Abstract
In this study, we evaluated the effect of low and high molecular weight polycyclic aromatic hydrocarbons (PAHs), i.e., Phenanthrene, Pyrene and Benzo[a]pyrene, on the radial growth and morphology of the PAH-degrading fungal strains Aspergillus nomius H7 and Trichoderma asperellum H15. The presence of PAHs in solid medium produced significant detrimental effects on the radial growth of A. nomius H7 at 4,000 and 6,000 mg L−1 and changes in mycelium pigmentation, abundance and sporulation ability at 1,000–6,000 mg L−1. In contrast, the radial growth of T. asperellum H15 was not affected at any of the doses tested, although sporulation was observed only up to 4,000 mg L−1 and as with the H7 strain, some visible changes in sporulation patterns and mycelium pigmentation were observed. Our results suggest that fungal strains exposed to high doses of PAHs significantly vary in their growth rates and sporulation characteristics in response to the physiological and defense mechanisms that affect both pigment production and conidiation processes. This finding is relevant for obtaining a better understanding of fungal adaptation in PAH-polluted environments and for developing and implementing adequate strategies for the remediation of contaminated soils.
Keywords: polycyclic aromatic hydrocarbons (PAHs), bioremediation, radial growth, sporulation, fungal physiology
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are an important group of persistent organic pollutants that are primarily produced as a consequence of thermal decomposition, incomplete combustion and pyrolysis of diverse organic molecules (Mrozik and Piotrowska-Seget, 2010). Due to their physicochemical properties, PAHs are highly hydrophobic compounds that persist in the environment and have potentially cytotoxic, carcinogenic, genotoxic and mutagenic effects on the flora and fauna of impacted habitats, resulting in the absorption and accumulation of several toxic products and active metabolic intermediaries in diverse organisms. Several natural and anthropogenic sources contribute to the release of PAHs into the environment. In particular, petrochemical activities and their related residues exert a strong negative impact on the environment and account for the majority of PAHs and other hydrocarbons released into the soil and bodies of water (Haritash and Kaushik, 2009).
The removal of low (LMW) and high molecular weight (HMW) PAHs from contaminated soils has become an environmental priority (US-EPA, 2008). Although chemical, physicochemical and thermal technologies are available for the remediation of contaminated soils, microbial degradation is considered the main natural degradation mechanism of PAHs. Many bacterial and fungal species are capable of degrading LMW or HMW-PAHs under aerobic or anaerobic conditions (Cerniglia and Sutherland, 2010; Seo et al., 2009), involving the action of mono and dioxygenase, laccase, and peroxidase enzymes among others (Haritash and Kaushik, 2009). Several fungal species possess the ability to degrade PAHs and have the potential to remediate polluted soils; however, one limiting factor in the success of these organisms is their inability to adapt and properly grow on extensively contaminated soils (Tabak et al., 2003). The use of native microorganisms that are capable of not only degrading PAHs but also having a high level of tolerance to PAHs would reduce the problems associated with adaptation, survival and activity on soils containing high amounts of heavy hydrocarbon fractions (Margesin and Schinner, 2001). However, the effect of aromatic hydrocarbons on the mycelial growth rate and sporulation of tolerant/degrading fungi could be an important factor not only for microbial adaptation but also for adequate cell development and degradation of PAHs. While in the soil, microbial populations are often surrounded by compounds that could serve as a source of carbon and energy, but they are toxic (Ramos et al., 2002). In this sense, the impact of PAHs on fungal populations is a key factor influencing its removal or persistence, particularly in soils with high amounts of toxic compounds. This is particularly important for selecting suitable microorganisms for the bioremediation of soils impacted by PAHs. Thus, the aim of this work was to evaluate the effect of PAHs on the growth of two PAH-degrading fungal strains, to better address the possible inhibitory effects and morphological changes caused in fungi at different PAH doses.
Materials and Methods
PAH-degrading microorganisms
Aspergillus nomius H7 and Trichoderma asperellum H15 were previously isolated from a heavy-crude oil contaminated-soil and are characterized as hydrocarbon-degrading strains, showing increased tolerance levels to 3 (Phenanthrene), 4 (Pyrene) and 5-ring (Benzo[a]pyrene) PAHs (Zafra et al., 2014). Fungi were maintained on potato dextrose agar (PDA) plates at 30 °C. Fresh spores were produced in 250 mL flasks containing 30 mL of PDA, inoculated with each of the strains and incubated at 30 °C. After 3 d of growth, spores were collected with the addition of 20 mL of 0.1% tween 80 solution and sterile glass beads.
