Abstract
Introduction
This study investigated whether neuropsychological testing in primary care (PC) offices altered physician-initiated interventions related to cognitive impairment (CI) or slowed the rate of CI progression.
Methods
This 24-month, cluster-randomized study included 11 community-based PC practices randomized to either treatment as usual (5 practices) or cognitive report (CR; 6 practices) arms. From 2005 to 2008, 533 patients aged ≥65 years and without a diagnosis of CI were recruited; 423 were retested 24 months after baseline.
Results
CR physicians were significantly more likely to order cognitive-related interventions (P = .02), document discussions about cognition (P = .003), and order blood tests to rule out reversible CI (P = .002). At follow-up, significantly more CR patients had a medication for cognition listed in their chart (P = .02). There was no difference in the rate of cognitive decline between the groups.
Discussion
Providing cognitive information to physicians resulted in higher rates of physician-initiated interventions for patients with CI.
Keywords: Age, Alzheimer's disease, Community-based, Cognitive impairment, Dementia, Mild cognitive impairment primary care, Primary care physicians
1. Introduction
Age is the single greatest risk factor for the development of dementia and disorders of cognition. As the proportion of older adults continues to grow, the coming decade will see a significant increase in the number of individuals living with impaired cognition. Trends in health care delivery suggest that, in the future, many older adults will obtain a majority of their health care from general practitioners and will not be referred to dementia specialists. However, identifying cognitive impairment (CI) in the primary care (PC) setting remains challenging [1].
Although it has been suggested that best practice care for older adults should include screening for cognitive disorders to facilitate early detection and treatment [2], the United States Preventive Services Task Force does not recommend universal screening in PC, citing performance characteristics of screening instruments and limited evidence of effectiveness [3]. Nevertheless, beginning in January 2011, in compliance with the Patient Protection and Affordable Care Act, the Centers for Medicare and Medicaid Services began covering the costs of an annual wellness visit, which calls for detection of CI by providers conducting the annual wellness visit [4].
A number of studies have investigated screening for dementia in PC [5], [6], [7] but fewer have examined the impact of screening for mild cognitive impairment (MCI) on older adults [8], [9], [10], [11]. With the current emphasis on earlier diagnosis of CI, the goal of this study was to determine whether identifying MCI in older PC patients, without a dementia diagnosis, would result in a change in physician practice or slow cognitive decline. Community-based PC practices were randomized to either treatment as usual (TAU) or cognitive report (CR) arms. We hypothesized that PC physicians (PCPs) in the CR group, who received CRs based on neuropsychological testing, would perform dementia screening tests, refer patients to specialists for diagnostic assessment, and prescribe anticholinesterase inhibitors more frequently than PCPs in the TAU group. We also hypothesized that patients of physicians in the CR group would have a slower rate of progression of cognitive deficits over 2 years than cognitively impaired patients in the TAU group (http://clinicaltrials.gov identifier: PCP-AG023129). We based the hypothesis on the rationale that patients with reversible CI would have improved cognition due to its spontaneous resolution or treatment of the underlying cause. If other causes of impairment were ruled out and the impairment was thought to be due to impending Alzheimer's disease (AD), the decline could be slowed if the physician prescribed cognitive-enhancing medications [12].
2. Methods
2.1. Design
This study was a 24-month, cluster-randomized trial with two parallel groups. The unit of randomization was PCP practice and not individual PCPs nor patients given that our primary outcome was physician-initiated interventions and that within practices, physicians frequently are called on to cover each other's patients and may exhibit similar practice patterns [13]. If randomization occurred at the PCP level, a given PCP could be called on to treat a patient from the other arm, leading to possible contamination between groups. Additionally, patients who share PCPs may share information about cognitive testing and subjective complaints that could lead to dilution of treatment effects [14]. Practices were recruited from October 2005 to January 2006; patients were recruited from January 2006 to January 2008. The University of Pittsburgh Institutional Review Board approved the study, and all physician and patient participants provided written informed consent.
2.2. Setting, participants, and randomization
We stratified 12 PC practices from southwestern Pennsylvania by geographic location (urban, suburban, and rural). Two of the 12 were classified as urban, and to ensure balance, they were randomly assigned to intervention or control with equal probability in a block size of two. Eight of the 12 were classified as suburban, and these sites were stratified by the number of physicians participating in the study. The eight suburban sites were also randomly assigned to intervention or control with equal probability in a block size of four. Two sites were classified as rural were randomly assigned to intervention or control with equal probability in a block size of two. Of the 12 practices, six were randomly assigned to the CR group and received the results of the patients' baseline and 24-month assessments. The remaining six practices were assigned to the TAU group and did not receive baseline CRs. After randomization, but before patient recruitment, one suburban practice (with one physician) dropped out because of perceived study burden, leaving five TAU practices in the study.
