Abstract
Background
Racial disparities in health outcomes after living donation have been reported, but generalizability is not known.
Methods
We linked Organ Procurement and Transplantation Network (OPTN) registry data for 4,007 living kidney donors in 1987 to 2008 with Medicare billing claims (2000–2008). Cox regression with left and right censoring was used to estimate the frequencies and relative risks of postdonation medical diagnoses according to race. Patterns were compared with findings from a previous linkage of OPTN donor records and private insurance claims.
Results
Among the Medicare-insured donors, 8% were African American and 5.7% were Hispanic. Diagnosis frequencies at 5 years after donation in the Medicare-versus privately insured donors included the following: malignant hypertension, 5.0% versus 0.9%; diabetes, 18.5% versus 4.1%; and chronic kidney disease, 21.8% versus 4.9%. After age and sex adjustment in the Medicare sample, African Americans, as compared with white donors, experienced higher risks of any hypertension diagnosis, including 2.4 times the likelihood of malignant hypertension (adjusted hazard ratio [aHR], 2.35; 95% confidence interval [CI], 1.40–3.93), and more common diabetes (aHR, 1.50; 95% CI, 1.12–2.04), chronic kidney disease (aHR, 1.84; 95% CI, 1.37–2.47), and proteinuria (aHR, 2.44; 95% CI, 1.45–4.11) diagnoses. Relative patterns for privately insured African American versus white donors were similar, including approximately three times the risk of malignant hypertension (aHR, 3.27; 95% CI, 1.82–5.88) and twice the relative risks of chronic kidney disease and proteinuria.
Conclusions
Consistent demonstration of racial variation in postdonation medical conditions regardless of sample/payer source supports the need for continued study of mediators and consequences of outcomes in non-white donors.
Keywords: Chronic kidney disease, Diabetes mellitus, Hypertension, Kidney transplantation, Living donors, Medicare
As policies for the informed consent, medical evaluation, and follow-up of living organ donors are receiving more scrutiny and formalization by the organizations that guide and regulate transplant practice (1, 2), detailed understanding of the evidence that underlies best practices applicable to donors with diverse demographic profiles is warranted. The largest reported cohort study of living kidney donors completed to date found no adverse impacts of donation on survival or end-stage renal disease (ESRD) risk compared with general population registry controls (3), but notably, more than 98% of donors in this cohort were of white race. Recent linkages of national Organ Procurement and Transplantation Network (OPTN) donation records with transplant waiting list registrations and Center for Medicare and Medicaid Services (CMS) ESRD reporting forms demonstrated that, while ESRD is an uncommon event after kidney donation, rates are markedly higher among African American compared with white donors (4). For example, among U.S. donors in 1987 to 2003 followed through 2009, the rate of ESRD in African American donors was nearly five times that of white donors (4).
As a reflection of the need for a better understanding of health outcomes specifically among non-white donors, a recent U.S. national consensus conference was convened to evaluate “Living Kidney Donor Follow-up: State-of-the-Art and Future Direction” (5). At this conference, non-white donors were identified as a leading subgroup deserving focused attention. Important postdonation outcomes named as focus areas included not only death and terminal renal failure but also chronic medical conditions that may cause kidney damage or bear bidirectional relationships with renal insufficiency, such as hypertension and diabetes mellitus (5). To that end, we recently linked administrative data from a private insurance provider to OPTN donor registration data and observed that, compared with white donors, African American donors had higher risks of postdonation morbidity (6). However, the generalizability of these findings to other samples and insurance systems is not known. In the current study, we constructed a similar linkage of OPTN-supplied donor identifiers with Medicare billing claims to identify and quantify postdonation medical diagnoses of hypertension, diabetes mellitus, chronic kidney disease, and subcategories of these conditions in a sample of Medicare-insured prior living kidney donors. Our aims were to estimate frequencies of these conditions according to donor race and to compare patterns to observations among privately insured donors.
RESULTS
Demographic Characteristics of the Donor Samples
There were 4,007 prior kidney donors in the linked Medicare data. Of these, 40% were men, 8% were African American, and 5.7% were Hispanic. The Medicare-insured donor sample was somewhat less racially diverse than the sample of privately insured and all U.S. donors in the period (Table 1). Donors with postdonation Medicare benefits were substantially older, with a mean age of 55 years at the time donation. The median time from donation to end of Medicare benefits was 6.0 years, with an average Medicare capture window of 2.1 years, similar to the average captured enrollment window of 2.1 years among the privately insured sample.
TABLE 1.
Demographic traits of the captured samples of Medicare-insured and privately insured living kidney donors
| Medicare-insured living donors, 1987–2008 (N=4,007) | Privately insured living donors, 1987–2007 (N=4,650)a | All living donors in OPTN, 1987–2008 (N=97,453) | |
|---|---|---|---|
| Male sex, % | 40.0 | 45.4 | 42.5 |
| Race, % | |||
| White (non-Hispanic) | 83.4 | 76.3 | 71.1 |
| African American (non-Hispanic) | 8.1 | 13.1 | 12.7 |
| Hispanic | 5.7 | 8.2 | 11.9 |
| Other | 2.8 | 2.4 | 4.3 |
| Related to recipient, % | 65.3 | 81.2 | 73.6 |
| Age at donor nephrectomy, mean (SD), yr | 54.8 (10.7) | 37.2 (10.0) | 39.3 (11.0) |
| Age at nephrectomy by race, mean (SD), yr | |||
| White (non-Hispanic) | 56.0 (9.9) | 38.2 (10.0) | 40.6 (10.9) |
| African American (non-Hispanic) | 46.0 (12.5) | 33.9 (9.0) | 35.6 (9.8) |
| Hispanic | 50.2 (10.9) | 34.3 (9.6) | 35.8 (10.4) |
| Other | 54.6 (11.0) | 34.8 (10.8) | 37.9 (11.3) |
| Time from donation to end of insurance eligibility, median, yr | 6.0 | 7.7 | — |
| Duration of insurance eligibility, median, yr | 2.1 | 2.1 | — |
As previously described in Lentine et al. (6).
