Abstract
Native rhizobia are ideal for use as commercial legume inoculants. The characteristics of the carrier used to store the inoculants are important for the survival and symbiotic potential of the rhizobia. The objective of this study was to investigate the effects of peat (PEAT), perlite sugarcane bagasse (PSB), carboxymethyl cellulose plus starch (CMCS), and yeast extract mannitol supplemented with mannitol (YEMM) on the survival, nodulation potential and N2 fixation capacity of the native strains Sinorhizobium mexicanum ITTG R7T and Rhizobium calliandrae LBP2-1T and of the reference strain Rhizobium etli CFN42T. A factorial design (4 × 3) with four repetitions was used to determine the symbiotic potential of the rhizobial strains. The survival of the strains was higher for PEAT (46% for strain LBP2-1T, 167% for strain CFN42T and 219% for strain ITTG R7T) than for the other carriers after 240 days, except for CFN42T kept on CMCS (225%). All the strains kept on the different carriers effectively nodulated common bean, with the lowest number of nodules found (5 nodules) when CFN42T was kept on CMCS and with the highest number of nodules found (28 nodules) when ITTG R7T was kept on PSB. The nitrogenase activity was the highest for ITTG R7T kept on PEAT (4911 μmol C2H4 per fresh weight nodule h−1); however, no activity was found when the strains were kept on YEMM. Thus, the survival and symbiotic potential of the rhizobia depended on the carrier used to store them.
Keywords: rhizobia, inoculants, nodulation, nitrogen fixation, carrier
Introduction
The inoculation of the plant rhizosphere or seeds with rhizobia, i.e., N2-fixing bacteria, to stimulate plant growth has been used for a long time (Lopez-Lopez et al., 2010). Symbiosis between the N2-fixing bacteria and the plant reduces the need for inorganic N fertilizer applications. The use of native rhizobia has been recommended because these bacteria adapt easily to the specific environmental conditions, which facilitates their survival and the successful nodulation of the host plant (Romdhane et al., 2008; Topre et al., 2011).
The rhizobial inoculants are kept on a support or a carrier so that these bacteria can be stored for a long period and used when required (Albareda et al., 2008). The material used as a carrier should allow the survival of the rhizobia and preserve their capacity to form nodules and to fix N2. A high-grade carrier should have high water retention and stable pH, and it should be inexpensive, constitutive, nontoxic for the strain or environment and easy to sterilize (Swelim et al., 2010). Peat is one of the most commonly used carriers (Albareda et al., 2008), although other substrates have been used. For instance, sugarcane bagasse and perlite were tested as carriers for Bradyrhizobium japonicum strain CB1809 (Khavazi et al., 2007). When the carrier was stored at 4 °C for six months, the bacterial inoculant survival was high, and a density of 109 cells g−1 was maintained. Mixtures of carboxymethyl cellulose and starch maintained a stable cellular concentration of B. japonicum strain BR3267 when stored at 20–26 °C for 180 days (Fernandes-Júnior et al., 2009). A stable rhizobia population of Sinorhizobium fredii SMH12 was also obtained when the cells where kept in yeast extract mannitol supplemented with 1% mannitol (YEMM) at 25 °C for 100 days (Albareda et al., 2008).
While these carriers were able to maintain the rhizobia population, considering other factors, such as the stability and viability of the biofertilizer, which might vary between the strains used as biofertilizer, is necessary (Fernandes-Júnior et al., 2012). Studying how the different carriers affect the biological activity of microorganisms, the infectivity of rhizobia inoculants and the symbiotic potential of the strains used as inoculants is also desirable (Hungria et al., 2005). Therefore, the objective of the present study was to determine the effects of peat (PEAT) and perlite sugarcane bagasse (PSB) as solid carriers and of carboxymethyl cellulose plus starch (CMCS) and YEMM as liquid carriers on the survival, potential for nodulation of the common bean (Phaseolus vulgaris L.) and capacity for N2 fixation of the native strains Sinorhizobium mexicanum ITTG R7Tand Rhizobium calliandrae LBP2-1T and of the reference strain Rhizobium etli CFN42T.
