Abstract
Lyme disease (LD) is a natural focal zoonotic disease caused by Borrelia burgdorferi, which is mainly transmitted through infected Ixodes ricinus tick bites. The presence and abundance of ticks in various habitats, the infectivity rate, as well as prolonged human exposure to ticks are factors that may affect the infection risk as well as the incidence of LD. In recent years, 20% to 25% of ticks infected with different borrelial species, as well as about 5,300 citizens with LD, have been registered in the Belgrade area. Many of the patients reported tick bites in city’s grassy areas. The aim of this study was to assess the seroprevalence of B. burgdorferi in high-risk groups (forestry workers and soldiers) in the Belgrade area, and to compare the results with healthy blood donors. A two-step algorithm consisting of ELISA and Western blot tests was used in the study. Immunoreactivity profiles were also compared between the groups. The results obtained showed the seroprevalence to be 11.76% in the group of forestry workers, 17.14% in the group of soldiers infected by tick bites and 8.57% in the population of healthy blood donors. The highest IgM reactivity was detected against the OspC protein, while IgG antibodies showed high reactivity against VlsE, p19, p41, OspC, OspA and p17. Further investigations in this field are necessary in humans and animals in order to improve protective and preventive measures against LD.
Keywords: Borrelia burgdorferi, Lyme disease, seroprevalence, forestry workers, soldiers
Introduction
Lyme disease (LD) is a multisystemic disorder caused by several genospecies of the Borrelia burgdorferi sensu lato (s.l.) complex. The vectors of these spirochetal bacteria are ticks of the genus Ixodes, although other hematophagous insects and some warm-blooded animals (rodents and birds) are also important in ecological transmission of the disease. Climate, economic and social changes have contributed to a higher incidence of the disease worldwide in the last decades (Fritz et al., 2003; Lindgren et al., 2000).
Clinical manifestations of LD are heterogenous and in the early phase mainly include erythema migrans (EM) and musculoskeletal symptoms. In later stages, the neurologic system, heart, joints and eyes become affected, and chronic skin changes appear. Different clinical manifestations, the possibility of overlapping symptoms and the high antigenic variability of Borrelia as a consequence of specific genomic organization (linear chromosome and many mobile genetic elements) make a reliable LD diagnosis difficult. The standard diagnostic protocol for laboratory confirmation of LD consists of a two-step algorithm including an enzyme-linked immunosorbent assay (ELISA) and Western blot (WB) testing (Wright et al., 2012). Timely diagnosis is important for the selection of adequate antibiotic treatment.
LD is currently the most frequent vector-borne disease reported in Europe and the US. In Europe, the borrelial infection of ticks ranges from 0% to 85%. LD was described in Serbia in 1987, and since then research on clinical, diagnostic, ecological, epidemiological and preventive aspects of the disease has started. Reporting of the disease is mandatory from 1989 (Drndarevic et al., 1992). In the period from 1991 to 2004, 7,774 people with LD were registered. According to the National Public Health Institute, 958 patiens were registered in 2012. About 47.8% of the patients were from the Belgrade area (Ilic, 2013).
B. burgdorferi s.l. was first isolated in 1992 from the spleen of an Apodemus flavicollis mouse in Belgrade and after that from Ixodes ricinus ticks (Ristanovic et al., 2007). Since the basic natural way for Borrelia infection is through the bite of an infected vector, the prevalence of these pathogens in ticks represents an important risk indicator for human populations (Radulovic et al., 2010). According to multi-year average data, the estimated tick infestation with B. burgdorferi has been 21.9%, excluding statistically significant differences by years of investigation. The lowest values of tick infection in the Belgrade area were recorded in city parks (17.9%). The values were higher in parks and woods (19.7% and 33.4%, respectively). The highest values (48%) were detected in localities similar to wooded areas (Cekanac et al., 2010).
Since different borrelial genospecies are associated with different clinical manifestations of LD, knowledge of strain diversity in a region is very important. According to molecular studies, the following Borrelia species have been detected in Serbia: B. lusitaniae, B. afzelii, B. burgdorferi sensu stricto, B. garinii and B. valaisiana (Tomanovic et al., 2014). The results obtained by other authors for the Belgrade area have showns dominance of B. afzelii strains (75%), followed by B. burgdorferi sensu stricto (22.2%), while B. garinii (2.8%) has been quite rare (Cekanac et al., 2010). These results are important because clinical manifestations of LD depend on the genospecies of pathogenic borrelial strains (Ristanovic et al., 2007; Cekanac et al., 2010).
The risk of the occurrence of LD correlates with potential exposure to tick bites and depends on the density of the tick population in an endemic area, the percentage of ticks infected with the cause of LD, the duration and the nature of activity of a susceptible population in a certain area (Potkonjak et al., 2013). Based on our results, it is clear that in our country, especially in the Belgrade area, where the most comprehensive survey was conducted, there exist optimal conditions for the maintenance of ixodid ticks and circulation of B. burgdorferi between various animal species.