Frequency and Variation in Postdonation Medical Diagnoses According to Race
Overall, medical diagnoses were more common at 5 years after donation among the Medicare-insured versus privately insured donor samples (Table 2). Hypertension diagnoses were very common among the Medicare-insured donors, occurring in 65.8% (95% confidence interval [CI], 52.0%–69.2%) by 5 years after donation, versus 17.8% (95% CI, 15.8%–20.2%) in the privately insured sample. However, these diagnoses were predominantly classified as benign and unspecified. Malignant hypertension was reported in 5.0% (95% CI, 3.2%–6.9%) of the Medicare-insured versus less than 1% of the privately insured donors. Diabetes mellitus was reported in 18.5% (95% CI, 15.0%–21.8%) of the Medicare-insured compared with 4.0% (95% CI, 2.7%–5.3%) of the privately insured donors, mainly reflecting type 2 diagnoses. Chronic kidney disease was coded in 21.8% (95% CI, 18.2 %–25.2%) of the Medicare-insured and 5.2% (95% CI, 3.7%–6.8%) of the privately insured donors with proteinuria in approximately 5% and 2%, respectively.
TABLE 2.
Cumulative frequencies of medical diagnoses at 5 years after donation among Medicare-insured and privately insured living kidney donors
| Medicare-insured living donors | Privately insured living donors | |
|---|---|---|
| Hypertension (any) | 65.8 (62.0–69.2) | 17.8 (15.8–20.2) |
| Benign hypertension | 38.3 (34.1–42.2) | 9.7 (7.8–11.6) |
| Malignant hypertension | 5.0 (3.2–6.9) | 0.9 (0.0–28.9) |
| Unspecified hypertension | 53.3 (49.1–57.2) | 10.8 (8.8–12.8) |
| Diabetes mellitus (any, including unspecified) | 18.5 (15.0–21.8) | 4.0 (2.7–5.3) |
| Type 1 diabetes | 1.9 (0.7–3.1) | 0.5 (0.0–19.2) |
| Type 2 diabetes | 18.5 (15.0–21.8) | 3.3 (2.1–4.4) |
| Chronic kidney disease | 21.8 (18.2–25.2) | 5.2 (3.7–6.8) |
| Proteinuria | 4.8 (2.9–6.7) | 2.3 (1.3–3.3) |
Values are given as percentages (95% confidence interval).
In multivariate regression including adjustment for donor age and sex, African American race was associated with a significantly higher risk of all categories of hypertension after donation in the Medicare-insured sample (Table 3). The association was particularly strong for malignant hypertension, with African American donors having 2.4 times the relative risk of white donors (adjusted hazard ratio [aHR], 2.35; 95% CI, 1.40–3.93). Hispanic donors with Medicare also had twice the relative risk of malignant hypertension after donation compared with white donors (aHR, 1.96; 95% CI, 1.04–3.69). The relative patterns for African American compared with white race were similar for postdonation hypertension in the privately insured sample. African American donors with private insurance had 52% higher relative risk of any hypertension diagnosis after donation (aHR, 1.52; 95% CI, 1.23–1.88) and more than three times the relative risk of malignant hypertension (aHR, 3.27; 95% CI, 1.82–5.88) as white donors. Among the privately insured sample, Hispanic donors had 36% higher relative risk of acquiring any hypertension diagnosis (aHR, 1.36; 95% CI, 1.04–1.78) but did not have higher risks of the hypertension subtypes.
TABLE 3.
Adjusted relative risks of postdonation medical diagnoses among Medicare-insured and privately insured living kidney donors according to demographic factors
| Medicare-insured | Any hypertension | Benign hypertension | Malignant hypertension | Unspecified hypertension |
|---|---|---|---|---|
| Age at donation (per year) | 1.02 (1.01–1.02)a | 1.02 (1.02–1.03)a | 1.02 (1.00–1.03) | 1.02 (1.01–1.02)a |
| Male sex | 1.12 (1.01–1.24)b | 1.20 (1.06–1.35)b | 1.10 (0.78–1.56) | 1.08 (0.96–1.20) |
| Race | ||||
| White | Reference | Reference | Reference | Reference |
| African American | 1.41 (1.17–1.70)a | 1.42 (1.13–1.77)b | 2.35 (1.40–3.93)b | 1.51 (1.25–1.83)a |
| Hispanic | 1.17 (0.95–1.46) | 1.11 (0.84–1.46) | 1.96 (1.04–3.69)b | 1.36 (1.08–1.70)b |
| Other | 1.03 (0.76–1.39) | 1.16 (0.82–1.64) | 1.20 (0.44–3.26) | 0.95 (0.68–1.34) |
| Privately insured | Any hypertensionc | Benign hypertension | Malignant hypertension | Unspecified hypertension |
|---|---|---|---|---|
| Age at donation (per year) | 1.06 (1.06–1.07)a | 1.06 (1.05–1.07)a | 1.03 (1.00–1.06)d | 1.06 (1.05–1.07)a |
| Male sex | 1.13 (0.98–1.31) | 1.14 (0.95–1.36) | 0.83 (0.49–1.41) | 1.09 (0.93–1.29) |
| Race | ||||
| White | Reference | Reference | Reference | Reference |
| African American | 1.52 (1.23–1.88)a | 1.56 (1.20–2.03)a | 3.27 (1.82–5.88)a | 1.69 (1.33–2.14)a |
| Hispanic | 1.36 (1.04–1.78)a | 1.30 (0.91–1.85) | 0.66 (0.16–2.74) | 1.29 (0.93–1.79) |
| Other | 1.13 (0.68–1.85) | 1.14 (0.95–1.36) | — | 0.90 (0.46–1.74) |