Materials and Methods
Bacterial strains
The native rhizobia used in this study were S. mexicanum ITTG R7T (Lloret et al., 2007) and R. calliandraeLBP2-1T (Rincón-Rosales et al., 2013) obtained from the Instituto Tecnológico de Tuxtla Gutiérrez (Chiapas, Mexico). These strains are characterized by rapid growth, high potentials for nitrogen fixation and activity under saline conditions and high aluminum concentrations. The reference strain R. etli CFN42Twas provided by the Centro de Ciencias Genómicas (Cuernavaca, México). R. etli CFN42T, which has been used commercially in Mexico as an inoculant, is characterized by a high nodulation potential (Martínez-Romero, 2003). The strains were grown in yeast extract mannitol (YEM) medium (Vincent, 1970) at 28 °C and then stored at 4 °C until use.
Preparation of the carriers
The carriers used in this study were 1) PSB and 2) PEAT as solid carriers and 3) CMCS and 4) YEMM as liquid carriers. Sphagnum peat (PROMIX 1100, Quebec, Canada) was used as a reference carrier (Albareda et al., 2008). The carriers PSB and PEAT were first ground and then sieved (mesh size 100). The carrier PSB had a 4:1 (sugarcane bagasse:perlite) ratio (Khavazi et al., 2007). The pH of the peat was adjusted to 6.7 using 35 g Na2CO3 for each 100 g material. The carriers PSB and PEAT were sterilized at 121 °C for 20 min.
The CMCS carrier was prepared by mixing carboxymethyl cellulose and starch (ratio 60:40 w/w) and amended with MgO at 1% to achieve a final concentration 1.28% w/v and a pH of 6.7 (Fernandes-Júnior et al., 2009). The YEMM carrier was prepared with 1.0 g yeast extract, 0.1 g NaCl, 0.5 g K2HPO4, 0.2 g MgSO47H2O, and 20 g mannitol L−1 distilled water and adjusted to pH 6.7. Both carriers were sterilized at 121 °C for 15 min (Albareda et al., 2008).
The physical characteristics of the carriers were determined each time a sample of the rhizobia was taken. The pH and water holding capacity (WHC) were determined for the solid carriers PEAT and PSB (Somasegaran and Hoben, 1985), and the pH and viscosity were determined for the CMCS and YEMM carriers (Fernandes-Júnior et al., 2009).
Biofertilizer production
The biofertilizers combined the four carriers and the three strains ITTG R7T, LBP2-1T and CFN42T. Erlenmeyer flasks (2 L) containing 800 mL YEM broth (Albareda et al., 2008) were inoculated with 10% (v/v) of the corresponding microbial suspension and aired with 2 L min−1sterile air for 27 h. All the carriers were inoculated with 1.5 mL inoculant g−1 under sterile conditions, i.e., > 2 × 109 cells g−1. Twenty-five grams solid and 25 mL liquid biofertilizer were added to sterilized polypropylene bags (20 cm × 30 cm) and stored at 25 °C until use.
Rhizobial survival determination and biofertilization test
The number of viable rhizobia was determined by serial dilution and plate counting on YEM agar (pH 6.7) after 14, 60, 120, 180 and 240 days (Vincent, 1970). Seeds of the common bean (Phaseolus vulgaris L.) cultivar Jamapa previously impregnated with 40% Arabic gum (Sigma-Aldrich, St. Louis, MO, USA) were inoculated with PSB or PEAT containing 8 g inoculant 100 g−1 seed. Biofertilizers formulated with CMCS or YEMM were directly applied to the seeds at 8 mL inoculant 100 g−1 seed. The treated seeds were sown in plastic pots containing sterilized vermiculite. Four replicate pots were used and arranged in a completely randomized design. The plants were grown in a climate chamber at 25 °C using photoperiods of 16 h light/8 h darkness (Rincón-Rosales et al., 2009) and watered with a N-free nutritive Fahraeus solution (Fahraeus, 1957). The shoot dry weight, number of nodules and shoot N content were determined after 240 days. The N2-fixing capacity was determined using the acetylene reduction assay. Ethylene was determined using a Varian-3300 (Pampa, TX, USA) gas chromatograph fitted with a flame ionization detector (FID) and a Porapak N column (300 × 0.1 cm) at 50–70 °C using nitrogen as carrier gas (50 mL min−1) (Ruiz-Valdiviezo et al., 2009).
Statistical analysis
Significant differences between the shoot dry weight, number of nodules, nitrogenase activity and shoot nitrogen content of the legume P. vulgaris L. because of the application of the different rhizobia kept on different carriers were determined using analysis of variance (ANOVA) with Tukey’s test. Relationships between physical characteristics of the carriers and strain viability were determined using the Pearson correlation coefficients. All analyses were performed using StatGraphics Centurion version XV.2 software (Warrenton, Virginia, USA).
