Abstract
Objective
Testing for human papillomavirus (HPV) 16 and 18 genotypes, which are known to cause more than 65-70% of invasive cervical cancer cases, may allow clinicians to identify women at highest risk for underlying high-grade dysplasia missed by Pap cytology. Our objective was to determine the cost-effectiveness of adding HPV-16 and 18 genotype triage to current cervical cancer screening strategies in the United States.
Methods
We developed a lifetime Markov model to assess the cost-effectiveness of adding HPV genotyping to current cervical cancer screening algorithms. All costs were estimated from a payer perspective in 2007 U.S. dollars. Outcome measures included lifetime risk of cervical cancer, quality-adjusted life-years saved (QALYs), and incremental cost-effectiveness ratios (ICERs).
Results
In our model, the use of HPV genotype triage prevented 51-73 deaths per 100,000 women screened compared to screening using liquid-based cytology (LBC) followed by HPV triage and 4-26 deaths compared to co-screening with LBC and HPV. Use of HPV genotyping to triage all high-risk HPV-positive women every three years had an ICER of $34,074 per QALY compared to HPV and LBC co-screening. HPV genotyping with co-screening was the most effective strategy and had an ICER of $33,807 per QALY compared to HPV genotyping for all high-risk HPV-positive women.
Conclusion
The addition of HPV-16 and -18 genotype triage to current adjunctive HPV screening with LBC is a cost-effective screening strategy in the United States.
Introduction
Virtually all cases of cervical cancer are caused by persistent infection with specific high-risk (HR) types of human papillomavirus (HPV). (i) The most common HPV genotypes detected in invasive cancers are HPV type 16 (HPV-16) and HPV-18, which are present in approximately 65-70% of invasive cervical cancer cases. (ii,iii) HPV-16 has been shown to be more persistent and more often associated with high-grade cervical lesions than other high-risk HPV types. (iv,v,vi) Although HPV-18 is the second most common type in invasive cervical cancer, precancerous lesions associated with HPV-18 are often underrepresented, possibly because these lesions are harder to detect with cervical screening. (iii,iv,v)
Current guidelines in the United States recommend screening annually with conventional cervical cytology or every two years using liquid-based cytology (LBC). (vii) For women 30 years of age or older, cervical screening can be performed every three years using conventional cytology or LBC combined with a HR HPV DNA test. (vii,viii) HPV DNA testing is considerably more sensitive than cytology at detecting high-grade cervical intraepithelial neoplasia (CIN) (ix,x,xi,xii,xiii) but is somewhat less specific due to the detection of transient HPV infections that may not progress to cervical lesions. (iv) As more women are vaccinated against HPV, the positive predictive value of current screening strategies is expected to decrease, resulting in additional testing and follow-up. (iii,xiv,xv) Several authors have proposed the use of HPV genotyping tests to address some of these concerns. (iv,v,vi,xiv,xvi) Using HPV genotyping to triage HPV-positive women may increase the specificity of HPV DNA testing thereby reducing referrals for colposcopies and treatment while still maintaining a high sensitivity. (iv,xiv) Women positive for HPV-16 or -18 could be followed more aggressively than women positive for other HR HPV types. (iv,vi)
Several studies have documented the cost-effectiveness of HPV DNA testing as a primary screening test or to triage equivocal cytology (atypical squamous cells of undetermined significance (ASC-US)) results. (xvii,xviii,xix) However, the cost-effectiveness of using HPV genotyping tests has not been established. As screening algorithms incorporating HPV genotyping tests are considered, it would be helpful to understand the economic implications of this approach. The purpose of our study was to determine the cost-effectiveness of adding HPV-16 and -18 genotype triage to current cervical cancer screening strategies in the United States.
Methods
We developed a lifetime Markov Monte Carlo model to assess the cost-effectiveness of adding HPV genotyping tests to current cervical cancer screening algorithms. We assumed that all women would undergo biennial LBC testing until age 30. After age 30, we compared the following screening strategies (refer Supplemental Digital Content 1 for detailed screening algorithms):
-
➢
Screening using LBC every 2 years;
-
➢
Screening using LBC every 2 years, followed by HPV DNA testing for all patients with equivocal results on cytology (ASC-US);
-
➢
Primary screening using HPV DNA testing every 3 years, followed by cytology for all patients with positive result on HPV;
-
➢
Screening using a combination of simultaneous cytology and HPV DNA testing every 3 years;
-
➢
Screening using a combination of simultaneous cytology and HPV DNA testing every 3 years, with reflux HPV genotyping and more intensive follow-up for HPV types 16/18;
-
➢
Screening using HPV DNA testing followed by reflux HPV genotyping for all HPV-positive women and more intensive follow-up for HPV types 16/18.
