Abstract
In recent years, there have been major advances in the development of novel nanoparticle and microparticle-based therapeutics. An emerging paradigm is the incorporation of biomimetic features into these synthetic therapeutic constructs to enable them to better interface with biological systems. Through the control of size, shape, and material consistency, particle cores have been generated that better mimic natural cells and viruses. In addition, there have been significant advances in biomimetic surface functionalization of particles through the integration of bio-inspired artificial cell membranes and naturally derived cell membranes. Biomimetic technologies enable therapeutic particles to have increased potency to benefit human health.
Keywords: biomimetic, micro/nanoparticle, biomaterial, drug delivery
Engineering Biomimetic Particles for Next Generation Therapeutics
Particle-based therapeutics have been investigated in recent years for diverse biomedical applications. Owing in part to their ability to circumvent biological delivery barriers, customizability to a desired therapeutic and application, and compatibility with biological systems, there has been significant progress in translating particle-based therapeutics to the clinic for diseases such as cancer and infectious disease. Through rational design, biomimetic particles, or particles that mimic biological function, can be developed as therapeutics. The particles are often on the nanoscale or are on the microscale with nanoscale features. Biomimetic particles can modulate cell function, encourage tissue regeneration, and provide for enhanced drug delivery over traditional particle therapeutics.
Biomimetic particles as next generation particle-based therapeutics replicate features from the biological systems and surfaces that the particles are designed to engage. By taking what has been learned through studies of cell and viral biology, particles can be engineered to mimic core cellular or viral aspects including physical properties such as material mechanical properties, particle size, and particle shape. In addition, these particles can be fabricated with surface features that also mimic the natural chemical and biological properties of biological surfaces including chemical composition, three-dimensional surface protein presentation, and membrane fluidity (Table 1). These strategies are increasingly being utilized and found to potentiate enhanced therapeutic action for the intended applications.
Table 1.
Summary of various biomimetic particle based therapeutics.
| Selected Biomimetic Focus |
Biomimetic Target* |
Biomimetic Feature** |
Material*** | Size | Application | Reference |
|---|---|---|---|---|---|---|
| Core Particle | Red blood cells | Low compressive modulus | HEA/PEGDA/CEA hydrogel | 6 μm | Extension of circulation half life | [24] |
| APCs | Magnetic assembly of APC/T-Cell sMAC | Iron-dextran | 50-100 nm | Antigen specific T-Cell activation | [13, 14] | |
| APCs | Ellipsoidal artificial cell shape | PLGA | 4-5 μm | Antigen specific T-Cell activation | [45, 46] | |
| Filamentous viruses | Non-spherical micellar DNA vector | PEG-b-PPA | 40-70 nm | Hepatic gene delivery | [60] | |
| Stromal cells | Paracrine delivery of LIF | PLGA | 100-200 nm | Dopaminergic cell regeneration | [65] | |
| APCs | Paracrine delivery of IL-2 | PLGA | 8 μm | Antigen specific T-Cell activation | [69] | |
| Surface Chemistry | “Self” | “Self” marker surface presentation | PS and CD47 mimicking peptides | 160 nm | Extension of circulation half life | [74] |
| Cell membranes | PEGylated lipid envelope | PLGA/PEG-DSPE/Combrestatin | 80-120 nm | Drug delivery for tumor treatment | [75] | |
| Cell membranes | Targeted particle supported lipid bilayer | Mesoporous silica and lipid bilayer | 120 nm | Cellular binding and intracellular delivery | [90-93] | |
| Red blood cells | RBC derived supported membrane | PLGA and RBC membrane | 70 nm | Extension of circulation half life | [97-100] | |
| White blood cells | WBC derived supported membrane | Silica and leukocyte membranes | 1-2 μm | Enhanced trafficking to inflamed areas | [101] | |
| Cancer cells | Melanoma derived supported membrane | PLGA and melanoma cell membranes | 100-200 nm | Cancer vaccination | [104] |
APC = antigen presenting cell.
sMAC = supramolecular activation cluster, LIF = leukemia inhibitory factor, RBC = red blood cell, WBC = white blood cell.
HEA/PEGDA/CEA = 2-hydroxyethyl acrylate/polyethylene glycol diacrylate/2-carboxyethyl acrylate, PLGA = poly(lactic-co-glycolic acid), PEG-b-PPA = polyethylene glycol-b-polyphosphoramidate, PS = polystryrene, PEG-DSPE = polyethylene glycol distearoylphosphatidylethanolamine
Many exciting new approaches are emerging from efforts to utilize bio-inspired design of new drug delivery systems. We will focus here on >100 nm particles which themselves directly mimic existing biological modalities for therapeutic applications. In addition to the biomimetic particles described here, many other approaches to bio-inspired drug delivery offer substantial promise [1, 2] such as apoferritin nanocages [3] and human serum albumin nanoparticles [4]. In addition, although the scope of this article includes a broad discussion of biomimetic particles, an area where biomimicry has particular impact is in the field of immunoengineering. As a result, many illustrative examples for engineering various biomimetic particles at the interface of chemistry, biology, and materials science are applications in this field. In describing these biomimetic particles, an inside-out approach is utilized starting with selection of core material properties, particle size, particle shape, and choice of encapsulated therapeutic. Subsequently, the design of biomimetic surfaces on these particles is described through the engineering of artificial cell membranes and the selection of surface bound ligands to mimic natural cells and viruses. Biomimetic particles can enable the realization of next generation therapeutics, where synthetic nanoparticles and microparticles emulate biological processes to enable highly efficacious and safe particle-based therapeutics.
Physical Properties: Size, Material, and Shape
Size
One important parameter that must be considered in the design of a biomimetic therapeutic is the size of the particle intended to interact with the biological system. In the case of particles intended for cellular mimicry, the literature suggests that the closer the particle is to cellular size, the more potent the effect on the target cell. One classic example of this concept is the artificial antigen presenting cell (aAPC). aAPCs mimic the natural function of the traditional antigen presenting cell through the presentation of APC surface proteins on an acellular substrate. These surface proteins include a T-Cell Receptor agonist (such a recombinant major histocompatibility complex loaded with antigen), and an agonist for a costimulatory receptor (such as a monoclonal antibody for CD-28 on the cell surface). Various platforms have been utilized for the artificial antigen cell including polystyrene [5], biodegradable polyesters [6], and lipid based formulations [7]. Classically, size is a critical factor for modulating T-Cell activity with larger 4-5 micron particles identified as optimal at T-Cell stimulation [5]. More recently, it has also been shown that for the same protein dose, nanodimensional aAPCs elicit 3-fold less cytokine production from T-Cells compared to micron-scaled aAPC counterparts [8]. One reason postulated for the relative ineffectiveness of nano aAPCs is that their individual areas of surface contact with the T-Cell are too small to permit T-Cell receptor clustering on the micron scale [9]. It has been shown that in a natural T-Cell/APC engagement, there is a dynamic rearrangement of surface receptors involved in signaling termed the immunological synapse (IS). [10] The net result is micron-scale clustering of T-Cell receptors and co-stimulatory receptors. Furthermore, this clustering has been shown to result in the enhanced activation of T-Cells.[11] Nanoengineering approaches may improve upon this paradigm [12]; in particular, one recent strategy used to circumvent this difficulty is the use of magnetic nano aAPCs and an externally applied magnetic field to drive receptor clustering. With this platform, the nano aAPCs demonstrated a 15-fold increase in antigen specific T-Cell proliferation by clustered magnetic nano aAPCs compared to non-clustered magnetic nano aAPCs [13, 14].