Results and Discussion
Physical characteristics of the carriers
The pH of the peat used in this study was 3.8. This carrier normally has a pH ranging from 3.5 to 4.5 (Somasegaran and Hoben, 1985). The pH was adjusted to 6.7 with 35 g Na2CO3 so that a near neutral pH was obtained, which maintains the viability of the rhizobia (Vincent, 1970). The WHC of peat in this study was 282%, which is higher than the 120% reported by Somasegaran and Hoben (1985). The origin of the peat might have resulted in a higher WHC (Tittabutr et al., 2007). The pH of the PSB was 7.7, which is appropriate for the growth of rhizobia (Albareda et al., 2008). The WHC of the PSB was 512%, which is higher than the value reported for perlite (400%) (Khavazi et al., 2007). The high WHC of both carriers favors the enzymatic processes involved in the degradation of the organic material that provide important nutrients such as phosphorus for the rhizobial bacteria.
The carrier CMCS, which is prepared by mixing carboxymethyl cellulose and starch, allowed the formation of a polymer with a viscosity of 414 cP. This viscosity favors the survival of the rhizobia. However, the initial pH was 10.8, which decreases the viability of the bacteria; thus, MgO was added. This addition adjusted the pH to 6.7, which is the pH recommended for rhizobia carriers (Fernandes-Júnior et al., 2009). The YEMM liquid carrier (pH 6.7) had a viscosity of only 1.11 cP. This result suggested that this liquid carrier might decrease the survival of the rhizobia as reported by Tittabutr et al. (2007) and by Albareda et al. (2008).
Survival of rhizobia strains in different carriers
The survival of strains S. mexicanum ITTG R7T, R. calliandrae LBP2-1T and R. etli CFN42T was determined in two solid and two liquid carriers kept at 25 °C for 240 days (Figure 1). The survival of the rhizobia strains differed between the carriers. After 240 days, the survival of the rhizobia was higher in the solid carriers PEAT and PSB than in the liquid carriers (Figure 1a and 1b). In the PEAT and PSB carriers, the cellular concentration of all strains remained at 2 × 109 cells g−1, which is recommended for the production of biofertilizers (Ben Rebah et al., 2007). PSB and PEAT contain high concentrations of organic material and essential nutrients (Khavazi et al., 2007), maintaining the viability of the rhizobia.
Figure 1. Survival of the ITTG R7T and LBP2-1T strains and of the reference strain CFN42T in the carriers a) PEAT, b) PSB, c) YEMM and d) CMCS stored at 25 °C for 240 days.
The cellular concentration of the R. etli CFN42Tstrain significantly decreased (p < 0.05) in the liquid carrier YEMM after 60 days (Figure 1c), while the cellular concentrations of the ITTG R7T and LBP2-1T strains remained at > 108 cells g−1. In the CMCS carrier, the strains maintained a viable population of > 108 bacteria g−1 until day 240 (Figure 1d), which is sufficient for use as biofertilizers (Fernandes-Júnior et al., 2009). The high survival of the rhizobia in this carrier might be due to its rheological and chemical characteristics, e.g., high viscosity and hygroscopicity. Flores da Silva et al. (2012) reported that survival of Gluconacetobacter diazotrophicus and Azospirillum amazonense was 109 cfu mL−1 in a medium of CMC-starch supplemented with MgO after 120 days.
Several researchers (e.g., Deaker et al., 2004; Fernandes-Júnior et al., 2009) reported that different physical factors affect the viability and survival of rhizobia in the carrier. In the present study, the pH, WHC and viscosity were monitored to determine their possible effects on the survival and viability of the rhizobia studied (Table 1). After 240 days, the pH of the YEMM liquid carrier decreased from 6.7 to 4.9 when inoculated with the CFN42T strain; to 6.4, with the ITTG R7T strain; and to 4.8, with the LBP2-1T strain. The viability of the CFN42T and ITTG R7T strains and their concentrations were negatively affected by a decrease in pH during storage, thus decreasing their symbiotic potentials.
