Abstract
Post-Traumatic Stress Disorder (PTSD) has major public health significance. Evidence that PTSD may be associated with premature senescence (early or accelerated aging) would have major implications for quality of life and healthcare policy. We conducted a comprehensive review of published empirical studies relevant to early aging in PTSD. Our search included the PubMed, PsycINFO and PILOTS databases for empirical reports published since the year 2000 relevant to early senescence and PTSD, including: (1) biomarkers of senescence (leukocyte telomere length (LTL) and pro-inflammatory markers), (2) prevalence of senescence-associated medical conditions, and (3) mortality rates. All six studies examining LTL indicated reduced LTL in PTSD (pooled Cohen’s d = 0.76). We also found consistent evidence of increased pro-inflammatory markers in PTSD (mean Cohen’s ds), including C-reactive protein = 0.18, Interleukin-1 beta = 0.44, Interleukin-6 = 0.78, and tumor necrosis factor alpha = 0.81. The majority of reviewed studies also indicated increased medical comorbidity among several targeted conditions known to be associated with normal aging, including cardiovascular disease, type 2 diabetes mellitus, gastrointestinal ulcer disease, and dementia. We also found seven of 10 studies indicated PTSD to be associated with earlier mortality (average HR = 1.29). In short, evidence from multiple lines of investigation suggests that PTSD may be associated with a phenotype of accelerated senescence. Further research is critical to understand the nature of this association. There may be a need to re-conceptualize PTSD beyond the boundaries of mental illness, and instead as a full systemic disorder.
INTRODUCTION
In recent years, there has been growing concern that psychiatric disorders such as schizophrenia, bipolar disorder, and major depression are associated with significant medical comorbidity, and that some of this morbidity may reflect an acceleration of the aging process (1–4). Post-traumatic stress disorder (PTSD) is an important public health concern in the US, particularly since 9/11/2001 and conflicts in Iraq and Afghanistan. Because of the importance of stress in various aspects of the aging process (5), we considered that PTSD may also show an association with early senescence. To investigate this hypothesis further, we performed a review of the relevant literature.
What constitutes evidence for premature or accelerated senescence has not been standardized, but in non-psychiatric conditions reportedly associated with early senescence, such as Hutchinson-Gilford Progeria Syndrome, Werner Syndrome, HIV Infection, and Down’s syndrome, the majority of the evidence falls into three categories: [1] biological indicators/biomarkers, [2] earlier occurrence or higher prevalence of medical conditions associated with advanced age, and [3] premature mortality (6–12). These categories formed the basis for our literature search into the possible association of PTSD with accelerated senescence.
The present review was conducted to evaluate evidence for early or accelerated senescence in PTSD. Given the diversity of outcomes and methods in individual studies, this was not intended as a formal meta-analysis. However, where possible, we calculated overall effect sizes for studies of targeted biomarkers and mortality for which there were multiple articles with sufficient overlap in methods and outcomes.
METHODS
We searched PubMed, PsycINFO, and PILOTS databases for papers published between January 1, 2000 and November 30, 2014. The year 2000 was chosen because it marked the publication of the DSM-IV-TR, which included the first major change in diagnostic criteria for PTSD since introduction of PTSD in DSM-III, and also included studies after 9/11/2001 and the wars in Iraq and Afghanistan, which represented the beginning of a period of intensified public and scientific interest in PTSD.
Targeted outcomes for the present review included: [1] biomarkers of senescence (leukocyte telomere length (LTL), blood pro-inflammatory indices, and oxidative stress), [2] comorbid medical conditions associated with aging (hypertension (HTN), heart/cardiovascular disease, metabolic syndrome, post-PTSD onset type 2 diabetes mellitus, gastrointestinal ulcer diseases, and dementia), and [3] mortality. Almost any medical condition could potentially be age-associated, but we focused on the above conditions because each has empirical evidence of increased incidence with advancing age, is potentially fatal, and tends to be worsened by stress (13). The last consideration, while ruling out some life-threatening conditions such as cancer, was felt important given the relationship of stress to PTSD.
The search of databases was an iterative process resulting in the following search query: (PTSD OR post-traumatic stress OR posttraumatic stress) AND (aging OR ageing OR allostatic OR allostasis OR dementia OR Alzheimer’s OR Alzheimer OR Alzheimers OR inflammation OR inflammatory OR longevity OR life expectancy OR length of life OR mortality OR mortalities OR oxidative OR oxidation OR senescence OR telomere OR telomeres OR vascular OR cardiovascular OR metabolic OR diabetes OR ulcer OR ulcers).
We examined the titles and abstracts of all citations returned by the above search criteria and selected the empirical English-language reports focused on comparing adults with PTSD, diagnosed with standard criteria, to one or more appropriate comparison groups in terms of aging-related outcomes relevant to this review. We excluded review papers and reports with duplicate data. Other than excluding studies of childhood trauma, we did not exclude studies based on the nature of the precipitating traumatic event. We also searched the bibliography of identified articles for additional relevant papers. Several of the co-authors (JBL, BWP, CAE, SA) participated in the review and selection of potential articles, as well as extraction of the study details and findings, which were agreed on by all the authors.
As noted above, the present review was not intended to serve as a formal meta-analyses, but we calculated pooled effect sizes for those targeted outcomes for which there were multiple articles with sufficient overlap in methods and measures to be amenable to such analyses. These included reports involving LTL and pro-inflammatory indices, as well as those for mortality. Given that we were able to calculate pooled effect sizes for most pro-inflammatory marker studies and hazard ratios for most mortality papers, we excluded a few isolated papers that had insufficient information for such calculations; for example, we excluded three mortality studies for which we could not derive hazard ratio information (14–16). The calculations of average effect sizes and hazard ratios were conducted using MIX 2.0 software (Biostat XL). Individual study effect sizes were synthesized to generate an overall effect size, weighted by the inverse of variance (the latter is partially a function of sample size, thereby, calculated effect sizes were weighted by sample size.)
RESULTS
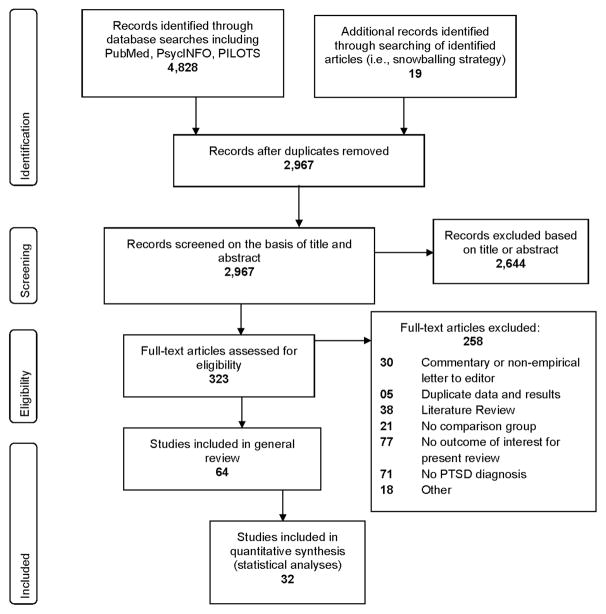
Our search yielded 64 studies that met inclusion and exclusion criteria for review (Figure 1). Of these, 22 were suitable for calculating overall effect sizes (Cohen’s d) for biomarkers and 10 for mortality (hazard ratio). Interestingly, only two articles that met our inclusion and exclusion criteria were published between 2000 and 2003 (17, 18), supporting our choice of the year 2000 as a cut-point for review. Except where otherwise indicated, our review focused on results comparing people with PTSD versus without PTSD. Given the high comorbidity between PTSD and Major Depressive Disorder (MDD) we also included and indicated those comparisons that involved people with PTSD and MDD. With a few isolated exceptions (e.g. (19, 20)) comparison of those with remitted PTSD versus non-remitted PTSD was not generally available in the published reports. Similarly, a few studies included comparison of people with “partial PTSD” (most symptoms of PTSD but not meeting diagnostic criteria for syndromal PTSD) (21–23). We have included the information on such participants within the more detailed online supplementary Tables, but our general focus was on the effects of full PTSD on the various outcome measures.
Figure 1.
PRISMA Flow Diagram for Selection of Published Articles for review
(1) Studies of Senescence-Related Biomarkers in PTSD
We found 22 studies of senescence-related biomarkers with sufficient information to calculate pooled effect sizes, which included six reports on LTL (24–29) and 16 on pro-inflammatory indices (17, 28, 30–44) (one of the reports included both LTL and pro-inflammatory markers (28)) (Table 1).
Table 1.