Effect of PAHs on radial growth
The effect of analytical grade Phenantrene (Phe), Pyrene (Pyr), and Benzo[a]pyrene (Bap) (Sigma-Aldrich, USA) on the radial growth of A. nomius H7 and T. asperellum H15 was tested by surface plate assays. A 2 mL mixture of Phe, Pyr and BaP (1:1:1) was dissolved in acetone (Sigma, USA), added to Petri dishes containing 20 mL of Toyama’s Medium (Wunder et al., 1994) and evaporated under sterile conditions to yield final superficial concentrations of 1,000, 2,000, 4,000 and 6,000 mgL−1. Plates were centrally inoculated with 1×104 spores and incubated at 30 °C for 7 d. Plates without PAHs that were inoculated with each of the fungal isolates were used as controls. Mycelium radial extension rate measurements (cm d−1) were made every 24 h by using a vernier digital caliper (Mitotuyo, Kawasaki, Japan).
Effect of PAHs on fungal morphology
The effect of PAHs on fungal morphology was monitored by direct macromorphological observations of both colony variations and microscopic changes in the mycelium and spores after 10 d of growth. Stereomicroscopic observations were made by using a Zoom 2000 Stereozoom Microscope (Leica, Wetzlar, Germany). Plate photographs and photomicrographs were taken with a PowerShot SX500 IS digital camera (Canon, New York, USA).
Statistical analysis
Fungal growth assays were structured by using a 2 × 5 factorial design (two strains and five PAH doses). Data from the radial extension rates were analyzed by Analysis of Variance (ANOVA) followed by a multiple comparison test (LSD) with SPSS Statistics Software version 19 (IBM). Differences with a p value < 0.05 were considered statistically significant. All assays were performed in triplicate.
Results and Discussion
Effect of PAHs on fungal radial growth
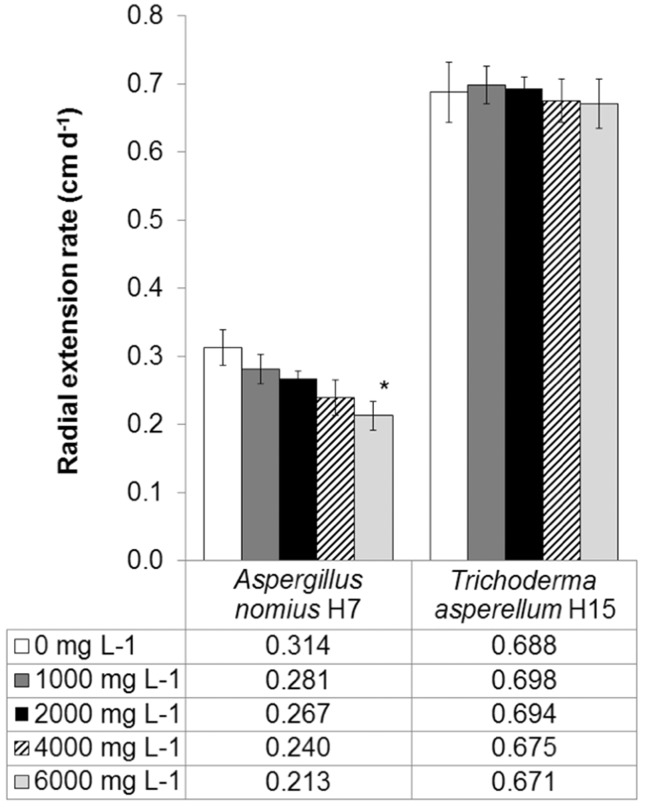
Because PAH-contaminated soils typically contain complex mixtures of LMW and HMW PAHs, we used high doses of a mixture of 3, 4 and 5-ring PAHs to better address the inhibitory effect caused on fungi in a hypothetical impacted soil. Figure 1 shows the ability of the fungal strains to grow in the presence of different doses of PAHs. Compared with control plates, the radial extension rates of A. nomius H7 were not significantly different at 1,000, 2,000 and 4,000 mg L−1, but they were different when growing in the presence of 6,000 mg L−1 (p = 0.047) of the PAH mixture. Although growth inhibition was observed at higher concentrations, the results showed that A. nomius spores could successfully germinate, even with 6,000 mg L−1 of PAHs in the medium, and could reach comparable extension rates at intermediate doses. The H7 strain was isolated from a highly hydrocarbon-polluted soil and previously showed the ability to grow with the same mixture of PAHs as their sole carbon source (Zafra et al., 2014), features that could confer a great adaptive advantage in PAH-contaminated soils (Hinga and Batchellor, 2005). In contrast, there were no apparent detrimental effects of PAHs on the radial growth of T. asperellum H15 because the growth rates showed no significant differences between controls without PAHs and the highest concentration tested (6,000 mg L−1). Unlike A. nomius, T. asperellum possess a complex enzymatic machinery capable of oxidizing and cleaving aromatic rings, including laccases, peroxidases and dioxygenases, among others (Cerniglia and Sutherland, 2010), which could actively contribute to adaptation and proper growth in the presence of PAHs. Moreover, T. asperellum produces a potent lignocellulolytic cocktail that favors the use of alternative carbon sources (including PAHs) (Marx et al., 2013). This is particularly relevant in soil, where a complex mixture of substrates can be found. The observed mycelium radial extension at extremely high concentrations of PAHs and the ability to use PAHs as a sole carbon source are good indications of the presumptive degradative ability of these strains in soils.