At the level of a virus, nanoparticles of controlled size have been fabricated for size-dependent mimicry of viral function. One of the features of the viral/host interaction that has been studied in great detail is the internalization rates and pathways of different sized particles. Nearly all eukaryotic cells engage in endocytosis and it has been shown that particles as large as 1 μm can be endocytosed in this process [15]. In addition, it has been shown that nanoparticle size can have a strong influence on the rate of internalization and the subsequent intracellular trafficking routes.[16]
One field where the size-dependent impact of endosomal trafficking of nanoparticles is gaining increasing attention is the area of nanoparticle vaccines. Nanoparticle vaccines attempt to mimic normal viral infection through the delivery of an antigen, or a gene encoding an antigen, and an adjuvant to accelerate the immune response to the antigen. As viruses exhibit a large diversity in size and immune response [17], this factor must be considered when designing a nanoparticle vaccine. Although the correlation between immunogenicity and nanoparticle size can depend on the application [18], it is clear that nanoparticle size can be used to tune the immune response. One potential explanation recently revealed is the preferential targeting of specific antigen presenting cell subsets. Numerous studies have been published on the impact of size on dendritic cell/macrophage cell internalization and subsequent immune phenotype of these cells [19-21]. Continued investigation into the effect of size on nanoparticle vaccine efficacy will greatly enhance our ability to mimic the natural capability of viruses to elicit immune responses.
Material
One of the most fundamental levels of biomimicry that can be achieved with a particle is through the selection of a core material. Traditionally, many therapeutic particles are synthesized from polymeric or inorganic metal precursors [22]. Although effective for many applications, these materials generally are not biomimetic owing in part to their synthetic nature. Consequently, there has been a focus to generate particles from materials that better mimic the natural biologic properties of cells and viruses.
For biomimicry at the cellular level, a key aspect is particles that have a low compressive modulus. One way this has been achieved is through the use of hydrogel-based microparticles synthesized from preformed template molds. Hydrogels have been shown to mimic red blood cell (RBC) compressibility, thus allowing them to squeeze through small flow channels [23]. By modulating the percentage of crosslinker used during hydrogel synthesis, particles of different rigidity can be synthesized ranging from 7.8 kPa to 63.9 kPa. Standard RBC modulus was noted to be 15-26 kPa. Low modulus microgels demonstrated a 45-fold increase in elimination half-life compared to the equivalent high modulus microgels [24]. This effect was further demonstrated to be maintained across a wide variety of particle sizes smaller than 8.9 μm [25]. Another way in which particles mimicking the mechanical properties of cells have been created is thorough chemical destabilization of particle cores. Doshl et. al. demonstrated a 10,000-fold reduction in particle modulus (from 106 kPa to 102 kPa) through core destabilization [26]. The resulting poly(lactic-co-glycolic acid) (PLGA) microparticles demonstrated deformability under flow through a size restricting capillary.
Viral mimicry can be achieved through direct use of biologically derived substances. For example, virus-like particles (VLPs) can be synthesized directly from viral capsid proteins. Due to their spontaneous self-assembly, and lack of genetic material required for viral reversion, VLPs are easy to assemble and safe to use compared to live viral counterparts [27]. In addition, since the process of VLP assembly retains the antigenic protein structure of the live virus, these nanomaterials have been well researched for their capability to act as adjuvants for vaccine therapies [28]. Owing in part to their safety and efficacy as vaccines, several VLP based platforms have been clinically approved including one for hepatitis B and human papilloma virus [29].
Material selection can also have implications for surface physiochemical properties such as topography and zeta potential. These surface properties can in turn have a broad impact on the way these materials interact with the biological system with which they are designed to interface. For example, surface charge can impact the affinity of a nanoparticle for the negatively charged membrane [30]. It was recently described by Cho et. al. using gold nanoparticles with different adsorbed molecules (containing cationic, anionic, and neutral surface moieties) that a positive surface charge on the particle enabled higher rates of internalization through the negatively charged membrane [31]. Surface roughness can also impact the way a cell interacts with a biomaterial. Returning to the aAPC as an example, there has been recent work by Fadel et. al. on the use of bundled carbon nanotubes as the core material for these biomimetic constructs [32]. Through modulation of chemistry in synthesis and subsequent introduction of surface defects, the authors were able to increase the accessible surface area for protein adsorption. The impact on T-Cell activation was dramatic with more than 10-fold higher levels of T-Cell activation by carbon nanotube aAPC with a rough surface topography compared to those without [32]. This finding was exploited in a later study to rapidly and efficiently generate large numbers of antitumor T-Cells for melanoma therapy [33].
Shape
One of the recently elucidated parameters to generate biomimetic particles is through the use of shape. At the micron scale, cells will present a dynamically non-spherical three dimensional shape when interacting with other cells and the extracellular matrix [34]. At the nanometer scale, viruses exhibit highly irregular 3D morphology which has been postulated to be correlated with cellular invasion [35]. To generate anisotropic particles, numerous approaches have been demonstrated including a thin film stretching method developed by Ho et. al. [36] and subsequently expanded for additional shapes [37] and automated [38]. Another well-researched method for the generation of particles with anisotropic shape is the particle replication in non-wetting templates method or PRINT [39]. This procedure has been recently expanded to produce particles of various shape and material composition [40, 41]. In addition to the PRINT and thin film stretching techniques, there are also a variety of microfluidic techniques and self-assembly based techniques for the generation of anisotropic particles as well [42, 43].
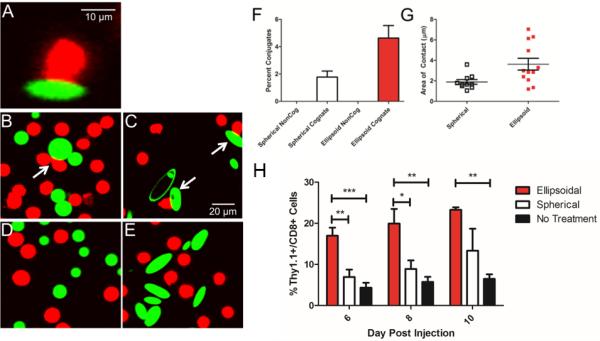
Through their biomimicry, non-spherical particles have been shown to have a clear advantage over spherical particles as artificial antigen presenting cells. Traditionally, these particles have been spherical and micron sized in nature [44]. Non-spherical aAPCs were shown to elucidate a 20-fold stronger T-Cell response compared to spherical counterparts [45]. In addition, these aAPCs mediated a stronger antitumor effect in vivo compared to spherical aAPC, and the effects of particle shape were separable from other critical factors like antigen density or antigen dose [45]. Confocal experiments showed that this effect was mediated by improved interaction along the long axis of the ellipsoidal aAPCs (Figure 1A-G) [45]. Despite the evidence suggesting the ineffectiveness of nano scale aAPCs [8] this shape effect was shown to be preserved at the nano-scale as well, with ellipsoidal nano aAPCs eliciting 15-fold stronger T-Cell activity compared to spherical nano aAPCs (Figure 1H) [46].
Figure 1.
Particle shape is a critical factor in antigen presenting cell biomimicry. (a) T-Cell (red) interacts with the long axis of an ellipsoidal artificial antigen presenting cell (green). Widefield view of (b,d) spherical and (c,e) ellipsoidal aAPC interacting with (b,c) cognate and (d,e) non-cognate T-Cells. (f) Ellipsoidal aAPC leads to greater frequency of conjugates between T-Cells and aAPC compared to spherical aAPC. (g) Ellipsoidal aAPC leads to greater length of contact between T-Cell and aAPC compared to spherical aAPC. (h) Nanoellipsoidal aAPC leads to greater in vivo proliferation and activation of T-Cells compared to equivalent spherical aAPC. Modified reproduction with permission from Sunshine et al, Biomaterials (2014) [45] and Meyer et al, Small (2014) [46].