Studies of Biomarkers of Senescence in PTSD
| Study | Sample Ns | Telomere Cohen’s d (95% CI) | CRP Cohen’s d (95% CI) | IL-6 Cohen’s d (95% CI) | IL-1β Cohen’s d 95% CI) | TNFα Cohen’s d (95% CI) |
|---|---|---|---|---|---|---|
| Baker, Ekhator et al. 2001 (17) | +PTSD: 11 −PTSD: 8 |
0.55 (−0.43 – 1.53) | ||||
| Von Kanel et al. 2007 (30) | +PTSD: 14 − PTSD: 14 |
0.1 (−0.67 – 0.87) | 0.18 (−0.59 – 0.95) | 0.68 (−0.11 – 1.47) | 0.58 (−0.21 – 1.37) | |
| Gill et al. 2008 (31) | +PTSD: 26 −PTSD/−Trauma: 21 |
7.3 (5.65 – 8.95) | 0.51 (−0.09 – 1.11) | 3.14 (2.25 – 4.03) | ||
| Hoge et al. 2009 (32) | +PTSD: 28 −PTSD: 28 |
1.08 / 0.51 / 1.65 | 0.28 (−0.26 – 0.82) | 0.3 (−0.24 – 0.84) | ||
| Vidovic et al. 2009 (33) | +PTSD: 39 −PTSD: 37 |
0.09 (−0.37 – 0.55) | 0.83 (0.26 – 1.40) | |||
| Gill et al. 2010 (34) | +PTSD: 9 −PTSD: 14 |
0 (−0.88 – 0.88) | ||||
| Spitzer et al. 2010 (35) | +PTSD: 55 −PTSD: 2,994 |
0.31 (0.04 – 0.58) | ||||
| Malan et al. 2011 (24) | +PTSD: 9 −PTSD: 53 |
0.65 (−0.08 – 1.38) | ||||
| O’Donovan et al. 2011 (25) | +PTSD: 43 −PTSD: 47 |
0.39 (−0.03 – 0.81) | ||||
| Guo 2012 (36) | +PTSD: 50 −PTSD: 50 |
3.42 (2.79 – 4.05) | 0.36 (−0.34 – 1.06) | |||
| Hammad et al. 2012 (37) | +PTSD: 8 −PTSD: 5 |
0.76 (− 0.50 – 2.02) | 1.63 (0.21 – 3.05) | |||
| Zimmerman et al. 2012 (38) | +PTSD: 37 −PTSD: 37 |
0.33 (−0.14 – 0.80) | 0.75 (0.27 – 1.23) | |||
| Baumert 2013 (39) | +PTSD: 51 −PTSD: 2,698 |
−0.09 (−0.37 – 0.19) | ||||
| Gill et al. 2013 (40) | +PTSD t: 26 −PTSD: 24 |
0.71 (0.13 – 1.29) | 0.67 (0.09 – 1.25) | |||
| Gola et al. 2013 (41) | +PTSD: 16 −PTSD: 18 |
0.12 (−0.57 – 0.81) | 2.54 (2.01 – 3.07) | |||
| Ladwig et al. 2013 (26) | +PTSD: 51 −PTSD: 2,687 |
0.21 (−0.07 – 0.49) | ||||
| Plantinga et al. 2013 (42) | +PTSD: 33 −PTSD: 33 |
0.33 (−0.16 – 0.82) | 0.03 (−0.46 – 0.52) | |||
| Spitzer et al. 2013 (43) | +PTSD: 12 −PTSD: 38 |
−0.61 (−1.28 – 0.06) | ||||
| Zhang et al. 2013 (27) | +PTSD: 84 −PTSD / Age-matched: 84 |
0.47 (0.16 – 0.78) | ||||
| Jergovic et al. 2014 (28) | +PTSD: 30 −PTSD: 17 |
4.85 (3.63 – 6.07) | −2.32 (−3.10 – − 1.54) | −2.39 (−3.18 – −1.60) | ||
| Lindqvist 2014 (44) | +PTSD: 51 −PTSD: 51 |
0.37 (−0.03 – 0.77) | 0.28 (− 0.11 – 0.67) | 0.22 (−0.17 – 0.61) | 0.42 (0.02 – 0.82) | |
| Shalev et al. 2014 (29) [MEN] |
+PTSD: 23 −PTSD: 288 |
0.47 (0.04 – 0.90) | ||||
| Shalev et al. 2014 (29) [WOMEN] |
+PTSD: 32 −PTSD: 236 |
0.01 (−0.36 – 0.38) |
Key to abbreviations: CI – confidence interval, CRP = C-Reactive Protien, IL = interleukin, TNF = tumor necrosis factor, +PTSD = diagnosted with Post-truamatic Stress Disorder, - PTSD = no PTSD.
Please note: 95% Cis not overlapping with zero are significant p<.05
LTL
All the six published studies of LTL in PTSD reported shorter LTL among people with PTSD compared to LTL among non-PTSD comparison groups. Shalev et al. (29) presented LTL data separately for men and women, so we incorporated these as separate samples. A positive effect size indicated the PTSD group had shorter telomeres compared to non-PTSD subjects. The pooled Cohen’s d was 0.76 (95% CI = 0.25 to 1.28; z = 2.90, p = .004), which falls in the medium-to-large effect size range.
Pro-inflammatory markers
There were at least five articles for each of four pro-inflammatory markers: C-reactive protein (CRP), interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNFα). Overall Cohen’s d values (positive effect sizes indicating an increase in the biomarker among people with PTSD relative to the comparison group) were as follows: CRP = 0.18 (95% CI = −0.07 to 0.44; z = 1.39, p = .16); IL-1β = 0.44 (95% CI = .21 to .67; z = 3.79, p < .001); IL-6 = 0.78 (95% CI = .09 to 1.48; z = 2.23, p = .026); and TNFα = 0.81 (95% CI = −0.09 to 1.71; z = 1.77, p = .08). Pooled effect size estimates for IL-1β and IL-6 showed higher values for PTSD vs control subjects, with all four biomarkers falling between small and large effect size ranges.
Oxidative Measures
Although many studies have examined effects of stress on oxidative parameters in animals and in non-PTSD human samples (45), and these studies have generally supported the hypothesis of increased oxidative measures in conditions of chronic stress (45), very few have actually examined this issue in PTSD. We found five studies relevant to PTSD and oxidative measures (18, 46–49). Tezcan et al. (18) compared 14 people with PTSD and 14 hospital staff used as comparison subjects. There were no significant group differences in any blood antioxidant enzyme activities (glutathione peroxidase, superoxide dismutase or catalase), but glutathione peroxidase and superoxide dismutase were significantly positively correlated with severity of PTSD symptoms (rs = .52 and .55, respectively, both p-values <.05). Ceprnja et al. (46) examined several potential oxidative markers among 46 Croatian combat Veterans and 28 healthy comparison subjects. The only statistically significant difference was diminished concentrations in PTSD of protein carbonyl (an oxidation by-product), but this finding did not adequately separate groups through receiver operating curve analyses, calling into question the clinical importance of the observed differences. Borovac Stefanovic et al. (47) studied Croatian war Veterans (50 with PTSD and 30 without PTSD); there were no group differences in serum malondialdehyde (an oxidation by-product), but the PTSD group had lower blood concentrations of erythrocyte superoxide dismutase and erythrocyte glutathione peroxidase, suggesting impaired antioxidant capacity and increased oxidative stress in the PTSD subjects. As part of a magnetic resonance spectroscopy (MRS) study, Michaels et al. (48) examined the dorsolateral prefrontal cortex and anterior cingulate cortex among 29 trauma-exposed individuals (12 with PTSD and 17 without PTSD), and found those with PTSD had significantly higher levels of the antioxidant, glutathione in both regions, which may represent a compensatory reaction to increased oxidation (it may also represent an excess of antioxidant activity for unclear reasons). Ozdemir et al. (49) recently reported a lack of significant group differences in total antioxidant or oxidative status among Turkish earthquake survivors with and without PTSD, and also did not find significant correlations between severity of PTSD symptoms and oxidative measures. Overall, the results from studies of PTSD and oxidative markers appear mixed at best, but given the limited availability of studies with overlapping methods or outcomes measures, and the small samples sizes within several of the available studies, it seems premature to draw firm conclusions regarding the presence or absence of an association of PTSD with oxidative stress.
(2) Studies of Earlier Onset of Senescence-Related Medical Conditions in PTSD
We found 30 studies of association of PTSD with one or more of the targeted medical conditions (Table 2). Some of the studies presented results in terms of more than one of the targeted health outcomes, and patterns sometimes differed among the specific conditions. To facilitate interpretation, results were tallied in terms of whether they provided positive, negative, or mixed/partial support of an associated of PTSD with specific disease categories, with some reports being included in more than one category. Specific references are provided in Table 2, organized by condition, and whether the findings offered positive, negative, or partial/mixed support for an association of PTSD with the targeted medical condition. Further details of each study (including sample sizes, gender, mean age, diagnostic methods, study design, key outcomes, and key findings) are available online in Supplemental Digital Content Tables 1 through 5.
Table 2.