Table 1. Viscosity (cP), WHC and pH of the carrier and viability (%) of strains R. etli CFN42T, S. mexicanum ITTG R7T, and R. calliandrae LBP2-1T kept on PEAT, PSB, CMCS, and YEMM for 240 days.
| Rhizobium etli CFN42T | Sinorhizobium mexicanum ITTG R7T | Rhizobium calliandrae LBP2-1T | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Carrier | Days | pH | Viscosity | Viability | pH | Viscosity | Viability | pH | Viscosity | Viability |
| YEMM | 0 | 6.7 Aa | 0.8 C | 100 A | 6.7 AB | 0.8 A | 100 A | 6.6 A | 0.8 A | 100 A |
| 14 | 5.5 B | 1.5 A | 12 B | 5.6 E | 0.9 A | 15 B | 5.8 AB | 1.1 A | 36 B | |
| 60 | 4.8 D | 0.9 BC | 5 C | 6.2 D | 1.1 A | 15 B | 5.7 AB | 1.2 A | 4 C | |
| 120 | 4.8 D | 0.8 C | 0 D | 6.5 BC | 1.2 A | 8 C | 5.3 BC | 1.2 A | 3 C | |
| 180 | 5.1 C | 0.9 BC | 0 D | 6.7 A | 1.5 A | 1 D | 6.1 A | 1.6 A | 1 C | |
| 240 | 4.9 CD | 1.0 B | 0 D | 6.4 CD | 1.5 A | 7 C | 4.8 C | 1.3 A | 1 C | |
| CMCS | 0 | 6.7 C | 190.5 A | 100 C | 6.8 B | 190.5 A | 100 A | 6.7 E | 190.5 A | 100 A |
| 14 | 6.7 C | 7.2 C | 295 B | 6.7 B | 16.7 BC | 30 B | 7.4 C | 24.3 BC | 16 B | |
| 60 | 6.7 C | 38.7 B | 269 B | 7.4 A | 33.2 B | 27 B | 7.7 B | 34.6 B | 20 B | |
| 120 | 7.1 AB | 9.0 C | 219 B | 7.6 A | 30.5 B | 6 C | 7.9 A | 30.6 BC | 4 C | |
| 180 | 7.4 A | 4.9 C | 507 A | 6.6 B | 7.7 C | 2 C | 7.7 AB | 21.3 BC | 5 C | |
| 240 | 7.6 A | 3.1 C | 225 B | 7.6 A | 7.7 C | 7 C | 7.2 D | 6.6 C | 16 B | |
|
| ||||||||||
| Carrier | Days | pH | WHC b | Viability | pH | WHC | Viability | pH | WHC | Viability |
|
| ||||||||||
| PSB | 0 | 7.7 A | 137 A | 100 A | 7.7 B | 123 A | 100 AB | 7.8 A | 134 A | 100 A |
| 14 | 7.5 B | 137 A | 206 A | 7.9 A | 123 A | 93 AB | 7.7 AB | 133 A | 29 CD | |
| 60 | 7.2 C | 113 AB | 106 A | 7.3 C | 150 A | 122 A | 7.0 D | 110 AB | 54 BC | |
| 120 | 7.2 C | 90 BC | 153 A | 7.3 C | 103 A | 114 AB | 7.0 D | 76 BC | 67 B | |
| 180 | 7.2 C | 67 C | 211 A | 7.3 C | 156 A | 71 B | 7.4 BC | 60 C | 37 CD | |
| 240 | 7.2 C | 23 D | 139 A | 7.3 C | 58 A | 21 C | 7.3 CD | 37 C | 13 D | |
| PEAT | 0 | 6.7 B | 120 A | 100 C | 6.7 D | 117 A | 100 C | 6.7 BC | 134 A | 100 A |
| 14 | 6.8 B | 120 A | 413 AB | 6.9 CD | 117 A | 116 C | 7.0 B | 134 A | 10 C | |
| 60 | 6.9 AB | 140 A | 492 A | 7.2 AB | 127 A | 204 AB | 6.7 C | 102 A | 37 BC | |
| 120 | 6.9 AB | 161 A | 553 A | 7.3 A | 107 A | 140 BC | 7.2 A | 113 A | 47 B | |
| 180 | 7.1 A | 122 A | 203 BC | 7.1 AB | 171 A | 163 ABC | 7.2 A | 93 A | 110 A | |
| 240 | 7.1 A | 102 A | 167 C | 7.1 BC | 109 A | 219 A | 7.3 A | 105 A | 46 B | |
Values with the same capital letter do not significantly differ over time, i.e., within the column (p < 0.05)
WHC: Water holding capacity (%).
Pearson correlation analysis showed that the pH and viscosity of the liquid carriers significantly affected the viability of strain LBP2-1T and that only the WHC of the PSB affected viability (p < 0.05) (Table 2). The viability of ITTG R7Tstrain significantly correlated with the viscosity of the liquid carrier and with the pH of the PEAT carrier (p < 0.05). The viability of the CFN42T strain significantly correlated only with the pH of the YEMM carrier and with the viscosity of the CMCS carrier (p < 0.05).