| Medicare-insured | Any diabetes | Type 1 diabetes | Type 2 diabetes | Chronic kidney disease | Proteinuria |
|---|---|---|---|---|---|
| Age at donation (per year) | 1.00 (1.00–1.01) | 0.99 (0.97–1.02) | 1.00 (1.00–1.01) | 1.03 (1.02–1.04)a | 1.00 (0.98–1.02) |
| Male sex | 1.38 (1.16–1.65)a | 0.95 (0.57–1.59) | 1.37 (1.15–1.64)a | 2.33 (1.95–2.78)a | 1.44 (1.00–2.09)d |
| Race | |||||
| White | Reference | Reference | Reference | Reference | Reference |
| African American | 1.50 (1.12–2.04)b | 1.11 (0.46–2.72) | 1.57 (1.16–2.12)b | 1.84 (1.37–2.47)a | 2.44 (1.45–4.11)a |
| Hispanic | 2.11 (1.54–2.89)a | 0.95 (0.29–3.09) | 2.13 (1.56–2.92)a | 1.13 (0.75–1.70) | 0.98 (0.40–2.44) |
| Other | 1.60 (1.00–2.57)d | 1.84 (0.57–5.91) | 1.76 (1.12–2.77)b | 1.18 (0.71–1.98) | 1.47 (0.54–4.02) |
| Privately insured | Any diabetesc | Type 1 diabetes | Type 2 diabetes | Chronic kidney diseasec | Proteinuria |
|---|---|---|---|---|---|
| Age at donation (per year) | 1.05 (1.03–1.06)a | 1.03 (0.99–1.06) | 1.05 (1.03–1.07)a | 1.04 (1.03–1.06)a | 1.01 (0.99–1.03) |
| Male sex | 0.91 (0.68–1.22) | 1.45 (0.73–2.86) | 0.97 (0.71–1.30) | 1.64 (1.16–2.34)a | 1.11 (0.72–1.71) |
| Race | |||||
| White | Reference | Reference | Reference | Reference | Reference |
| African American | 1.52 (1.00–2.30)a | 2.06 (0.88–4.82) | 1.64 (1.07–2.51)b | 2.32 (1.48–3.62)a | 2.27 (1.32–3.89)b |
| Hispanic | 1.65 (1.00–2.74)a | 1.04 (0.24–4.43) | 1.86 (1.12–3.10)b | 1.90 (1.05–3.43)a | 1.47 (0.67–3.26) |
| Other | 1.35 (0.50–3.67) | — | 1.17 (0.37–3.69) | 1.74 (0.66–4.76) | 2.77 (0.99–7.72)d |
Values are presented as adjusted hazard ratios (95% confidence interval).
Adjusted hazard ratios for medical diagnoses estimated in the donor samples by multivariate Cox regression with left and right-censoring.
P<0.001.
P<0.05 to 0.001.
As previously reported in Lentine et al. (6).
P=0.05.
African American donors with Medicare had 50% higher relative risk of postdonation diabetes mellitus than white donors (aHR, 1.50; 95% CI, 1.12–2.04), driven by type 2 diabetes diagnoses (Table 3). Hispanic donors in Medicare had twice the relative risk of diabetes after donation as white donors (aHR, 2.11; 95% CI, 1.54–2.89). Similar relative associations of race and ethnicity with type 2 diabetes were seen among the privately insured donors, with 64% (aHR, 1.64; 95% CI, 1.07–2.51) and 86% (aHR, 1.86; 95% CI, 1.12–3.10) higher relative risks among African American and Hispanic compared with white donors, respectively.
Medicare-insured African American donors had 1.8 times the likelihood of postdonation chronic kidney disease diagnoses (aHR, 1.84; 95% CI, 1.37–2.47) and 2.4 times the relative risk of proteinuria diagnosis (aHR, 2.44; 95% CI, 1.45–4.11) as white donors (Table 3). Similar associations of African American race with approximately twice the relative risk of chronic kidney disease (aHR, 2.32; 95% CI, 1.48–3.62) and proteinuria (aHR, 2.27; 95% CI, 1.32–3.89) diagnoses compared with white race were observed among the privately insured donors. Privately insured Hispanic donors had nearly twice the relative risk of chronic kidney disease diagnoses as white donors (aHR, 1.90; 95% CI, 1.05–3.43), but Hispanic ethnicity was not significantly associated with chronic kidney disease diagnoses or proteinuria in the sample of Medicare-insured donors.
Sensitivity Analyses With Censoring for Early Medicare Enrollment
Among the Medicare-insured donor sample, 1,278 donors began enrollment for disability younger than age 65 and 31 for ESRD before age 65. Sensitivity analyses incorporating censoring of risk time incurred before age 65 in the Medicare sample produced a somewhat higher point estimate for the frequency of any hypertension diagnosis (75%; 95% CI, 69.3%–79.9%) by 5 years after donation but a slightly lower estimate of the 5-year frequency of malignant hypertension (4.4%; 95% CI, 2.0%–6.8%) (see Table S1, SDC, http://links.lww.com/TP/A882). With censoring of early Medicare enrollment time, the estimated frequency of diabetes mellitus at 5 years after donation was slightly higher (20.0%; 95% CI, 14.2%–25.3%), that of chronic kidney disease was stable (22.2%; 95% CI, 16.8%–27.2%), and proteinuria was slightly lower (3.1%; 95% CI, 1.2%–5.0%) than in the primary analyses. Patterns of relative risk for postdonation medical outcomes according to donor race and ethnicity were similar in the sensitivity analyses (Table 4). There was loss of statistical significance for the associations of African American race with higher relative risks of postdonation malignant hypertension and proteinuria diagnoses but the point estimates were similar and the impact of censoring for early enrollment was mainly a widening of the CIs.