Association of PTSD with Cardiovascular Diseases, Type II Diabetes, Metabolic Syndrome and Dementia.
| Age-associated Cardiovascular Conditions | ||
|---|---|---|
|
| ||
| Hypertension | ||
|
| ||
| Positive (support senescence model) | Negative (do not support senescence model) | Mixed (partial or mixed support for senescence model) |
| Lauterbach et al. 2005 (50) | David et al. 2004 (56) | Walczewska et al. 2011 (60) |
| +PTSD: N=429; −PTSD: N= 5448 | PTSD: N=55 | +PTSD: N=80 |
| Gender: 50% women | Alcohol dependence: 38 | −PTSD: N=70 |
| Gender: all men | Gender: 50% women | |
|
| ||
| Kang et al. 2006 (55) | Muhtz et al. 2011 (5) | |
| +PTSD/+POW: 3,254 | ||
| −PTSD/+POW: N=16,188 | Chronic PTSD: N=25 Trauma-exposed/−PTSD: | |
| +PTSD/−POW: N=133 | N=25 | |
| −PTSD/−POW: N=9,595 | Gender: 64% women | |
| Gender: all men | ||
|
| ||
| Andersen et al. 2010 (51) | Spiro et al. 2006 (58) | |
| +PTSD: N=1,258 | +PTSD: N=456 | |
| − PTSD: N=3,158 | −PTSD N=1455 | |
| Gender: 89% mean | MDD: N=351 | |
| Gender: all men | ||
|
| ||
| Glaesmer et al. 2011 (52) | Dobie et al. 2004 (59) | |
| +PTSD: N=67 | +PTSD in past month: N=266 | |
| +trauma/−PTSD: N=423 | −PTSD: N=940 | |
| − trauma: N=966 | Gender: All women | |
| Gender: | Mean (SD) age years: | |
| +PTSD = 53.7% women | +PTSD = 42 (11) | |
| +trauma/−PTSD = 52.7% women | −PTSD = 47 (15) | |
| − trauma = 52.2 % women | ||
|
| ||
| KIbler et al. 2009 (53) | ||
| +PTSD/−MDD: N=220 | ||
| +PTSD/+MDD: N=209 | ||
| +MDD/−PTSD: N=785 | ||
| HC: N=2794 | ||
| Gender: 55% women | ||
|
| ||
| Pietrzak et al. 2012 (21) | ||
| Full PTSD: N=469 | ||
| Partial PTSD:545 | ||
| Trauma exposed/−PTSD: N=7519 | ||
| Gender: | ||
| Full PTSD = 69.7% women | ||
| Partial PTSD = 65.7% women | ||
| Trauma exposed/−PTSD = 53.2 % women | ||
|
| ||
| Paulus et al 2013 (54) | ||
| +PTSD: N=88 | ||
| −PTSD/+trauma: N=27 | ||
| −PTSD/−trauma: N=150 | ||
| Gender: all men | ||
|
| ||
| Heart/Cardiovascular Disease | ||
|
| ||
| Positive | Negative | Mixed |
|
| ||
| Lauterbach et al. 2005 (50) | Dobie et al. 2004 (59) | Walczewska et al. 2011 (60) |
| +PTSD: N=429; −PTSD: | +PTSD in past month: N=266 | +PTSD: N=80 |
| N=5448 | −PTSD: N=940 | −PTSD: N=70 |
| Gender: 50% women | Gender: All women | Gender: 50% women |
|
| ||
| Sawchuk et al. 2005 (61) | Crum-Cianflone et al. 2014 (66) | |
| +Lifetime PTSD: N=208 | +PTSD: N=3,331 | |
| −PTSD: N=1206 | − PTSD: N=56,694 | |
| Gender: +PTSD 34.4%, −PTSD 23.4% women | ||
|
| ||
| Kang et al. 2006 (55) | ||
| +PTSD/+POW: N=3,254 | ||
| −PTSD/+POW: N=16,188 | ||
| +PTSD/−POW: N=133 | ||
| −PTSD/−POW: N=9,595 | ||
| Gender: all men | ||
|
| ||
| Spiro et al. 2006 (58) | ||
| +PTSD: N=456 | ||
| −PTSD: N=1455 | ||
| MDD: N=351 | ||
| Gender: all men | ||
|
| ||
| Sledjeski et al. 2008 (62) | ||
| +PTSD: N=574 | ||
| +trauma/−PTSD: N=4,054 | ||
| −trauma: N=738 | ||
| Gender : | ||
| +PTSD = 75.0% women | ||
| +trauma/−PTSD = 49.3% women | ||
| − trauma = 59.1% women | ||
|
| ||
| Spitzer et al. 2009 (63) | ||
| +PTSD: N=62 | ||
| +trauma/−PTSD: N=1,669 | ||
| −trauma/−PTSD: N=1,440 | ||
| Gender: | ||
| +PTSD = 67.7% women | ||
| +trauma/−PTSD = 50.1% | ||
| women | ||
| − trauma = 53.6% women | ||
|
| ||
| Glaesmer et al. 2011(52) | ||
| +PTSD: N=67 | ||
| +trauma/−PTSD: N=423 | ||
| − trauma: N=966 | ||
| Gender: | ||
| +PTSD = 53.7% women | ||
| +trauma/−PTSD = 52.7% women | ||
| − trauma = 52.2 % women | ||
|
| ||
| Pietrzak et al. 2012 (21) | ||
| Full PTSD: N=469 | ||
| Partial PTSD: N=545 | ||
| Trauma exposed/−PTSD: N=7519 | ||
| Gender: | ||
| Full PTSD = 69.7% women | ||
| Partial PTSD = 65.7% women | ||
| Trauma exposed/−PTSD = 53.2 % women | ||
|
| ||
| Vaccarino et al. 2013 (64) | ||
| +PTSD: N=137 | ||
| −PTSD: N=425 | ||
| Gender: all men | ||
|
| ||
| Turner et al. 2013 (65) | ||
| +PTSD: N=433 | ||
| −PTSD: N=230 | ||
| Gender (% women): | ||
| +PTSD: 10.4% | ||
| −PTSD: 3.2% | ||
|
| ||
| Metabolic Conditions | ||
|
| ||
| Metabolic Syndrome | ||
|
| ||
| Positive | Negative | Mixed |
|
| ||
| Heppner et al. 2009 (22) | Linnville et al. (19) | Weiss et al. 2011 (20) |
| +PTSD: N=139 | + current PTSD/+Repatriated Prisoner of War (RPW): N=61 | +current PTSD: N=46 |
| Subthreshold PTSD: N=60 | − current PTSD/+lifetime PTSD/+ RPW: N=29 | − current PTSD: N=199 |
| −PTSD: N=54 | − current or lifetime PTSD/+RPW: N=196 | Gender: 69.6% women |
| Gender: 8% women | − PTSD (compater experience but not POWs): N=65 | |
| Gender: All men | ||
|
| ||
| Jin et al. 2009 (67) | ||
| PTSD: N=33 | ||
| Schizophrenia: N=65 | ||
| Dementia: N=56 | ||
| Mood disorder: N=49 | ||
| Gender: | ||
| PTSD = 12% women | ||
| Schizophrenia = 26% women | ||
| Dementia = 45% women | ||
| Mood disorder = 39% women | ||
|
| ||
| Type 2 Diabetes Mellitus | ||
|
| ||
| Positive | Negative | Mixed |
|
| ||
| Boyko et al. 2010 (69) | Spiro et al. 2006 (58) | None |
| +PTSD: N=1,595 | +PTSD: N=456 | |
| − PTSD: N=42,115 | −PTSD: N=1455 | |
| Gender: 74% men | MDD: N=351 | |
| Gender: all men | ||
|
| ||
| Agyemang et al 2012 (68) | ||
| +PTSD men: N=2,681 | ||
| + PTSD women: N=1,967 | ||
| −PTSD men: N=66066 | ||
| −PTSD women: N=32,466 | ||
| Gender: see above | ||
|
| ||
| Lukaschek et al. 2013 (23) | ||
| +PTSD: N=50 | ||
| Partial PTSD: N=261 | ||
| Gender: N=48.4% men | ||
|
| ||
| Gastrointestinal Ulcer Diseases | ||
|
| ||
| Positive | Negative | Mixed |
|
| ||
| Pietrzak et al. 2012 (21) | None | None |
| Full PTSD: N=469 | ||
| Partial PTSD: N=545 | ||
| Trauma exposed/−PTSD: N=7519 | ||
| Gender: | ||
| Full PTSD = 69.7% women | ||
| Partial PTSD = 65.7% women | ||
| Trauma exposed/−PTSD = 53.2 % women | ||
|
| ||
| Sledjeski et al. 2008 (62) | ||
| +PTSD:c N=574 | ||
| +trauma/−PTSD: N=4,054 | ||
| −trauma: N=738 | ||
| Gender : | ||
| +PTSD = 75.0% women | ||
| +trauma/−PTSD = 49.3% women | ||
| − trauma = 59.1% women | ||
| N=3108 (52.8 weighted %) females and N=2258 (47.2 weighted %) males | ||
|
| ||
| Scott et al. 2013 (70) | ||
| A number of mental health conditions were examined, but among these were PTSD (sample size, gender composition, and mean age not provided) | ||
|
| ||
| Weisberg et al. 2002 (71) | ||
| +PTSD: N=185 | ||
| −PTSD/+trauma: N=233 | ||
| −trauma: N=233 | ||
| Gender and mean age : uncertain – unable to access tables which may provide this information. | ||
|
| ||
| Dementia | ||
|
| ||
| Positive | Negative | Mixed |
|
| ||
| Qureshi et al. 2010 (72) | None | None |
| +PTSD/−PH: N=3,660 (n=3,616 men, n=44 women) | ||
| −PTDS/+PH: N=1,503 (n=1,502 men, n=1 woman) | ||
| +PTSD/−PH: N=153 (all men) | ||
| −PTSD/−PH: N=5,165 (n=5,123 men, n=42 women) | ||
|
| ||
| Yaffe et al. 2010 (73) | ||
| +PTSD: N=53,155 (n=51,986 men and n=1,169 women) | ||
| −PTSD: N=127,938 (n=122,820 men and n=5,118 women) | ||
|
| ||
| Meziab et al. 2014 (74) | ||
| +PTST/+POW: N=150 | ||
| +PTSD/−POW: N=5,964 | ||
| −PTSD/+POW: N=334 | ||
| −PTSD/−POW: N=176,431 | ||
| Gender: not specified | ||
Note: Studies categorized as “Positive” are those for which the study results indicated PTSD was associated with increased prevalence or earlier onset of the medical condition under investigation. “Negative” studies were those for which no significant effects of PTSD on prevalence or onset of the comorbid condition was found. “Mixed” studies were those in which a significant effect of PTSD on medical comorbidity was found in some but not all subset of participants or analyses.
