase pathway may prove efficacious and safe for patients with RASopathies, there are concerns that the longer term therapy that might be necessary (relative to the length of typical chemotherapy courses for cancers) might not be safe, particularly in infants and growing children. Until drugs such as trametinib and MEK162 are given to pediatric patients for extended periods, this will remain moot. Of note, it is possible that lower dosing might achieve the desired balance between efficacy and toxicity. In addition, children with RASopathy disorders may respond differently to these drugs since their increased RAS pathway signaling is broader than in patients with cancers where the abnormal signaling is restricted to the somatically mutated tumor. Future trials could also include measures to look for added benefits to growth, development, and learning that may result.
An alternative approach to therapy for the RASopathies is to seek drugs that alter RAS pathway signaling in less dramatic fashion. Rather than seeing a potent inhibitor of the canonical central RAS-mediated signaling pathways, one might consider small molecules that alter the signaling more subtly, perhaps including some altering crosstalk or feedback mechanisms. There is precedent for that. In a recent paper, Alcino Silva’s research team demonstrated that a mouse model of Noonan syndrome with the PTPN11 D61G missense mutation exhibits learning and memory deficits that could be rescued by treatment with the HMG-Co reductase inhibitor lovastatin [39]. Lovostatin, which is approved by the Food and Drug Administration, is believed to tamp down signaling through RAS by reducing RAS isoprenylation, which, in turn, reduces localization of those proteins to the cell membrane from where signaling occurs. In such manner, lovastatin is believed to decrease RAS signaling rather than inhibiting it entirely. While the efficacy of lovastatin for treating hypertrophic cardiomyopathy in the RASopathies remains untested, the data regarding its impact on neurocognition provides strong proof-of-principal. Statins, while not without side effects, have ones that are not generally life threatening and have been used to treat children for extended periods of time.
Concluding remarks
The RASopathies comprise several autosomal dominant disorders, Noonan syndrome being the most common, with overlapping cardiac and non-cardiac features. While hypertrophic cardiomyopathy is observed for all of these diagnoses, there are important differences in their relative prevalence. The elucidation of the genetic mutations for Noonan syndrome in particular has made clear that hypertrophic cardiomyopathy risk is dependent upon genotype. Therefore, diagnostic genetic testing may be useful, particularly in an infant suspected of having a RASopathy. Ongoing studies of animal models suggest that treatment of RASopathy-associated hypertrophic cardiomyopathy is possible, but will likely need to be gene/mutation-specific. The use of a rapalogue to treat the hypertrophic cardiomyopathy associated with Noonan syndrome with multiple lentigines is likely to undergo testing with a clinical trial in the not-too-distant future. This approach may also be useful for treating the recently described RAF1 mutation-related dilated cardiomyopathy. A MEK inhibitor is in an early phase clinical trial for adults with Noonan syndrome and hypertrophic cardiomyopathy. Whether direct inhibition of the RAS/mitogen-activated protein kinase pathway or subtler approaches to altering signaling, such as through HMG-CoA reductase inhibitors, will be both safe and efficacious for longer-term treatment in infants and children with RASopathies and hypertrophic cardiomyopathy remains to be determined.
Acknowledgments
This manuscript was prepared with grant support from the National Institutes of Health (R01 HL071207) to B.D.G. and Telethon-Italy (GGP13107) to M.T.