Belgrade, the capital and the largest Serbian city, has about two million people. It is located at the confluence of the Sava and Danube rivers, where the Pannonian Plain meets the Balkans. Belgrade lies in the humid subtropical climate zone, with four seasons. It has a large number of green and forest areas which serve as picnic areas and city parks. In addition, there are population groups occupationally exposed to the attack of LD vectors and, therefore, having an increased risk of the disease. This study was conducted in order to assess the seroprevalence of B. burgdorferi in the high-risk groups (forestry workers and soldiers) in the Belgrade area, and compare the results with healthy blood donors.
Materials and Methods
The study was carried out on 104 blood samples of people divided into three groups. The first one consisted of 34 forestry workers from the Belgrade area who were potentially exposed to tick bites. They were chosen from the national forestry directory. The group included 33 men and one woman, 25–45 years of age, working as foresters for as long as 15 years. The second group consisted of 35 professional soldiers, 26 men and nine women, 25–45 years of age. They were in military service for 5–15 years, and all were exposed to I. ricinus tick bites. The ticks were removed professionally at the Institute of Epidemiology of the Military Medical Academy in Belgrade and examined by the PCR method (Sacace Biotechnologies, Italy) using an ECO Illumina Real-Time PCR system (San Diego, CA, USA) in our Institute of Microbiology. The serum samples of the soldiers with infected tick bites were taken six weeks after tick removal. The samples of 35 healthy blood donors (25 men and 10 women) who lived in the city center and had no risk factors for infection were used as a control group. None of them reported any history of a tick bite nor any symptoms involving skin, nervous and osteoarticular systems. All participants completed a questionnaire about residence, age, gender, profession, tick bite history and dermatological, neurological, rheumatological and heart problems. The serum samples of all the groups were preserved at − 20 °C until testing was performed at the Institute of Microbiology. The protocol for the serodiagnosis of LD involved ELISA, as a screening test. If it was equivocal or positive, a confirmatory WB test was performed. If the screening test was negative, no further testing was necessary. All samples were screened using an ELISA test (Euroimmun, Germany) for detection of specific IgM and IgG antibodies, according to the manufacturer’s instructions. The optical density was measured on an ELISA reader (URIT-660, China). The results were evaluated directly and interpreted according to the following recommendations: concentrations of IgM and IgG antibodies higher than 22 RU/mL (relative units per milliliter) were considered seropositive, while concentrations from 16 to 22 RU/mL were considered borderline. In order to avoid cross-reactivity and false positive results, all positive or equivocal serum samples were tested for antistreptolysin-O (Dade Behring, Germany), rheumatoid factor (RF) (Omega Diagnostics, UK), Treponema pallidum hemagglutination (TPHA) (Omega Diagnostics, UK) and antinuclear antibodies (ANA) (Euroimmun, Germany). The ELISA-positive samples were then tested using Anti-Borrelia EUROLINE WB (Euroimmun, Germany) for IgM and IgG antibodies, according to the producer’s recommendations, to confirm positive results and perform detection of antibodies against individual antigenic fractions (p17, p19, p21, p25 (OspC), p30, p31 (OspA), p39, p41, p83, and VlsE). EUROLINE-WB is a combination of western blot and line blot techniques. Proteins from a whole Borrelia antigen extract are electrophoretically separated and transferred onto a nitrocellulose membrane. An additional membrane chip coated with recombinant VlsE is printed onto the membrane. The interpretation of the results was done according to the scheme proposed by the producer. For statistical evaluation of the results we used χ2 test in the SPSS program package.
Results
The presence of specific antiborrelial antibodies in the serum samples examined was detected by the ELISA test and the WB method. The results obtained are shown in Table 1.
Table 1. Detection of IgM and IgG antibodies to B. burgdorferi in the examined groups of forestry workers, soldiers and healthy blood donors by serological examinations using two-step protocol, ELISA test and confirmatory Western blot method.
| Results of serological examinations | IgM + IgG + | IgM + IgG − | IgM +/− IgG + | IgM +/− IgG +/− | IgM + IgG +/− | IgM +/− IgG − | IgM − IgG + | IgM − IgG +/− | IgM − IgG − | Total number of samples | The obtained seroprevalence (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I -group (forestry workers) | ||||||||||||
| 1. | ELISA | - | - | - | - | - | - | 4 | - | 30 | 34 | |
| 2. | Western blot | - | - | - | - | - | - | 4 | - | 30 | 34 | |
| 11.76% | ||||||||||||
| II-group (soldiers) | ||||||||||||
| 1. | ELISA | 1 | 2 | - | - | 1 | 1 | 2 | 1 | 29 | 35 | |
| 2. | Western blot | 1 | 1 | 1 | 3 | 29 | 35 | |||||
| 17.14% | ||||||||||||
| III-group (healthy blood donors) | ||||||||||||
| 1. | ELISA | 1 | 2 | 32 | 35 | |||||||
| 2. | Western blot | 1 | 2 | 32 | 35 | |||||||
| 8.57% |
Interpretation of the results: + positive result, − negative result, +/− borderline (equivocal) result-“grey zone“.