Key to abbreviations + PTSD = diagnosed with Post-Traumatic Stress Disorder; − PTSD = no PTSD; POW = Prisoner of War; MDD = Major Depressive Disorder; HC = Health Comparison subject; RPW= Repatriated POW; PH = Purple Heart.
Of 12 HTN studies, seven (58%) were positive (21, 50–55), four (33%) negative (56–59), and one (8%) reported mixed results (60) (Table 2 and Online Data Supplement Table 1). Of 13 CVD studies, 10 (77%) were positive (21, 50, 52, 55, 58, 61–65), two (15%) mixed (60, 66), and one (8%) was negative (59) (see Table 2 and Online Data Supplement Table 2). Of four studies reporting the association of PTSD with metabolic syndrome, two (50%) provided positive results (22, 67), one (25%) negative results (19), and one (25%) mixed support (20) (Table 2 and Online Data Supplement Table 3). Of four studies reporting the association of PTSD with type 2 diabetes mellitus, three (75%) provided positive findings (23, 68, 69), one (25%) provided negative findings (58), and none reported mixed findings (Table 2 and Online Data Supplement Table 3). All four studies that reported on the association of PTSD with gastrointestinal ulcer diseases were positive (21, 62, 70, 71) (Table 2 and Online Data Supplement Table 4). Finally, all three studies (100%) that examined PTSD as a risk factor for dementia reported positive findings (72–74). Therefore, the reviewed studies most consistently showed an association between PTSD and an increased incidence of CVD, gastrointestinal ulcers and dementia, with moderate support for an association with type 2 diabetes mellitus, and less support for a relationship with HTN.
(3) Studies of Mortality in PTSD
We found 10 studies (Table 3) of comparative mortality rates in PTSD for which hazard ratio data could be calculated. In the context of the present review, the mortality hazard ratio is the risk of a death among those with PTSD at a given point in time relative to the risk of a death in the non-PTSD group at that same point in time. For example, if the hazard ratio is 2.0, then the risk of death for individuals with PTSD at any given point in time is twice the risk of death in individuals without PTSD. Seven studies (70%) reported an increased mortality hazard ratio in the PTSD group compared to comparison subjects. Overall, the pooled hazard ratio (weighted by sample size) was 1.29 (95% CI = 1.11 to 1.5; Z = 3.32, p < .001), indicating a 29% increased risk for mortality in those with PTSD, corresponding to a small to medium effect size.
Table 3.
Studies of Mortality in PTSD
| Study | N | HR (95% CI) | Reported Findings |
|---|---|---|---|
| Boscarino 2006 (85) | +PTSD: 1,050 −PTSD: 14,238 |
2.1 (1.69 – 2.6) | Increase in all-cause mortality associated with PTSD |
| Bramsen et al. 2007 (94) | +PTSD: 65 Non-PTSD: 1,383 |
1.54 (1.02 – 2.32) | Higher mortality rate associated with PTSD symptoms |
| Boscarino 2008 (86) | +PTSD: 311 −PTSD: 4,017 |
2.25 (1.02 – 4.92) | Increase in mortality due to heart disease |
| Kinder et al. 2008 (87) | +PTSD: 748 −PTSD: 24,329 |
0.92 (0.82 – 1.04) | No increase in all-cause mortality for PTSD alone, but not after adjusting for depression |
| Chwastiak et al. 2010 (88) | +PTSD: 34,719 −PTSD: 525,266 |
1.02 (1.00 – 1.04) | Not significant after adjusting for medical and psychiatric comorbidity |
| Flood et al., 2010 (89) | +PTSD: 1,176 −PTSD: 4,072 |
1.54 (1.12 – 2.10) | Increased risk of all-cause and behavioral-cause (e.g. homicide, suicide) mortality |
| Ahmadi et al. 2011 (90) | +PTSD: 88 −PTSD: 549 |
1.82 (1.05 – 3.15) | Higher risk of coronary artery disease and resultant mortality associated with PTSD |
| Kimbrell et al. 2011 (91) | +PTSD: 3,593 −PTSD: 5,010 |
1.11 (1.00 – 1.22) | Significantly higher mortality rate (in non- Purple Hearth groups) for +PTSD |
| Xue et al. 2012 (92) | +PTSD: 91 −PTSD: 800 |
1.79 (1.14 – 2.80) | Abnormal cardiac biological indicators and increased mortality associated with PTSD |
| Zohar and Fostick 2014 (93) | +PTSD: 2,457 −PTSD: 2,457 |
0.91 (0.67 – 1.25) | No increased mortality with treated PTSD |
Key to Abbreviations: HR = Hazard Ratio; CI = Confidence Interval; + PTSD = diagnosed with Post-traumatic Stress Disorder; − PTSD = no PTSD
DISCUSSION
We reviewed 64 studies representing three different categories of evidence linking PTSD with accelerated aging (biomarkers of senescence, senescence-associated medical comorbidities, and mortality rates). We found at least partial evidence of an association in each category discussed in detail below.
Biomarkers related to senescence
A majority of studies of LTL and pro-inflammatory markers supported a model of early senescence in PTSD, although not all individual studies supported this, and conclusions must be tempered due to the relatively small number of studies available for comparison. The results for oxidative stress were equivocal as only five studies were recovered and the methods and outcomes were too diverse to permit firm conclusions about the presence or absence of an association of PTSD with oxidative stress (18, 46–49), although there are theoretical reasons to expect such an association (45). Pooled effect sizes of LTL and pro-inflammatory biomarkers were generally consistent with an association of PTSD with senescent-like changes. The nature of this relationship is not known. Some investigators suggest that shorter LTL may be a risk factor for PTSD (24), while others contend that LTL may be shortened as a result of a traumatic event or the onset of PTSD (25–27). Prospective longitudinal research, either measuring LTL among those pre- and post-trauma, or repeatedly measuring LTL among people with PTSD would be helpful in disentangling these possibilities. The biochemical/molecular mechanisms underlying a possible decrease in LTL in PTSD are not fully known. LTL is approximately 70% genetically determined (75), and telomeres are also subject to epigenetic modifications acquired over the lifespan (76). Such processes could explain the possibility of LTL shortening preceding (and possibly being associated with risk of acquiring) PTSD. A major non-genetic or epigenetic determinant of LTL is repeated cell division, as might occur in leukocytes responding to chronic antigen exposure, in the absence of adequate telomerase activity (77). The other major effectors of LTL shortening are oxidative stress (78), which, as reviewed here, has equivocal evidence of increases in PTSD, and chronic inflammation. A large number of studies have now reported increased inflammation in PTSD (79), which offers one plausible explanation for LTL shortening.
Earlier Onset of Senescence-Associated Conditions
The reviewed evidences also suggest associations between PTSD and most of the age-associated medical illnesses targeted in this review, with the possible exception of HTN. The association of PTSD with such distinct medical conditions, which nevertheless share an association with aging, supports an accelerated aging model of PTSD.