Table 2. Pearson’s correlation coefficient and p value between viability and viscosity, WHC or pH for the strains R. etliCFN42T, S. mexicanum ITTG R7T, and R. calliandrae LBP2-1Tkept on PEAT, PSB, CMCS, and YEMM for 240 days.
| Viability | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| R. etli CFN42T | S. mexicanum ITTG R7T | R. calliandrae LBP2-1T | |||||
|
|
|
|
|||||
| Carrier | R value | p value | R value | p value | R value | p value | |
| YEMM | pH | 0.969 | < 0.001 | 0.324 | 0.189 | 0.737 | < 0.001 |
| Viscosity | −0.280 | 0.261 | −0.560 | 0.019 | −0.498 | 0.036 | |
| CMCS | pH | 0.335 | 0.175 | −0.198 | 0.432 | −0.837 | < 0.001 |
| Viscosity | −0.609 | 0.007 | 0.942 | < 0.001 | 0.966 | < 0.001 | |
| PSB | pH | −0.323 | 0.191 | 0.053 | 0.835 | 0.361 | 0.141 |
| WHC | −0.084 | 0.740 | 0.254 | 0.310 | 0.495 | 0.037 | |
| PEAT | pH | −0.140 | 0.579 | 0.626 | 0.006 | 0.159 | 0.526 |
| WHC | 0.253 | 0.310 | −0.097 | 0.702 | −0.128 | 0.613 | |
Several strains of Rhizobium produce exopolysaccharides (EPSs), which facilitate symbiosis (Luque-Castellane et al., 2014). In the present study, EPS production was higher for the CFN42T strain than for the native ITTG R7T and LBP2-1T strains when kept in the YEMM carrier (data not shown). Rhizobial EPSs are composed of different types of monosaccharides and secreted into the environment. Rhizobium leguminosarum strains produce acidic EPSs that are composed of glucose, glucuronic acid and galactose, which may cause changes in the pH of the culture medium (Skorupska et al., 2006). Our results indicated that the exopolysaccharides produced by the rhizobia strains used in the present study decreased the pH, thereby reducing the viability of the bacteria in the carrier.
When the CMCS support was inoculated with the CFN42T, ITTG R7T or LBP2-1T strain, the pH was maintained between 6.7 and 7.2 (Table 1), which is suitable for the growth of rhizobia cells. In the CMCS carrier, the viscosity decreased significantly from 190.5 cP to 3.1 cP for the CFN42T strain, to 7.7 cP for ITTG R7T and to 6.6 cP for LBP2-1T (p < 0.05). These results indicated that the viscosity affected the viability of the ITTG R7 T and LBP2-1T strains, which could be because the rhizobial cells used the carrier as a C source (Fernandes-Júnior et al., 2009).
The WHC and pH are the factors that primarily affect the survival of rhizobia strains in carriers (Ben Rebah et al., 2002). In the present study, the pH remained stable in the inoculated PSB carrier. However, the WHC decreased significantly from an initial value of 137% to 23% when the CFN42T strain was added, to 58% when the ITTG R7T strain was added and to 37% when the LBP2-1T strain was added (Table 1). The high amount of water retained in the PSB carrier favored the enzymatic processes involved in the degradation of organic matter that generates nutrients required for bacterial growth (Ben Rebah et al., 2007).
Peat is the most commonly used carrier to store rhizobia used as biofertilizer (Khavazi et al., 2007; Kaira et al., 2010). This carrier is excellent for storing microorganisms because peat has a high WHC, chemical and physical uniformity, a lack of toxic compounds and no environmental risk (Ferreira and Castro, 2005; Ferreira et al., 2010). In the present study, the WHC values remained constant when peat was inoculated with the ITTG R7T strain; the viability of this strain increased significantly compared with the other evaluated strains during 240 days of storage (p < 0.05) (Table 1).
Plant growth, nodulation and nitrogen fixation of Phaseolus vulgaris inoculated with different biofertilizers
Plants treated with strain CFN42T stored in the CMCS carrier had the highest dry weight (0.83 g) (Figure 2). The CMCS carrier is a polymer blend, which favors the survival of the bacteria and the nodulation of different legumes, while the starch served as a C substrate (Fernandes-Júnior, 2009). Aeron et al. (2012)reported that Mucuna pruriens plants inoculated with Ensifer meliloti RMP6 and Bradyrhizobiumsp. BMP7T strains kept in a CMC carrier significantly increased plant biomass, the number of nodules and other plant growth parameters. The R. etli strain CFN42T, in combination with other diazotrophic bacteria, has a positive effect on the development of various legume and non-legume plants (Rosenblueth and Martinez-Romero, 2004).