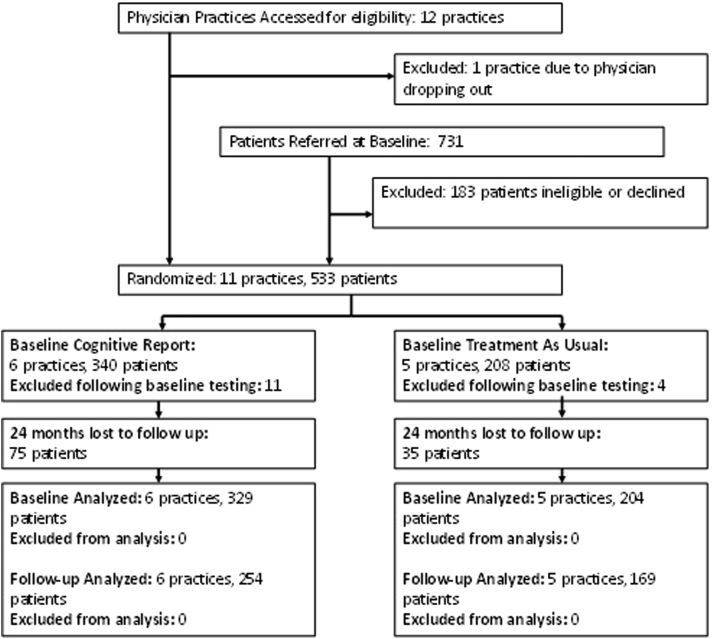
Patients were first approached by physicians who were instructed to refer all patients aged ≥65 years without a dementia diagnosis to the study coordinator. Patients with a diagnosis of dementia on their medical record, or with mini-mental state examination (MMSE) [15] scores of 18 or below, which indicates the presence of unrecognized dementia, were excluded from this study. However, patients with complaints of memory loss who did not have a diagnosis of dementia were not excluded. A total of 731 patients were referred. Among those, 183 (25%) declined participation or were ineligible (e.g., <65 years old, had a notation of “dementia or Alzheimer's disease” somewhere within the medical record, died before enrollment; Fig. 1). Comparisons between patients who did and did not participate demonstrated no significant differences regarding PC office, physician, geographic location, or group assignment. A total of 548 patients completed the baseline assessment; 15 (2.7%) were subsequently excluded because they did not meet study entry criteria (e.g., MMSE <18, dementia diagnosis, or the referring physician was not a participating PCP). The final sample included 533 patients (TAU = 204, 38.3%; CR = 329, 61.7%).
Fig. 1.
Study timeline.
Of the initial 533 enrolled participants, 423 (79.4%) returned for the 2-year assessment (TAU = 169, 82.8%; CR = 254, 77.2%). Among the 110 (20.6%) not included in the 2-year assessment (TAU = 35, 17.2%; CR = 75, 22.8%), 18 (16.4%) died (TAU = 12, CR = 6), 15 (13.6%) changed PCP (TAU = 5, CR = 10), 30 (27.3%) cited poor health (TAU = 10, CR = 20), 27 (24.5%) cited lack of interest (TAU = 5, CR = 22), and 20 (18.1%) gave no reason (TAU = 3, CR = 17).
2.3. Study measurements
The study included multiple measures, including participant demographics, neuropsychological tests, self-rated questionnaires, and data extracted from PC medical records by registered nurses (Table 1. The study nurses extracted data using a structured chart abstraction tool over four periods: (1) 12-months before baseline; (2) baseline to 12-months after baseline; (3) 13–24 months; and (4) 25–30 months. Data extracted from the medical record included clinical and utilization outcomes, including the medical problem list, family history of dementia, number of falls, PCP visits and phone calls, emergency department visits, hospitalizations, number of medications, number of new medical problems, and newly initiated advance directives. For all data collection time points after baseline, physician-initiated outcomes were recorded from the medical record. For the purposes of this analysis, the following outcomes were designated as physician-initiated interventions: discussions about memory or cognitive problems or dementia; administration of cognitive or functional assessments; referrals to dementia specialists; brain imaging; blood work to rule out reversible causes; referrals to social services; prescription and over-the-counter (OTC) medications; notations of memory or functional impairments from patient, family, or PCP; contact or discussion with family member; concern of deviation from medical instructions or noncompliance with medications; concern of unsafe driving; presence of advanced directive; and note of any formal or informal cognitive or functional assessments.
Table 1.