The findings of senescence-related medical comorbidities are consistent with prior reviews focused on the elevated incidence and prevalence of cardiovascular disease in PTSD (80, 81). The studies of dementia are consistent with many reports indicating worse cognitive performance in patients with PTSD. A recent meta-analysis conducted by members of the current research team revealed older adults with PTSD have greater than age-expected deficits in a range of cognitive domains, with particularly strong effects noted for processing speed, learning/memory, and executive functions (82). Note that these are also among the domains most commonly affected by normal aging (83). The increased cognitive impairment may also impact risk of dementia due to lowered cognitive reserve. While combat-related traumatic brain injury (TBI) could conceivably explain some of the increased risk of dementia in PTSD, such an increase was also reported in one non-combat sample (84).
Mortality
Seven of 10 studies reported increased mortality in PTSD, with the statistical analysis suggesting a mild to moderate association. This is consistent with an early onset or acceleration of senescence in PTSD.
Limitations
Retrospective mortality analyses tend to be easier to conduct in military Veteran settings (e.g. VA hospital systems) because of the availability of long-term retrospective administrative or clinical datasets. Nine of the 10 mortality studies included in the present review were conducted in military Veteran samples (85–93); the other study included Veterans and other World War II survivors in a community sample in the Netherlands (94). As mortality among military Veterans may differ in form or overall rate from the general population, the observed overall mortality hazard ratio may not fully generalize to non-Veteran PTSD populations. On the other hand, the non-PTSD comparison subjects in each of these studies were also war Veterans, so general military/war experience was not a systematic bias or unilaterally confounded with PTSD status within these studies. In terms of mortality, it is difficult to say which causes of death should be included in any analysis. Suicides and accidents should perhaps be excluded, but if one is looking at mortality as an indicator of physiological aging, it is not clear that such exclusions are appropriate, e.g. suicides of individuals who are suffering from severe impairments in functioning, or accidents due to age-related physical problems are relevant, but this information is usually not available.
Other potential interpretative limitations reflect the fact that the present review required synthesis across a broad spectrum of studies that varied in outcomes using divergent methods. There was large variability among included studies in the subject selection criteria, nature of the comparison samples, nature of the index traumas, age at the index trauma, assessment methods, and the statistical analyses and reported results. Indeed, the variability in outcomes and available data for the medical comorbidity studies made calculation of meaningful pooled effect sizes questionable, so we focused that part of our review on whether the findings of each study supported an association of PTSD with the targeted medical condition rather than conducting meta-analytic estimates of pooled effect sizes. On the other hand, there was sufficient overlap to calculate pooled effect size estimates for several biomarkers (LTLs, CRP, IL-6, IL-1 β, and TNFα) as well as in the mortality reports to calculate an average hazard ratio. Thus, the standard limitations of any meta-analyses are relevant, including publication bias toward studies with positive findings, and heterogeneity in the specific design and nature of component studies (95, 96). In addition, many of these findings could reflect associations of PTSD with other risk factors such as smoking and alcohol consumption, lack of exercise, and poor nutrition. A number of the reviewed studies were limited in terms of their assessment of some potential risk factors such as the number and severity of traumas, duration of PTSD, concomitant injuries and types of treatment received by the patients. Another limitation is that any individual increase in a senescence-associated biomarker does not necessarily prove an association with senescence per se, as the biomarker may be altered in other conditions as well.
This review intentionally focused on comorbidity of several specific conditions that are potentially fatal, known to increase in incidence with normal aging, and worsened by stress. However, it is possible that PTSD is associated with increased general medical comorbidity and not just age-related morbidity. That is, PTSD may be bad for one’s biological and neurocognitive health, irrespective of a specific age-related process. On the other hand, most medical health problems are adversely affected by normal aging, making it difficult to identify comparator conditions that are definitively not age-related.
Is there evidence for early or accelerated senescence in PTSD?
Overall, the reviewed empirical literature suggested early senescence in PTSD. There is a question of whether the mortality rate curve, usually modeled with the exponential Gompertz mortality function (97), is left-shifted toward a younger age - premature senescence - resulting in senescence occurring earlier due to a higher initial mortality rate, vs. whether the exponential curve rises at a faster rate - a true acceleration of senescence. [The Gompertz function is an empirical population model for mortality rates which is usually expressed as an exponential function; the slope of the line represents the acceleration of mortality rate with age, which gives an estimate of the rate of senescence.] In both of these cases, which are not mutually exclusive, there would be a higher mortality rate at a given age. To our knowledge, no studies have been performed in PTSD or any other psychiatric disorders in which the Gompertz rate has been estimated. Thus, there is no direct evidence of “accelerated” senescence in any psychiatric condition at this time. But, if PTSD were associated with not just earlier but accelerated aging, comorbid medical conditions could continue to evolve at an accelerated pace. Furthermore, one would expect that any intervention affecting core aging processes might open the field to a new set of therapeutic approaches. For PTSD, this could mean using drugs that have been considered for their possible anti-aging potential, such as anti-inflammatory medications.
What are the potential protective or risk factors for early senescence in PTSD?
Is any increase in senescence-related morbidity associated with PTSD itself; with other health-related behavioral changes associated with PTSD, such as increased smoking, drug use, or suicidal behavior, or with other factors such as gender or treatment? This question cannot be answered with certainty at this time. Few studies specifically focused on gender differences in PTSD-related early senescence (29). Treatment or improvement of PTSD may also influence senescence effects, which is supported by the study of Gill et al. (40), in which CRP and IL-6 were elevated in women who had current PTSD, but not in those who had recovered from PTSD, and the study of Zohar et al. (93), which did not report an association of PTSD with increased mortality, but in which all the participants had received aggressive monitoring and treatment for PTSD.
What are the possible mechanisms of early senescence in PTSD?
Mechanisms of early senescence in PTSD may depend on the type of association between the two, and there are several possibilities, again not mutually exclusive. First, individuals vulnerable to early senescence processes may also be more vulnerable to develop PTSD (24). Second, traumatic events that precipitate PTSD may affect the initial mortality rate of individuals. For example, survivors of trauma may have earlier mortality due to complications from physical injuries rather than PTSD. Third, PTSD may be associated with ongoing physiological processes that increase the rate of senescence over time, e.g., inflammation.
The pathways by which inflammatory or other senescence-related processes could be maintained after the initial trauma are unknown, but could involve increased allostatic load, where neural mechanisms are utilized to maintain optimal functioning under changing stressors. Allostatic mechanisms are hypothesized to be utilized to anticipate the effects of stressors and address them to preserve optimal functioning with the least cost (98). The sum of the changes in allostatic mechanisms (including blood concentrations of cortisol, norepinephrine, epinephrine, cholesterol, and glycosylated hemoglobin, as well as blood pressure and heart rate variability) in response to anticipated stressors is called allostatic load. An increase in allostatic load may be associated with obesity, HTN, hyperlipidemia, and atherosclerosis, and may be involved in mechanisms for the development of ulcer disease and cardiovascular disorders (99, 100). Studies have reported abnormalities in allostatic load in PTSD (101), and it may be important to determine how these relate to biological indicators of senescence and to medial morbidity and mortality.
Suggestions for Future Research
The present review does not speak to whether accelerated aging is specific to PTSD. As was noted above, there have been suggestions that medical comorbidity in some other psychiatric conditions, including schizophrenia, bipolar disorder, and major depression, reflect an acceleration of the aging process (1–4). Among studies in the present review there were isolated reports that, in addition to normative comparison samples, included other psychiatric conditions as potential comparison groups, but we intentionally focused our literature search and review on comparisons of people with PTSD to psychiatrically healthy comparison subjects. But even if PTSD is only one of several psychiatric conditions associated with accelerated aging, the relative precision with which one can identify the time of the proximal causal traumatic event in PTSD affords a relatively unique research opportunity. That is, the relative precision with which onset can be estimated among people with PTSD relative to other psychiatric conditions may allow researchers and clinicians to better characterize the trajectory of such change, such as whether the added comorbidity occurs soon after illness onset or continues to accelerate in the course of years living with the disorder, as well as whether treatment of the psychiatric symptoms of PTSD alters that course.
We recommend that future studies focus on issues of premature versus accelerated senescence, senescence-associated versus non-senescence-associated medical comorbidities, and greater specifics about the nature of inflammatory, oxidative, cellular aging, and allostatic processes that may be involved in PTSD, as well as about the nature of the trauma—type, intensity, recurrence, and developmental timing. To address one of the potential mechanisms we noted above, that earlier mortality may be due to physical injuries rather than PTSD, studies could compare individuals with PTSD who incurred injuries during the traumatic event to those with PTSD who did not. In addition, it would be important to learn exactly how biological indicators of senescence may be relevant to future therapeutic efforts, perhaps enabling more personalized treatment. As noted above, only a few isolated studies considered remitted versus non-remitted PTSD (see discussion of Gill et al. (40) and Zohar et al. (93) above), and/or directly compared those with full versus “partial” PTSD. Thus, another set of key questions for further research is the importance and timing of PTSD syndromal status, i.e. does accelerated aging occur even after the psychiatric symptoms have remitted and/or among those who show most but not full PTSD symptoms? Finally, we urge scientists to investigate the public health consequences of early aging and PTSD.