Seroprevalence was determined in relation to Western blot positivity.
In the first group that included forestry workers, 30 samples were LD-seronegative, whereas four samples were seropositive. All four samples were IgM-negative and IgG-positive in the ELISA test. When these samples were tested by the WB confirmatory test, three samples were IgM-negative, while the OspC fraction of one sample was IgM-positive. All four samples were IgG-positive in the WB test, with the highest reactivity registered in the fractions of 19 kDa, OspA, 41 kDa and VlsE antigens. The LD seroprevalence in this group was found to be 11.76%.
Among 35 samples collected from soldiers, with previous infected I. ricinus tick bites, 29 samples were negative, while six samples were positive. Three of the 6 donors had clinical manifestations of LD. Among the six samples, one sample was IgM and IgG-positive in both tests, two samples were IgM-positive and IgG-negative in the ELISA test, while WB registered weak reactivity of IgG antibodies to the borrelial fraction of 39 kDa in one of them. Only IgG reactivity was detected in two other samples by ELISA and confirmed by WB. In the remaining sample, the ELISA test showed a borderline IgG titer, whereas it was IgG-positive based on the WB data. The LD seroprevalence in this group was found to be 17.14%. The highest IgM reactivity was registered against the specific OspC protein. IgG reactivity was observed against the following proteins: OspC, p17, OspA, p83 and VlsE.
In the third group of 35 samples from healthy blood donors who denied any history of either tick bite or clinical manifestations of LD, seropositivity was detected in 3 samples. Two of them were IgM-negative and IgG-positive, while only IgM reactivity (borderline titer in ELISA, OspC reactivity in WB) was detected in one sample. The highest IgG reactivity was shown by the 19 and 41 kDa proteins and by the VlsE antigen. The LD seroprevalence in the healthy blood donor group was found to be 8.57%.
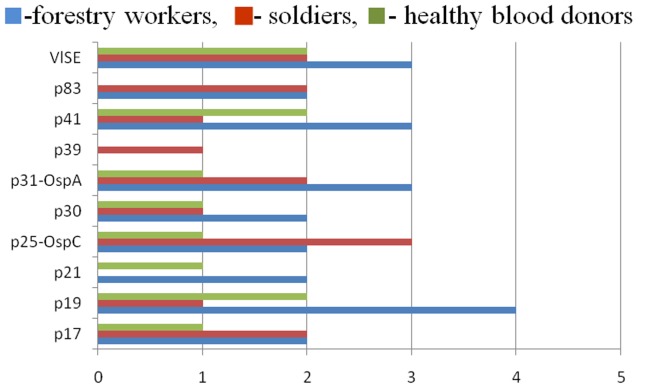
The incidences of antigenic reactivity of particular B. burgdorferifractions to specific IgM and IgG antibodies in the analyzed samples from all three groups are shown in Figures 1 and 2. Immunological characteristics of the antigenic fractions obtained and their correspondence with specific clinical stages of LD are shown in Table 2.
Figure 1. IgM reactivity against specific antigenic fractions of B. burgdorferi detected by Western blot.
Figure 2. IgG reactivity against specific antigenic fractions of B. burgdorferi detected by Western blot.
Table 2. Immunological characteristics of the obtained antigenic bands of B. burgdorferi in correlation with clinical stages of LD.
| Band | Antigen | Diagnostic importance |
|---|---|---|
| VlsE | Variable surface-exposed lipoprotein species specific antigen | Highly immunogenic; main antigen of early and late antibody response to LD |
| p83 | Main extracellular protein; antigen of the protoplasmic cylinder; degradation product of p100 | Late antibody response antigen; highly immunoreactive antigen, typical of neuroborreliosis |
| p41 | Inner part of flagellin | Highly specific antigen of early antibody response |
| p39 (BmpA) | Glycosaminopeptide receptor | Antigen of late antibody response; Significant antigen for advanced disseminated form of LB, often associated with Lyme arthritis |
| OspA | Outer surface protein A | Antigen of late antibody response, typical of neuroborreliosis |
| p30 | Unknown | Antibody response more frequent in later stages of infection |
| OspC | Outer surface protein C | Antigen of early antibody response |
| p21 | Unknown | Antigen of early and late antibody response |
| p17 | Outer surface protein, DbpA (Decorin-Binding protein A) | Antigen of early and late antibody response |
Statistical evaluation of the results with χ2 test using the SPSS program package did not show significant differences between the groups (p < 0.05).