Non-spherical nanoparticles have also gained interest for their drug delivery potential and enhanced mimicry of viral function for intracellular delivery. Recent evidence has suggested that non-spherical nanoparticles avoid non-specific clearance by the reticuloendothelial system [47]. For macrophage clearance, it has been demonstrated that ellipsoids can be internalized 2-fold less than their spherical counterparts [48]. Furthermore, aspect ratio plays a role in this inhibition of cellular uptake. Using MSCs and HeLa cells, Florez et. al. demonstrated that particle with an aspect ratio of 4 were taken up 2-5 times less than particles with aspect ratio of 2 [49]. In an attempt to elucidate this phenomenon, several groups have investigated the mechanism behind this differential uptake based on cell shape. Champion et. al. demonstrated that the angle in which the particle approaches the membrane has a profound impact on whether or not it is internalized [50]. For spherical particles, the angle of approach has no effect on internalization of the particle due to isotropy. For non-spherical micro and nanoparticles, it was found that the rod-shaped particle could only be successfully internalized if the particle approached the membrane perpendicular to its long axis. Otherwise, the particle would not be internalized [50]. This observation could potentially be linked to the increased strain energy required for the membrane to wrap around the particle. Agarwal et. al. investigated internalization rates of rod and disc shaped particles of different shapes and aspect ratios. The authors found that discs were internalized more readily than rods and this was linked to the calculated 2 fold increase in strain energy of the membrane wrapping around the rod shaped particles as opposed to the disc shaped particles [51].
In addition to resistance of non-specific cellular uptake, non-spherical particles have been shown to have increased targeted binding to cells in vitro. Barua et. al. reported that conjugation of a targeting antibody increased binding specificity and targeted uptake of prolate ellipsoids by a factor of 3 compared to spheres [52]. This effect translated in vivo as well. Kolhar et. al. demonstrated 2-fold more accumulation of targeted ellipsoids compared to spheres in lung endothelium (through conjugation of an antibody for ICAM for lung endothelial cells), and up to 7.5-fold more accumulation of ellipsoids compared to spheres in brain endothelium (through conjugation of an antibody for the transferrin receptor on brain endothelial cells) [53]. The mechanism behind the increased targeted binding to cells is more intuitive then that of the reduced non-specific uptake [54]. Non-spherical particles such as rods and discs generally have regions where the radius of curvature at the surface is much higher than that of spherical particle of equivalent volume. Assuming a fixed length of the targeting ligand, this increases the available functionalized area on the particle with which the cell can interact. As a result, there are more protein/ligand interactions between a particle with a larger radius of curvature than that of a smaller one. This increased avidity of non-spherical particles for targets in turn increases the binding affinity compared to volumetrically equivalent spherical particles [54].
There have also been many reports of successful intracellular drug and genetic therapeutic delivery with anisotropic nanoparticles. PRINT based rod shaped particles have been used extensively for applications including siRNA delivery [55], replicon vaccine delivery [56], and chemotherapeutic delivery [57, 58]. In addition, cylindrically shaped PRINT based particles have been used to investigate the impact of drug weight percentage in drug loading. Through direct templating of particles with the use of a single solvent, Chu et.al. was able to fabricate nanocylinders with a precise weight ratio of docetaxel content [59]. The authors demonstrated for the same amount of drug administered, particle with a lower weight ratio of the therapeutic demonstrated more effective delivery of the drug to a tumor site, and subsequent therapeutic benefit in an adenocarcinoma model. Such studies illustrate the importance of drug loading as a variable to be controlled in the fabrication of biomimetic particles for drug delivery.[59]
Self-assembled anisotropic nanoparticles have also been utilized in the past decade for gene and chemotherapeutic delivery. One prominent example in the literature of the use of shape to enhance the delivery of genetic therapeutics is the use of PEG-PPA block copolymers self-complexed with a plasmid to generate particles with anisotropic shape for gene delivery [60]. These rod-like micellar nanoparticles outperformed equivalent spherical nanoparticles for hepatic gene delivery in vivo by 10,000-fold [61]. Anistotropic self-assembled nanoparticles have also shown promise as vectors for intracellular delivery of doxorubicin. Karagoz et. al. demonstrated that block copolymer worm-like micelle formulations of doxorubicin were able to mediate 7-fold increased killing of cancer cells compared to spherical micelles [62]. These promising results motivate continued research in the application biomimetic nanoparticles for intracellular delivery.
Soluble Mediators
Aside from mimicking the physical form of cells and viruses, there has been significant effort into designing particle therapeutics that mimic the secretory function of cells as well. One of the most widely studied methods to achieve this goal is the controlled release of small molecules and proteins for a paracrine effect on other cells. Mimicking the localized paracrine delivery of small molecules exhibited by cells is desirable for many therapeutic applications. It allows for spatial control over small molecule or protein therapeutic concentration to ensure only the target cell/cells are affected. One example of a natural, biological situation where this paracrine release occurs is in the engagement of a helper CD4+ T-Cell with its target. After activation by an APC, a CD4+ cell will polarize its granules for directional secretion of soluble mediators.[63] This ensures that only the subsequent target cells to which the CD4+ T-Cell binds will be effected by the secretion of these potent immunostimulatory cytokines such as IFN-γ. Biodegradable polymers remain a popular material of choice for this application due to their capability to interface with biological systems and permit controlled release of a therapeutic. Three applications involving mimetic biodegradable particles for paracrine delivery of soluble mediators include the delivery of growth factors for tissue regeneration, the delivery of chemokines for directed cell migration, and delivery of immune cytokines for enhanced immune cell activation.
The first application, particulate systems for controlled growth factor delivery, has been investigated for stem cell research and regenerative medicine due to the capability to mimic the temporal control over release seen in natural tissue regeneration. As an example, hydrogel microrods can deliver an insulin like growth factor mimicking peptide to prevent unwanted cardiac remodeling and enhance survival in a post myocardial infarction mouse model [64]. Additionally, for neural regeneration, PLGA nanoparticles have been shown to deliver leukemia inhibitory factor to support the growth of dopaminergic cells for the regeneration of lost tissue observed in Parkinson's disease [65].
Controlled release of chemokines for control over cell migration has been second area of interest in the application of biodegradable nano- and microparticles for paracrine delivery of soluble mediators. One area where establishment of a chemokine gradient has been of importance is the administration of particulate-based vaccines. To enhance the efficacy of dendritic cell targeting vaccines, Zhao et. al. designed PLGA microspheres designed to establish a chemokine gradient of chemoattractant molecules for DCs [66]. For recruitment of immune cells to the lymph nodes, Popova et.al. took advantage of the natural tendency of nanoparticles of sufficiently small size to drain to the lymph nodes upon injection. Once at their intended site, the nanoparticles would release a chemokine to attract a target immune cell to the lymph nodes [67]. Biomimetic particles have also been used to direct the migration of mesenchymal stem cells as well. Huang et. al. encapsulated stromal cell-derived factor-1 in a chitosan based nanoparticle and demonstrated a 5-fold increase in MSC migration in a transwell assay over blank nanoparticle controls or soluble growth factor alone [68].
Finally, paracrine delivery of soluble mediators has been applied in the delivery of cytokines in the context of aAPC technology. During the intimate contact between the APC and T-Cell, the APC can release soluble mediators known as “Signal 3” to alter target T-Cell function. Owing in part to the tight contact and minimal distance between the two cells, these soluble mediators primary affect the target T-Cell. Taking this cue, Steenblock et. al. designed an aAPC with paracrine delivery of IL-2, the T-Cell growth factor, through the use of biodegradable PLGA. As a result of this loading there was an enhancement of T-Cell proliferation compared to soluble IL-2 [69]. In a follow up study, the same group explored this effect with computational modeling of release from a biodegradable particle juxtaposed against a cell. With reduced contact space between the aAPC and the T-Cell, there was a 2-fold increase in surface concentrations of the released therapeutic and through local release this stimulation could occur with significantly lower doses IL-2 (Figure 2) [70]. In a melanoma tumor treatment model, Fadel et. al. reported similar therapeutic effect of the aAPC with paracrine delivery of IL-2 compared to aAPC administration with 1000-fold higher amounts of soluble IL-2 [33]. These results motivate further studies of local paracrine delivery of soluble mediators from targeted nanoparticle and microparticle therapeutics.
Figure 2.