Figure 1. Radial extension rates of fungal strains in presence of different concentrations of a mixture of Phe, Pyr and BaP.
Effect of PAHs on mycelium pigmentation
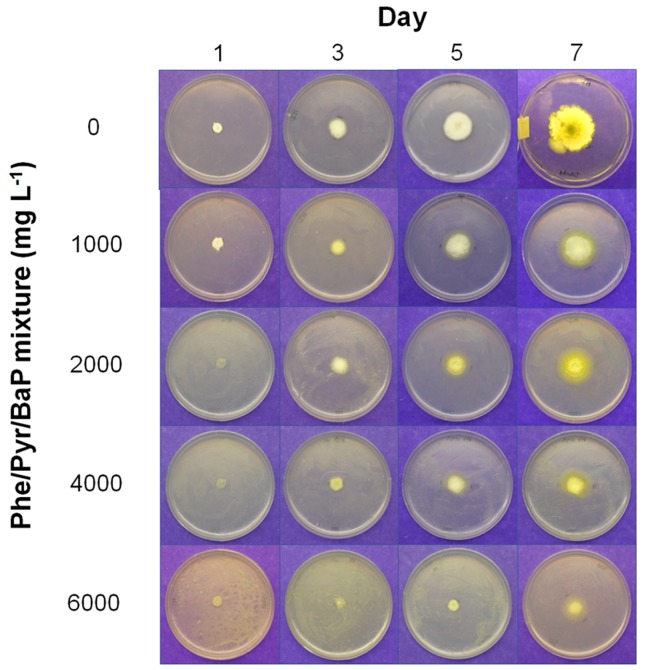
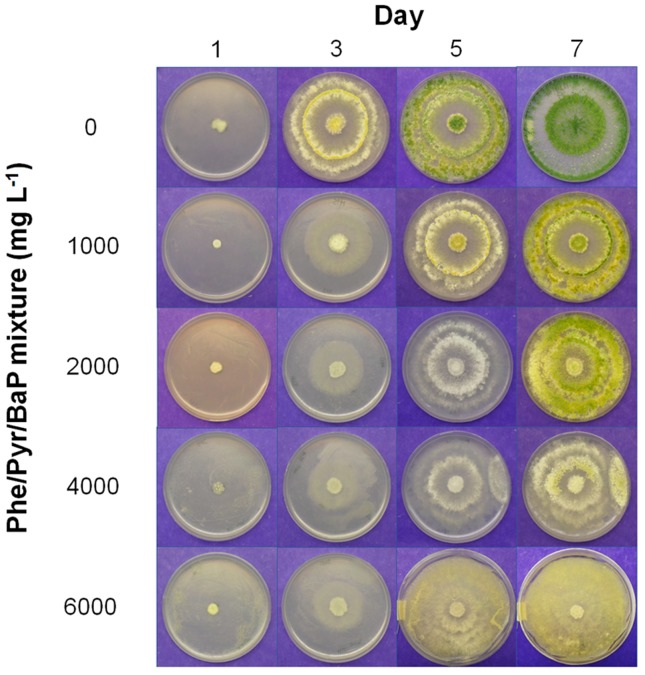
Previous studies on PAH metabolism indicated that fungal growth could be significantly impaired in the presence of PAHs (Brodkorb and Legge, 1992). Our results showed that fungal exposure to increased doses of PAHs caused drastic changes in the morphology of both strains compared with uncontaminated controls, particularly on A. nomius H7. PAHs produced evident alterations on the mycelium pigmentation of strain H7, even when growing at the lowest dose (Figure 2). A slight yellow pigmentation was observed at the periphery of the colonies, and this effect was more evident when the contamination levels increased. Conversely, although the radial growth of T. asperellum H15 was not affected at any of the PAH doses, mycelium pigmentation was affected particularly at 6,000 mg L−1(Figure 3).