Discussion
According to the City Institute of Public Health, LD was identified in about 5300 residents of Belgrade by the end of 2013. Among those, more than 70% reported a tick bite in city’s green spaces (Pavlovic and Begovic-Lazarevic, 2014). For this reason, we started exploring seroprevalence of LD in the populations occupationally exposed to tick bites in the Belgrade area. The serologic tests were performed using a two-stage diagnostic algorithm, and only WB-positive results were considered.
The first group examined consisted of 34 forestry workers, and four of them showed seropositivity for LD. Three persons confirmed a previous tick bite, while one person denied it. Clinical manifestations of LD in the form of EM were present in one person who reported the tick bite approximately 4 weeks before the symptoms appeared. The WB method detected IgM reactivity against the OspC protein fraction, which corresponds to an early infection stage. At the time of testing, the person did not receive antibiotics. He removed the tick himself and did not know for how long the tick was in the skin before it was removed. It should be noted that the risk of developing an early phase of LD is statistically significantly higher in patients who removed the tick unprofessionally compared to those who did it in a medical institution (Mladenovic et al., 2010). As has been mentioned, the LD seroprevalence in this group was 11.76%.
Surveys of LD seroprevalence among forestry workers have been performed in different parts of the world, and the results obtained have been different. Thus, Polish authors (Niscigorska et al., 2003) recorded seropositivity in more than 60% of forestry workers, while Morgan-Capner et al.(1989) registered seropositivity in 14.0% of forestry workers in the UK. Also, Serbian authors registered a seroprevalence of 23.5% in the population of forestry workers in Belgrade (Krstic and Stajkovic, 2007), but the tests were conducted only by ELISA, without confirmation by the WB method, which may explain the difference with our data. Schwartz and Goldstein (1990) recorded a seroprevalence of 18.7% and Kuiper et al.(1991) recorded a seroprevalence of 20.0% among foresters. These data are comparable to our results and suggest that in most European countries there is a similar risk of infection with B. burgdorferi among the population of foresters. Thus, the largest numbers of ticks occur in forest areas, as well as at the borders between forests and other environments. The numbers may be lower in park areas which are mainly developed and have less vegetation along the paths, thus creating unfavorable conditions for tick survival (Mannelli et al., 1999; Junttila et al., 1999; Talleklint-Eisen and Lane, 1999). Our results should be interpreted in this context, and further research is necessary that would examine a larger number of samples with an accurate ecological description of work sites in terms of the conditions for ticks as well as their infection rate.
By the nature of their work, soldiers are professionally exposed to tick bites, which increases the risk of LD. In our study, we used serum samples of 35 soldiers who were exposed to I. ricinus tick bite in the previous year. In our area, 98.9% of the ticks removed from the skin of people belong to I. ricinus, indicating the dominant presence of this species. The remaining 1.1% of the ticks removed belong to species of the genera Dermacentor, Hyalomma, Rhipicephalus, Haemaphysalis and Boophilus, which are not primary LD vectors (Drndarevic et al., 1993). The ticks were professionally removed, and none of the soldiers in our study gave accurate information on how long the ticks remained in the skin. The results of Mladenovic et al. (2010)showed that subjects in which ticks remained in the skin for less than 24 hours did not develop EM, indicating that removal of a tick within this period may have a protective effect. However, if a tick remains attached longer, the risk of developing B. burgdorferi infection increases proportionally (Mladenovic et al., 2010). Some authors found though that migration of spirochetes in ticks and their transmission to susceptible hosts was recorded in less than 24 hours after a tick bite (Kahl et al., 1998; Crippa et al., 2002). Some studies have shown that partially fed ticks can transmit B. burgdorferi to sensitive hosts much faster than hungry ticks (Shih and Spielman, 2003; Steere et al., 2004).
We registered six seropositive samples among the sera samples of the soldiers tested. Based on this, the seroprevalence in this group was found to be 17.14%. According to the literature, seroprevalence in military populations ranged from 0.27% in the Greek Navy up to a very high value ??recorded by Polish researchers (Stamouli et al., 2000). Oksi and Viljanen (1995) revealed the presence of antibodies to B. burgdorferi in 16.9% of Finnish recruits exposed to vectors, which is comparable to our data. All seropositive samples in our study were collected from soldiers who previously had a bite of I. ricinus ticks infected with B. burgdorferi as determined by the PCR method. The ticks were of different gender and at different developmental stages (larva, nymph, adult), which is very important in terms of ecological characterization of LD vectors for possible transstadial and transovarial transmission of B. burgdorferi (Kahl et al., 1998).
Exactly 50% of seropositive soldiers (3/6) previously had clinical signs and symptoms of EM, which is the most common manifestation of early stages of LD. Our national experts have reported that in 68% of all cases the disease ends at the first stage, while the development of the second and third LD stages has been registered in a significantly smaller number of patients, 20% and 5%, respectively (Pavlovic and Begovic-Lazarevic, 2014).