Detailed methods regarding our core cervical cancer screening model have been published previously. (xvii) Briefly our model follows a hypothetical cohort of 100,000 U.S. women over their lifetimes, starting at age 13 years. The natural history of cervical neoplasia was modeled using 18 distinct Markov health states representing type-specific HPV infection, CIN, and invasive cervical cancer (Figure 1). Women could transition between health states at the end of each month. All modeling was conducted using TreeAge Pro 2007 release 1.5 (TreeAge Software, Williamstown, MA).
Figure 1.
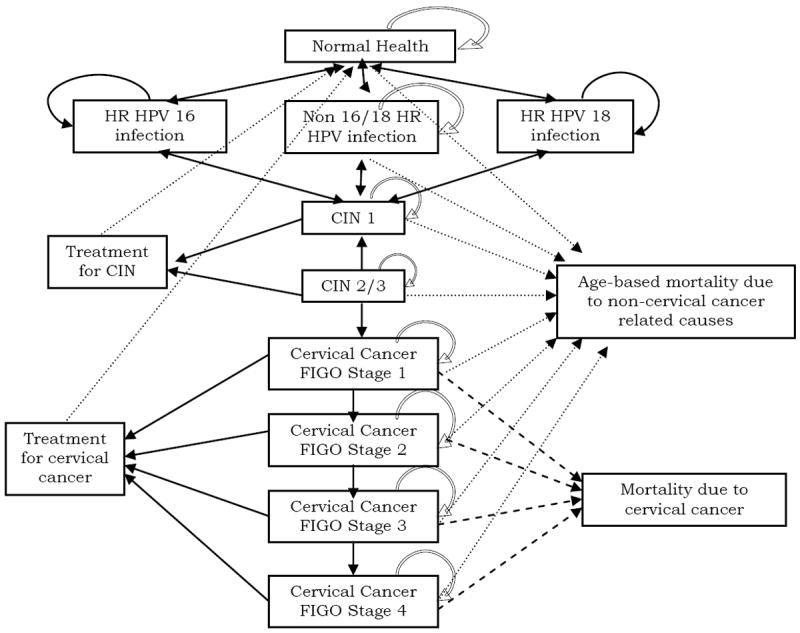
Schematic representation of the model.
The natural history of HPV infection, CIN, and cervical cancer was based on data from the published literature. The type-specific incidence of HPV infection was based on data from a recent study among 18- to 70-year-old women in the United States.(xx) During the course of the model, women with persistent HPV infection could develop CIN or invasive cervical cancer based on conditional probabilities that depended on the woman’s age and underlying HPV type (Table 1). (ii,vi,xxi,xxii,xxiii,xxiv,xxv,xxvi,xxvii,xxviii,xxix)
Table 1.
Input variables and sources*
| Clinical variable | Base-case value | Range | Source |
|---|---|---|---|
| Population variables | |||
| Annual hysterectomy rate† (%) | 0.02 – 1.17 | Keshavarz (2002) | |
| Age-specific prevalence of HR HPV infection†‡(%) | 4 – 31 | 0.5 – 2x baseline | Apple |
|
| |||
| Probability of disease progression | |||
| HPV infection progressing to CIN 1 (%) | 8.1 | 5.4-15.0 | Ho (1998) |
| Prevalence of LSIL among patients with non 16/18 HR HPV infection (%) | 14 | 0.5 – 2x baseline | Herrero (2005) |
| RR of LSIL among patients with HPV-16 infection§ | 1 | “ | Assumption |
| RR of LSIL among patients with HPV-18 infection§ | 1 | “ | Assumption |
| HPV infection progressing to CIN 2,3 (%) | 0.56 | 0.54-1.5 | Ho (1998), Myers (2000) |
| Prevalence of CIN 2,3 among patients with non 16/18 HR HPV infection (%) | 4 | 0.5 – 2x baseline | Khan (2005) |
| Progression from CIN 1 to CIN 2,3† (%) | 1.7 – 5.7 | 1.7-8.3 | Myers (2000) |
| Annual rate of progression among patients with non 16/18 HR HPV infection | 0.09 | 0.5 -2x baseline | Khan (2005) |
| RR of CIN 2,3 in patients with HPV-16 infection§ | 4.6 | “ | Khan (2005) |
| RR of CIN 2,3 in patients with HPV-18 infection§ | 2.5 | “ | ∥ |
| Progression from CIN 2,3 to cervical cancer (%) | 3.8 | 3.0-6.2 | Sanders (2003) |
| Incidence rate of cervical cancer in the United States (per 100,000 women) | 9.4 | 0.5 – 2x baseline | Saraiya (2007) |
| Prevalence of HPV-16 in patients with cervical cancer (%) | 59 | 55-63 | Clifford (2003), Munoz (2003) |
| Prevalence of HPV-18 in patients with cervical cancer (%) | 13 | 11-15 | |
| Prevalence of non 16/18 HR HPV types in patients with cervical cancer (%) | 28 | 22-34 | |
|
| |||
| Probability of cervical cancer progression (%) ¥ | 43.7-68.3 | 40-70 | Myers (2000), Sanders (2003) |
|
| |||
| Probability of disease regression | |||
| Age-specific probability of regression of HPV infection† (%) | 3.3 – 37.3 | 1.7-60.0 | Myers (2000), Ho (1998) |
| Rate of clearance of non 16/18 HR HPV infection | 1.29 | 0.5 – 2x baseline | Trottier (unpublished manuscript) |
| RR of clearance of HPV-16 infection§ | 0.85 | “ | Trottier |
| RR of clearance of HPV-18 infection§ | 0.96 | “ | Trottier |
| Regression of CIN 1† (%) | 2.7 – 14.2 | 2 – 16 | Sanders (2003) |
| Patients regressing to HPV infection without lesion (%) | 10 | 0 – 20 | Sanders (2003), Myers (2000) |
| Regression rate of LSIL among patients with non 16/18 HR HPV infection | 0.98 | 0.5 – 2x baseline | Schlecht (2003) |
| RR of regression of LSIL in patients with HPV-16§ | 0.91 | “ | “ |
| RR of regression of LSIL in patients with HPV-18§ | 0.91 | “ | Assumption |