Neuropsychological test battery and self-rated questionnaires administered at baseline and 24 months
|
Neuropsychological test battery: Memory: Word list learning test (WLL) [16] with delayed recall, Wechsler memory scale-revised (WMS-R) logical memory (LM) I and II [17], and the modified Rey-Osterrieth (mR-O) figure immediate and delayed recall [18] Attention/psychomotor speed: WMS-R digit span forward, trail making test part A [19], and Wechsler adult intelligence scale-revised (WAIS-R) digit symbol [20] Visuospatial ability: modified WAIS-R block design and copy of the mR-O figure Language abilities: Boston naming test [21] and semantic fluency (animals) [22] Executive functions: Trail making test part B, WMS-R digit span backward, letter fluency (F,A,S), clock drawing, and WAIS-R digit symbol Self-rated questionnaires: Structured interview to assess subjective memory abilities [23] Activities of daily living (ADLs) and instrumental ADLs [24], [25] Center for Epidemiological Studies-Depression (CES-D) [26], [27] Structured interview documenting self-reported prescription and over-the-counter (OTC) medications |
2.4. Adjudication procedure
Participants were first classified as having cognition within normal, MCI, or dementia ranges using the following criteria from the University of Pittsburgh Alzheimer Disease Research Center [28], [29]: (1) dementia, scores ≥2 standard deviations (SDs) below age norms on two cognitive domains, one of which must be memory; (2) MCI, at least two scores 1–2 SDs below age norms; (3) and normal, individuals not meeting either (1) or (2). The final diagnosis was determined after adjudication by three expert neuropsychologists (all licensed psychologists) based on cognitive scores together with a review of participants' demographic, functional, behavioral, and medical information. Adjudications were conducted blind to study group status.
2.5. Physician CR
The CR included a graphical display of neuropsychological scores, a clinical interpretation of the scores ranges (normal, MCI, or dementia), a review of medical status and medications, and recommendations. For individuals meeting criteria for MCI or dementia, the panel recommended performing a work-up for possible dementia and provided the PCP with a copy of the American Academy of Neurology (AAN) guidelines [30]. In cases where the panel felt that other factors might adversely affect cognition, additional recommendations were made, including a work-up for depression and anxiety for patients who endorsed symptoms on study questionnaires, and a review of medications prompted by possible anticholinergic side effects or when patient's self-reported medication list and the physician's chart list did not agree, or when there was a question of medication compliance or inappropriate use. CR physicians were required to sign, date, and return a receipt stating they had reviewed the report.
2.6. Patient report
CR participants received a letter describing the results in lay language and instructing them to discuss the results with their physician. Given the design of the study, neither patients nor physicians were blind to the treatment arm.
2.7. Statistical analysis
Baseline data were examined for outliers and missing values. Comparisons between study groups were made using t tests and chi-squared tests, as appropriate, and nonparametric procedures were employed using Mann-Whitney U or Fisher's exact tests for analysis.
Cognitive test scores from baseline and 24-month follow-up were converted to z-scores using baseline scores of participants with normal cognition as a reference group (e.g., mean and SD). Average z-scores were calculated for each of the five cognitive domains and a global z-score calculated from the mean of the five domains. Finally, the global z-score at baseline was subtracted from the global z-score at follow-up for a global z-change score, and a larger positive value indicates improvement. Z-scores were analyzed as a continuous variable and a binary variable (e.g., improvement or decline).
Models were fit with improvement in cognitive status (z-score) as the dependent variable and randomization group as the independent. Adjusted models included age and number of medical problems as covariates. To test whether improvement in cognitive status was associated with receiving a CR, we used a generalized estimating equation [31] model with a random intercept to adjust for the clustering of patients with physicians and for the clustering of physicians within practices. Statistical significance was defined as a P value of <.05. Analyses were conducted with IBM SPSS Statistics version 19.0 (SPSS, Inc., Chicago, IL).
The study was powered so that a sample size of approximately 120 patients in each group (equivalent to an effective sample size of 240 patient per group after accounting for clustering) had a >90% power to determine if there is an effect on the level of cognitive function between the two groups over a 2-year period.
3. Results
3.1. Practice and physician characteristics
The PC practices were primarily in suburban locations (63.6%) and were relatively small (1–5 physicians). The 24 physicians were mostly male (83.3%), aged 55–69 years (58.3%), and Caucasian (83.3%), and most (62.5%) reported a panel of more than 200 patients who were at least 65-years-old (Table 2).
Table 2.
Baseline physician and primary care practice characteristics
| Practice characteristics (n = 11) | n (%) |
|---|---|
| Randomization group | |
| Cognitive report | 6 (54.5) |
| Treatment as usual | 5 (45.5) |
| Total number of physicians in practice (median, min–max) | 6.0 (1–26) |
| Number of study subjects per practice (median, min–max) | 31.0 (8–100) |
| Geographic location | |
| Urban | 2 (18.2) |
| Suburban | 7 (63.6) |
| Rural | 2 (18.2) |
| Practice size | |
| 1–5 physicians in practice | 6 (54.5) |
| >5 physicians in practice |
5 (45.5) |
| Physician characteristics (n = 24) | n (%) |
| Randomization group | |
| Cognitive report | 13 (54.2) |
| Treatment as usual | 11 (45.8) |
| Age group, y | |
| 25–39 | 2 (4.2) |
| 40–54 | 7 (25.0) |
| ≥55 | 15 (58.3) |
| Male | 20 (83.3) |
| White | 20 (83.3) |
| Number of patients PCP sees in practice ≥65-years old | |
| 1–200 | 4 (16.7) |
| ≥200 | 15 (62.5) |
| Unknown | 1 (20.8) |
| Study patients per PCP (mean ± SD) | 17.63 ± 13.33 (1–56) |
| Cognitive report | 19.54 ± 15.58 (2–56) |
| Treatment as usual | 15.36 ± 10.36 (1–36) |
Abbreviations: PCP, primary care physicians; SD, standard deviation.