Timely introduction of appropriate antibiotic therapy successfully prevents the development of the late phase of the disease. Doxycycline is often the preferred agent for oral treatment because of its activity against other tick-borne illnesses (Wright et al., 2003). Serbian as well as many foreign authors recommend prophylactic use of antibiotics after tick bite in people with a higher risk of LD, e.g. if a tick was in the skin for over 24 hours, or if the time is unknown, or if a tick was removed unprofessionally or incompletely (Mladenovic et al., 2010; Patey, 2007).
The LD seroprevalence detected in the group of healthy blood donors from the Belgrade area, who were not occupationally exposed to tick bites, was equal to 8.57%. Based on our results, persons with occupational exposure to B. burgdorferi including soldiers and forestry workers showed a higher seroprevalence of specific antibodies (17.14% and 11.76% respectively) compared to healthy blood donors. The differences are not significant likely due to the great tick infection rate in the Belgrade area and high exposure of the general population. Thus, the findings may suggest that exposure to B. burgdorferi mostly occurs without giving rise to clinical LD in the population. In particular, other authors have shown that although the incidence of LD after a tick bite may be very low, 0.6% to 0.8%, seroconversion in the individuals affected may be as much as 10 times higher (Fahrer et al., 1991).
Since the WB method offers the possibility of examining the reactivity of individual antigenic fractions, the initial results obtained for our groups were further analyzed and compared. Based on the immunoreactivity profiles of particular antigenic fractions, certain conclusions can be drawn about the stage and duration of the infection. However, the data must be interpreted with caution, because they may be affected by antibiotic treatment, a possible presence of other diseases, as well as by individual immune response.
The highest IgM reactivity was detected against the OspC protein (p25) and registered in 4 cases in all three groups. The flagellar protein, p41, was immunoreactive in three cases of soldiers with tick bites and clinical signs of EM, while the reactivity of the OspA protein (p31) and p83 was noted in one case also in the group of soldiers.
It is known that at early stages of LD IgM antibodies can only be detected against few B. burgdorferi antigens such as p41 and outer surface proteins (Osp). Highly immunogenic OspC lipoprotein is the main virulence factor of B. Burgdorferi. Antibodies to this protein are synthesized early in the infection, but also exist in the late LD phase. Bacterial strains isolated in Europe express this protein while isolates from the United States do not express it (Wilske et al., 1989, 1993). Flagellar protein p41 is a strong immunogen and provokes the early synthesis of antibodies, but due to cross-reactivity it is not a specific diagnostic marker (Collins and Peltz, 1991; Craft et al., 1986). Lipoproteins OspA and OspB are important spirochetal surface antigens and bind antibodies inhibiting in vitro growth of B. burgdorferi (Coleman et al., 1992) or kill the bacterium in the presence of complement (Coleman et al., 1992, Moskophidis and Luther, 1993). Antibodies to these antigens appear rather late during infection (Fikrig et al., 1992), although some authors have demonstrated the presence of anti-OspA early in the infection (Schutzer et al., 1994). Antibodies to p83, whose presence is characteristic of chronic disease, are found in our area in a higher percentage during an early stage of LD (Atanasievska, 2012).
IgG antibodies usually appear several weeks after an infected tick bite, and their levels can increase after the disappearance of clinical symptoms. In our study, the highest IgG reactivity was obtained against the VlsE and p19 protein fractions detected in seven samples. The reactivity against p41, p31 (OspA) and p25 (OspC) was detected in six samples, against p17 in five samples, while fractions p30 and p83 showed reactivity in four samples. Antibodies against fraction p21 were detected in three samples, and p39 was reactive in one sample. The reactivity profiles differed between the groups examined. Thus, in the group of forestry workers we recorded the highest IgG reactivity against proteins p19, p31 (OspA), p41 and VlsE. In the group of soldiers the most reactive were p25 (OspC), p17, p31 (OspA) and VlsE, while in the group of healthy blood donors the highest reactivity was detected against the p19, p41 and VlsE proteins.
VlsE is considered to be the most sensitive recombinant antigen of B. burgdorferi and is used in diagnostics (Stajkovic et al., 1993; Anderson, 1999). Antibodies to this protein can be found in serum even 6 months after successful antibiotic treatment (Peltoma et al., 2003).
Reactivity of the p19 protein was detected only with IgG antibodies, while anti-OspC antibodies, as noted above, can occur early in the immune response and also in the chronic phase of LD (Wilske et al., 1989, 1993). OspA protein (p31) showed high reactivity to IgG antibodies, while IgM antibodies against it appeared in only one sample, as has been mentioned. Literature reports have shown that antibodies to this protein are characteristic of the late disease stage, although they can be present in the early LD phase as well (Schutzer et al., 1994; Magnarelli et al., 1996; Kalish et al., 1993). Flagellar protein p41 is highly reactive with IgG antibodies, and studies have shown that protein p17 is a strong immunogen. Hauser et al. (1998) believe that it is important to differentiate between an asymptomatic infection and active disease. In the literature, there are data that p30 reactivity is characteristic of the post Lyme syndrome (Chandra et al., 2011). Antibodies to p83 are generally typical for the chronic disease (Rasiah et al., 1994), whereas antibodies to protein p39 can be detected in the early and late LD stages (Scriba et al., 1993). Immunoreactivity of proteins p21 and p39 was only registered in our study with IgG antibodies. In the literature, it is stated that antibodies to the highly specific borrelial protein of 39kDa can be detected in the early and late stages of the disease (Scriba et al., 1993; Simpson et al., 1990).