Figure 2. Effect of different concentrations of Phe, Pyr and BaP on the radial growth and morphology of Aspergillus nomius H7.
Figure 3. Effect of different concentrations of Phe, Pyr and BaP on the radial growth and morphology of Trichoderma asperellumH15.
Our findings are in agreement with previous studies describing yellow pigment formation and mycelial changes in ascomycetes grown on hydrocarbons, including Aspergillus versicolor, Aspergillus ochraceous, Aspergillus alliaceous, Aspergillus niger and Aspergillus terreus(Nyns et al., 1968; Zajic and Kuehn, 1962). Similar effects have also been observed in Chrysosporium merdarium and Trichoderma harzianum grown on lubricants, which caused the presence of a thick mycelium and intense mycelial development (Lugauskas et al., 2008). Mycelial yellow pigment production has also been linked to aflatoxin production in A. nomius and A. flavus (Abbas et al., 2004; Shier et al., 2005) as a response against abiotic stress and limiting growth conditions (Yu et al., 1996). The alterations observed in A. nomius and T. asperellummycelium could therefore be related to an accumulation of hydrocarbons in the central part of the cell membrane, causing dramatic changes in the structure, modifications of membrane fluidity, increased mycelium area and an eventual swelling of the bilayer (Sikkema et al., 1995). Furthermore, hydrocarbon-induced changes could lead to altered membrane function, disrupted cellular homeostasis and impaired energy transduction (Pope et al., 1984).
Effect of PAHs on sporulation
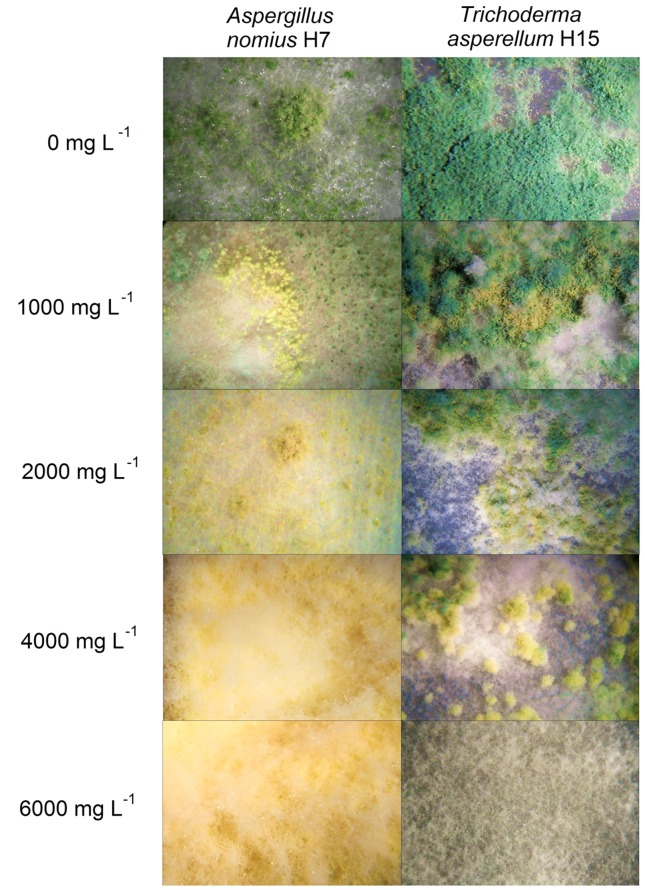
We found that the presence of PAHs affected the sporulation process in Aspergillus nomius and Trichoderma asperellum to a different extent. Whereas sporulation in A. nomius H7 was observed from day 5 in control plates, the presence of 1,000 and 2,000 mg L−1 of PAHs retarded the start of sporulation until day 10, was minimal at 2,000 mg L−1 and was completely absent at 4,000 and 6,000 mg L−1 (Figure 4). In addition, a gradual change in conidia pigmentation from green to yellow was observed from 1,000 mg L−1 of PAHs and was evident at 2,000 mg L−1 (Figure 4). This effect appeared to be dose-dependent and stronger in A. nomiusH7. Conversely, T. asperellum H15 showed similar effects in sporulation as those observed in A. nomius H7. However, there was a difference regarding the doses at which sporulation was inhibited, as strain H15 was able to sporulate even at 4,000 mg L−1 and the conidial pigmentation changes were less pronounced (Figure 4). The impact of the PAH mixture on fungal sporulation was evident, as both strains presented delayed sporulation and showed changes in conidial pigmentation.