3.2. Patient characteristics
The mean age of study participants at entry was 73.6 years, 50.0% had a high school degree or equivalent education, 58.9% were female, and 63.8% were married. The average number of prescription medications at baseline was 7.89 (SD, 4.16), and 1.7% were taking a prescription cognitive-enhancing medication (Table 3). The mean number of medical problems was 8.13 (SD, 3.81). Arthritis (72.6%) represented the most frequently listed problem, followed by hypertension (69%), and diabetes (62.9%).
Table 3.
Patient participant baseline characteristics by randomization group
| Total (n = 533) | TAU (n = 204) | CR (n = 329) | Stat, P value∗ | |
|---|---|---|---|---|
| Age, y (mean, SD) | 73.57 ± 6.06 | 73.51 ± 5.76 | 73.60 ± 6.25 | t = 0.17, P = .87 |
| Female, n (%) | 314 (58.9) | 107 (52.5) | 207 (62.9) | χ2 = 5.7, P = .02 |
| White, n (%) | 515 (96.6) | 202 (99.0) | 313 (95.1) | χ2 = 5.8, P = .02 |
| High school or equivalent, n (%) | 288 (54.1) | 107 (52.5) | 181 (55.2) | χ2 = 0.4, P = .54 |
| Years of education (mean, SD) | 13.83 ± 2.84 | 13.99 ± 2.74 | 13.73 ± 2.90 | t = 1.01, P = .31 |
| Marital status, n (%) | χ2 = 5.2, P = .08 | |||
| Married | 340 (63.8) | 142 (69.6) | 198 (60.2) | |
| Widowed | 130 (24.4) | 40 (19.6) | 90 (27.4) | |
| Other | 63 (11.8) | 22 (10.8) | 41 (12.5) | |
| Family history dementia, n (%) | 105 (30.8) | 38 (28.8) | 67 (32.1) | χ2 = 0.4, P = .52 |
| Subjective memory less well than 1-y ago, n (%) | 177 (33.2) | 65 (31.9) | 112 (34.0) | χ2 = 0.3, P = .60 |
| Instrumental ADL impairment (range 0–30) (mean ± SD) | 0.45 ± 1.26 | 0.37 ± 1.02 | 0.50 ± 1.39 | t = 1.22, P = .22 |
| CES-D depression score (range 0–21) (mean ± SD) | 2.17 ± 2.88 | 2.03 ± 2.92 | 2.25 ± 2.86 | t = 0.83, P = .41 |
| Number of prescription medications | 7.89 ± 4.16 | 7.70 ± 4.35 | 8.02 ± 4.04 | t = 0.85, P = .39 |
| Use of at least 1 anti-dementia prescription medication, n (%) | 1 (0.2) | 0 (0) | 1 (0.3) | FET, P = 1.00 |
| Use of at least one anti-dementia over-the-counter medication, n (%) | 2 (0.4) | 0 (0) | 2 (0.6) | FET, P = .53 |
| Number of medical problems (mean, SD) | 8.13 ± 3.81 | 8.59 ± 3.66 | 7.85 ± 3.87 | t = 2.17, P = .03 |
| Hyperlipidemia/cholesterol, n (%) | 139 (26.1) | 50 (24.5) | 89 (27.1) | χ2 = 0.4 P = .52 |
| Hypertension, n (%) | 368 (69.0) | 133 (65.2) | 235 (71.4) | χ2 = 2.3, P = .13 |
| Arthritis, n (%) | 387 (72.6) | 151 (74.0) | 236 (71.7) | χ2 = 0.3, P = .57 |
| Diabetes, n (%) | 335 (62.9) | 138 (67.6) | 197 (59.9) | χ2 = 3.3, P = .07 |
| Coronary artery disease, n (%) | 60 (11.3) | 17 (8.3) | 43 (13.1) | χ2 = 2.8, P = .09 |
| Myocardial infarct, n (%) | 131 (24.6) | 39 (19.1) | 92 (28.0) | χ2 = 5.3, P = .02 |
| Chronic obstructive pulmonary disease, n (%) | 50 (9.4) | 16 (7.8) | 34 (10.3) | χ2 = 0.9, P = .34 |
| Stroke or TIA, n (%) | 62 (11.6) | 25 (12.3) | 37 (11.2) | χ2 = 0.1, P = .72 |
| Cancer, n (%) | 128 (24.0) | 47 (23.0) | 81 (24.6) | χ2 = 0.2, P = .70 |
| Baseline cognitive diagnosis, n (%) | χ2 = 2.4, P = .30 | |||
| Normal status | 307 (57.6) | 114 (55.9) | 193 (58.7) | |
| MCI | 206 (38.6) | 85 (41.7) | 121 (36.8) | |
| Demented | 20 (3.8) | 5 (2.5) | 15 (4.6) |
Abbreviations: TAU, treatment as usual; CR, cognitive report; SD, standard deviation; ADL, activities of daily living; FET, Fisher's exact test; CES-D, Center for Epidemiological Studies-Depression; TIA, transient ischemic attack; MCI, mild cognitive impairment.