| Regression of CIN 2,3† (%) | 3.7 – 5.8 | 3-7 | Sanders (2003), Myers (2000) |
| Patients regressing to normal (%) | 45 | 40-50 | “ |
| Patients regressing to HPV infection without lesion (%) | 5 | 0-10 | “ |
| Patients regressing to CIN 1 (%) | 50 | 40-60 | “ |
| Regression rate of HSIL among patients with non 16/18 HR HPV infection | 0.77 | 0.5 – 2x baseline | Schlecht (2003) |
| RR of regression of HSIL in patients with HPV-16§ | 0.27 | “ | “ |
| RR of regression of HSIL in patients with HPV-18§ | 0.27 | “ | “ |
|
| |||
| Annual symptom detection probability for cervical cancer (%) ¥ | 15-90 | 12-93 | Sanders (2003) |
|
| |||
| 5-year survival rates (% alive at 5 years) ¥ | 85-12 | 84-10 | NCDB commission on cancer (2006) |
|
| |||
| Disease-specific utilities | Sanders (2003) | ||
| HPV infection | 1 | 0.8-1 | |
| CIN 1 | 0.97 | 0.8-1 | |
| CIN 2,3 | 0.97 | 0.5-1 | |
| Invasive cervical cancer ¥ | 0.79-0.62 | 0.25-1 | |
|
| |||
| Initial efficacy of treatment (%) | Karyn Goodman (personal communication), Assumption | ||
| CIN 1 | 98 | 95-100 | |
| CIN 2 | 95 | 90-98 | |
| Invasive cervical cancer¥ | 90-15 | 10-100 | |
| Probability HPV persists after effective treatment (%) | Sanders (2003) | ||
| CIN 1 | 10 | 0-25 | |
| CIN 2,3 | 10 | 0-25 | |
| Invasive cervical cancer | 0 | --- | Assumption |
|
| |||
| Screening tests (%) | Sensitivity | Specificity | |
| Liquid-based cytology (ASCUS or worse) | |||
| CIN 1 or worse | 43 | 85 | Ratnam (2000) |
| CIN 2,3 or worse | 53 | 96 | Cuzick (2006) |
| HPV positive | |||
| CIN 1 or worse | 57 | 96 | Bigras (2005) |
| CIN 2,3 or worse | 96 | 91 | Cuzick (2006) |
All variables are annual unless otherwise noted. HR denotes high risk, HPV human papillomavirus, CIN cervical intraepithelial neoplasia, LSIL low-grade squamous intraepithelial lesion, RR relative risk, FIGO International Federation of Gynecology and Obstetrics, HSIL high-grade squamous intraepithelial lesion, NCDB National Cancer Database, and ASCUS atypical squamous cells of undetermined significance.
These data vary based on age. The range of values is shown.
These data also vary by HPV type.
Compared to patients with non 16/18 HR HPV infection
Estimated using a Markov model to match the incidence of CIN 2,3 and cervical cancer in the United States
These data vary based on cervical cancer stage (FIGO I, II, III or IV)
We stratified the 13 known HR HPV types into three categories: HPV-16 only, HPV-18 only, and other HR HPV types (including 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68). We combined data from several studies to evaluate the risk of progression and regression of HPV and CIN based on the underlying HPV category (ii,vi,xxiii,xxvii,xxviii). (Additional data are available from the authors). Given the limited data on the impact of HPV co-infection, we assumed that the risk of disease progression and regression for patients with co-infection was the same as patients infected with the most aggressive HPV type only. For instance, a patient infected with HPV-16 and HPV-18 had a risk of progression and regression similar to a patient infected with HPV-16 only. We also assumed that in patients with non-16/18 HR HPV infection, disease progression and regression rates were identical for patients infected with single versus multiple HPV types. The rate at which women progress from FIGO (International Federation of Gynecology and Obstetrics) stage I through stages II, III, and IV invasive cervical cancer was assumed to be independent of the type of the underlying HPV infection. In a few cases, we used cytology test results as a proxy for the histological diagnosis. In these cases, we have assumed that a cytological diagnosis of low-grade squamous intraepithelial lesions (LSIL) correlates with CIN 1 and high-grade squamous intraepithelial lesions (HSIL) with CIN 2/3.