Figure 4. Stereomicroscopic evaluation of the effect of PAHs on the morphology of fungal strains (magnification 20×).
Fungal sporulation is a complex process involving the action of many regulatory genes affecting cell specialization and intercellular communication. The central regulatory pathway of conidiation has been described in Aspergillus nidulans, with at least three essential modulatory genes identified (brlA, abaA and wetA) (Adams and Yu, 1998). Previous work by our group showed that concentrations as low as 200 mg L−1 of Phe can delay the expression of essential genes in the central regulatory pathway of Aspergillus niger, particularly wetA (Vasquez, 2010). The wetA gene is required in the late phase of conidiation for the synthesis of cell wall layers (Tao and Yu, 2011). Mutants lacking this gene produce normal conidiophores, but the conidia never become pigmented and autolyse (Sewall et al., 1990). Furthermore, wetA orthologues have been identified in several Trichoderma species as key genes in the conidiation process (Carreras-Villasenor et al., 2012). Thus, it is likely that a PAH-induced delay in the expression of central genes involved in conidiation may be responsible, at least in part, for the observed alterations in delayed sporulation and the changes in pigmentation in both strains, particularly A. nomius.
Conclusions
Our data suggest that fungal strains exposed to high doses of PAHs significantly vary in their growth rate and sporulation characteristics due to alterations in cell membrane structure and the partial inhibition of the central conidiation pathway. The results are relevant to better understand fungal adaptation in PAH-polluted environments, to select suitable fungal strains able to tolerate, grow and degrade high amounts of PAHs and to develop and implement adequate strategies for the remediation of contaminated soils.
Acknowledgments
This work was supported by the Consejo Nacional de Ciencia y Tecnología (CONACYT) project CB2008-105643, the Instituto Politécnico Nacional project SIP20152025 and CONACYT grant 269828.
References
- Abbas HK, Shier WT, Horn BW, et al. Cultural methods for aflatoxin detection. J Toxicol Toxin Rev. 2004;23:295–315. [Google Scholar]
- Adams TH, Yu JH. Coordinate control of secondary metabolite production and asexual sporulation in Aspergillus nidulans . Curr Opin Microbiol. 1998;1:674–677. doi: 10.1016/s1369-5274(98)80114-8. [DOI] [PubMed] [Google Scholar]
- Brodkorb TS, Legge RL. Enhanced biodegradation of phenanthrene in oil tar-contaminated soils supplemented with Phanerochaete chrysosporium . Appl Environ Microbiol. 1992;58:3117–3121. doi: 10.1128/aem.58.9.3117-3121.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreras-Villasenor N, Sanchez-Arreguin JA, Herrera-Estrella AH. Trichoderma: sensing the environment for survival and dispersal. Microbiology. 2012;158:3–16. doi: 10.1099/mic.0.052688-0. [DOI] [PubMed] [Google Scholar]
- Cerniglia CE, Sutherland GR. Degradation of Polycyclic Aromatic Hydrocarbons by Fungi. In: Timmis KN, editor. Handbook of hydrocarbon and lipid microbiology. Springer; Berlin; London: 2010. pp. 2079–2110. [Google Scholar]
- Haritash AK, Kaushik CP. Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater. 2009;169:1–15. doi: 10.1016/j.jhazmat.2009.03.137. [DOI] [PubMed] [Google Scholar]
- Hinga KR, Batchellor A. Waste Processing and Detoxification. In: Hassan RM, Scholes R, Ash N, editors. Ecosystems and human well-being : current state and trends : findings of the Condition and Trends Working Group of the Millennium Ecosystem Assessment. Island Press; Washington, DC: 2005. xxi. 917 [Google Scholar]
- Lugauskas A, Griguceviciene A, Asadauskas S, et al. Selection of micromycetes capable of developing on technical lubricants. Ekologija. 2008;54:186–194. [Google Scholar]