Antibody reactivity against specific B. burgdorferi proteins is still a subject of research. Based on previous findings (Magnarelli et al., 1996), the major immunodominant proteins are OspC, VlsE, p41, p39, OspA, and p83, which is comparable to our results. It is particularly important for improved diagnostics to investigate the presence of antibodies to individual antigenic fractions.
The following criteria are important for the assessment of LD risk: the density of tick populations, infection rate, seasonal vector activity, and the length of human exposure (Fish, 1995). The size of a tick population varies with the latitude, the composition of the habitat, as well as with environmental factors such as outside temperature, humidity, light, air flow, and the presence and abundance of various host species (rodents, deer, birds, stray dogs) (Krstic and Stajkovic, 2007). Due to this, further research on the LD seroprevalence in exposed human groups, as well as in animals, is needed for better health protection of the population. Also, adequate preventive measures are necessary, which include avoiding areas with high tick burdens, wearing protective clothing, using tick repellants, conducting frequent body checks as well as performing environmental landscape modifications in endemic zones.
Thus, the tick infection rate, as well as the incidence of LD, have increased in the Belgrade area in the last years. Our results suggest that the risk of LD is higher in professionally exposed persons, although due to the high tick infection rate LD seroprevalence in the general population is also high. Considering the importance of the problem, it is necessary to implement preventive and diagnostic measures and to continue research in this area.
Acknowledgments
This paper is part of the activity under the research project MNTR BI 173006: Enzootic and transmission cycles of pathogens transmitted by ticks. 2011–2014 financially supported by Ministry of Sciences and Technological Development, Republic of Serbia.
References
- Anderson JF. Epizootology of Lyme borreliosis. Scand J Infect Dis. 1999;77:23–34. [PubMed] [Google Scholar]
- Atanasievska S. Problems and challenges of modern microbiological diagnotic of lyme disease. University of Belgrade; Belgrade: 2012. M.Sc. Dissertation. [Serbian] [Google Scholar]
- Cekanac R, Pavlovic N, Grugurecic A, et al. Prevalence of Borrelia burgdorferi in Ixodes ricinus ticks in Belgrade area. Vector Borne Zoonotic Dis. 2010;10:447–452. doi: 10.1089/vbz.2009.0139. [DOI] [PubMed] [Google Scholar]
- Chandra A, Wormser GP, Marques AR, et al. Anti-Borrelia burgdorferi antibody profile in post-Lyme disease syndrome. Clin Vaccine Immunol. 2011;18:767–771. doi: 10.1128/CVI.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JL, Rogers RC, Benach JL. Selection of an escape variant of borrelia burgdorferi by use of bactericidal monoclonal antibodies of OspB. Infect Immun. 1992;60:3098–3104. doi: 10.1128/iai.60.8.3098-3104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C, Peltz G. Immunoreactive epitopes on an expressed recombination flagellar protein of Borrelia burgdorferi . Infect Immun. 1991;59:514–520. doi: 10.1128/iai.59.2.514-520.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft JE, Fisher DK, Shimamoto GT, et al. Antigens of Borrelia burgdorferi recognized during Lyme disease: appearance of new IgM response and expansion of the IgM response late in the illness. J Clin Invest. 1986;78:934. doi: 10.1172/JCI112683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippa M, Rais O, Gern L. Investigations on the mode and dynamics of transmission and infectivity of Borrelia burgdorferi sensu stricto and Borrelia afzelii in Ixodes ricinus ticks. Vector Borne Zoonotic Dis. 2002;2:3–9. doi: 10.1089/153036602760260724. [DOI] [PubMed] [Google Scholar]
- Drndarevic D, Lako B, Stojanovic N, et al. Ixodes ricinus dokazan vektor lajm borelioze i u Jugoslaviji (Ixodes ricinus-confirmed vector of Lyme borreliosis in Yugoslavia) Vojnosanit pregl. 1992;49:8. (Serbian) [PubMed] [Google Scholar]
- Drndarevic D, Stajkovic N, Dmitrovic R, et al. Ecology of Borrelia burgdorferi. Glas Srp Akad Nauka Med. 1993;43:33–44. [Serbian] [PubMed] [Google Scholar]
- Fahrer H, van der Linden SM, Sauvin MJ, et al. The prevalence and incidence of clinical and asymptomatic Lyme borreliosis in a population at risk. J Infect Dis. 1991;163:305–310. doi: 10.1093/infdis/163.2.305. [DOI] [PubMed] [Google Scholar]