Screening and treatment protocols were based on recently published consensus guidelines. (vii, viii) The sensitivity and specificity of LBC and HPV tests were based on clinical studies and meta-analyses in the published literature. (xxx, xxxi, xxxii) In the genotyping strategies, we assumed that patients who have normal cytology, a positive HPV result, and are HPV-16 or -18 positive will receive immediate colposcopy and biopsy, while those who are HPV-16 and -18 negative will have repeat LBC and HPV tests in one year.
All costs were estimated from a health-care payer perspective in 2007 U.S. dollars. Detailed micro-costing methods were used to identify the total direct medical cost of screening and treating CIN and invasive cervical cancer. Unit costs were obtained from the 2007 Medicare fee schedules (Table 2). Primary outcome measures included quality-adjusted life-years saved (QALYs), incremental cost-effectiveness ratios (ICERs), and lifetime risk of cervical cancer. Disease-specific utilities were used to incorporate quality-of-life decrements. (xxv)
Table 2.
Cost variables*
| Variable | Base-case value |
|---|---|
| Cost per clinic visit for routine screening | $66 |
| Cost per clinic visit for repeat screening | $97 |
|
| |
| Diagnostic test costs | |
| Liquid-based cytology | $28 |
| HPV DNA testing | $49 |
| Linear array† | $100 |
| Colposcopy only | $172 |
| Colposcopy with biopsy | $399 |
| Colposcopy with endocervical curettage | $424 |
|
| |
| Treatment costs | |
| LLETZ | $1,279 |
| Conization | $1,768 |
| Cryosurgery | $212 |
| Total abdominal hysterectomy | $35,402 |
| Vaginal hysterectomy | $35,241 |
| Radical abdominal hysterectomy | $36,176 |
| Chemotherapy | $5,062 |
| Radiotherapy (EBRT) | $10,509 |
| Radiotherapy (EBRT with brachytherapy) | $17,778 |
|
| |
| Treatment costs based on stage of disease | |
| CIN 1 and CIN 2,3 | $1,086 |
| FIGO stage I cancer | $53,621 |
| FIGO stage II cancer | $26,206 |
| FIGO stage III and stage IV cancer | $26,014 |
All costs are in 2007 USD and are based on 2007 Medicare reimbursement unless otherwise noted. HPV denotes human papillomavirus, LLETZ large loop excision of the transformation zone, EBRT external beam radiation therapy, CIN cervical intraepithelial neoplasia, and FIGO International Federation of Gynecology and Obstetrics.
The cost of the linear array is an assumption.
We conducted extensive sensitivity analyses to assess the impact of all parameters on the overall cost-effectiveness results. The range of values used for sensitivity analysis was based on published literature and clinician input as shown in Table 1.
Results
The model was calibrated to match the age-specific prevalence of HPV infection to within +/- 0.5 percentage points of the data in the literature (Figure 2). We then matched model predications to the overall prevalence of HPV-type-specific CIN and cervical cancer (Table 3). The margin of error (standard deviation) was less than 0.02% of total lifetime cost per patient and less than 0.01% of the average life expectancy. The model predicted an average annual incidence of nine cervical cancer cases per 100,000 women screened using LBC every 2 years. (xxvi)
Figure 2.
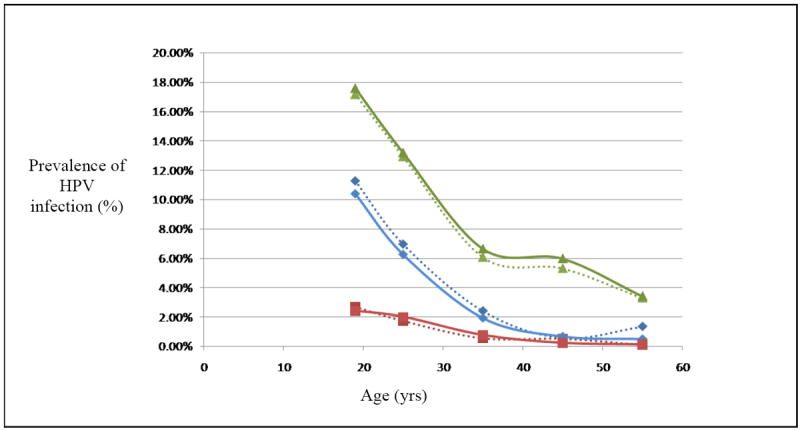
Prevalence of HPV infection in the US. Dotted line denotes data from the literature & solid line denotes model prediction
Table 3.