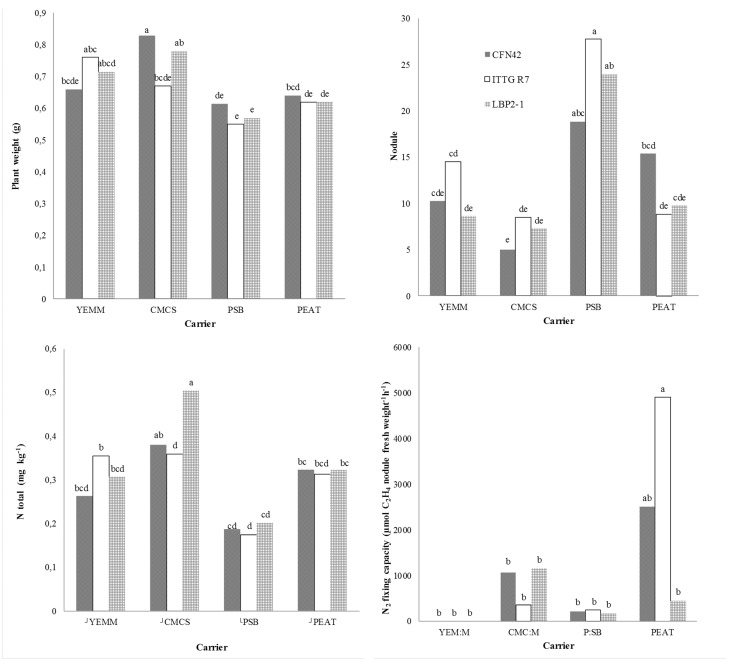
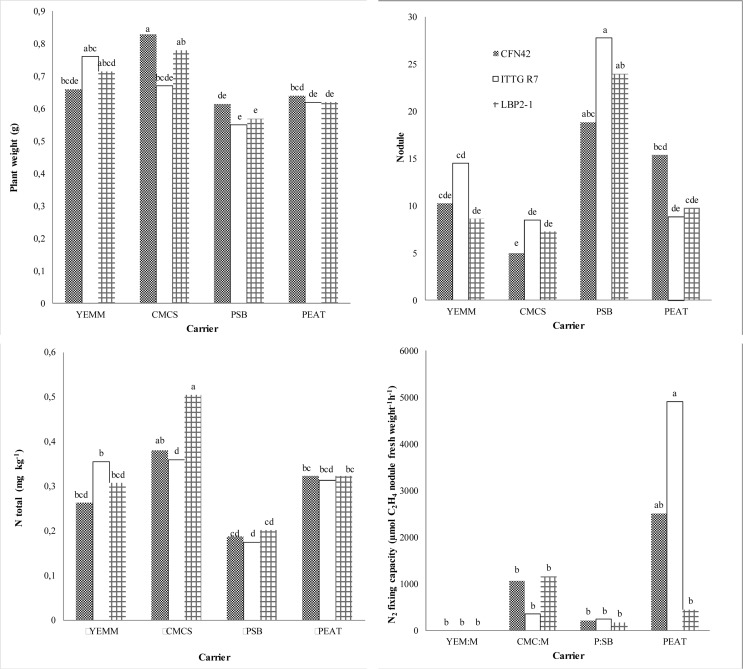
Figure 2. Plant growth, nodulation and nitrogen fixation of Phaseolus vulgaris cv. Jamapa inoculated with different rhizobial strains.
All the strains kept on the different carriers effectively nodulated bean plants; however, the number of nodules formed was highest when inoculated with strain ITTG R7T kept on the PSB carrier (p < 0.05). PSB is a carrier that provides stability to the bacterial cells and essential nutrients for symbiotic N2 fixation while maintaining cell viability during infection and nodule formation on the host plant (Khavazi et al., 2007). The N fixed by inoculated plants was significantly different between the treatments (p < 0.05). Plants inoculated with LBP2-1T kept on CMCS fixed the largest amount of N (0.50 mg kg−1). Rincón-Rosales et al. (2013) reported similar findings and found that the R. calliandrae strain LBP2-1Tisolated from the tropical legume Calliandra grandiflora grown in acidic soil and with high amounts of aluminum had a high potential for nodulation and nitrogen fixation.