Footnotes
Conflicts of Interest:
The authors (B.D.G. and M.T.) declare the following conflicts of interest: they receive royalty payments for genetic testing for Noonan syndrome from Correlegan, LabCorp, GeneDx, Prevention Genetics, Baylor College of Medicine, and Harvard/Partners; B.D.G. received a sponsored-research award from Shire.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Roberts AE, Allanson JE, Tartaglia M, Gelb BD. Noonan syndrome. Lancet. 2013;381:333–42. doi: 10.1016/S0140-6736(12)61023-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807–16. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 3.Burch M, Mann JM, Sharland M, Shinebourne EA, Patton MA, McKenna WJ. Myocardial disarray in Noonan syndrome. Br Heart J. 1992;68:586–8. doi: 10.1136/hrt.68.12.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hickey EJ, Mehta R, Elmi M, Asoh K, McCrindle BW, Williams WG, et al. Survival implications: hypertrophic cardiomyopathy in Noonan syndrome. Congenit Heart Dis. 2011;6:41–7. doi: 10.1111/j.1747-0803.2010.00465.x. [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson JD, Lowe AM, Salbert BA, Sleeper LA, Colan SD, Cox GF, et al. Outcomes in children with Noonan syndrome and hypertrophic cardiomyopathy: A study from the Pediatric Cardiomyopathy Registry. Am Heart J. 2012;164:442–8. doi: 10.1016/j.ahj.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 6.Shaw AC, Kalidas K, Crosby AH, Jeffery S, Patton MA. The natural history of Noonan syndrome: a long-term follow-up study. Arch Dis Child. 2007;92:128–32. doi: 10.1136/adc.2006.104547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Limongelli G, Pacileo G, Marino B, Digilio MC, Sarkozy A, Elliott P, et al. Prevalence and clinical significance of cardiovascular abnormalities in patients with the LEOPARD syndrome. Am J Cardiol. 2007;100:736–41. doi: 10.1016/j.amjcard.2007.03.093. [DOI] [PubMed] [Google Scholar]
- 8.Lin AE, Alexander ME, Colan SD, Kerr B, Rauen KA, Noonan J, et al. Clinical, pathological, and molecular analyses of cardiovascular abnormalities in Costello syndrome: A Ras/MAPK pathway syndrome. Am J Med Genet A. 2011 doi: 10.1002/ajmg.a.33857. [DOI] [PubMed] [Google Scholar]
- 9.Tomita H, Fuse S, Ikeda K, Matsuda K, Chiba S. An infant with Costello syndrome complicated with fatal hypertrophic obstructive cardiomyopathy. Acta Paediatr Jpn. 1998;40:608–11. doi: 10.1111/j.1442-200x.1998.tb02000.x. [DOI] [PubMed] [Google Scholar]
- 10.Roberts A, Allanson J, Jadico SK, Kavamura MI, Noonan J, Opitz JM, et al. The cardiofaciocutaneous syndrome. J Med Genet. 2006;43:833–42. doi: 10.1136/jmg.2006.042796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazzanti L, Cacciari E, Cicognani A, Bergamaschi R, Scarano E, Forabosco A. Noonan-like syndrome with loose anagen hair: a new syndrome? Am J Med Genet A. 2003;118A:279–86. doi: 10.1002/ajmg.a.10923. [DOI] [PubMed] [Google Scholar]
- 12.Gripp KW, Zand DJ, Demmer L, Anderson CE, Dobyns WB, Zackai EH, et al. Expanding the SHOC2 mutation associated phenotype of Noonan syndrome with loose anagen hair: structural brain anomalies and myelofibrosis. Am J Med Genet A. 2013;161:2420–30. doi: 10.1002/ajmg.a.36098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komatsuzaki S, Aoki Y, Niihori T, Okamoto N, Hennekam RC, Hopman S, et al. Mutation analysis of the SHOC2 gene in Noonan-like syndrome and in hematologic malignancies. J Hum Genet. 2010;55:801–9. doi: 10.1038/jhg.2010.116. [DOI] [PubMed] [Google Scholar]