Conclusions and Implications
We believe these findings suggest a need to re-conceptualize PTSD as being more than a mental illness. Early senescence and increased medical morbidity and premature mortality in PTSD have implications for healthcare beyond simply the treatment of PTSD symptoms, and warrant a more integrated medical-psychiatric approach. At a time when people are generally aging better (delaying social security, living longer, etc.), there may be a subgroup of people that is moving in the opposite direction. This has obvious humanitarian and healthcare cost implications.
Supplementary Material
Acknowledgments
This work was supported, in part, by the VA Center of Excellence for Stress and Mental Health (CESAMH), National Institutes of Health grants R01 MH099987, R01 MH094151, T32 MH019934, Department of Defense grant W81XWH-12-1-0614, and by the University of California, San Diego Center for Healthy Aging, and the Sam and Rose Stein Institute for Research on Aging.
Footnotes
Financial Disclosure: All authors report no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jeste DV, Wolkowitz OM, Palmer BW. Divergent trajectories of physical, cognitive, and psychosocial aging in schizophrenia. Schizophr Bull. 2011;37:451–455. doi: 10.1093/schbul/sbr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolkowitz OM, Reus VI, Mellon SH. Of sound mind and body: depression, disease, and accelerated aging. Dialogues Clin Neurosci. 2011;13:25–39. doi: 10.31887/DCNS.2011.13.1/owolkowitz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirkpatrick B, Messias E, Harvey PD, et al. Is schizophrenia a syndrome of accelerated aging? Schizophr. Bull. 2008;34:1024–1032. doi: 10.1093/schbul/sbm140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizzo LB, Costa LG, Mansur RB, et al. The theory of bipolar disorder as an illness of accelerated aging: implications for clinical care and research. Neurosci Biobehav Rev. 2014;42:157–169. doi: 10.1016/j.neubiorev.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Epel ES. Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones. 2009;8:7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa N, Nakamura K, Izumiyama-Shimomura N, et al. Accelerated in vivo epidermal telomere loss in Werner syndrome. Aging (Albany NY) 2011;3:417–429. doi: 10.18632/aging.100315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zigman WB. Atypical aging in down syndrome. Dev Disab Res Rev. 2013;18:51–67. doi: 10.1002/ddrr.1128. [DOI] [PubMed] [Google Scholar]
- 8.Pollex RL, Hegele RA. Hutchinson-Gilford progeria syndrome. Clin Genet. 2004;66:375–381. doi: 10.1111/j.1399-0004.2004.00315.x. [DOI] [PubMed] [Google Scholar]
- 9.Pathai S, Bajillan H, Landay AL, et al. Is HIV a Model of Accelerated or Accentuated Aging? The Journals of Gerontology. 2013 doi: 10.1093/gerona/glt168. Series A, Biological Sciences and Medical Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerhard-Herman M, Smoot LB, Wake N, et al. Mechanisms of premature vascular aging in children with Hutchinson-Gilford progeria syndrome. Hypertension. 2012;59:92–97. doi: 10.1161/HYPERTENSIONAHA.111.180919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farriols Danés C. Specific aspects of ageing in Down’s syndrome. International Medical Review on Down Syndrome. 2012;16:3–10. [Google Scholar]
- 12.Decker ML, Chavez E, Vulto I, et al. Telomere length in Hutchinson-Gilford progeria syndrome. Mech Ageing Dev. 2009;130:377–383. doi: 10.1016/j.mad.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Akushevich I, Kravchenko J, Ukraintseva S, et al. Age patterns of incidence of geriatric disease in the U.S. elderly population: Medicare-based analysis. J Am Geriatr Soc. 2012;60:323–327. doi: 10.1111/j.1532-5415.2011.03786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson DR, Fontana A, Lubin H, et al. Long-term course of treatment-seeking Vietnam veterans with posttraumatic stress disorder: mortality, clinical condition, and life satisfaction. J Nerv Ment Dis. 2004;192:35–41. doi: 10.1097/01.nmd.0000105998.90425.6a. [DOI] [PubMed] [Google Scholar]
- 15.Crawford EF, Drescher KD, Rosen CS. Predicting mortality in veterans with posttraumatic stress disorder thirty years after Vietnam. J Nerv Ment Dis. 2009;197:260–265. doi: 10.1097/NMD.0b013e31819dbfce. [DOI] [PubMed] [Google Scholar]
- 16.O’Toole BI, Catts SV, Outram S, et al. Factors associated with civilian mortality in Australian Vietnam veterans three decades after the war. Mil Med. 2010;175:88–95. doi: 10.7205/milmed-d-09-00071. [DOI] [PubMed] [Google Scholar]
- 17.Baker DG, Ekhator NN, Kasckow JW, et al. Plasma and cerebrospinal fluid interleukin-6 concentrations in posttraumatic stress disorder. Neuroimmunomodulation. 2001;9:209–217. doi: 10.1159/000049028. [DOI] [PubMed] [Google Scholar]
- 18.Tezcan E, Atmaca M, Kuloglu M, et al. Free radicals in patients with post-traumatic stress disorder. Eur Arch Psychiatry Clin Neurosci. 2003;253:89–91. doi: 10.1007/s00406-003-0413-x. [DOI] [PubMed] [Google Scholar]
- 19.Linnville S, Hoyt RE, Moore JL, et al. Posttraumatic stress disorder and metabolic syndrome: retrospective study of repatriated prisoners of war. Mil Med. 2011;176:369–374. doi: 10.7205/milmed-d-10-00367. [DOI] [PubMed] [Google Scholar]
- 20.Weiss T, Skelton K, Phifer J, et al. Posttraumatic stress disorder is a risk factor for metabolic syndrome in an impoverished urban population. Gen Hosp Psychiatry. 2011;33:135–142. doi: 10.1016/j.genhosppsych.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pietrzak RH, Goldstein RB, Southwick SM, et al. Physical health conditions associated with posttraumatic stress disorder in U.S. older adults: results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Am Geriatr Soc. 2012;60:296–303. doi: 10.1111/j.1532-5415.2011.03788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heppner PS, Crawford EF, Haji UA, et al. The association of posttraumatic stress disorder and metabolic syndrome: a study of increased health risk in veterans. BMC Med. 2009;7:1. doi: 10.1186/1741-7015-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukaschek K, Baumert J, Kruse J, et al. Relationship between posttraumatic stress disorder and type 2 diabetes in a population-based cross-sectional study with 2970 participants. J Psychosom Res. 2013;74:340–345. doi: 10.1016/j.jpsychores.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Malan S, Hemmings S, Kidd M, et al. Investigation of telomere length and psychological stress in rape victims. Depress Anxiety. 2011;28:1081–1085. doi: 10.1002/da.20903. [DOI] [PubMed] [Google Scholar]
- 25.O’Donovan A, Epel E, Lin J, et al. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatry. 2011;70:465–471. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ladwig KH, Brockhaus AC, Baumert J, et al. Posttraumatic stress disorder and not depression is associated with shorter leukocyte telomere length: findings from 3,000 participants in the population-based KORA F4 study. PLoS One. 2013;8:e64762. doi: 10.1371/journal.pone.0064762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Hu XZ, Benedek DM, et al. The interaction between stressful life events and leukocyte telomere length is associated with PTSD. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.141. [DOI] [PubMed] [Google Scholar]
- 28.Jergovic M, Tomicevic M, Vidovic A, et al. Telomere shortening and immune activity in war veterans with posttraumatic stress disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:275–283. doi: 10.1016/j.pnpbp.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Shalev I, Moffitt TE, Braithwaite AW, et al. Internalizing disorders and leukocyte telomere erosion: a prospective study of depression, generalized anxiety disorder and post-traumatic stress disorder. Mol Psychiatry. 2014 doi: 10.1038/mp.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Kanel R, Hepp U, Kraemer B, et al. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res. 2007;41:744–752. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Gill J, Vythilingam M, Page GG. Low cortisol, high DHEA, and high levels of stimulated TNF-alpha, and IL-6 in women with PTSD. J Traum Stress. 2008;21:530–539. doi: 10.1002/jts.20372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoge EA, Brandstetter K, Moshier S, et al. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety. 2009;26:447–455. doi: 10.1002/da.20564. [DOI] [PubMed] [Google Scholar]