The nitrogenase activity was higher in bean plants inoculated with the ITTG R7T strain kept on peat. As mentioned before, peat is a commonly used carrier due to its favorable characteristics (Khavazi et al., 2007; Ferreira et al., 2010). Lloret et al. (2007)reported that the strain ITTG R7T has high nitrogenase activity and nodulation capacity when used to inoculate P. vulgaris and other tropical legumes.
Conclusions
Peat and perlite sugarcane bagasse were shown to be the most appropriate carriers to store the rhizobial strains (2 × 109 cells g−1) and to maintain their survival. Strains Sinorhizobium mexicanum ITTG R7T, R. calliandrae LBP2-1T and R. etli CFN42T kept on perlite sugarcane bagasse induced the largest number of nodules in the common bean; however, their N2 fixation capacity was lower than when the strains were kept on peat. Consequently, no direct relationship existed between nodule formation in the host plant and N2 fixation capacity.
Acknowledgments
We thank M.A. Rogel-Hernández for technical assistance. This research was funded by Consejo Nacional de Ciencia y Tecnología (CONACyT, Mexico) and by Dirección General de Educación Superior Tecnológica (DGEST-SEP, Mexico). L.A.C.S. received grant-aided support from CONACyT.
References
- Albareda M, Rodríguez-Navarro DN, Camacho M, et al. Alternatives to peat as a carrier for rhizobia inoculants: Solid and liquid formulations. Soil Biol Biochem. 2008;40:2771–2779. [Google Scholar]
- Aeron A, Khare E, Arora NK, et al. Practical use of CMC-amended rhizobial inoculant for Mucuna pruriens cultivation to enhance the growth and protection against Macrophomina phaseolina . J Gen Appl Microbiol. 2012;58:121–127. doi: 10.2323/jgam.58.121. [DOI] [PubMed] [Google Scholar]
- Ben Rebah F, Tyagi RD, Prevost D. Wastewater sludge as a substrate for growth and carrier for rhizobia: the effect of storage conditions on survival of Sinorhizobium meliloti . Bioresour Technol. 2002;83:145–151. doi: 10.1016/s0960-8524(01)00202-4. [DOI] [PubMed] [Google Scholar]
- Ben Rebah F, Prevost D, Yezza A, et al. Agro-industrial waste materials and wastewater sludge for rhizobial inoculant production: a review. Bioresour Technol. 2007;98:3535–3546. doi: 10.1016/j.biortech.2006.11.066. [DOI] [PubMed] [Google Scholar]
- Deaker R, Roughley RJ, Kennedy IR. Legume seed inoculation technology a review. Soil Biol Biochem. 2004;36:1275–1288. [Google Scholar]
- Fahraeus G. The infection of clover root hair by nodule bacteria studied by a single glass slide technique. J Gen Microbiol. 1957;16:374–381. doi: 10.1099/00221287-16-2-374. [DOI] [PubMed] [Google Scholar]
- Fernandes-Júnior PI, Gusmão RT, Jansen de Oliveira P, et al. Polymers as carriers for rhizobial inoculant formulations. Pesqui Agropecu Bras. 2009;44:1184–1190. [Google Scholar]
- Fernandes-Júnior PI, da Silva Júnior EB, da Silva Júnior S, et al. Performance of polymer compositions as carrier to cowpea rhizobial inoculant formulations: Survival of rhizobia in pre-inoculated seeds and field efficiency. Afr J Biotechnol. 2012;11:2945–2951. [Google Scholar]
- Ferreira EM, Castro IV. Residues of the cork industry as carriers for the production of legumes inoculants. Silva Lusitana. 2005;13:159–167. [Google Scholar]
- Ferreira JS, Baldani JI, Baldani VLD. Selection of peats inoculants with diazotrophic bacteria in two rice varieties. Acta Scientiarum Agronomy. 2010;32:179–185. [Google Scholar]
- Flores da Silva M, de Souza AC, Jansen de Oliveira P, et al. Survival of endophytic bacteria in polymer-based inoculants and efficiency of their application to sugarcane. Plant Soil. 2012;356:231–243. [Google Scholar]
- Hungria M, Loureiro MF, Mendes JC, et al. Inoculant preparation, production and application. In: Werner D, Newton WE, editors. Nitrogen Fixation in Agriculture, Forestry, Ecology and the Environment. Springer; Netherlands: 2005. pp. 223–253. [Google Scholar]