- 14.Mazzanti L, Tamburrino F, Scarano E, Perri A, Vestrucci B, Guidetti M, et al. GH Therapy and first final height data in Noonan-like syndrome with loose anagen hair (Mazzanti syndrome) Am J Med Genet A. 2013;161A:2756–61. doi: 10.1002/ajmg.a.36255. [DOI] [PubMed] [Google Scholar]
- 15.Cordeddu V, Di Schiavi E, Pennacchio LA, Ma’ayan A, Sarkozy A, Fodale V, et al. Mutation of SHOC2 promotes aberrant protein N-myristoylation and causes Noonan-like syndrome with loose anagen hair. Nat Genet. 2009;41:1022–6. doi: 10.1038/ng.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tartaglia M, Mehler EL, Goldberg R, Zampino G, Brunner HG, Kremer H, et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29:465–8. doi: 10.1038/ng772. [DOI] [PubMed] [Google Scholar]
- 17.Fragale A, Tartaglia M, Wu J, Gelb BD. Noonan syndrome-associated SHP2/PTPN11 mutants cause EGF-dependent prolonged GAB1 binding and sustained ERK2/MAPK1 activation. Hum Mutat. 2004;23:267–77. doi: 10.1002/humu.20005. [DOI] [PubMed] [Google Scholar]
- 18.Tartaglia M, Gelb BD. Disorders of dysregulated signal traffic through the RAS-MAPK pathway: phenotypic spectrum and molecular mechanisms. Ann N Y Acad Sci. 2010;1214:99–121. doi: 10.1111/j.1749-6632.2010.05790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Digilio MC, Conti E, Sarkozy A, Mingarelli R, Dottorini T, Marino B, et al. Grouping of multiple-lentigines/LEOPARD and Noonan syndromes on the PTPN11 gene. Am J Hum Genet. 2002;71:389–94. [Google Scholar]
- 20.Legius E, Schrander-Stumpel C, Schollen E, Pulles-Heintzberger C, Gewillig M, Fryns JP. PTPN11 mutations in LEOPARD syndrome. J Med Genet. 2002;39:571–4. doi: 10.1136/jmg.39.8.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tartaglia M, Martinelli S, Stella L, Bocchinfuso G, Flex E, Cordeddu V, et al. Diversity and functional consequences of germline and somatic PTPN11 mutations in human disease. Am J Hum Genet. 2006;78:279–90. doi: 10.1086/499925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kontaridis MI, Swanson KD, David FS, Barford D, Neel BG. PTPN11 (Shp2) mutations in LEOPARD syndrome have dominant negative, not activating, effects. J Biol Chem. 2006;281:6785–92. doi: 10.1074/jbc.M513068200. [DOI] [PubMed] [Google Scholar]
- 23.Hanna N, Montagner A, Lee WH, Miteva M, Vidal M, Vidaud M, et al. Reduced phosphatase activity of SHP-2 in LEOPARD syndrome: consequences for PI3K binding on Gab1. FEBS Lett. 2006;580:2477–82. doi: 10.1016/j.febslet.2006.03.088. [DOI] [PubMed] [Google Scholar]
- 24.Aoki Y, Niihori T, Kawame H, Kurosawa K, Ohashi H, Tanaka Y, et al. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat Genet. 2005;37:1038–40. doi: 10.1038/ng1641. [DOI] [PubMed] [Google Scholar]
- 25.Tartaglia M, Kalidas K, Shaw A, Song X, Musat DL, van der Burgt I, et al. PTPN11 mutations in Noonan syndrome: molecular spectrum, genotype-phenotype correlation, and phenotypic heterogeneity. Am J Hum Genet. 2002;70:1555–63. doi: 10.1086/340847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lepri F, De Luca A, Stella L, Rossi C, Baldassarre G, Pantaleoni F, et al. SOS1 mutations in Noonan syndrome: molecular spectrum, structural insights on pathogenic effects, and genotype-phenotype correlations. Hum Mutat. 2011;32:760–72. doi: 10.1002/humu.21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandit B, Sarkozy A, Pennacchio LA, Carta C, Oishi K, Martinelli S, et al. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet. 2007;39:1007–12. doi: 10.1038/ng2073. [DOI] [PubMed] [Google Scholar]