Comparisons between cognitive report and treatment as usual groups.
There were no significant baseline differences between study groups in the proportion of individuals meeting criteria for normal (61.5%), MCI (36.9%), and dementia (1.6%; P = .30) either for the total baseline sample of 533 (Table 3) or for the 423 who subsequently returned for the 24-month follow-up testing (P = .36).
3.3. Patient outcomes
3.3.1. Clinical and utilization outcomes
We found no difference between study groups in any of the clinical and utilization outcomes (e.g., falls, PCP visits and phone calls, emergency department visits, hospitalizations, number of medications, number of new medical problems, and newly initiated advance directives).
3.3.2. Cognitive outcomes
There was no significant difference between study groups in change in cognitive status at 24 months (Table 4). The proportion of individuals meeting criteria for normal, MCI, and dementia at follow-up was 72.1%, 24.1%, and 3.8%, respectively. There was no difference in z-score change between TAU and CR for each of the diagnostic groups. Among the 163 cognitively impaired participants (MCI = 156; dementia = 7) from the TAU and CR groups who returned for the 24-month assessment, there was no significant difference in cognitive outcomes between those who received a physician-initiated intervention (n = 106) and those who did not (n = 57). Additionally, among the 106 who received an intervention, there was no significant difference in cognitive outcomes between study groups (TAU = 39; CR = 67) (χ2 = 0.44, P = .80).
Table 4.
Change in cognitive diagnosis of patient participants from baseline to follow-up by randomization group (n = 423)
| Baseline diagnosis | Randomization group |
||||
|---|---|---|---|---|---|
| 24-month diagnosis | Total | Treatment as usual | Cognitive report | Stat, P value | |
Normal, n (%)
|
Normal | 235 (90.4) | 89 (90.8%) | 146 (90.1) | χ2 = 0.03, P = 1.00 |
| MCI | 25 (9.6) | 9 (9.2%) | 16 (9.9) | ||
| Total | 260 (100) | 98 (100) | 162 (100) | ||
MCI, n (%)
|
Normal | 70 (44.9) | 33 (47.8) | 37 (42.5) | χ2 = 0.44, P = .80 |
| MCI | 72 (46.2) | 30 (43.5) | 42 (48.3) | ||
| Dementia | 14 (9.0) | 6 (8.7) | 8 (9.2) | ||
| Total | 156 (100) | 69 (100) | 87 (100) | ||
Dementia, n (%)
|
MCI | 5 (71.4) | 1 (50) | 4 (80) | FET, P = 1.00 |
| Dementia | 2 (28.6) | 1 (50) | 1 (20) | ||
| Total | 7 (100) | 2 (100) | 5 (100) | ||
Abbreviations: MCI, mild cognitive impairment; FET, Fisher's exact test.
3.4. Physician-initiated interventions
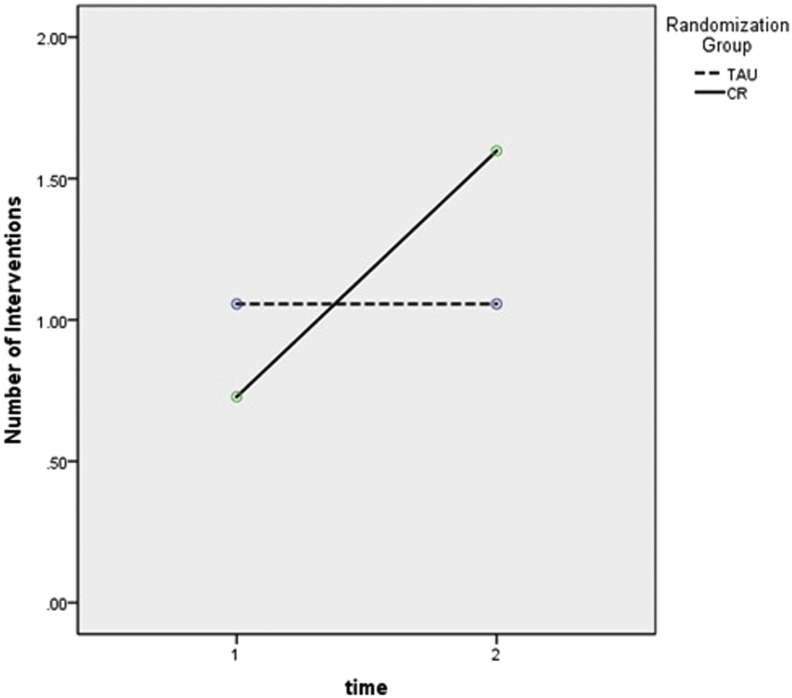
All physicians had some patients for whom no intervention was ordered and some with at least one intervention. Among cognitively impaired patients, the average number of PCP-initiated interventions per subject increased significantly between baseline and 24 months in the CR group as compared with the TAU group (Fig. 2). Overall, CR physicians were more likely than TAU physicians to intervene. Specifically, CR physicians were significantly more likely to order blood tests as part of a dementia work-up (P = .002) and document discussions about cognition/dementia with the patient (P = .001). Although there was no difference in prescription rates for cognitive-enhancing medications, individuals in the CR group were more likely at follow-up to have either a prescription or OTC medication for cognition listed on their medical chart (TAU = 4; CR = 17; P = .02). Finally, CR physicians were more likely than TAU physicians to document a discussion about the study (TAU = 0, 0%; CR = 61, 24.0%; χ2 = 47.4, P < .001).