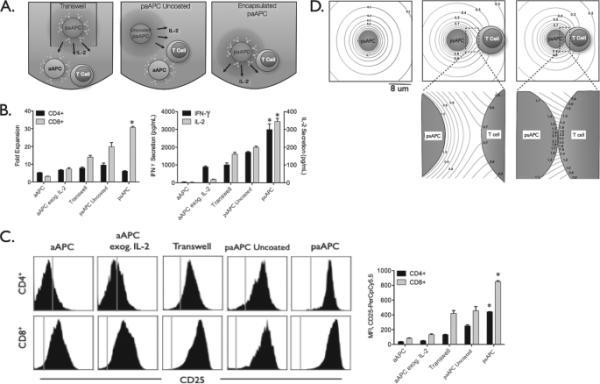
Paracrine release of IL-2 by a targeted nanoparticle therapeutic enhances T-Cell proliferation and activation. (a) Schematic illustration of experimental conditions to test the efficacy of paracrine delivery of IL-2. In the transwell condition, the particle and cells are separated by a transwell barrier. In the uncoated condition, the particle and cells are mixed together but there is no targeted delivery of the IL2. (b) The highest T-Cell fold expansion is observed in the groups with targeted paracrine delivery of IL-2 by artificial antigen presenting cell particles. (c) CD-25 (IL-2 receptor) expression is highest in the group with targeted paracrine delivery of IL-2. (d) Computational modeling of local juxtapositional concentration effects. With the narrowing of the gap between the aAPC and the cell, there is an increase in the available local concentration of the released therapeutic. Reproduced with permission from Steenblock et al, J Biol Chem (2011) [70].
Surface Chemistry Mimicry
Biological cells are surrounded by highly complex membranes composed of lipid bilayers and glycated cell surface proteins, which are highly organized and transmit and receive critical signals that direct normal and abnormal cellular function. Recently, there has been increased attention in taking cues from this biology to design therapeutic particles with greater biomimicry. There are two main approaches towards development of biomimetic particles in general and for particles that mimic cell surfaces in particular. Bottom-up approaches begin with molecular components and then build these up through physical and chemical approaches into larger structures. Bottom-up approaches include many microfluidic and self-assembly-based techniques for the generation of supramolecular assemblies and particles. To emulate cells, bottom up approaches typically include the molecular components of cells such as lipids, as well as desired surface proteins, which are synthetically incorporated either by surface functionalization of an intact lipid bilayer or by formulating the proteins with lipid linkers and adding them during a particle coating step. Top-down approaches, on the other hand, are either directly engineered from the macroscale (such as PRINT technology) or directly utilize macroscale biological components that are reformulated. For example, to cause a particle to more closely emulate a cell, cell membranes can be harvested from biological cells and directly added to the particle cores.
Bottom up approaches
Surface conjugation
A major goal in particle-based drug delivery involves the avoidance of non-specific uptake and rapid clearance from the blood by immune cells such as macrophages. Classical approaches towards this problem involve densely conjugating hydrophilic polymers such polyethylene glycol (PEG) to the particle surface in an effort to minimize interactions with biomolecules and cells based on hydrophobic interactions and charge [71, 72]. Although there is significant research on the use of polymers to make nanomedicines more amenable to in vivo use, our focus in these sections is to summarize some of the strategies that utilize design elements from cell membranes to avoid foreign designation by the body during circulation. For a more comprehensive review on some of these surface chemistries, we refer the reader to a more comprehensive review.[73]
One approach to improve on the PEGylated particle model is identification and use of biological “self” signals. CD47 has recently been identified as perhaps the most potent “self” marker and Rodriguez et al developed “self” peptides, computationally derived from human CD47, that optimally bind to the same binding partner as CD47 (CD172a) [74]. These “stealth” self peptides strongly inhibit nanoparticle uptake by macrophages at far lower densities than are required for effective protection by PEG, requiring only a single CD47 molecule per 60 nm particle [74].
Lipid coating
Cellular membranes are formed from fluid lipid bilayers and present a variety of protein signals in addition to being shielded by a dense glycocalyx. Sengupat et al developed anticancer “nanocells” (NC) [75] composed of a core PLGA nanoparticle with an encapsulated chemotherapeutic that was surrounded by a peglyated-lipid envelope that trapped an anti-angiogenic agent. This allowed for rapid release of the antiangiogenic agent followed by slower release of the chemotherapeutic, enabling vascular shutdown prior to direct tumor killing. NC showed significant tumor accumulation, tumor growth inhibition, mouse survival, tumor cell apoptosis, and reduced systemic toxicity.
Polymer-supported lipid bilayers (SLBs) have been used for a long time as cell-surface models [76-89]. Advantages of SLBs over polymer-support free systems such as free standing lipid membranes or liposomes include improved mechanical stability and maintenance of membrane fluidity [83, 84]. They have been developed for phase-transition chromatography [85, 86] as well as isolation of cell membranes on particle surfaces [87-89]. In a biomimetic approach, the Brinker group developed “protocells” with mesoporous silica cores surrounded by fully synthetic lipid bilayers [90-93]. These fully synthetic protocells can encapsulate a variety of chemically drugs and present ligands on the supported lipid bilayer surface, including short peptides and larger antibodies or glycoproteins (Figure 3A). This enables presentation of a variety of important biological signals on the cell-like artificial surface, including targeting moieties to enhance specific cell targeting and fusogenic peptides to enhance endosomal escape on the surface of the nanocarrier [93]. Compared to liposomes presenting the same targeting peptides, these protocells show increased membrane fluidity and as a result show enhanced specific binding due to the ability to recruit additional peptides to the particle-cell interface, increasing the multivalency of the interaction [93].
Figure 3.
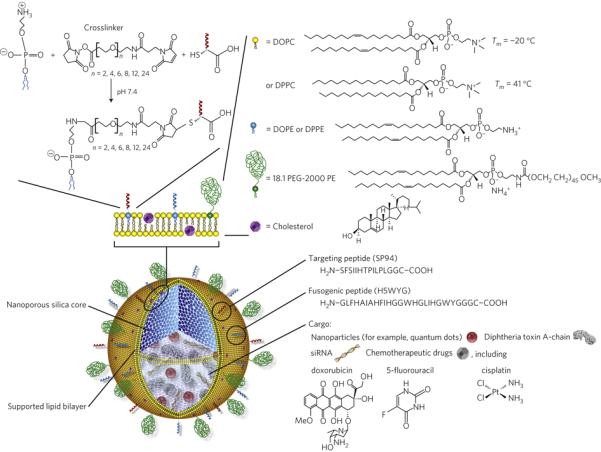
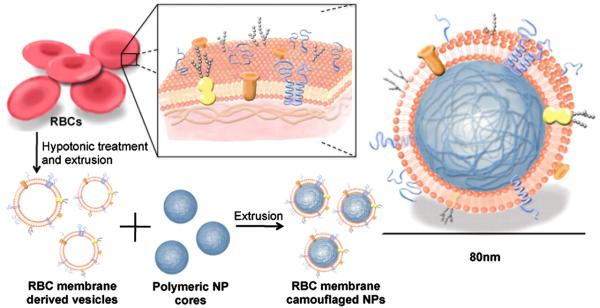
Schematics of (A) bottom-up, fully synthetic, biomimetic particle supported lipid bilayers, reprinted with permission from Ashley et al., Nat Mater. (2011)[93] and (B) top-down particle coating with biological membranes, reprinted with permission from Hu et al., PNAS (2011)[97].
Top down approaches: Cell membrane coated particles
Rather than synthesizing an artificial membrane out of fully synthetic components, several groups have recently explored the concept of harvesting and purifying biological membranes from cells and then using the purified membrane to coat particles. Red blood cells (RBCs) offer a number of advantages as potential targets for biomimetic therapeutic particles as they have extremely long circulating half-lives, possess a discoidal-shape, and have the flexibility to squeeze through capillaries. Multiple groups have purified RBC membranes via hypotonic lysis and centrifugation and generated RBC vesicles either via sonication or physical extrusion of the purified membrane through filters with defined pore sizes.These RBC vesicles, also named resealed erythrocytes, can then be loaded with a variety of hydrophilic drugs in the same fashion as with synthetic liposomes [94-96]. Challenges with this approach include early removal from the circulation, early drug release for smaller or more lipophillic molecules, and variability with particle fabrication and stability.