TABLE 4.
Sensitivity analysis: Adjusted relative risks of postdonation medical diagnoses among Medicare-insured living kidney donors according to demographic factors by multivariate Cox regression, including left censoring of observation time before age 65 (the universal Medicare eligibility time)
| Medicare-insured | Any hypertension | Benign hypertension | Malignant hypertension | Unspecified hypertension |
|---|---|---|---|---|
| Age at donation (per year) | 0.98 (0.97–1.00) | 0.98 (0.97–1.00) | 1.04 (0.99–1.09) | 1.01 (0.99–1.03) |
| Male sex | 1.16 (1.03–1.31)a | 1.30 (1.13–1.50)b | 1.10 (0.73–1.65) | 1.07 (0.94–1.22) |
| Race | ||||
| White | Reference | Reference | Reference | Reference |
| African American | 1.53 (1.15–2.02)a | 1.53 (1.11–2.10)a | 2.11 (0.97–4.59) | 1.44 (1.07–1.92)a |
| Hispanic | 1.34 (1.01–1.78)a | 1.18 (0.82–1.69) | 1.80 (0.73–4.46) | 1.41 (1.03–1.91)a |
| Other | 0.98 (0.69–1.40) | 1.29 (0.88–1.88) | 1.59 (0.58–4.35) | 0.93 (0.63–1.37) |
| Medicare-insured | Any diabetes | Type 1 diabetes | Type 2 diabetes | Chronic kidney disease | Proteinuria |
|---|---|---|---|---|---|
| Age at donation (per year) | 0.99 (0.96–1.01) | 0.95 (0.87–1.03) | 0.98 (0.95–1.01) | 1.03 (1.01–1.06)a | 1.04 (0.99–1.10) |
| Male sex | 1.54 (1.25–1.92)b | 1.27 (0.69–2.34) | 1.56 (1.26–1.93)b | 2.44 (2.00–2.99)b | 1.86 (1.16–2.96)a |
| Race | |||||
| White | Reference | Reference | Reference | Reference | Reference |
| African American | 1.72 (1.08–2.74)a | 1.24 (0.30–5.16) | 1.98 (1.28–3.06)a | 1.55 (1.01–2.39)b | 1.93 (0.77–4.84) |
| Hispanic | 2.53 (1.65–3.87)b | 2.18 (0.67–7.14) | 2.64 (1.73–4.01)b | 1.38 (0.83–2.28) | 1.41 (0.44–4.49) |
| Other | 1.89 (1.13–3.19)a | 2.73 (0.84–8.90) | 1.89 (1.13–3.19)a | 1.44 (0.85–2.47) | 1.74 (0.54–5.56) |
Values are presented as adjusted hazard ratios (95% confidence interval).
P<0.001.
P<0.05 to 0.001.
DISCUSSION
Better understanding of postdonation medical outcomes among racially and ethnically diverse samples of living kidney donors is needed to improve donor counseling and selection and may improve care by focusing long-term follow-up and preventative measures on higher-risk groups. We previously examined a linkage of OPTN donor registration data and administrative billing claims from a private health insurer and observed racial variation in the risk of postdonation medical outcomes (6). In the current study, we used similar methods to link OPTN donor registration data with Medicare billing claims and found that overall, postdonation diagnoses of hypertension, diabetes mellitus, and chronic kidney disease were more common among Medicare-insured compared with privately insured donors. Event frequencies at 5 years after donation in the Medicare-insured sample included malignant hypertension in 5%, diabetes in 18.5%, and proteinuria in 4.8%. Importantly, however, consistent patterns of racial variation in long-term diagnosis rates were seen regardless of sample and payer source. Similar to findings in the privately insured sample, compared with white donors, African American donors with Medicare had significantly higher risk of any hypertension diagnosis including more than twice the likelihood of malignant hypertension, as well as higher risks of diabetes, chronic kidney disease and proteinuria diagnoses. Hispanic living donors with Medicare insurance had higher relative risks of malignant hypertension and diabetes mellitus. Patterns were generally consistent after censoring for Medicare enrollment before age 65 years. Thus, even if donation has no direct impact, screening and selection do not eliminate higher relative risks of postdonation medical diagnoses in non-white living kidney donors.
Racial differences in the burden and consequences of medical conditions among non-white persons in the general U.S. population are well documented (7, 8), but disparities in medical outcomes including hypertension among non-white donors have only recently raised attention. A retrospective study from one U.S. transplant center reported drug-treated hypertension in 25% of 255 white donors assessed at an average of 12 years after donation (3) while in contrast, a notably higher prevalence of hypertension was identified in 41% of 39 African American donors at one urban center at an earlier average assessment time of 7 years after donation (9). Among a cohort of 38 Canadian Aboriginal donors evaluated at an average of 14 years after donation, 42% were hypertensive compared with 14% of Caucasian donor controls (10).