Fig. 2.
Repeated measures ANOVA comparing number of physician outcomes per patient at baseline and follow-up (n = 163). Group: F = 0.30, P = .58; time: F = 14.08, P < .001; group by time: F = 14.08, P < .001. Abbreviations: ANOVA, analysis of variance; TAU, treatment as usual; CR, cognitive report.
4. Discussion
The cognitive assessment intervention had no significant effect on patient cognitive status; however, our findings suggest that when physicians are provided with data and recommendations regarding the cognitive status of older patients, they use this information to make decisions about treatment strategies aimed at remediating cognitive decline. Although there were statistically significant differences between study groups in the proportion of physician-initiated interventions, the actual number of medical interventions was surprisingly small. After receipt of the CR, physicians ordered blood tests as part of a dementia work-up for 17 of the 92 CR participants who were reported as having CI. We found such a low rate surprising given that AAN guidelines recommend screening for reversible causes of CI and CR physicians documented that they were aware of the CI in 30 (32.6%) cases by noting “memory problems” in the medical records (compared with 21% of TAU cases).
Several studies have suggested that impaired cognition in PC patients is under-recognized [32], [33] and poor recognition has been attributed to a number of factors, including the difficulty of identifying subtle cognitive symptoms or changes overtime, the length of time and perceived complexity of a dementia evaluation, and the lack of physician confidence in making cognitive diagnoses [2], [3], [34], [35]. In this study, experts conducted the cognitive assessments without any time burden for the PCP or staff; yet, few PCPs took action when a CR indicated possible MCI or dementia. We considered a number of reasons that may explain this. We looked first to see if physicians had conducted their own cognitive assessment from which they determined that the evidence of CI was not substantiated. However, our data showed that most physicians did not conduct, or at least did not document, cognitive assessments or cognitive screening in the record. Physicians may have decided to take a “watchful waiting” approach, perhaps, as a general treatment strategy, but perhaps also because physicians knew the patients would undergo retesting, and they may have considered the CR to be an expert consultation, making additional referral unnecessary. Additionally, the possibility exists that physicians interpreted the CR as indicating the presence of early AD and considered further investigation unnecessary, believing that potentially treatable causes of CI had been ruled out and that progressive decline is inevitable. Finally, physicians may have been reluctant to conduct further work-up regarding cognitive deficits if the patient did not make subjective complaints, suggesting that physicians may wish to see evidence of more significant decline. Support for this possibility comes from our finding that physicians were more likely to intervene when MMSE scores were lower. Many dementia experts believe that identification at the very earliest stage of impairment has the greatest impact, especially in identifying those whose cognitive deficits put them at risk for medication mismanagement that may lead to increased emergency department and PCP visits and greater utilization of services that may be unnecessary, unwanted, or even harmful.
In addition to the CR, a letter was sent to each patient recommending that they discuss the results with their physician. It is not possible from our data to determine how much of the physicians' treatment response was driven directly by the CR or by a discussion initiated by the patient. We note that physicians documented general discussions about cognition and memory with 43.5% of CR patients as compared with 21.1% of TAU patients.
A follow-up period of 2 years is relatively short given the long prodromal stage of AD and, thus, it is possible that we may have seen an effect in the rate of cognitive decline between the groups with longer follow-up. Although we controlled for receipt of a CR, we did not control the PCP's response to receiving the CR and the clinical determination of whether to initiate an intervention. We found that PCPs were more likely to order interventions for older, more cognitively impaired patients with the greatest number of medical problems; therefore, it is possible that these individuals may be less likely to benefit from an intervention than younger, healthier patients.
Our findings cannot be used to infer prevalence of MCI in PC settings as this was not an epidemiologic study. Moreover, although the protocol instructed physicians to refer all patients, they may have referred only patients for whom they had concerns, thus over-representing MCI in this sample.
This study has a number of strengths as compared with previous studies of cognitive assessments in PC patients. First, to the best of our knowledge, this is the only study to use a cluster-randomized controlled design in which study physicians in one group were randomized to receive the results of a neuropsychological assessment of their patients, and physicians in the practices randomized to TAU did not. Second, primary measures were collected over a 24-month period, which allowed us to compare multiple PCP-patient interactions. Third, the assessment included a comprehensive cognitive evaluation, rather than a brief screening measure, resulting in more confidence in the diagnosis of MCI and dementia.