Hu et al [97] fused RBC vesicles with nanoparticle cores to form RBC membrane camouflaged nanoparticles (Figure 3B) by mixing nanoparticles cores with preformed RBC vesicles and then extruding the mixture repeatedly through polycarbonate filters. These RBC membrane camouflaged nanoparticles showed superior in vivo half-life compared to PEGylated nanoparticles. The process coats the nanoparticles in a unilamellar fashion, preserves the majority of the membrane proteins, including CD47, and presents them in a correct-side out orientation [98]. These RBC-coated particles were used as RBC decoys to protect against RBC lysis from pore forming toxins (PFTs) [99]. When formulated with PFTs and injected, these particles engender effective immune responses against the PFTs [100].
Leukocytes or white blood cells use their cell surface interactions to bind to inflamed endothelium and respond to infection in the tissues. Parodi et al [101] encapulated nanoporous silica nanoparticles with white blood cell (leukocyte) membranes and demonstrated that these “leukolike vectors” (LLVs) recapitulate functions of white blood cells, including avoiding immunological clearance, interacting with endothelial cells, and transporting drugs across a model of inflamed endothelium. In the presence of TNF-alpha, LLVs are transported across an inflamed endothelial barrier 4-fold more efficiently than bare particles, showing the same propensity to cross-inflamed endothelium as biologic leukocytes. These properties allowed doxorubicin-loaded LLVs to show enhanced tumor cell killing with relative protection of the endothelium, delayed liver clearance, and tumor accumulation in mice.
Nanoparticle biomimicry may be extended to other cell types as well through the harvesting of cellular “ghosts” such as from platelets [102] or mesenchymal stem cells (MSCs) [103]. MSCs show intriguing active tumor-targeting properties, and Furman et al. showed that MSC vesicles or nanoghosts loaded with sTRAIL could substantially inhibit prostate tumor growth in vivo [103]. Of particular note, this effect was restricted to nanoghosts derived from MSCs, as nanoghosts derived from human smooth muscle cells, which were of similar size and physical properties, showed no therapeutic effect [103]. This type of biomimicry is not restricted to use of purified membranes from healthy cells alone. Cancer cell membrane coated nanoparticles (CCNPs) [104] have also been produced and are efficiently taken up by dendritic cells and useful as cancer nanovaccines. CCNPs may also show enhanced tumor targeting for direct delivery of anticancer drugs.
Concluding remarks and future perspectives
The biomimetic strategies discussed in this review can be combined to enable next-generation particles for cellular engineering. On the microscale, such particles could closely mimic biological cells by precisely matching multiple aspects such as size, shape, membrane fluidity, presentation of protein extracellularly, receptor clustering, and release of soluble signaling factors. In some cases such “artificial cells” could have added benefits as therapeutics compared to biological cells as they can be precisely programmed and can resist extracellular signals from the microenvironment that are antagonistic to this precise programming. On the nanoscale, next-generation biomimetic particles could have enhanced stealth and “self” properties for prolonged circulation, enhanced avidity to their target through the design of shape, and the ability to signal on multiple biological levels simultaneously through the release of varied biomolecular cargos.
In one example of a potential future technology, off-the-shelf biomimetic biodegradable plastic particles, such as next-generation aAPCs, could be manufactured in large batches and stored lyophilized for long periods of time. Such particles could incorporate novel protein antigens, such as those discovered from a biopsy or by DNA/RNA sequencing, via synthetic peptide synthesis of the amino acid sequences followed by simple mixing with the off-the-shelf aAPCs to load the novel antigen on the surface. These aAPCs could also encapsulate synergistic molecules, such as monoclonal antibodies for checkpoint inhibitors, for in vivo release. Such an immunotherapy would be non-cellular and may have certain advantages compared to cellular therapy in terms of manufacturing and regulatory hurdles. This particle-based therapy would also have a direct advantage over a traditional cell based therapy in that it could both resist suppression by the host immune system as well as have a biological impact on checkpoint inhibitors that the traditional antigen presenting cell could not effect.
There are varied potential advantages of biomimetic particle systems. They can enable a more precise and potent biological response while theoretically also improving specificity and safety. They may also be able to replace cellular therapies in a manner that could be potentially more controlled, safe, and efficacious. However, as with all new technologies, significant challenges remain before clinical translation. Many of these particle systems are complex and involve biological therapies. Combination products encompassing biologics, drugs, and devices (the particle) are more challenging from a regulatory and clinical trials standpoint than conventional small molecules. Scaling up manufacturing can be a significant hurdle as well, and continuous-based processes can have certain advantages over batch synthesis processes. Although there are challenges, the future of biomimetic particles, precisely designed particles that mimic biological systems, is promising.
In this review, current approaches at extending the therapeutic efficacy of nanoparticle and microparticle systems were discussed in the context of designing the particles to be more biomimetic. Bio-inspired design of nanoparticles enables long circulation times and stealth interaction with off-target molecules and cells and simultaneous high affinity interactions with target cells. Such biomimetic particles can encapsulate and release soluble small molecules and proteins extracellularly or intracellularly and can also present biological molecules in specific orientations to trigger cell signaling of target cells. These approaches can be used to combat many diseases, including treating cancer in new ways including through nanoparticle-based vaccines and construction of artificial antigen presenting cells. The future of particle-based therapeutics involves learning from natural biological systems and designing the needed physical, chemical, and biological parameters to emulate them. Such biomimetic therapeutic particles have a promising outlook to benefit human health.
Biomimetic Particles as Therapeutics – Highlights.
➢ Engineering particle material, size, and shape enables enhanced biomimicry.
➢ Particles with paracrine delivery of soluble mediators mimic secretory cells.
➢ Artificial cell membranes mimic the surface chemistry of cells and viruses.
➢ Particles can be coated with naturally derived cell membranes for cell mimicry.
Table 2.
Summary of the various advantages and disadvantages of bottom up and top down surface biomimicry strategies.