Model validation results – prevalence of CIN 2,3 and invasive cervical cancer among women screened using LBC every 2 years*
| Parameter | Data from: | Source | |
|---|---|---|---|
| Literature | Model | ||
| Prevalence of CIN 1 cases among patients with HR HPV infection (%) | 14 | 13 | Herrero (2005) |
| Prevalence of CIN 2,3 cases among patients with HR HPV infection (%) | 4 | 4.5 | Khan (2005) |
| CIN 2,3 cases caused by HPV-16 (%) | 41 | 43 | Khan (2005) |
| CIN 2,3 cases caused by HPV-18 (%) | 10 | 10 | Khan (2005) |
| CIN 2,3 cases caused by non 16/18 HR HPV (%) | 49 | 47 | Khan (2005) |
| Annual incidence of cervical cancer in the United States (per 100,000 women) | 9.4 | 9.2 | Saraiya (2007) |
CIN denotes cervical intraepithelial neoplasia, HR high-risk, and HPV human papillomavirus.
Table 4 shows the lifetime incidence of cervical cancer cases and cervical cancer-related deaths per 100,000 women. Screening for cervical cancer and its precursors prevented 770-940 cervical cancer cases and 640-740 cervical cancer-related deaths per 100,000 women screened. The average annual incidence of cervical cancer ranged from six to nine cases per 100,000 women for the various screening strategies. Compared to screening using LBC followed by HPV triage every two years, HPV genotyping reduced the incidence of cervical cancer by 12-23% and prevented an additional 51-73 deaths per 100,000 women depending upon genotyping strategy. Compared to co-screening with both LBC and HPV, use of the genotyping test prevented an additional 4-26 deaths per 100,000 patients.
Table 4.
Health and economic outcomes – Lifetime incidence of disease per 100,000 women, costs, QALE, and ICER compared to next most effective strategy*
| Strategy | Cervical cancer cases | Cervical cancer deaths | Annual incidence of cervical cancer | Costs ($) | QALE (years) | ICER ($/QALY) |
|---|---|---|---|---|---|---|
| No screening | 1,383 | 894 | 20.64 | $86,700 | 28.5866 | -- |
| LBC only (2 year interval) | 615 | 259 | 9.17 | $88,162 | 28.6623 | $19,321 |
| LBC with HPV triage (2 year interval) | 574 | 231 | 8.56 | $88,221 | 28.6651 | $21,304 |
| HPV with LBC triage (3 year interval) | 527 | 193 | 7.86 | $88,226 | 28.6670 | $2,618 |
| HPV and LBC co-screening (3 year interval) | 502 | 184 | 7.49 | $88,303 | 28.6714 | $17,204 |
| HPV genotyping for HR HPV-positive women (3 year interval) | 507 | 180 | 7.57 | $88,340 | 28.6725 | $34,074 |
| HPV genotyping with co-screening (3 year interval) | 444 | 158 | 6.62 | $88,407 | 28.6745 | $33,807 |
ICER denotes incremental cost-effectiveness ratio, QALE quality-adjusted life expectancy, QALY quality-adjusted life year, LBC liquid-based cytology, HPV human papillomavirus, and HR high-risk.
In the absence of screening, women lived an average of 28.59 discounted QALYs (Table 5). Screening using LBC only every two years, increased life expectancy by 28 days at an incremental cost of $19,321 per QALY. Current clinical practice of LBC with HPV triage for equivocal pap smears was the next most effective strategy, with an incremental cost of $21,304 per QALY. HPV testing as a primary screening test with LBC triage for all HPV-positive women was a highly cost-effective strategy with an ICER of $2,618 per QALY. Compared to HPV with LBC triage, co-screening with LBC and HPV testing every three years increased quality-adjusted life expectancy (QALE) to 28.67 years resulting in an ICER of $17,204 per QALY. Use of HPV genotyping for all HR HPV-positive women every three years was more effective and had an ICER of $34,074 per QALY compared to co-screening. Adding HPV genotyping to HPV and LBC co-screening was the most effective strategy and had an ICER of $33,807 per QALY, compared to using HPV genotyping for all HR HPV-positive women in the absence of cytology screening.
We compared current clinical practice with HPV and LBC co-screening to HPV genotyping with HPV and LBC co-screening in one-way sensitivity analysis. The tornado diagram in Figure 3 shows the range of ICERs for key variables in the model. The vertical line represents the base-case analysis ICER. A wide horizontal bar indicates that the associated variable has a large effect on the results of the model. Model results were sensitive to HPV incidence rate, relative risk of progression of CIN for HPV-16 and -18, and the risk of progression to cervical cancer for all HPV types. Cost of the HPV tests and the cost of colposcopy and biopsy also had a significant impact on the ICERs. Overall, the HPV genotyping strategies remained cost-effective. The model results were also sensitive to the sensitivity and specificity of LBC and HPV tests. Increasing the sensitivity of the cytology test by more than 10% or decreasing the sensitivity of the HPV test by more than 15% increased the ICER of the genotyping strategy to more than $100,000.