In the current study, the frequency of hypertension diagnoses 5 years after donation was notably higher among living donors enrolled in Medicare compared with privately insured donors at 65.8% versus 17.8%. In almost all contemporary societies, blood pressure rises with aging and the risk of becoming hypertensive in later life is considerable. For example, a community-based cohort study of participants in the Framingham Heart Study aged 55 to 65 years and free of hypertension at baseline found that the residual lifetime risks for developing stage 1 hypertension (blood pressure ≥140/90 mm Hg regardless of treatment) was 90% and the lifetime probability of receiving antihypertensive medication was 60% (11). The high frequency of hypertension in the Medicare-insured donor sample, which increased with censoring for early enrollment for indications other than age, is consistent with expectations for a sample of elderly Americans even in the absence of hypertension at the time of donation.
Here we also expanded previous studies of postdonation hypertension diagnoses by identifying medically coded subcategories. The most common category of postdonation hypertension diagnosis was unspecified, particularly among the Medicare beneficiaries, of whom 53.3% had an unspecified hypertension diagnosis at 5 years compared with 10.8% of privately insured donors, while the 5-year frequencies of malignant hypertension diagnoses were approximately 10-fold lower (5.0% and 0.9% among the Medicare and privately insured donors, respectively). Further study comparing integrated claims with records of clinically recorded blood pressure is needed to define the correlation of coded hypertension categories with actual blood pressure levels. However, despite current uncertain precision of the severity of hypertension categories defined by billing claims, the consistently higher relative risks of hypertension diagnoses among African American compared with white donors across subcategories and across samples are important findings that include particularly strong racial variation for reports of malignant hypertension.
Regarding the attributable impact of donation on post-donation hypertension, data from predominantly Caucasian cohorts suggest increased risk of hypertension after kidney donation that exceeds the risk from normal aging, which may reflect physiological alterations (hyperfiltration in the remaining kidney, changes in vascular tone, and renin-angiotensin-aldosterone regulation) and/or heightened clinical follow-up (12, 13). A study of 1,278 primarily white race living donors in Canada found a 40% higher relative incidence of claims-based hypertension diagnoses among donors compared with matched nondonor controls (13). Recently, a small study of 103 African American donors at two centers found that hypertension was more common among donors compared with African American controls matched for age, sex, baseline systolic blood pressure, and duration of follow-up, 40.8% versus 17.9% at an average of 6.4 years (14). The direct impact of donation on hypertension risk warrants further study, particularly among nonwhite donors.
Although there is no evidence to suggest a causal association of donor nephrectomy with diabetes mellitus, recent studies have suggested racial variation in the onset of diabetes after donation. For example, diabetes was identified in 3.1% of 255 donors from the University of Minnesota at an average of 12 years after donation (3). In contrast, 19% of a small cohort of Canadian Aboriginal donors evaluated at an average of 14 years after donation were diabetic compared with 2% of white donor controls (10). In the current study, we found that diabetes diagnoses were more common in donors with Medicare versus private insurance at approximately 18.5% versus 4% at 5 years after donation, consistent with the known association of aging with the development of type 2 diabetes due to factors including weight gain, decreased activity, and reductions in muscle mass and muscle mitochondrial activity with consequent insulin resistance (15). The estimated prevalence of diabetes among Americans aged 65 years and older in the general population in 2010 was 27% (16). Notably, however, consistent associations of African American race and Hispanic ethnicity with increased relative risks of postdonation diabetes were seen in both the Medicare- and privately insured donor samples.
As the presence of diabetes mellitus at the time of donor evaluation should exclude living donation by clinical practice guidelines and policy requirements (2, 17, 18) these patterns suggest the influence of race-related factors (possibly genetic or environmental) that predispose to development of diabetes over time after donation. Such racial variation also illustrates the variable predictive value of a “normal” evaluation before donation for all aspects of long-term health. Obesity is more common among non-white donors (19, 20) and, in turn, is a strong risk factor for diabetes (21). We lacked sufficient body mass index data to investigate obesity as a mediator of diabetes risk in the current study. Further investigation of possible associations of predonation obesity, postdonation weight gain, genetic/familial, and environmental factors with postdonation diabetes, and other medical outcomes are needed.
With respect to associations of race with postdonation renal outcomes, while one study of OPTN survey data for live donors in 2000 to 2005 showed no appreciable differences in early estimated glomerular filtration rate (eGFR) among African American compared with white donors at an average of 5 months after donation (22), preliminary data suggest that race may interact with other risk factors in impacting long-term renal function. In a small study 36 obese living kidney donors assessed at an average of 7 years postdonation, the absolute average decrement in eGFR was greater among African American obese donors compared with non-African American obese donors (33.3 versus 22.7 mL/min/1.73 m2, respectively; P=0.016) (23). In the current study, we observed that chronic kidney disease diagnoses were more common among donors with Medicare versus private insurance (22% even with censoring for disease-specific Medicare eligibility versus 5% at 5 years after donation), again consistent with the older average age of Medicare-insured donors. Nearly 45% of Americans aged 65 years and older in the general U.S. population are estimated to have chronic kidney disease, reflecting increased prevalence of comorbidities that affect renal function with aging as well as age-related decline in glomerular filtration (24). Notably, African Americans had approximately twice the relative risk of postdonation chronic kidney disease and more than twice the likelihood of proteinuria diagnoses as white donors regardless of sample. Because ESRD offers disease-specific Medicare entitlement, our study data were not directly suitable for quantifying postdonation ESRD risk. Sensitivity analyses censoring early Medicare enrollment for ESRD or disability were performed, yielding persistently significant association of African American race with the risk of postdonation chronic kidney disease. After censoring for early Medicare enrollment, a similar point estimate was also observed for the association of African American race with proteinuria as in the primary analysis, albeit with loss of statistical significance.