| Advantages | Disadvantages | |
|---|---|---|
| Top Down | • Complete control over contents • Fully characterized • Readily customizable • Versatile |
• Often requires multiple input sources and many steps to synthesize |
| Bottom Up | • Can recapitulate complex properties • May offer properties whose mechanisms have not been fully elucidated as yet • Potentially highly biocompatible |
• Limited membrane sources • Incomplete control over content • Varying difficulty and cost in membrane purification depending on cell type |
Acknowledgements
RAM thanks the NIH Cancer Nanotechnology Training Center (R25CA153952) at the JHU Institute for Nanobiotechnology for fellowship support. JCS thanks the NIH MSTP program for support. The authors also thank the NIH (R01-EB016721) for support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, Irvine DJ. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507:519–522. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoo JW, Irvine DJ, Discher DE, Mitragotri S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat Rev Drug Discov. 2011;10:521–535. doi: 10.1038/nrd3499. [DOI] [PubMed] [Google Scholar]
- 3.Yan F, Zhang Y, Yuan H.-k., Gregas MK, Vo-Dinh T. Apoferritin protein cages: a novel drug nanocarrier for photodynamic therapy. Chem Commun. 2008:4579–4581. doi: 10.1039/b810949d. DOI. [DOI] [PubMed] [Google Scholar]
- 4.Kratz F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J Control Release. 2008;132:171–183. doi: 10.1016/j.jconrel.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Mescher M. Surface contact requirements for activation of cytotoxic T lymphocytes. The J Immunol. 1992;149:2402–2405. [PubMed] [Google Scholar]
- 6.Shalaby WS, Yeh H, Woo E, Corbett JT, Gray H, June CH, Shalaby SW. Absorbable microparticulate cation exchanger for immunotherapeutic delivery, J Biomed Mater Res Part B. Applied biomaterials. 2004;69:173–182. doi: 10.1002/jbm.b.20040. [DOI] [PubMed] [Google Scholar]
- 7.Giannoni F, Barnett J, Bi K, Samodal R, Lanza P, Marchese P, Billetta R, Vita R, Klein MR, Prakken B. Clustering of T cell ligands on artificial APC membranes influences T cell activation and protein kinase C θ translocation to the T cell plasma membrane. J Immunol. 2005;174:3204–3211. doi: 10.4049/jimmunol.174.6.3204. [DOI] [PubMed] [Google Scholar]
- 8.Steenblock ER, Fahmy TM. A comprehensive platform for ex vivo T-cell expansion based on biodegradable polymeric artificial antigen-presenting cells. Mol Ther. 2008;16:765–772. doi: 10.1038/mt.2008.11. [DOI] [PubMed] [Google Scholar]
- 9.Perica K, Kosmides AK, Schneck JP. Linking form to function: Biophysical aspects of artificial antigen presenting cell design. Biochim Biophys Acta-Mol Cell Res. 2014 doi: 10.1016/j.bbamcr.2014.09.001. DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alarcon B, Mestre D, Martinez-Martin N. The immunological synapse: a cause or consequence of T-cell receptor triggering? Immunology. 2011;133:420–425. doi: 10.1111/j.1365-2567.2011.03458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prakken B, Wauben M, Genini D, Samodal R, Barnett J, Mendivil A, Leoni L, Albani S. Artificial antigen-presenting cells as a tool to exploit the immunesynapse'. Nat Med. 2000;6:1406–1410. doi: 10.1038/82231. [DOI] [PubMed] [Google Scholar]
- 12.Sunshine JC, Green JJ. Nanoengineering approaches to the design of artificial antigen-presenting cells. Nanomedicine. 2013;8:1173–1189. doi: 10.2217/nnm.13.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perica K, De Leon Medero A, Durai M, Chiu YL, Bieler JG, Sibener L, Niemoller M, Assenmacher M, Richter A, Edidin M, Oelke M, Schneck J. Nanoscale artificial antigen presenting cells for T cell immunotherapy. Nanomedicine. 2014;10:119–129. doi: 10.1016/j.nano.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perica K, Tu A, Richter A, Bieler JG, Edidin M, Schneck JP. Magnetic field-induced T cell receptor clustering by nanoparticles enhances T cell activation and stimulates antitumor activity. ACS Nano. 2014;8:2252–2260. doi: 10.1021/nn405520d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci U S A. 2008;105:11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao F, Zhao Y, Liu Y, Chang X, Chen C, Zhao Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small. 2011;7:1322–1337. doi: 10.1002/smll.201100001. [DOI] [PubMed] [Google Scholar]
- 17.Fenner FJ, McAuslan B, Mims C. The biology of animal viruses. Elsevier; 2013. [Google Scholar]
- 18.Oyewumi MO, Kumar A, Cui Z. Nano-microparticles as immune adjuvants: correlating particle sizes and the resultant immune responses. 2010 doi: 10.1586/erv.10.89. DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foged C, Brodin B, Frokjaer S, Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int J Pharm. 2005;298:315–322. doi: 10.1016/j.ijpharm.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 20.Vallhov H, Gabrielsson S, Strømme M, Scheynius A, Garcia-Bennett AE. Mesoporous silica particles induce size dependent effects on human dendritic cells. Nano Lett. 2007;7:3576–3582. doi: 10.1021/nl0714785. [DOI] [PubMed] [Google Scholar]
- 21.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38:1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 22.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 23.Haghgooie R, Toner M, Doyle PS. Squishy Non-Spherical Hydrogel Microparticles. Macromol Rapid Comm. 2010;31:128–134. doi: 10.1002/marc.200900302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merkel TJ, Jones SW, Herlihy KP, Kersey FR, Shields AR, Napier M, Luft JC, Wu H, Zamboni WC, Wang AZ, Bear JE, DeSimone JM. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc Natl Acad Sci U S A. 2011;108:586–591. doi: 10.1073/pnas.1010013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merkel TJ, Chen K, Jones SW, Pandya AA, Tian S, Napier ME, Zamboni WE, DeSimone JM. The effect of particle size on the biodistribution of low-modulus hydrogel PRINT particles. J Control Release. 2012;162:37–44. doi: 10.1016/j.jconrel.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doshi N, Zahr AS, Bhaskar S, Lahann J, Mitragotri S. Red blood cell-mimicking synthetic biomaterial particles. Proc Natl Acad Sci U S A. 2009;106:21495–21499. doi: 10.1073/pnas.0907127106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noad R, Roy P. Virus-like particles as immunogens. Trends Microbiol. 2003;11:438–444. doi: 10.1016/s0966-842x(03)00208-7. [DOI] [PubMed] [Google Scholar]
- 28.Rosenthal JA, Chen L, Baker JL, Putnam D, DeLisa MP. Pathogen-like particles: biomimetic vaccine carriers engineered at the nanoscale. Curr Opin Biotech. 2014;28:51–58. doi: 10.1016/j.copbio.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Roldão A, Mellado MCM, Castilho LR, Carrondo MJ, Alves PM. Virus-like particles in vaccine development. 2010 doi: 10.1586/erv.10.115. DOI. [DOI] [PubMed] [Google Scholar]
- 30.Verma A, Stellacci F. Effect of surface properties on nanoparticle–cell interactions. Small. 2010;6:12–21. doi: 10.1002/smll.200901158. [DOI] [PubMed] [Google Scholar]
- 31.Cho EC, Xie J, Wurm PA, Xia Y. Understanding the role of surface charges in cellular adsorption versus internalization by selectively removing gold nanoparticles on the cell surface with a I2/KI etchant. Nano Lett. 2009;9:1080–1084. doi: 10.1021/nl803487r. [DOI] [PubMed] [Google Scholar]
- 32.Fadel TR, Steenblock ER, Stern E, Li N, Wang X, Haller GL, Pfefferle LD, Fahmy TM. Enhanced cellular activation with single walled carbon nanotube bundles presenting antibody stimuli. Nano Lett. 2008;8:2070–2076. doi: 10.1021/nl080332i. [DOI] [PubMed] [Google Scholar]
- 33.Fadel TR, Sharp FA, Vudattu N, Ragheb R, Garyu J, Kim D, Hong E, Li N, Haller GL, Pfefferle LD. A carbon nanotube–polymer composite for T-cell therapy. Nat Nanotechnol. 2014;9:639–647. doi: 10.1038/nnano.2014.154. [DOI] [PubMed] [Google Scholar]
- 34.Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature. 2010;463:485–492. doi: 10.1038/nature08908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho C, Keller A, Odell J, Ottewill R. Preparation of monodisperse ellipsoidal polystyrene particles. Colloid Polym Sci. 1993;271:469–479. [Google Scholar]