- Margesin R, Schinner F. Biodegradation and bioremediation of hydrocarbons in extreme environments. Appl Microbiol Biotechnol. 2001;56:650–663. doi: 10.1007/s002530100701. [DOI] [PubMed] [Google Scholar]
- Marx IJ, van Wyk N, Smit S, et al. Comparative secretome analysis of Trichoderma asperellum S4F8 and Trichoderma reesei Rut C30 during solid-state fermentation on sugarcane bagasse. Biotech Biofuels. 2013;6:172. doi: 10.1186/1754-6834-6-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrozik A, Piotrowska-Seget Z. Bioaugmentation as a strategy for cleaning up of soils contaminated with aromatic compounds. Microbiol Res. 2010;165:363–375. doi: 10.1016/j.micres.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Nyns EJ, Auquiere JP, Wiaux AL. Taxonomic value of the property of fungi to assimilate hydrocarbons. Antonie Van Leeuwenhoek. 1968;34:441–457. doi: 10.1007/BF02046466. [DOI] [PubMed] [Google Scholar]
- Pope JM, Walker LW, Dubro D. On the ordering of N-alkane and N-alcohol solutes in phospholipid bilayer model membrane systems. Chem Phys Lipids. 1984;35:259–277. [Google Scholar]
- Ramos JL, Duque E, Gallegos MT, et al. Mechanisms of solvent tolerance in gram-negative bacteria. Ann Rev Microbiol. 2002;56:743–768. doi: 10.1146/annurev.micro.56.012302.161038. [DOI] [PubMed] [Google Scholar]
- Seo JS, Keum YS, Li QX. Bacterial degradation of aromatic compounds. Int J Environ Res Public Health. 2009;6:278–309. doi: 10.3390/ijerph6010278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewall TC, Mims CW, Timberlake WE. Conidium differentiation in Aspergillus nidulanswild-type and wet-white (wetA) mutant strains. Dev Biol. 1990;138:499–508. doi: 10.1016/0012-1606(90)90215-5. [DOI] [PubMed] [Google Scholar]
- Shier WT, Lao Y, Steele TW, et al. Yellow pigments used in rapid identification of aflatoxin-producing Aspergillus strains are anthraquinones associated with the aflatoxin biosynthetic pathway. Bioorg Chem. 2005;33:426–438. doi: 10.1016/j.bioorg.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Sikkema J, de Bont JA, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev. 1995;59:201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak HH, Lazorchak JM, Lei L, et al. Studies on bioremediation of polycyclic aromatic hydrocarbon-contaminated sediments: bioavailability, biodegradability, and toxicity issues. Environ Toxicol Chem. 2003;22:473–482. [PubMed] [Google Scholar]
- Tao L, Yu JH. AbaA and WetA govern distinct stages of Aspergillus fumigatus development. Microbiology. 2011;157:313–326. doi: 10.1099/mic.0.044271-0. [DOI] [PubMed] [Google Scholar]
- US-EPA Polycyclic Aromatic Hydrocarbons (PAHs) [online] 2008. [[accessed March 2013]]. Available from http://www.epa.gov/wastes/hazard/wastemin/minimize/factshts/pahs.pdf.
- Vasquez A. Estudio de la expresión de un gen suicida en Aspergillus niger para su aplicación en sistemas de biorremediación. Instituto Politécnico Nacional; Tlaxcala, México: 2010. M.Sc. Thesis. [Google Scholar]
- Wunder T, Kremer S, Sterner O, et al. Metabolism of the polycyclic aromatic hydrocarbon pyrene by Aspergillus niger SK 9317. Appl Microbiol Biotechnol. 1994;42:636–641. doi: 10.1007/BF00173932. [DOI] [PubMed] [Google Scholar]
- Yu JH, Butchko RA, Fernandes M, et al. Conservation of structure and function of the aflatoxin regulatory gene aflR from Aspergillus nidulans and A. flavus . Curr Genet. 1996;29:549–555. doi: 10.1007/BF02426959. [DOI] [PubMed] [Google Scholar]
- Zafra G, Absalón AE, Cuevas MC, et al. Isolation and Selection of a Highly Tolerant Microbial Consortium with Potential for PAH Biodegradation from Heavy Crude Oil-Contaminated Soils. Water Air Soil Poll. 2014;225:1826. [Google Scholar]
- Zajic JE, Kuehn HH. Biosynthesis of yellow pigments by Aspergillus niger . Mycopathol Mycol Appl. 1962;17:149–158. [Google Scholar]