- Fikrig E, Barthold SW, Marcantonio N, et al. Roles of OspA, OspB and flagellin in protective immunity to Lyme borreliosis in laboratory mice. Infect Immun. 1992;60:657–661. doi: 10.1128/iai.60.2.657-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish D. Environmental Risk and Prevention of Lyme Disease. Am J Med. 1995;98:4A2S–4A-9S. doi: 10.1016/s0002-9343(99)80038-2. [DOI] [PubMed] [Google Scholar]
- Fritz CL, Kjemptrup AM. Lyme borreliosis. J Am Vet Med Assoc. 2003;2239:1261–1270. doi: 10.2460/javma.2003.223.1261. [DOI] [PubMed] [Google Scholar]
- Hauser U, Krahl H, Peters H, et al. Impact of strain heterogeneity of Lyme-disease serology in Europe-comparison of enzyme-linked immunosorbent using different species of Borrelia burgdorferi sensu lato. J Clin Microbiol. 1998;36:427–436. doi: 10.1128/jcm.36.2.427-436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic D. Report on infectious diseases in 2012 in the Republic of Serbia. Institute of Public Health of Serbia; 2013. Available at: http://www.batut.org.rs/download/izvestaji[Serbian] [Google Scholar]
- Junttila J, Peltomaa M, Soini H, et al. Prevalence of Borrelia burgdorferi in Ixodes ricinus ticks in urban recreational areas of Helsinki. J Clin Microbiol. 1999;37:1361–1365. doi: 10.1128/jcm.37.5.1361-1365.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl O, Janetzky-Mittmann C, Gray JS, et al. Risk of infection with Borrelia burgdorferisensu lato for a host in relation to the duration of nymphal ixodes ricinus and the method of tick removal. Zentralbl Bakteriol. 1998;287:41–45. doi: 10.1016/s0934-8840(98)80142-4. [DOI] [PubMed] [Google Scholar]
- Kalish RA, Leong JM, Steere AC. Association of treatment-resistant chronic Lyme arthritis with HLA-DR4 and antibody reactivity to OspA and OspB of borrelia burgdorferi . Infect Immun. 1993;61:2774–2779. doi: 10.1128/iai.61.7.2774-2779.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krstic M, Stajkovic N. Risk for infection by Lyme disease cause in green surfaces maintenance workers in Belgrade. Vaojnosanit pregl. 2007;64:313–318. doi: 10.2298/vsp0705313k. [DOI] [PubMed] [Google Scholar]
- Kuiper H, Jongh BM, Nauta AP, et al. Lyme borreliosis in Dutch forestry workers. J Infect. 1991;23:279–286. doi: 10.1016/0163-4453(91)92936-y. [DOI] [PubMed] [Google Scholar]
- Lindgren E, Talleklint L, Polfeald T. Impact of climatic change on the northern latitude limit and population density od the disease-transmitting European tick Ixodes ricinus . Environ Health Perspect. 2000;108:119. doi: 10.1289/ehp.00108119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnarelli LA, Fikrig E, Padula SJ, et al. Use of recombinant antigens of borrelia burgdorferi in serologic test for diagnosis of Lyme borreliosis. J Clin Microbiol. 1996;34:237–240. doi: 10.1128/jcm.34.2.237-240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannelli A, Cerri D, Buffrini L, et al. Low risk of Lyme borreliosis in a protected area on the Tyrrhenian coast, in central Italy. Eur J Epidemiol. 1999;15:371–377. doi: 10.1023/a:1007535313763. [DOI] [PubMed] [Google Scholar]
- Mladenovic J, Cekanac R, Stajkovic N, et al. Risk of Lyme disease development after a tick bite. Vojnosanit pregl. 2010;67:369–374. doi: 10.2298/vsp1005369m. [Serbian] [DOI] [PubMed] [Google Scholar]
- Morgan-Capner P, Cutler SJ, Wright DJM. Borrelia burgdorferi infection in UK workers at risk of tick bites. Lancet. 1989;1:789–790. [PubMed] [Google Scholar]
- Moskophidis M, Luther B. Monoclonal antibodies with in vitro borreliacidal activities define the outer surface proteins A and B of Borrelia burgdorferi . Zbl Bakt. 1993;279:201–213. doi: 10.1016/s0934-8840(11)80398-1. [DOI] [PubMed] [Google Scholar]
- Niscigorska J, Skotarczak B, Wodecka B. Borrelia burgdorferi infection among forestry workers-assessed with an immunoenzymatic method (ELISA), PCR and correlated with the clinical state of the patients. Ann Agric Environ Med. 2003;10:15–19. [PubMed] [Google Scholar]
- Oksi J, Viljanen MK. Tick bites, clinical symptoms of Lyme borreliosis, and Borrelia antibody responses in Finnish army recruits training in an endemic region during summer. Mil Medn. 1995;160:453–456. [PubMed] [Google Scholar]
- Patey O. Lyme disease: prophylaxis after tick bite. Med Mal Infect. 2007;37:446–455. doi: 10.1016/j.medmal.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Pavlovic N, Begovic-Lazarevic I. Lyme disease. 2014. (Serbian) Available on: http://www.zdravlje.org.rs/radovi/Lajmska%20bolest.pdf.