- 28.Razzaque MA, Nishizawa T, Komoike Y, Yagi H, Furutani M, Amo R, et al. Germline gain-of-function mutations in RAF1 cause Noonan syndrome. Nat Genet. 2007;39:1013–7. doi: 10.1038/ng2078. [DOI] [PubMed] [Google Scholar]
- 29.Aoki Y, Niihori T, Banjo T, Okamoto N, Mizuno S, Kurosawa K, et al. Gain-of-function mutations in RIT1 cause Noonan syndrome, a RAS/MAPK pathway syndrome. Am J Hum Genet. 2013;93:173–80. doi: 10.1016/j.ajhg.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gripp KW, Hopkins E, Sol-Church K, Stabley DL, Axelrad ME, Doyle D, et al. Phenotypic analysis of individuals with Costello syndrome due to HRAS p.G13C. Am J Med Genet A. 2011;155A:706–16. doi: 10.1002/ajmg.a.33884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allanson JE, Anneren G, Aoki Y, Armour CM, Bondeson ML, Cave H, et al. Cardio-facio-cutaneous syndrome: does genotype predict phenotype? Am J Med Genet C Semin Med Genet. 2011;157C:129–35. doi: 10.1002/ajmg.c.30295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abe Y, Aoki Y, Kuriyama S, Kawame H, Okamoto N, Kurosawa K, et al. Prevalence and clinical features of Costello syndrome and cardio-facio-cutaneous syndrome in Japan: findings from a nationwide epidemiological survey. Am J Med Genet A. 2012;158A:1083–94. doi: 10.1002/ajmg.a.35292. [DOI] [PubMed] [Google Scholar]
- 33.Wu X, Simpson J, Hong JH, Kim KH, Thavarajah NK, Backx PH, et al. MEK-ERK pathway modulation ameliorates disease phenotypes in a mouse model of Noonan syndrome associated with the Raf1(L613V) mutation. J Clin Invest. 2011;121:1009–25. doi: 10.1172/JCI44929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen PC, Wakimoto H, Conner D, Araki T, Yuan T, Roberts A, et al. Activation of multiple signaling pathways causes developmental defects in mice with a Noonan syndrome-associated Sos1 mutation. J Clin Invest. 2010;120:4353–65. doi: 10.1172/JCI43910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marin TM, Keith K, Davies B, Conner DA, Guha P, Kalaitzidis D, et al. Rapamycin reverses hypertrophic cardiomyopathy in a mouse model of LEOPARD syndrome-associated PTPN11 mutation. J Clin Invest. 2011;121:1026–43. doi: 10.1172/JCI44972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edouard T, Combier JP, Nedelec A, Bel-Vialar S, Metrich M, Conte-Auriol F, et al. Functional effects of PTPN11 (SHP2) mutations causing LEOPARD syndrome on epidermal growth factor-induced phosphoinositide 3-kinase/AKT/glycogen synthase kinase 3beta signaling. Mol Cell Biol. 2010;30:2498–507. doi: 10.1128/MCB.00646-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuhmacher AJ, Guerra C, Sauzeau V, Canamero M, Bustelo XR, Barbacid M. A mouse model for Costello syndrome reveals an Ang II-mediated hypertensive condition. J Clin Invest. 2008;118:2169–79. doi: 10.1172/JCI34385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhandapany PS, Razzaque MA, Muthusami U, Kunnoth S, Edwards JJ, Mulero-Navarro S, et al. RAF1 mutations in childhood-onset dilated cardiomyopathy. Nat Genet. 2014;46:635–9. doi: 10.1038/ng.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee YS, Ehninger D, Zhou M, Oh JY, Kang M, Kwak C, et al. Mechanism and treatment for learning and memory deficits in mouse models of Noonan syndrome. Nat Neurosci. 2014;17:1736–43. doi: 10.1038/nn.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marino B, Digilio MC, Toscano A, Giannotti A, Dallapiccola B. Congenital heart diseases in children with Noonan syndrome: An expanded cardiac spectrum with high prevalence of atrioventricular canal. J Pediatr. 1999;135:703–6. doi: 10.1016/s0022-3476(99)70088-0. [DOI] [PubMed] [Google Scholar]