Figure 3.
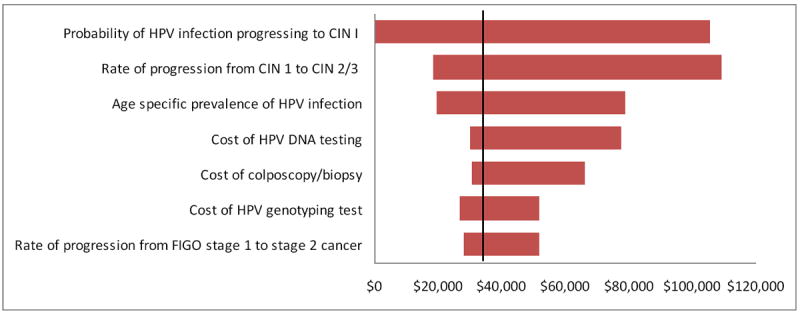
Tornado Diagram: One-way sensitivity analysis showing the range of incremental cost-effectiveness ratios comparing LBC and HPV co-screening with genotyping and LBC and HPV co-screening. The vertical dotted line represents the base-case analysis ICER.
Discussion
We found that adding HPV-16 and -18 genotype triage to current cervical cancer screening strategies in the United States resulted in fewer cervical cancer-related deaths, longer QALE, and higher costs. Using the 2007 U.S. per capita gross domestic product as a threshold for defining cost-effectiveness (xxxiii), HPV genotyping was very cost-effective when used to triage HR HPV-positive women.
Results from a large cohort study demonstrated that identification of women who were positive for HPV-16 was a better predictor of progression to CIN3 or cervical cancer than a cytological diagnosis of LSIL. (vi) The authors have suggested that HPV genotyping may identify women at the greatest risk of developing CIN3 and cancer and may allow less aggressive management of women with non-16/18 HR HPV infections and have called for evaluations of the cost-effectiveness of this approach. Based on a Pub Med search (1966-May 2009; English language; search terms: “HPV”, “genotyping”, and “cost-effectiveness”), we believe this is the first study to assess the cost-effectiveness of cervical cancer screening algorithms incorporating HPV genotyping. One of the strengths of the modeling approach that we used is the ability to determine the impact of HPV genotyping on survival and cost-effectiveness over a woman’s lifetime, a length of follow-up that is impractical for a clinical trial.
Although HPV DNA testing in the United States is currently used either to triage equivocal cytology or as a primary screening test in conjunction with cytology, the International Agency for Research on Cancer (xxxiv) and other authors (xxxi) have endorsed the use of HR HPV testing alone as an option for primary cervical cancer screening, and ongoing clinical studies are investigating the effectiveness of this approach. (xvi) One of the strengths of our model is that it compares the current use of HPV DNA testing as an adjunct to cytology to the potential use of HPV DNA testing as a primary screening tool as well as HPV DNA testing in conjunction with HPV genotyping.
Our study has several limitations. First, because the use of HPV genotyping has not yet been incorporated into current cervical cancer screening guidelines in the United States, we chose two possible HPV genotyping scenarios to include in the model – as a triage test for HPV-positive, cytology-negative women and as a triage test for HPV-positive women who received HPV testing alone for primary screening. These screening algorithms are consistent with other recently proposed HPV genotyping algorithms (iv,vi). However, there are other potential screening algorithms and other possible applications of HPV genotyping, such as its use as a primary screening test or for post-treatment monitoring for recurrence (xvi), which were not included in this model. Second, we have not incorporated the impact of HPV vaccination on the screening strategies evaluated in our model. However, given the recent introduction of HPV vaccinaton in the United States for women aged 11-26 years, the cohort in our model that is eligible for HPV genotype triage (women age 30+) today represents an unvaccinated group in the US. In addition, our results probably reflect a conservative evaluation of the cost-effectiveness of HPV testing given the likely negative impact that HPV vaccination will have on the performance of cytology relative to that of HPV testing, though this hypothesis will require further research. (xv)
Third, there are limited data available on the risk of progression and regression of HPV and CIN for specific HPV genotypes and for patients infected with more than one HPV genotype. As shown in Table 3, the prevalence of CIN 2,3 and invasive cervical cancer predicted by the model closely match the data in the literature. In addition, we tested all assumptions through extensive sensitivity analysis and our results remained consistent over a range of plausible values. Finally, because a standard reimbursement for the HPV genotyping test has not yet been established, we assumed a cost of $100 for the test in the model, which is approximately two times the cost of the standard HPV test available today. Although the model results were sensitive to the cost of both the standard HPV test and the HPV genotyping test, we found that the HPV genotyping strategies remained cost-effective throughout the range of test costs we evaluated in sensitivity analyses (standard HPV test cost ranged from $37 to $61 and HPV genotyping test cost ranged from $75 to $125).