- Peltoma M, McHugh G, Steere AC. Persistence of the Antibody Response to the VlsE Sixth Invariant Region (IR6) Peptide of Borrelia burgdorferi after Successful Antibiotic Treatment of Lyme Disease. The Journal of Infectious Diseases. 2003;187:1178. doi: 10.1086/374376. [DOI] [PubMed] [Google Scholar]
- Potkonjak A, Jurusic A, Petrovic A, Obrenovic S, et al. Entomological and ecological index for risk of infection causing lyme disease in territory of Vojvodina, Serbia. Vet glasnik. 2013;67:3–14. [Serbian] [Google Scholar]
- Radulovic Z, Milutinovic M, Tomanovic S, et al. Detection of Borrelia-specific 16S rRNA sequence total RNA extracted from Ixodes ricinusticks. Arq Bras Med Vet Zootec. 2010;62:862–867. [Google Scholar]
- Rasiah C, Rauer S, Gassmann GS, et al. Use of hybrid protein cosisting of the variable region of the Borelia burgdorferi flagellin and part of the 83-kDa protein as antigen for serodiagnosis of Lyme disease. J Clin Microbiol. 1994;32:1011–1017. doi: 10.1128/jcm.32.4.1011-1017.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristanovic E, Kitamura K, Masuzawa T, et al. Molecular characterization of Borrelia burgdorferi sensu lato strains isolated in the area of Belgrade, Serbia. Sao Paolo: Braz J Microbiol. 2007;38:1. [Google Scholar]
- Stajkovic N, Drndarevic D, Lako B, et al. Vectors of Borelia burgdorferi . Glas SANU. 1993;43:45–56. [Serbian] [PubMed] [Google Scholar]
- Schutzer SE, Coyel OK, Dunn JJ, et al. Early and specific antibody response to ospA in Lyme disease. J Clin Invest. 1994;94:454–457. doi: 10.1172/JCI117346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz BS, Goldstein MD. Lyme disease in outdoor workers: risk factors, preventive measures, and tick removal methods. Am J Epidemiol. 1990;131:877–885. doi: 10.1093/oxfordjournals.aje.a115578. [DOI] [PubMed] [Google Scholar]
- Scriba M, Ebrahim JS, Schlott T, et al. The 39-kilodalton protein of Borrelia burgdorferi; a targer for bactericidal monoclonal autibodies. Infect Immun. 1993:4523–4526. doi: 10.1128/iai.61.10.4523-4526.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih CM, Spielman A. Accelerated transmission of Lyme disease spirochetes by partially fed vector ticks. J Clin Microbiol. 1993;31:2878–2881. doi: 10.1128/jcm.31.11.2878-2881.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson WJ, Schrumpf ME, Schwan TG. Reactivity of human Lyme borreliosis with a 39-kilodalton antigen specific to Borrelia burgdorferi. J Clin Microbiol. 1990;28:1329–1337. doi: 10.1128/jcm.28.6.1329-1337.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamouli M, Totos G, Braun HB, et al. Very low seroprevalence of Lyme borreliosis in young Greek males. Eur J Epidemiol. 2000;16:495–496. doi: 10.1023/a:1007657430880. [DOI] [PubMed] [Google Scholar]
- Steere AC, Coburn J, Glickstein L. The emergence of Lyme disease. J Clin Invest. 2004;113:1093–1101. doi: 10.1172/JCI21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talleklint-Eisen L, Lane RS. Variation in the density of questing Ixodes pacificus (Acari:Ixodidae) nymphs infected with Borrelia burgdorferiat different spatial scales in California. J Parasitol. 1999;85:824–831. [PubMed] [Google Scholar]
- Tomanovic S, et al. Strain diversity of Borrelia burgdorferi sensu lato in Serbia. Parasites and Vectors. 2014;7:O25. [Google Scholar]
- Wilske B, Preac-Mursic V, Schirez G, et al. Detection of IgM and IgG antibodies to Borrelia burgdorferi using different strains as antigen. Zentralbl Bactriol. 1989;18:299–309. [Google Scholar]
- Wilske B, Preac-Mursic V, Jauris S, et al. Immunological and molecular polymorphisms of OspC and immunodominant major surface protien of borelia burgdorferi. Infect Immun. 1993;61:2182–2191. doi: 10.1128/iai.61.5.2182-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright F, Riedel D, Talwain R, et al. Diagnosis and managment of Lyme disease. American Family Physician. 2012;185:1086–1093. [PubMed] [Google Scholar]