- 33.Vidovic A, Vilibic M, Sabioncello A, et al. Changes in immune and endocrine systems in posttraumatic stress disorder - prospective study. Acta neuropsychiatrica. 2009;21 (Suppl 2):46–50. doi: 10.1017/S0924270800032725. [DOI] [PubMed] [Google Scholar]
- 34.Gill J, Luckenbaugh D, Charney D, et al. Sustained elevation of serum interleukin-6 and relative insensitivity to hydrocortisone differentiates posttraumatic stress disorder with and without depression. Biol Psychiatry. 2010;68:999–1006. doi: 10.1016/j.biopsych.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 35.Spitzer C, Barnow S, Volzke H, et al. Association of posttraumatic stress disorder with low-grade elevation of C-reactive protein: evidence from the general population. J Psychiatr Res. 2010;44:15–21. doi: 10.1016/j.jpsychires.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Guo M, Liu T, Guo J-C, et al. Study on serum cytokine levels in posttraumatic stress disorder patients. Asian Pac J Trop Med. 2012;5:323–325. doi: 10.1016/S1995-7645(12)60048-0. [DOI] [PubMed] [Google Scholar]
- 37.Hammad SM, Truman JP, Al Gadban MM, et al. Altered blood sphingolipidomics and elevated plasma inflammatory cytokines in combat Veterans with Post-traumatic Stress Disorder. Neurobiol Lipids. 2012;10:2. [PMC free article] [PubMed] [Google Scholar]
- 38.Zimmerman G, Shaltiel G, Barbash S, et al. Post-traumatic anxiety associates with failure of the innate immune receptor TLR9 to evade the pro-inflammatory NFkappaB pathway. Transl Psychiatry. 2012;2:e78. doi: 10.1038/tp.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baumert J, Lukaschek K, Kruse J, et al. No evidence for an association of posttraumatic stress disorder with circulating levels of CRP and IL-18 in a population-based study. Cytokine. 2013;63:201–208. doi: 10.1016/j.cyto.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 40.Gill JM, Saligan L, Lee H, et al. Women in recovery from PTSD have similar inflammation and quality of life as non-traumatized controls. J Psychosom Res. 2013;74:301–306. doi: 10.1016/j.jpsychores.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 41.Gola H, Engler H, Sommershof A, et al. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry. 2013;13:40. doi: 10.1186/1471-244X-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plantinga L, Bremner JD, Miller AH, et al. Association between posttraumatic stress disorder and inflammation: a twin study. Brain Behav Immun. 2013;30:125–132. doi: 10.1016/j.bbi.2013.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spitzer C, Wibisono D, Terfehr K, et al. C-reactive protein, pre- and postdexamethasone cortisol levels in post-traumatic stress disorder. Nord J Psychiatr. 2013 doi: 10.3109/08039488.2013.844271. [DOI] [PubMed] [Google Scholar]
- 44.Lindqvist D, Wolkowitz OM, Mellon S, et al. Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 45.Miller MW, Sadeh N. Traumatic stress, oxidative stress and post-traumatic stress disorder: neurodegeneration and the accelerated-aging hypothesis. Mol Psychiatry. 2014;19:1156–1162. doi: 10.1038/mp.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ceprnja M, Derek L, Unic A, et al. Oxidative stress markers in patients with post-traumatic stress disorder. Coll Antropol. 2011;35:1155–1160. [PubMed] [Google Scholar]
- 47.Borovac Stefanovic L, Kalinic D, Mimica N, et al. Oxidative status and the severity of clinical symptoms in patients with post-traumatic stress disorder. Ann Clin Biochem. 2014 doi: 10.1177/0004563214528882. [DOI] [PubMed] [Google Scholar]
- 48.Michels L, Schulte-Vels T, Schick M, et al. Prefrontal GABA and glutathione imbalance in posttraumatic stress disorder: preliminary findings. Psychiatry Res. 2014;224:288–295. doi: 10.1016/j.pscychresns.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Ozdemir PG, Kaplan I, Uysal C, et al. Serum total oxidant and antioxidant status in earthquake survivors with post-traumatic stress disorder. Acta Neuropsychiatr. 2015:1–6. doi: 10.1017/neu.2014.47. [DOI] [PubMed] [Google Scholar]
- 50.Lauterbach D, Vora R, Rakow M. The relationship between posttraumatic stress disorder and self-reported health problems. Psychosom Med. 2005;67:939–947. doi: 10.1097/01.psy.0000188572.91553.a5. [DOI] [PubMed] [Google Scholar]
- 51.Andersen J, Wade M, Possemato KA, et al. Association between posttraumatic stress disorder and primary care provider-diagnosed disease among Iraq and Afghanistan veterans. Psychosom Med. 2010;72:498–504. doi: 10.1097/PSY.0b013e3181d969a1. [DOI] [PubMed] [Google Scholar]
- 52.Glaesmer H, Brahler E, Gundel H, et al. The association of traumatic experiences and posttraumatic stress disorder with physical morbidity in old age: a German population-based study. Psychosom Med. 2011;73:401–406. doi: 10.1097/PSY.0b013e31821b47e8. [DOI] [PubMed] [Google Scholar]
- 53.Kibler JL, Joshi K, Ma M. Hypertension in relation to posttraumatic stress disorder and depression in the US National Comorbidity Survey. Behavioral medicine (Washington, D C) 2009;34:125–132. doi: 10.3200/BMED.34.4.125-132. [DOI] [PubMed] [Google Scholar]
- 54.Paulus EJ, Argo TR, Egge JA. The impact of posttraumatic stress disorder on blood pressure and heart rate in a veteran population. J Trauma Stress. 2013;26:169–172. doi: 10.1002/jts.21785. [DOI] [PubMed] [Google Scholar]
- 55.Kang HK, Bullman TA, Taylor JW. Risk of selected cardiovascular diseases and posttraumatic stress disorder among former World War II prisoners of war. Ann Epidemiol. 2006;16:381–386. doi: 10.1016/j.annepidem.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 56.David D, Woodward C, Esquenazi J, et al. Comparison of comorbid physical illnesses among veterans with PTSD and veterans with alcohol dependence. Psychiatr Serv. 2004;55:82–85. doi: 10.1176/appi.ps.55.1.82. [DOI] [PubMed] [Google Scholar]
- 57.Muhtz C, Godemann K, von Alm C, et al. Effects of chronic posttraumatic stress disorder on metabolic risk, quality of life, and stress hormones in aging former refugee children. J Nerv Ment Dis. 2011;199:646–652. doi: 10.1097/NMD.0b013e318229cfba. [DOI] [PubMed] [Google Scholar]
- 58.Seeman TE, Crimmins E, Huang M-H, et al. Cumulative biological risk and socioeconomic differences in mortality: MacArthur Studies of Successful Aging. Soc Sci Med. 2004;58:1985–1997. doi: 10.1016/S0277-9536(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 59.Dobie DJ, Kivlahan DR, Maynard C, et al. Posttraumatic stress disorder in female veterans: association with self-reported health problems and functional impairment. Arch Intern Med. 2004;164:394–400. doi: 10.1001/archinte.164.4.394. [DOI] [PubMed] [Google Scholar]
- 60.Walczewska J, Rutkowski K, Wizner B, et al. Stiffness of large arteries and cardiovascular risk in patients with post-traumatic stress disorder. Eur Heart J. 2011;32:730–736. doi: 10.1093/eurheartj/ehq354. [DOI] [PubMed] [Google Scholar]
- 61.Sawchuk CN, Roy-Byrne P, Goldberg J, et al. The relationship between post-traumatic stress disorder, depression and cardiovascular disease in an American Indian tribe. Psychol Med. 2005;35:1785–1794. doi: 10.1017/S0033291705005751. [DOI] [PubMed] [Google Scholar]
- 62.Sledjeski EM, Speisman B, Dierker LC. Does number of lifetime traumas explain the relationship between PTSD and chronic medical conditions? Answers from the National Comorbidity Survey-Replication (NCS-R) J Behav Med. 2008;31:341–349. doi: 10.1007/s10865-008-9158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spitzer C, Barnow S, Volzke H, et al. Trauma, posttraumatic stress disorder, and physical illness: findings from the general population. Psychosom Med. 2009;71:1012–1017. doi: 10.1097/PSY.0b013e3181bc76b5. [DOI] [PubMed] [Google Scholar]
- 64.Vaccarino V, Goldberg J, Rooks C, et al. Post-traumatic stress disorder and incidence of coronary heart disease: a twin study. J Am Coll Cardiol. 2013;62:970–978. doi: 10.1016/j.jacc.2013.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Turner JH, Neylan TC, Schiller NB, et al. Objective evidence of myocardial ischemia in patients with posttraumatic stress disorder. Biol Psychiatry. 2013;74:861–866. doi: 10.1016/j.biopsych.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crum-Cianflone NF, Bagnell ME, Schaller E, et al. Impact of combat deployment and posttraumatic stress disorder on newly reported coronary heart disease among US active duty and reserve forces. Circulation. 2014;129:1813–1820. doi: 10.1161/CIRCULATIONAHA.113.005407. [DOI] [PubMed] [Google Scholar]