- 37.Champion JA, Katare YK, Mitragotri S. Making polymeric micro- and nanoparticles of complex shapes. Proc Natl Acad Sci U S A. 2007;104:11901–11904. doi: 10.1073/pnas.0705326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer RA, Meyer RS, Green JJ. An automated multidimensional thin film stretching device for the generation of anisotropic polymeric micro-and nanoparticles. J Biomed Mater Res Part A. 2015 doi: 10.1002/jbm.a.35399. DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J Am Chem Soc. 2005;127:10096–10100. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Merkel TJ, Chen K, Fromen CA, Betts DE, DeSimone JM. Generation of a library of particles having controlled sizes and shapes via the mechanical elongation of master templates. Langmuir. 2011;27:524–528. doi: 10.1021/la1045095. [DOI] [PubMed] [Google Scholar]
- 41.Morton SW, Herlihy KP, Shopsowitz KE, Deng ZJ, Chu KS, Bowerman CJ, Desimone JM, Hammond PT. Scalable manufacture of built-to-order nanomedicine: spray-assisted layer-by-layer functionalization of PRINT nanoparticles. Adv Mater. 2013;25:4707–4713. doi: 10.1002/adma.201302025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo G, Du L, Wang Y, Lu Y, Xu J. Controllable preparation of particles with microfluidics. Particuology. 2011;9:545–558. [Google Scholar]
- 43.Meyer RA, Green JJ. Shaping the future of nanomedicine: anisotropy in polymeric nanoparticle design. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2015 doi: 10.1002/wnan.1348. DOI 10.1002/wnan.1348 n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oelke M, Krueger C, Giuntoli RL, II, Schneck JP. Artificial antigen-presenting cells: artificial solutions for real diseases. Trends Mol Med. 2005;11:412–420. doi: 10.1016/j.molmed.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 45.Sunshine JC, Perica K, Schneck JP, Green JJ. Particle shape dependence of CD8+ T cell activation by artificial antigen presenting cells. Biomaterials. 2014;35:269–277. doi: 10.1016/j.biomaterials.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer RA, Sunshine JC, Perica K, Kosmides AK, Aje K, Schneck JP, Green JJ. Biodegradable Nanoellipsoidal Artificial Antigen Presenting Cells for Antigen Specific T-Cell Activation. Small. 2014 doi: 10.1002/smll.201402369. DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toy R, Peiris PM, Ghaghada KB, Karathanasis E. Shaping cancer nanomedicine: the effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine. 2013;9:121–134. doi: 10.2217/nnm.13.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma G, Valenta DT, Altman Y, Harvey S, Xie H, Mitragotri S, Smith JW. Polymer particle shape independently influences binding and internalization by macrophages. J Control Release. 2010;147:408–412. doi: 10.1016/j.jconrel.2010.07.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Florez L, Herrmann C, Cramer JM, Hauser CP, Koynov K, Landfester K, Crespy D, Mailander V. How shape influences uptake: interactions of anisotropic polymer nanoparticles and human mesenchymal stem cells. Small. 2012;8:2222–2230. doi: 10.1002/smll.201102002. [DOI] [PubMed] [Google Scholar]
- 50.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci U S A. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agarwal R, Singh V, Jurney P, Shi L, Sreenivasan S, Roy K. Mammalian cells preferentially internalize hydrogel nanodiscs over nanorods and use shape-specific uptake mechanisms. Proc Natl Acad Sci U S A. 2013;110:17247–17252. doi: 10.1073/pnas.1305000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barua S, Yoo J-W, Kolhar P, Wakankar A, Gokarn YR, Mitragotri S. Particle shape enhances specificity of antibody-displaying nanoparticles. Proc Natl Acad Sci U S A. 2013;110:3270–3275. doi: 10.1073/pnas.1216893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kolhar P, Anselmo AC, Gupta V, Pant K, Prabhakarpandian B, Ruoslahti E, Mitragotri S. Using shape effects to target antibody-coated nanoparticles to lung and brain endothelium. Proc Natl Acad Sci U S A. 2013;110:10753–10758. doi: 10.1073/pnas.1308345110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toy R, Peiris PM, Ghaghada KB, Karathanasis E. Shaping cancer nanomedicine: the effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine. 2014;9:121–134. doi: 10.2217/nnm.13.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hasan W, Chu K, Gullapalli A, Dunn SS, Enlow EM, Luft JC, Tian S, Napier ME, Pohlhaus PD, Rolland JP, DeSimone JM. Delivery of multiple siRNAs using lipid-coated PLGA nanoparticles for treatment of prostate cancer. Nano Lett. 2012;12:287–292. doi: 10.1021/nl2035354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu J, Luft JC, Yi X, Tian S, Owens G, Wang J, Johnson A, Berglund P, Smith J, Napier ME, DeSimone JM. RNA replicon delivery via lipid-complexed PRINT protein particles. Mol Pharm. 2013;10:3366–3374. doi: 10.1021/mp400190z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chu KS, Hasan W, Rawal S, Walsh MD, Enlow EM, Luft JC, Bridges AS, Kuijer JL, Napier ME, Zamboni WC, DeSimone JM. Plasma, tumor and tissue pharmacokinetics of Docetaxel delivered via nanoparticles of different sizes and shapes in mice bearing SKOV-3 human ovarian carcinoma xenograft. Nanomedicine. 2013;9:686–693. doi: 10.1016/j.nano.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chu KS, Finniss MC, Schorzman AN, Kuijer JL, Luft JC, Bowerman CJ, Napier ME, Haroon ZA, Zamboni WC, DeSimone JM. Particle replication in nonwetting templates nanoparticles with tumor selective alkyl silyl ether docetaxel prodrug reduces toxicity. Nano Lett. 2014;14:1472–1476. doi: 10.1021/nl4046558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chu KS, Schorzman AN, Finniss MC, Bowerman CJ, Peng L, Luft JC, Madden AJ, Wang AZ, Zamboni WC, DeSimone JM. Nanoparticle drug loading as a design parameter to improve docetaxel pharmacokinetics and efficacy. Biomaterials. 2013;34:8424–8429. doi: 10.1016/j.biomaterials.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang X, Leong D, Ren Y, Li Z, Torbenson MS, Mao HQ. String-like micellar nanoparticles formed by complexation of PEG-b-PPA and plasmid DNA and their transfection efficiency. Pharm Res. 2011;28:1317–1327. doi: 10.1007/s11095-011-0436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang X, Qu W, Pan D, Ren Y, Williford JM, Cui H, Luijten E, Mao HQ. Plasmidtemplated shape control of condensed DNA-block copolymer nanoparticles. Adv Mater. 2013;25:227–232. doi: 10.1002/adma.201202932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karagoz B, Esser L, Duong HT, Basuki JS, Boyer C, Davis TP. Polymerization-Induced Self-Assembly (PISA) – control over the morphology of nanoparticles for drug delivery applications. Polym Chem. 2014;5:350. [Google Scholar]
- 63.Valitutti S, Coombs D, Dupre L. The space and time frames of T cell activation at the immunological synapse. FEBS letters. 2010;584:4851–4857. doi: 10.1016/j.febslet.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 64.Peña JR, Pinney JR, Ayala P, Desai TA, Goldspink PH. Localized delivery of mechano-growth factor E-domain peptide via polymeric microstructures improves cardiac function following myocardial infarction. Biomaterials. 2015;46:26–34. doi: 10.1016/j.biomaterials.2014.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Su M. Evaluation of PLGA Nanoparticles Carrying Leukaemia Inhibitory Factor for Stromal-Like Support of Rat Fetal Dopaminergic Cells. J Nanomater Mol Nanotechnol. 2014 DOI. [Google Scholar]
- 66.Zhao X, Jain S, Larman HB, Gonzalez S, Irvine DJ. Directed cell migration via chemoattractants released from degradable microspheres. Biomaterials. 2005;26:5048–5063. doi: 10.1016/j.biomaterials.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 67.Popova TG, Teunis A, Magni R, Luchini A, Espina V, Liotta LA, Popov SG. Chemokine-Releasing Nanoparticles for Manipulation of the Lymph Node Microenvironment. Nanomaterials. 2015;5:298–320. doi: 10.3390/nano5010298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang Y-C, Liu T-J. Mobilization of mesenchymal stem cells by stromal cell-derived factor-1 released from chitosan/tripolyphosphate/fucoidan nanoparticles. Acta Biomater. 2012;8:1048–1056. doi: 10.1016/j.actbio.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 69.Steenblock ER, Fahmy TM. A comprehensive platform for ex vivo T-cell expansion based on biodegradable polymeric artificial antigen-presenting cells. Mol Ther. 2008;16:765–772. doi: 10.1038/mt.2008.11. [DOI] [PubMed] [Google Scholar]