Data designed to quantify the risk of postdonation ESRD have been recently assembled in a linkage of OPTN donor registration data with CMS ESRD reporting forms by Cherikh et al. (4) who found that while African Americans comprised 13.0% of donors in 1987 to 2003, 47% of the 126 prior donors who developed ESRD after donating were African Americans. The overall postdonation ESRD rate was 0.134 per 1,000 years at risk, but this rate was significantly higher in African American donors compared with white donors (0.423 vs. 0.086 per 1,000 years at risk; relative risk, 4.92; 95% CI, 2.79–8.66). While African American donors seem to develop chronic kidney disease and ESRD more frequently than white donors, African American individuals in the general population also develop renal failure at higher rates than white individuals (25), and it remains unknown whether donating a kidney directly increases the risk of developing ESRD.
How do available demonstrations of racial variation in postdonation medical outcomes translate to clinical practice? Based on the rationale that “the risk of CKD [chronic kidney disease] and CVD [cardiovascular disease] is increased in individuals from certain racial backgrounds or ethnic groups and in those with elements of the metabolic syndrome” and that “the risk of developing hypertension in a normotensive kidney donor is greater with black and Hispanic donors compared to Caucasians,” a recent consensus document from the AST/ASTS/NATCO/United Network for Organ Sharing (UNOS) Joint Societies Work Group on Evaluation of the Living Kidney Donor recommended that hypertension in a non-white person at any age should be considered a relative contraindication to live kidney donation (18). However, “relative contraindications” are not permissible in OPTN/UNOS policy, and this recommendation is not formalized in the recently adopted national medical evaluation policy (2). Further research is needed to determine whether donor selection polices should differ according to race, as well as to improve risk discrimination among non-white donors. Provocative new research has identified coding variants in the apolipoprotein L1 (APOL1) gene that are strongly associated with nondiabetic ESRD risk among African Americans in an autosomal recessive pattern (26). The presence of two APOL1 risk alleles has also been associated with increased risks of focal segmental glomerulosclerosis/HIV-associated nephropathy histopathologies, proteinuria, low eGFR, and younger age at dialysis among African Americans in the general population (27–29), and the presence of two APOL1 risk alleles in a deceased donor confers nearly four times the relative risk of allograft loss compared with zero or one risk allele (30). While more data and follow-up are needed to evaluate how APOL1 genotyping for the purposes of risk stratification and selection of potential living donors impacts rates of donor candidacy and postdonation outcomes, more studies of APOL1 screening as an approach to attenuate the current disparities in renal failure among African Americans compared with white persons after living donation are warranted.
The observation that approximately 32% of captured donors received Medicare before age 65 is interesting and concerning. While we found that the observed patterns of racial variation in outcomes, the topic of interest in the current study, were robust to censoring for early Medicare enrollment before age 65, the matter of postdonation disability in previously healthy living donors deserves further attention. Medicare files capture eligibility as related to age, disability, or ESRD; thus, delineating the underlying causes of disability through detailed claims analyses and/or linkages to other information sources warrants focused exploration in future studies.
Limitations of the current study include factors related to the samples and outcome measures. The outcomes measures are derived from insurance data, and uninsured living donors are not captured. Claims are surrogate measures for diagnoses and coding errors are possible. The precision of claims-based hypertension severity subcategories among live donors is also not defined. Billing claims have been demonstrated to provide sensitive measures of diabetes and cardiovascular diagnoses in other populations (31, 32) but to underrepresent the burden of kidney dysfunction compared to laboratory-based measures (33). Predonation benefits were captured for only a minority of the donors (7.7%), and thus, information on predonation diagnoses was not adequate for inclusion. Because of the nature of OPTN collection of donor registration data, we also lacked baseline information on relevant clinical parameters such as body mass index sufficient for inclusion. This study was specifically designed to perform within-donor comparisons, and future work is needed to compare outcomes among donors to comparable nondonor controls.
In conclusion, we found that postdonation medical conditions are more common in Medicare-insured compared with privately insured living donors even with censoring for early Medicare enrollment owing to disability or ESRD and thus likely reflect the impact of aging on comorbidity burden. Importantly, however, racial variation is consistently present regardless of sample and payer source. To tailor counseling and informed consent, ongoing attention to long-term medical outcomes among demographically diverse living kidney donors is needed. These efforts should include assembly of controls for assessment of long-term health consequences directly attributable to donation as an important priority.
METHODS
Data Source and Sample
Study data were assembled by linking OPTN/UNOS records for prior living kidney donors with administrative data from Medicare. OPTN data include information on all donors and transplant recipients in the U.S. as submitted by OPTN member centers. After approval by the Health Resources and Services Administration and the Saint Louis University Institutional Review Board, beneficiary identifier numbers from Medicare’s electronic databases were linked using Social Security Number, sex, and birthdates to unique OPTN identifiers for living kidney donors. Because of the large sample size, the anonymity of the patients studied, and the non-intrusive nature of the research, a waiver of informed consent was granted per the Department of Health and Human Services Code of Federal Regulations (Title 45, Part 46, Paragraph 46.116). Analyses were performed using Health Information Portability and Accountability Act-compliant limited datasets with all direct identifiers removed. People were eligible if they had OPTN records of serving as a living kidney donor in October 1987 through July 2008 and Medicare benefits after donor nephrectomy at some point in 2004 to 2008 (the period of available Medicare claims data).
Definitions of Outcomes and Covariates
The outcomes of interest were postdonation medical diagnoses, as certained by billing claims with International Classification of Disease, Ninth Revision, Clinical Modification diagnosis codes for hypertension, diabetes mellitus, and chronic kidney disease, similar to previously described algorithms (6, 31, 34, 35). We also subclassified hypertension based on coding as benign, malignant, or unspecified (see Appendix, SDC, http://links.lww.com/TP/A882). Diabetes was subclassified as types 1 and 2, and we also examined coded proteinuria.