Additional clinical studies are needed to refine our understanding of the effect of different HPV genotypes on the risk of progression and regression of HPV and CIN, as well as to determine the clinical effectiveness of using HPV DNA testing alone and using HPV genotyping in conjunction with HPV DNA testing and/or cytology for primary cervical cancer screening. Our analysis provides evidence of the cost-effectiveness of HPV genotyping based on currently available data. These findings will require confirmation when additional clinical and cost data become available.
In our model, the addition of HPV-16 and -18 genotype triage to current cervical cancer screening strategies in the United States was clinically effective and very cost-effective. Policymakers should consider the use of HPV genotyping triage as one strategy to increase the positive predictive value and cost-effectiveness of current cervical cancer screening algorithms.
Supplementary Material
Acknowledgments
Financial support: This study was funded by a grant from Roche Molecular Systems, Inc., Pleasanton, CA, USA (Roche). All authors have received honoraria or consultancy fees from Roche. Representatives from Roche were allowed to review model results as well as a draft of the manuscript, but all final decisions regarding model calculations and manuscript content were made by the authors.
References
- i.Lowy DR, Solomon D, Hildesheim A, Schiller JT, Schiffman M. Human papillomavirus infection and the primary and secondary prevention of cervical cancer. Cancer. 2008;113(7 suppl):1980–93. doi: 10.1002/cncr.23704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ii.Munoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al. for the International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- iii.Wheeler CM, Hunt WC, Joste NE, Key CR, Quint WGV, Castle PE. Human papillomavirus genotype distributions: implications for vaccination and cancer screening in the United States. J Natl Cancer Inst. 2009;101(7):475–87. doi: 10.1093/jnci/djn510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- iv.Cuzick J, Arbyn M, Sankaranarayanan R, Tsu V, Ronco G, Mayrand MH, et al. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. 2008;26S:K29–41. doi: 10.1016/j.vaccine.2008.06.019. [DOI] [PubMed] [Google Scholar]
- v.Safaeian M, Schiffman M, Gage J, Solomon D, Wheeler CM, Castle PE. Detection of precancerous cervical lesions is differential by human papillomavirus type. Cancer Res. 2009;69(8):3262–6. doi: 10.1158/0008-5472.CAN-08-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vi.Khan MJ, Castle PE, Lorincz AT, Wacholder S, Sherman M, Scott DR, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005 Jul 20;97(14):1072–9. doi: 10.1093/jnci/dji187. [DOI] [PubMed] [Google Scholar]
- vii.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 American Society for Colposcopy and Cervical Pathology-sponsored Consensus Conference. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007 Oct;197(4):346–55. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- viii.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 American Society for Colposcopy and Cervical Pathology-sponsored Consensus Conference. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol. 2007 Oct;197(4):340–5. doi: 10.1016/j.ajog.2007.07.050. [DOI] [PubMed] [Google Scholar]
- ix.Bulkmans NW, Berkhof J, Rozendaal L, van Kemenade FJ, Boeke AJ, Bulk S, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. 2007 Nov 24;370(9601):1764–72. doi: 10.1016/S0140-6736(07)61450-0. [DOI] [PubMed] [Google Scholar]
- x.Mayrand MH, Duarte-Franco E, Rodrigues I, Walter SD, Hanley J, Ferenczy A, et al. for the Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007 Oct 18;357(16):1579–88. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- xi.Naucler P, Ryd W, Törnberg S, Strand A, Wadell G, Elfgren K, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007 Oct 18;357(16):1589–97. doi: 10.1056/NEJMoa073204. [DOI] [PubMed] [Google Scholar]
- xii.Naucler P, Ryd W, Törnberg S, Strand A, Wadell G, Elfgren K, et al. Efficacy of HPV DNA testing with cytology triage and/or repeat HPV DNA testing in primary cervical cancer screening. J Natl Cancer Inst. 2009;101(2):88–99. doi: 10.1093/jnci/djn444. [DOI] [PubMed] [Google Scholar]
- xiii.Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, et al. New Technologies for Cervical Cancer Screening Working Group. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst. 2008 Apr 2;100(7):492–501. doi: 10.1093/jnci/djn065. [DOI] [PubMed] [Google Scholar]
- xiv.Gravitt PE, Coutlée F, Iftner T, Sellors JW, Quint WGV, Wheeler CM. New technologies in cervical cancer screening. Vaccine. 2008;26S:K42–52. doi: 10.1016/j.vaccine.2008.05.002. [DOI] [PubMed] [Google Scholar]