- 70.Steenblock ER, Fadel T, Labowsky M, Pober JS, Fahmy TM. An artificial antigen-presenting cell with paracrine delivery of IL-2 impacts the magnitude and direction of the T cell response. J Biol Chem. 2011;286:34883–34892. doi: 10.1074/jbc.M111.276329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lai SK, O'Hanlon DE, Harrold S, Man ST, Wang YY, Cone R, Hanes J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc Natl Acad Sci U S A. 2007;104:1482–1487. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gref R, Luck M, Quellec P, Marchand M, Dellacherie E, Harnisch S, Blunk T, Muller RH. 'Stealth' corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf B Biointerfaces. 2000;18:301–313. doi: 10.1016/s0927-7765(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 73.Mout R, Moyano DF, Rana S, Rotello VM. Surface functionalization of nanoparticles for nanomedicine. Chem Soc Rev. 2012;41:2539–2544. doi: 10.1039/c2cs15294k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, Discher DE. Minimal “Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science. 2013;339:971–975. doi: 10.1126/science.1229568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sengupta S, Eavarone D, Capila I, Zhao G, Watson N, Kiziltepe T, Sasisekharan R. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature. 2005;436:568–572. doi: 10.1038/nature03794. [DOI] [PubMed] [Google Scholar]
- 76.Tanaka M, Sackmann E. Polymer-supported membranes as models of the cell surface. Nature. 2005;437:656–663. doi: 10.1038/nature04164. [DOI] [PubMed] [Google Scholar]
- 77.Roder F, Wilmes S, Richter CP, Piehler J. Rapid transfer of transmembrane proteins for single molecule dimerization assays in polymer-supported membranes. ACS Chem Biol. 2014;9:2479–2484. doi: 10.1021/cb5005806. [DOI] [PubMed] [Google Scholar]
- 78.Roder F, Birkholz O, Beutel O, Paterok D, Piehler J. Spatial organization of lipid phases in micropatterned polymer-supported membranes. J Am Chem Soc. 2013;135:1189–1192. doi: 10.1021/ja310186g. [DOI] [PubMed] [Google Scholar]
- 79.Waichman S, Roder F, Richter CP, Birkholz O, Piehler J. Diffusion and interaction dynamics of individual membrane protein complexes confined in micropatterned polymer-supported membranes. Small. 2013;9:570–577. doi: 10.1002/smll.201201530. [DOI] [PubMed] [Google Scholar]
- 80.Roder F, Waichman S, Paterok D, Schubert R, Richter C, Liedberg B, Piehler J. Reconstitution of membrane proteins into polymer-supported membranes for probing diffusion and interactions by single molecule techniques. Anal Chem. 2011;83:6792–6799. doi: 10.1021/ac201294v. [DOI] [PubMed] [Google Scholar]
- 81.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: A molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 82.Chan PY, Lawrence MB, Dustin ML, Ferguson LM, Golan DE, Springer TA. Influence of Receptor Lateral Mobility on Adhesion Strengthening between Membranes Containing Lfa-3 and Cd2. J Cell Biol. 1991;115:245–255. doi: 10.1083/jcb.115.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Groves JT, Dustin ML. Supported planar bilayers in studies on immune cell adhesion and communication. J Immunol Methods. 2003;278:19–32. doi: 10.1016/s0022-1759(03)00193-5. [DOI] [PubMed] [Google Scholar]
- 84.Sackmann E. Supported membranes: Scientific and practical applications. Science. 1996;271:43–48. doi: 10.1126/science.271.5245.43. [DOI] [PubMed] [Google Scholar]
- 85.LoidlStahlhofen A, Kaufmann S, Braunschweig T, Bayerl TM. The thermodynamic control of protein binding to lipid bilayers for protein chromatography. Nat Biotechnol. 1996;14:999–1002. doi: 10.1038/nbt0896-999. [DOI] [PubMed] [Google Scholar]
- 86.Galneder R, Kahl V, Arbuzova A, Rebecchi M, Radler JO, McLaughlin S. Microelectrophoresis of a bilayer-coated silica bead in an optical trap: Application to enzymology. Biophys J. 2001;80:2298–2309. doi: 10.1016/S0006-3495(01)76201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jacobson BS, Branton D. Plasma-Membrane - Rapid Isolation and Exposure of Cytoplasmic Surface by Use of Positively Charged Beads. Science. 1977;195:302–304. doi: 10.1126/science.831278. [DOI] [PubMed] [Google Scholar]
- 88.Cohen CM, Kalish DI, Jacobson BS, Branton D. Membrane Isolation on Polylysine-Coated Beads - Plasma-Membrane from Hela-Cells. J Cell Biol. 1977;75:119–134. doi: 10.1083/jcb.75.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaufmann S, Tanaka M. Cell adhesion onto highly curved surfaces: One-step immobilization of human erythrocyte membranes on silica beads. Chemphyschem. 2003;4:699–704. doi: 10.1002/cphc.200200537. [DOI] [PubMed] [Google Scholar]
- 90.Liu J, Stace-Naughton A, Jiang X, Brinker CJ. Porous nanoparticle supported lipid bilayers (protocells) as delivery vehicles. J Am Chem Soc. 2009;131:1354–1355. doi: 10.1021/ja808018y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu JW, Stace-Naughton A, Brinker CJ. Silica nanoparticle supported lipid bilayers for gene delivery. Chem Commun. 2009:5100–5102. doi: 10.1039/b911472f. DOI Doi 10.1039/B911472f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu JW, Jiang XM, Ashley C, Brinker CJ. Electrostatically Mediated Liposome Fusion and Lipid Exchange with a Nanoparticle-Supported Bilayer for Control of Surface Charge, Drug Containment, and Delivery. J Am Chem Soc. 2009;131:7567. doi: 10.1021/ja902039y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ashley CE, Carnes EC, Phillips GK, Padilla D, Durfee PN, Brown PA, Hanna TN, Liu J, Phillips B, Carter MB, Carroll NJ, Jiang X, Dunphy DR, Willman CL, Petsev DN, Evans DG, Parikh AN, Chackerian B, Wharton W, Peabody DS, Brinker CJ. The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers. Nat Mater. 2011;10:389–397. doi: 10.1038/nmat2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Patel PD, Dand N, Hirlekar RS, Kadam VJ. Drug loaded erythrocytes: as novel drug delivery system. Curr Pharm Des. 2008;14:63–70. doi: 10.2174/138161208783330772. [DOI] [PubMed] [Google Scholar]
- 95.Zarrin A, Foroozesh M, Hamidi M. Carrier erythrocytes: recent advances, present status, current trends and future horizons. Expert Opin Drug Del. 2014;11:433–447. doi: 10.1517/17425247.2014.880422. [DOI] [PubMed] [Google Scholar]
- 96.Muzykantov VR. Drug delivery by red blood cells: vascular carriers designed by mother nature. Expert Opin Drug Del. 2010;7:403–427. doi: 10.1517/17425241003610633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hu CM, Zhang L, Aryal S, Cheung C, Fang RH. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A. 2011;108:10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hu CM, Fang RH, Luk BT, Chen KN, Carpenter C, Gao W, Zhang K, Zhang L. 'Marker-of-self' functionalization of nanoscale particles through a top-down cellular membrane coating approach. Nanoscale. 2013;5:2664–2668. doi: 10.1039/c3nr00015j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hu CM, Fang RH, Copp J, Luk BT, Zhang L. A biomimetic nanosponge that absorbs pore-forming toxins. Nat Nanotechnol. 2013;8:336–340. doi: 10.1038/nnano.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hu CM, Fang RH, Luk BT, Zhang L. Nanoparticle-detained toxins for safe and effective vaccination. Nat Nanotechnol. 2013;8:933–938. doi: 10.1038/nnano.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Parodi A, Quattrocchi N, van de Ven AL, Chiappini C, Evangelopoulos M, Martinez JO, Brown BS, Khaled SZ, Yazdi IK, Enzo MV, Isenhart L, Ferrari M, Tasciotti E. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol. 2013;8:61–68. doi: 10.1038/nnano.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Menter DG, Steinert BW, Sloane BF, Gundlach N, Ogara CY, Marnett LJ, Diglio C, Walz D, Taylor JD, Honn KV. Role of Platelet Membrane in Enhancement of Tumor-Cell Adhesion to Endothelial-Cell Extracellular-Matrix. Cancer Res. 1987;47:6751–6762. [PubMed] [Google Scholar]
- 103.Furman NET, Lupu-Haber Y, Bronshtein T, Kaneti L, Letko N, Weinstein E, Baruch L, Machluf M. Reconstructed Stem Cell Nanoghosts: A Natural Tumor Targeting Platform. Nano Lett. 2013;13:3248–3255. doi: 10.1021/nl401376w. [DOI] [PubMed] [Google Scholar]
- 104.Fang RH, Hu CM, Luk BT, Gao W, Copp JA, Tai Y, O'Connor DE, Zhang L. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014;14:2181–2188. doi: 10.1021/nl500618u. [DOI] [PMC free article] [PubMed] [Google Scholar]