At the same time, this study also had limitations. First, an overwhelming majority of patient participants identified themselves as white. Second, the method of recruitment through physician referral could have led to an increase in the number of patients for whom the physician already had concerns about cognitive abilities. The small number of physician practices is also of concern in that there may be confounding factors not controlled by randomization that could have influenced our findings. Finally, 25% of individuals referred by their physicians were not eligible or declined to participate. However, no definitive conclusions can be made regarding potential bias as data were not collected for subjects who did not participate.
5. Conclusions
In conclusion, we found that physicians who received CRs were more likely to order diagnostic tests and discuss memory problems with patients, and patients were more likely to be taking either a prescription or OTC cognitive-enhancing medication at follow-up. These findings are relevant to the current controversy regarding cognitive screening in PC in that we have demonstrated that having available cognitive information will change physician behavior. Although the physician response was modest, it is possible a more significant response will be seen once a more effective treatment becomes available and once the Medicare annual wellness visit is more widely adopted, and more potential cases of CI are detected
Research in context.
-
1.
Systematic review: It has been suggested that optimal care for older adults should include screening for cognitive disorders to facilitate early detection and treatment. Yet, the United States Preventive Services Task Force does not recommend dementia screening in primary care, citing performance characteristics of screening instruments and limited evidence of effectiveness.
-
2.
Interpretation: Our study showed that primary care physicians who receive cognitive data about their patient initiated interventions that may improve the quality of care, but there was no impact on overall rate of patient's cognitive decline.
-
3.
Future directions: Test the long-term patient and caregiver outcomes after the cognitive assessments in primary care.
.
Acknowledgments
This study was supported by funds from the National Institute on Aging (R01 AG023129). The authors gratefully acknowledge and thank the primary care physicians and patients who participated in this study and the research staff who conducted the assessments. Trial registration: http://clinicaltrials.gov; PCP-AG023129.
Footnotes
N.R.F., B.S., E.R., K.H., and L.C. have no relevant financial interests related to the article. J.S. and L.M. have received salary support through grant RC3 AG037357 from the National Institute on Aging to Psychology Software Tools. J.S. received payment for consultant services between the years 2005 and 2011 from Forest Pharmaceuticals and between 2005 and 2009 from Pfizer Inc.
References
- 1.Valcour V.G., Masaki K.H., Curb J.K., Blanchette P.L. The detection of dementia in the primary care setting. Arch Intern Med. 2000;160:2964–2968. doi: 10.1001/archinte.160.19.2964. [DOI] [PubMed] [Google Scholar]
- 2.Brayne C., Fox C., Boustani M. Dementia screening in primary care: Is it time? JAMA. 2007;298:2409–2411. doi: 10.1001/jama.298.20.2409. [DOI] [PubMed] [Google Scholar]
- 3.Boustani M., Petersen B., Hanson L., Harris R., Lohr K.N. Screening for dementia in primary care: A summary of the evidence for the U.S. Prevention Services Task Force. Ann Intern Med. 2003;138:927–937. doi: 10.7326/0003-4819-138-11-200306030-00015. [DOI] [PubMed] [Google Scholar]
- 4.Cordell C.B., Borson S., Boustani M., Chodosh J., Reuben D., Verghese J., Medicare Detection of Cognitive Impairment Work Group Alzheimer's Association recommendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting. Alzheimers Dementia. 2013;9:141–150. doi: 10.1016/j.jalz.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Borson S., Scanlan J., Hummel J., Gibbs K., Leddig M., Zuhr E. Implementing routine cognitive screening of older adults in primary care: Process and impact on physician behavior. J Gen Intern Med. 2007;22:811–817. doi: 10.1007/s11606-007-0202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarten J.R., Anderson P., Kuskowski M.A., McPherson S.E., Borson S. Screening for cognitive impairment in an elderly veteran population: Acceptability and results using different versions of the mini-cog. J Am Geriatr Soc . 2011;59:309–313. doi: 10.1111/j.1532-5415.2010.03249.x. [DOI] [PubMed] [Google Scholar]
- 7.McCarten J.R., Anderson P., Kuskowski M.A., McPherson S.E., Borson S., Dysken M.W. Finding dementia in primary care: The results of a clinical demonstration project. J of the Am Geriatr Soc . 2012;60:210–217. doi: 10.1111/j.1532-5415.2011.03841.x. [DOI] [PubMed] [Google Scholar]