Demographic data available in the OPTN registry and Medicare administrative records included age, race, and sex. Time from donation to the start of captured insurance benefits (at or after donation) and age at benefits start were computed using Medicare enrollment records.
Comparison Data: Privately Insured Donors
For comparison, we used our prior linkage of OPTN living donor registration data with administrative claims data (2000–2007) from a private health insurance provider. The comparison used parallel outcome definitions and parallel demographic information from the OPTN. Assembly and analysis of the database of 4,650 privately insured living donors have been previously described (36), and no new data were acquired from the private payer for the current report. Analyses among the privately insured donors were expanded to include subcategories of hypertension, diabetes types, and coded proteinuria.
Statistical Analyses
Data sets were merged and analyzed with SAS for Windows software, version 9.2 (SAS Institute, Inc., Cary, NC). A schematic of the OPTN-Medicare data linkage and analyses is shown in Figure 1. Because windows of captured insurance benefits varied across the sample, Cox regression with left and right censoring was used to estimate the cumulative frequency (%) of diagnoses over time after donation and associations (aHR) between donor traits (particularly race) and the study outcomes. Censoring was applied from donation to claims enrollment and after the end of an individual’s claims. Body mass index was reported to the OPTN for only 10.2% and 5.3% of the linked Medicare-insured and privately insured donors, respectively, and so was inadequate for inclusion in analytic models. Because Medicare eligibility may result not only from age but also from disability or ESRD, and because reasons for Medicare enrollment could be informative for medical outcomes, we performed sensitivity analyses including left censoring from donation to age 65 years (the universal Medicare eligibility time in the United States). Thus, in the sensitivity analyses, periods of early enrollment due to disability or ESRD before age 65 were censored and patients whose observed claims ended before age 65 were entirely excluded.
FIGURE 1.
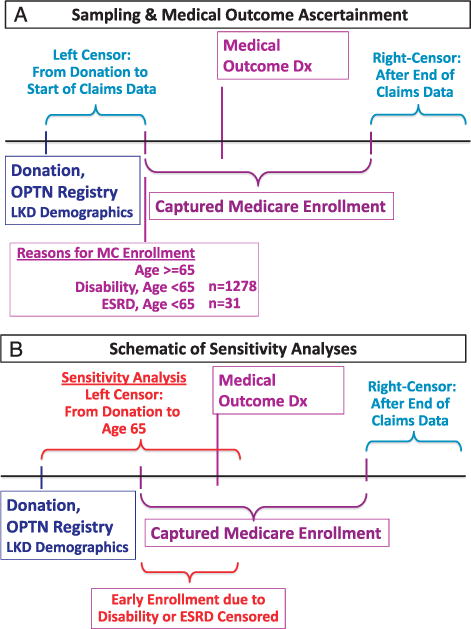
Schematic of the study data aggregation and analytic design.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases RC1-DK086450 and R01-DK096008.
Footnotes
K.L.L. and M.A.S. participated in study design, data acquisition, data analysis, and writing of the article. H.X. participated in study design, data analysis, and writing of the article. D.A., A.X.G., J.T.N., D.C.B., and D.L.S. participated in study design, interpretation, and writing of the article. All authors agreed to publish the article. K.L.L. wrote the first draft of the article.
The authors declare no conflicts of interest.
An abstract describing portions of this work was presented at the 2013 American Transplant Congress in Seattle, WA.
Data reported here have been supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the OPTN, the U.S. Government, the National Institute of Diabetes and Digestive and Kidney Diseases, or the National Institutes of Health.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
References
- 1.European Parliament C. Directive 2010/45/EU of the European Parliament and of the Council of 7 July 2010 on standards of quality and safety of human organs intended for transplantation. Available at: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32010L0053:en:NOT. Accessed March 15, 2013.
- 2.US Department of Health and Human Services. Organ Procurement and Transplantation Network (OPTN) Policies. Policy 12.0. Available at: http://optn.transplant.hrsa.gov/PoliciesandBylaws2/policies/pdfs/policy_172.pdf. Accessed March 15, 2013.
- 3.Ibrahim HN, Foley R, Tan L, et al. Long-term consequences of kidney donation. N Engl J Med. 2009;360:459. doi: 10.1056/NEJMoa0804883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherikh WS, Young CJ, Kramer BF, et al. Ethnic and gender related differences in the risk of end-stage renal disease after living kidney donation. Am J Transplant. 2011;11:1650. doi: 10.1111/j.1600-6143.2011.03609.x. [DOI] [PubMed] [Google Scholar]
- 5.Leichtman A, Abecassis M, Barr M, et al. Living kidney donor followup: state-of-the-art and future directions, conference summary and recommendations. Am J Transplant. 2011;11:2561. doi: 10.1111/j.1600-6143.2011.03816.x. [DOI] [PubMed] [Google Scholar]
- 6.Lentine KL, Schnitzler MA, Xiao H, et al. Racial variation in medical outcomes among living kidney donors. N Engl J Med. 2010;363:724. doi: 10.1056/NEJMoa1000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter JS, Pugh JA, Monterrosa A. Non–insulin-dependent diabetes mellitus in minorities in the United States. Ann Intern Med. 1996;125:221. doi: 10.7326/0003-4819-125-3-199608010-00011. [DOI] [PubMed] [Google Scholar]
- 8.Cutler JA, Sorlie PD, Wolz M, et al. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52:818. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 9.Nogueira JM, Weir MR, Jacobs S, et al. A study of renal outcomes in African American living kidney donors. Transplantation. 2009;88:1371. doi: 10.1097/TP.0b013e3181c1e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Storsley LJ, Young A, Rush DN, et al. Long-term medical outcomes among Aboriginal living kidney donors. Transplantation. 2010;90:401. doi: 10.1097/TP.0b013e3181e6e79b. [DOI] [PubMed] [Google Scholar]
- 11.Vasan RS, Beiser A, Seshadri S, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. JAMA. 2002;287:1003. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 12.Boudville N, Prasad GV, Knoll G, et al. Meta-analysis: risk for hypertension in living kidney donors. Ann Intern Med. 2006;145:185. doi: 10.7326/0003-4819-145-3-200608010-00006. [DOI] [PubMed] [Google Scholar]
- 13.Garg AX, Prasad GV, Thiessen-Philbrook HR, et al. Cardiovascular disease and hypertension risk in living kidney donors: an analysis of health administrative data in Ontario, Canada. Transplantation. 2008;86:399. doi: 10.1097/TP.0b013e31817ba9e3. [DOI] [PubMed] [Google Scholar]
- 14.Doshi MD, Goggins MO, Li L, et al. Medical outcomes in African American live kidney donors: a matched cohort study. Am J Transplant. 2013;13:111. doi: 10.1111/j.1600-6143.2012.04303.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee HY, Choi CS, Birkenfeld AL, et al. Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell Metab. 2010;12:668. doi: 10.1016/j.cmet.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Diabetes Information Clearinghouse (NDIC) National Diabetes Statistics. 2011 Available at: http://diabetes.niddk.nih.gov/dm/pubs/statistics/#fast. Accessed February 1, 2013.