- xv.Franco EL, Cuzick J. Cervical cancer screening following prophylactic human papillomavirus vaccination [review] Vaccine. 2008 Mar 14;26(suppl 1):A16–23. doi: 10.1016/j.vaccine.2007.11.069. [DOI] [PubMed] [Google Scholar]
- xvi.Castle PE. The potential utility of HPV genotyping in screening and clinical management. JNCCN. 2008;6(1):83–95. doi: 10.6004/jnccn.2008.0008. [DOI] [PubMed] [Google Scholar]
- xvii.Vijayaraghavan A, Efrusy M, Lindeque G, Dreyer G, Santas C. Cost effectiveness of high-risk HPV DNA testing for cervical cancer screening in South Africa. Gynecol Oncol. 2009 Feb;112(2):377–83. doi: 10.1016/j.ygyno.2008.08.030. [DOI] [PubMed] [Google Scholar]
- xviii.Goldhaber-Fiebert JD, Stout NK, Salomon JA, Kuntz KM, Goldie SJ. Cost-effectiveness of cervical cancer screening with human papillomavirus DNA testing and HPV-16,18 vaccination. J Natl Cancer Inst. 2008 Mar 5;100(5):308–20. doi: 10.1093/jnci/djn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xix.Goldie SJ, Kim JJ, Myers E. Chapter 19: Cost-effectiveness of cervical cancer screening. Vaccine. 2006 Aug 31;24(Suppl 3):S3/164–70. doi: 10.1016/j.vaccine.2006.05.114. [DOI] [PubMed] [Google Scholar]
- xx.Apple et al; Prevalence of High-Risk (HR) HPV Genotypes in a US General Screening Population with Normal Cytology
- xxi.Keshavarz H, Hillis SD, Kieke BA, Marchbanks PA. Hysterectomy surveillance --- United States, 1994-1999. CDC surveillance summaries (July 12) MMWR. 2002 Jul 12;51(SS05):1–8. [PubMed] [Google Scholar]
- xxii.Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998 Feb 12;338(7):423–8. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- xxiii.Herrero R, Castle PE, Schiffman M, Bratti C, Hildesheim A, Morales J, et al. Epidemiologic profiles of type-specific human papillomavirus infection and cervical neoplasia in Guanacaste, Costa Rica. JID. 2005 Jun 1;191:1796–807. doi: 10.1086/428850. [DOI] [PubMed] [Google Scholar]
- xxiv.Myers ER, McCrory DC, Nanda K, Bastian L, Matchar DB. Mathematical model for the natural history of human papillomavirus infection and cervical carcinogenesis. Am J Epidemiol. 2000;151(12):1158–71. doi: 10.1093/oxfordjournals.aje.a010166. [DOI] [PubMed] [Google Scholar]
- xxv.Sanders GD, Taira AV. Cost effectiveness of a potential vaccine for human papillomavirus. Emerg Infect Dis. 2003 Jan;9(1):37–48. doi: 10.3201/eid0901.020168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxvi.Saraiya M, Ahmed F, Krishnan S, Richards TB, Unger ER, Lawson HW. Cervical cancer incidence in a prevaccine era in the United States, 1998-2002. Obstet Gynecol. 2007 Feb 1;109(2 Pt 1):360–70. doi: 10.1097/01.AOG.0000254165.92653.e8. [DOI] [PubMed] [Google Scholar]
- xxvii.Clifford GM, Smith JS, Plummer M, Muñoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003;88(1):63–73. doi: 10.1038/sj.bjc.6600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxviii.Schlecht NF, Platt RW, Duarte-Franco E, Costa MC, Sobrinho JP, Prado JCM, et al. Human papillomavirus infection and time to progression and regression of cervical intraepithelial neoplasia. J Natl Cancer Inst. 2003 Sep 3;95(17):1336–43. doi: 10.1093/jnci/djg037. [DOI] [PubMed] [Google Scholar]
- xxix.National Cancer Database (NCDB) [2006 Nov];Commission on Cancer, ACoS/ACS. Survival Reports [online]. Available at: http://web.facs.org/ncdbbmr/sas6/surv/GRAPHS/OY97S38XaT00000B.html.
- xxx.Ratnam S, Franco EL, Ferenczy A. Human papillomavirus testing for primary screening of cervical cancer precursors. Cancer Epidemiol Biomarkers Prev. 2000 Sep 9;:945–51. [PubMed] [Google Scholar]
- xxxi.Cuzick J, Clavel C, Petry KU, Meijer CJ, Hoyer H, Ratnam S, et al. Overview of the European and North American studies on HPV testing in primary cervical screening. Int J Cancer. 2006 Sep 1;119(5):1095–101. doi: 10.1002/ijc.21955. [DOI] [PubMed] [Google Scholar]
- xxxii.Bigras G, de Marval F. The probability for a Pap test to be abnormal is directly proportional to HPV viral load: results from a Swiss study comparing HPV testing and liquid-based cytology to detect cervical cancer precursors in 13 842 women. Br J Cancer. 2005 Sep 5;93(5):575–81. doi: 10.1038/sj.bjc.6602728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxxiii.World Health Organization. Macroeconomics and health: investing in health for economic development: report of the Commission on Macroeconomics and Health. Geneva: World Health Organization; 2001. [Google Scholar]
- xxxiv.International Agency for Research on Cancer, World Health Organization. IARC handbooks of cancer prevention : Cervix cancer screening. Vol. 10. Lyon, France: IARC Press; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