- 67.Jin H, Lanouette NM, Mudaliar S, et al. Association of posttraumatic stress disorder with increased prevalence of metabolic syndrome. J Clin Psychopharmacol. 2009;29:210–215. doi: 10.1097/JCP.0b013e3181a45ed0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Agyemang C, Goosen S, Anujuo K, et al. Relationship between post-traumatic stress disorder and diabetes among 105,180 asylum seekers in the Netherlands. European journal of public health. 2012;22:658–662. doi: 10.1093/eurpub/ckr138. [DOI] [PubMed] [Google Scholar]
- 69.Boyko EJ, Jacobson IG, Smith B, et al. Risk of diabetes in U. S. military service members in relation to combat deployment and mental health. Diabetes Care. 2010;33:1771–1777. doi: 10.2337/dc10-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scott KM, Alonso J, de Jonge P, et al. Associations between DSM-IV mental disorders and onset of self-reported peptic ulcer in the World Mental Health Surveys. J Psychosom Res. 2013;75:121–127. doi: 10.1016/j.jpsychores.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weisberg RB, Bruce SE, Machan JT, et al. Nonpsychiatric illness among primary care patients with trauma histories and posttraumatic stress disorder. Psychiatr Serv. 2002;53:848–854. doi: 10.1176/appi.ps.53.7.848. [DOI] [PubMed] [Google Scholar]
- 72.Qureshi SU, Kimbrell TA, Pyne JM, et al. Greater prevalence and incidence of dementia in older veterans with posttraumatic stress disorder. Journal of the American Geriatrics Society. 2010;58:1627–1633. doi: 10.1111/j.1532-5415.2010.02977.x. [DOI] [PubMed] [Google Scholar]
- 73.Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67:608–613. doi: 10.1001/archgenpsychiatry.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meziab O, Kirby KA, Williams B, et al. Prisoner of war status, posttraumatic stress disorder, and dementia in older veterans. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2014;10:S236–241. doi: 10.1016/j.jalz.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 75.Broer L, Codd V, Nyholt DR, et al. Meta-analysis of telomere length in 19,713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. European journal of human genetics: EJHG. 2013;21:1163–1168. doi: 10.1038/ejhg.2012.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aviv A, Levy D. Telomeres, atherosclerosis, and the hemothelium: the longer view. Annu Rev Med. 2012;63:293–301. doi: 10.1146/annurev-med-050311-104846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Calado RT, Young NS. Telomere diseases. The New England journal of medicine. 2009;361:2353–2365. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saretzki G, Von Zglinicki T. Replicative aging, telomeres, and oxidative stress. Ann N Y Acad Sci. 2002;959:24–29. doi: 10.1111/j.1749-6632.2002.tb02079.x. [DOI] [PubMed] [Google Scholar]
- 79.Baker DG, Nievergelt CM, O’Connor DT. Biomarkers of PTSD: neuropeptides and immune signaling. Neuropharmacology. 2012;62:663–673. doi: 10.1016/j.neuropharm.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 80.Edmondson D, Kronish IM, Shaffer JA, et al. Posttraumatic stress disorder and risk for coronary heart disease: a meta-analytic review. Am Heart J. 2013;166:806–814. doi: 10.1016/j.ahj.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dedert EA, Calhoun PS, Watkins LL, et al. Posttraumatic stress disorder, cardiovascular, and metabolic disease: a review of the evidence. Annals of behavioral medicine: a publication of the Society of Behavioral Medicine. 2010;39:61–78. doi: 10.1007/s12160-010-9165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schuitevoerder S, Rosen JW, Twamley EW, et al. A meta-analysis of cognitive functioning in older adults with PTSD. J Anxiety Disord. 2013 doi: 10.1016/j.janxdis.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 83.Palmer BW, Dawes SE. Cognitive aging: From basic skills to scripts and schemata. In: Jeste DV, Depp CD, editors. Handbook of Successful Cognitive and Emotional Aging. Arlington, VA: American Psychiatric Publishing, Inc; 2010. pp. 37–54. [Google Scholar]
- 84.Sperling W, Kreil SK, Biermann T. Posttraumatic stress disorder and dementia in Holocaust survivors. J Nerv Ment Dis. 2011;199:196–198. doi: 10.1097/NMD.0b013e31820c71e0. [DOI] [PubMed] [Google Scholar]
- 85.Boscarino JA. Posttraumatic stress disorder and mortality among U. S. Army veterans 30 years after military service. Ann Epidemiol. 2006;16:248–256. doi: 10.1016/j.annepidem.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 86.Boscarino JA. A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: implications for surveillance and prevention. Psychosom Med. 2008;70:668–676. doi: 10.1097/PSY.0b013e31817bccaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kinder LS, Bradley KA, Katon WJ, et al. Depression, posttraumatic stress disorder, and mortality. Psychosom Med. 2008;70:20–26. doi: 10.1097/PSY.0b013e31815aac93. [DOI] [PubMed] [Google Scholar]
- 88.Chwastiak LA, Rosenheck RA, Desai R, et al. Association of psychiatric illness and all-cause mortality in the National Department of Veterans Affairs Health Care System. Psychosom Med. 2010;72:817–822. doi: 10.1097/PSY.0b013e3181eb33e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Flood AM, Boyle SH, Calhoun PS, et al. Prospective study of externalizing and internalizing subtypes of posttraumatic stress disorder and their relationship to mortality among Vietnam veterans. Compr Psychiatry. 2010;51:236–242. doi: 10.1016/j.comppsych.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ahmadi N, Hajsadeghi F, Mirshkarlo HB, et al. Post-traumatic stress disorder, coronary atherosclerosis, and mortality. Am J Cardiol. 2011;108:29–33. doi: 10.1016/j.amjcard.2011.02.340. [DOI] [PubMed] [Google Scholar]
- 91.Kimbrell TA, Pyne JM, Kunik ME, et al. The impact of Purple Heart commendation and PTSD on mortality rates in older veterans. Depress Anxiety. 2011;28:1086–1090. doi: 10.1002/da.20850. [DOI] [PubMed] [Google Scholar]
- 92.Xue Y, Taub PR, Iqbal N, et al. Cardiac biomarkers, mortality, and post-traumatic stress disorder in military veterans. Am J Cardiol. 2012;109:1215–1218. doi: 10.1016/j.amjcard.2011.11.063. [DOI] [PubMed] [Google Scholar]
- 93.Zohar J, Fostick L. Mortality rates between treated post-traumatic stress disorder Israeli male veterans compared to non-diagnosed veterans. Eur Neuropsychopharmacol. 2014;24:117–124. doi: 10.1016/j.euroneuro.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 94.Bramsen I, Deeg DJ, van der Ploeg E, et al. Wartime stressors and mental health symptoms as predictors of late-life mortality in World War II survivors. J Affect Disord. 2007;103:121–129. doi: 10.1016/j.jad.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 95.Flather MD, Farkouh ME, Pogue JM, et al. Strengths and limitations of meta-analysis: Larger studies may be more reliable. Control Clin Trials. 1997;18:568–579. doi: 10.1016/s0197-2456(97)00024-x. [DOI] [PubMed] [Google Scholar]
- 96.Walker E, Hernandez AV, Kattan MW. Meta-analysis: Its strengths and limitations. Cleve Clin J Med. 2008;75:431–439. doi: 10.3949/ccjm.75.6.431. [DOI] [PubMed] [Google Scholar]
- 97.Boxenbaum H. Gompertz mortality analysis: aging, longevity hormesis and toxicity. Archives of Gerontology and Geriatrics. 1991;13:125–137. doi: 10.1016/0167-4943(91)90055-u. [DOI] [PubMed] [Google Scholar]
- 98.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 99.McFarlane AC. The long-term costs of traumatic stress: intertwined physical and psychological consequences. World Psychiatry. 2010;9:3–10. doi: 10.1002/j.2051-5545.2010.tb00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kibler JL, Joshi K, Ma M. Hypertension in relation to posttraumatic stress disorder and depression in the US National Comorbidity Survey. Behav Med. 2009;34:125–132. doi: 10.3200/BMED.34.4.125-132. [DOI] [PubMed] [Google Scholar]
- 101.Vidovic A, Gotovac K, Vilibic M, et al. Repeated assessments of endocrine- and immune-related changes in posttraumatic stress disorder. Neuroimmunomodulation. 2011;18:199–211. doi: 10.1159/000322869. [DOI] [PubMed] [Google Scholar]
- 102.Lukaschek K, Baumert J, Kruse J, et al. Relationship between posttraumatic stress disorder and type 2 diabetes in a population-based cross-sectional study with 2970 participants. J Psychosom Res. 2013;74:340–345. doi: 10.1016/j.jpsychores.2012.12.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.