- 17.Delmonico F. A report of the Amsterdam forum on the care of the live kidney donor: data and medical guidelines. Transplantation. 2005;79(6 Suppl):S53. [PubMed] [Google Scholar]
- 18.AST/ASTS/NATCO/UNOS Joint Societies Work Group. Evaluation of the Living Kidney Donor – a consensus document from the AST/ASTS/NATCO/UNOS Joint Societies Work Group. Available at: www.asts.org/Tools/Download.aspx?fid=1427. Accessed September 12, 2012.
- 19.Davis CL, Cooper M. The state of U.S. living kidney donors. Clin J Am Soc Nephrol. 2010;5:1873. doi: 10.2215/CJN.01510210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taler SJ, Messersmith EE, Leichtman AB, et al. Demographic, metabolic, and blood pressure characteristics of living kidney donors spanning five decades. Am J Transplant. 2013;13:390. doi: 10.1111/j.1600-6143.2012.04321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van ’t Riet E, Dekker JM, Sun Q, et al. Role of adiposity and lifestyle in the relationship between family history of diabetes and 20-year incidence of type 2 diabetes in U.S. women. Diabetes Care. 2010;33:763. doi: 10.2337/dc09-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doshi M, Garg AX, Gibney E, et al. Race and renal function early after live kidney donation: an analysis of the United States Organ Procurement and Transplantation Network Database. Clin Transplant. 2010;24:E153. doi: 10.1111/j.1399-0012.2010.01209.x. [DOI] [PubMed] [Google Scholar]
- 23.Nogueira JM, Weir MR, Jacobs S, et al. A study of renal outcomes in obese living kidney donors. Transplantation. 2010;90:993. doi: 10.1097/TP.0b013e3181f6a058. [DOI] [PubMed] [Google Scholar]
- 24.Center for Disease Control and Prevention. National Chronic Kidney Disease Fact Sheet. 2010 Available at: http://www.cdc.gov/diabetes/pubs/factsheets/kidney.htm. Accessed February 1, 2013.
- 25.U.S. Renal Data System. Atlas of ESRD. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Chapter One. Available at: http://www.usrds.org/2012/pdf/v2_ch1_12.pdf. Accessed February 1, 2013. [Google Scholar]
- 26.Genovese G, Friedman DJ, Ross MD, et al. Association of ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 2011;22:2129. doi: 10.1681/ASN.2011040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman DJ, Kozlitina J, Genovese G, et al. Population-based risk assessment of APOL1 on renal disease. J Am Soc Nephrol. 2011;22:2098. doi: 10.1681/ASN.2011050519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanji Z, Powe CE, Wenger JB, et al. Genetic variation in APOL1 associates with younger age at hemodialysis initiation. J Am Soc Nephrol. 2011;22:2091. doi: 10.1681/ASN.2010121234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reeves-Daniel AM, Depalma JA, Bleyer AJ, et al. The APOL1 Gene and Allograft Survival after Kidney Transplantation. Am J Transplant. 2011;11:1025. doi: 10.1111/j.1600-6143.2011.03513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hebert PL, Geiss LS, Tierney EF, et al. Identifying persons with diabetes using Medicare claims data. Am J Med Qual. 1999;14:270. doi: 10.1177/106286069901400607. [DOI] [PubMed] [Google Scholar]
- 32.Lentine KL, Schnitzler MA, Abbott KC, et al. Sensitivity of billing claims for cardiovascular disease events among kidney transplant recipients. Clin J Am Soc Nephrol. 2009;4:1213. doi: 10.2215/CJN.00670109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevens LA, Fares G, Fleming J, et al. Low rates of testing and diagnostic codes usage in a commercial clinical laboratory: evidence for lack of physician awareness of chronic kidney disease. J Am Soc Nephrol. 2005;16:2439. doi: 10.1681/ASN.2005020192. [DOI] [PubMed] [Google Scholar]
- 34.Kasiske BL, Snyder JJ, Gilbertson D, et al. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3:178. doi: 10.1034/j.1600-6143.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 35.U.S. Renal Data System. USRDS 2012 Annual Data Report, Methods Appendix. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Available at: http://www.usrds.org/2012/pdf/v1_z_appendix_12.pdf. Accessed January 28, 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