- 8.Petersen R.C. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–184. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 9.Winblad B., Palmer K., Kivipelto M., Jelic V., Fratiglioni L., Wahlun L.O. Mild cognitive impairment—beyond controversies, towards a consensus: Report of the International Working Group on mild cognitive impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 10.Petersen R.C., Smith G.E., Waring S.C., Ivnik R.J., Tangalos E.G., Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 11.Larrieu S., Letenneur L., Orgogozo J.M., Fabrigoule C., Amieva H., Le Carret N. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59:1594–1599. doi: 10.1212/01.wnl.0000034176.07159.f8. [DOI] [PubMed] [Google Scholar]
- 12.Kaduszkiewicz H., Zimmermann T., Beck-Bornholdt H.P., van den Bussche H. Cholinesterase inhibitors for patients with Alzheimer's disease: Systematic review of randomized clinical trials. BMJ. 2005;331:321–327. doi: 10.1136/bmj.331.7512.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flocke S.A., Litaker D. Physician practice patterns and variation in the delivery of preventive services. J Gen Intern Med. 2007;22:191–196. doi: 10.1007/s11606-006-0042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glynn R.J., Brookhart M.A., Stedman M., Avorn J., Solomon D.H. Design of cluster-randomized trials of quality improvement interventions aimed at medical care providers. Med Care. 2007;45:S38–S43. doi: 10.1097/MLR.0b013e318070c0a0. [DOI] [PubMed] [Google Scholar]
- 15.Folstein M.F., Folstein S.E., McHugh P.R., “Mini mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Morris J.C., Heyman A., Mohs R.C., Hughes J.P., van Belle G., Fillenbaum G. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 17.Wechsler D. The Psychological Corporation; San Antonio: 1987. Wechsler memory scale-revised. [Google Scholar]
- 18.Becker J.T., Boller F., Saxton J., McGonigle-Gibson K.L. Normal rates of forgetting of verbal and non-verbal material in Alzheimer's disease. Cortex. 1987;23:59–72. doi: 10.1016/s0010-9452(87)80019-9. [DOI] [PubMed] [Google Scholar]
- 19.Reitan R.M. Validity of the trail-making tests as an indication of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 20.Wechsler D. The Psychological Corporation; San Antonio: 1981. Wechsler adult intelligence scale-revised manual. [Google Scholar]
- 21.Kaplan E., Goodglass H., Weintraub S. 2nd ed. Lippincott Williams & Wilkins; Philadelphia: 2001. Boston naming test. [Google Scholar]
- 22.Spreen O., Strauss E. Oxford University Press; New York: 1998. A Compendium of neuropsychological tests. [Google Scholar]
- 23.Ganguli M., Dodge H.H., Shen C., DeKosky S.T. Mild cognitive impairment, amnestic type: An epidemiologic study. Neurology. 2004;63:115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- 24.McDowell I., Newell C. Oxford University Press; New York: 1987. Measuring health: A guide to rating scales and questionnaires. [Google Scholar]
- 25.Galasko D., Bennett D., Sano M., Ernesto C., Thomas R., Grundman M. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11:S33–S39. [PubMed] [Google Scholar]
- 26.Radloff L.S. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1997;1:385–401. [Google Scholar]
- 27.Ganguli M., Gilby J., Seaberg E., Belle S. Depressive symptoms and associated factors in a rural older population: the MoVIES Project. Am J Geriatr Psychiatry. 1995;3:144–160. doi: 10.1097/00019442-199500320-00006. [DOI] [PubMed] [Google Scholar]
- 28.Lopez O.L., Becker J.T., Klunk W., Saxton J., Hamilton R.L., Kaufer D.I. Research evaluation and diagnosis of possible Alzheimer's disease over the last two decades: II. Neurology. 2000;55:1863–1869. doi: 10.1212/wnl.55.12.1863. [DOI] [PubMed] [Google Scholar]
- 29.Lopez O.L., Becker J.T., Klunk W., Saxton J., Hamilton R.L., Kaufer D.I. Research evaluation and diagnosis of probable Alzheimer's disease over the last two decades: I. Neurology. 2000;55:1854–1862. doi: 10.1212/wnl.55.12.1854. [DOI] [PubMed] [Google Scholar]
- 30.Doody R.S., Stevens J.C., Beck C., Dubinsky R.M., Kaye J.A., Gwyther L. Practice parameter: Management of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1154–1166. doi: 10.1212/wnl.56.9.1154. [DOI] [PubMed] [Google Scholar]
- 31.Liang K., Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 32.Boustani M., Callahan C.M., Unverzagt F.W., Austrom M.G., Perkins A.J., Fultz B.A. Implementing a screening and diagnosis program for dementia in primary care. J Gen Intern Med. 2005;20:572–577. doi: 10.1111/j.1525-1497.2005.0126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Callahan C.M., Hendrie H.C., Tierney W.M. Documentation and evaluation of cognitive impairment in older primary care patients. Ann Intern Med. 1995;122:422–429. doi: 10.7326/0003-4819-122-6-199503150-00004. [DOI] [PubMed] [Google Scholar]
- 34.Boise L., Neal M.B., Kaye J. Dementia assessment in primary care: results from a study in three managed care systems. J Gerontol. 2004;59A:621–626. doi: 10.1093/gerona/59.6.m621. [DOI] [PubMed] [Google Scholar]
- 35.Boise L., Eckstrom E., Fagnan L., King A., Goubaud M., Buckley D.I. The Rural Older Adult Memory (ROAM) Study: A practice-based intervention to improve dementia screening and diagnosis. J Am Board Fam Med. 2010;23:486–498. doi: 10.3122/jabfm.2010.04.090225. [DOI] [PMC free article] [PubMed] [Google Scholar]