- Kaira A, Chandra M, Awasthi A, et al. Natural compounds enhancing growth and survival of rhizobial inoculants in vermicompost-based formulations. Biol Fertil Soils. 2010;46:521–524. [Google Scholar]
- Khavazi K, Rejali F, Seguin P, et al. Effects of carrier, sterilization method, and incubation on survival of Bradyrhizobium japonicum in soybean (Glycine max L.) inoculants. Enzyme Microb Tech. 2007;41:780–784. [Google Scholar]
- Lloret L, Ormeño-Orrillo E, Rincón R, et al. Ensifer mexicanum sp. nov. a new species nodulating Acacia angustissima (Mill.) Kuntze in Mexico. Syst Appl Microbiol. 2007;30:280–290. doi: 10.1016/j.syapm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Lopez-Lopez A, Rogel MA, Ormeno-Orrillo E, et al. Phaseolus vulgaris seed-borne endophytic community with novel bacterial species such as Rhizobium endophyticum sp. nov. Syst Appl Microbiol. 2010;33:322–327. doi: 10.1016/j.syapm.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Luque-Castellane TC, Franco-Lemos MV, de Macedo-Lemos EG. Evaluation of the biotechnological potential of Rhizobium tropici strains for exopolysaccharide production. Carbohydr Polym. 2014;111:191–197. doi: 10.1016/j.carbpol.2014.04.066. [DOI] [PubMed] [Google Scholar]
- Martínez-Romero E. Diversity of Rhizobium-Phaseolus vulgarissymbiosis: overview and perspectives. Plant Soil. 2003;252:11–23. [Google Scholar]
- Rincón-Rosales R, Villalobos-Escobedo JM, Rogel MA, et al. Rhizobium calliandrae sp. nov., Rhizobium mayense sp. nov. and Rhizobium jaguaris sp. nov., rhizobial species nodulating the medicinal legume Calliandra grandiflora . Int J Syst Evol Microbiol. 2013;63:3423–3429. doi: 10.1099/ijs.0.048249-0. [DOI] [PubMed] [Google Scholar]
- Rincón-Rosales R, Lloret L, Ponce RE, et al. Rhizobia with different symbiotic efficiencies nodulate Acaciella angustissima in Mexico including Sinorhizobium chiapanecum sp. nov. that has common symbiotic genes with S. mexicanum . FEMS Microbiol Ecol. 2009;64:1–14. doi: 10.1111/j.1574-6941.2008.00590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romdhane SB, Aovani ME, Trabelsi M, et al. Selection of high nitrogen-fixing rhizobia nodulating chickpea (Cicer arietinum) for semi-arid Tunisia. J Agron Crop Sci. 2008;194:413–420. [Google Scholar]
- Rosenblueth M, Martínez-Romero E. Rhizobium etli maize populations and their competitiveness for root colonization. Arch Microbiol. 2004;181:337–344. doi: 10.1007/s00203-004-0661-9. [DOI] [PubMed] [Google Scholar]
- Ruiz-Valdiviezo VM, Ayora-Talavera TR, Gutiérrez-Miceli FA, et al. Effects of inorganic fertilizers and rhizobial inoculation on growth, nodulation and tannin content of Acaciella angustisima (Mill.) Britton & Rose. Gayana Bot. 2009;66:206–217. [Google Scholar]
- Skorupska A, Janczarek M, Marczak M, et al. Rhizobial exopolysaccharides: genetic control and symbiotic functions. Microb Cell Fact. 2006;5:1–19. doi: 10.1186/1475-2859-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasegaran P, Hoben HJ. Methods in legume-rhizobium technology. United States Agency for International Development; USA: 1985. [Google Scholar]
- Swelim DM, Nassef MA, Elkhatib EI. Survival and shelf life of leguminous trees rhizobia as affected by sterilization, culture dilution and maltose and trace elements enriched carrier. J Appl Sci Res. 2010;6:1366–1372. [Google Scholar]
- Tittabutr P, Payakapong W, Teaumroong N, et al. Growth, Survival and field performance of bradyrhizobial liquid inoculant formulations with polymeric additives. Sci Asia. 2007;33:69–77. [Google Scholar]
- Topre SD, Panikar SS, Mahajani SU, et al. Biofertilizer: A novel approach for agriculture. J Agric Biotech Sustain Dev. 2011;3:205–208. [Google Scholar]
- Vincent JM. A manual for the practical study of root nodule bacteria. Oxford: Blackwell Scientific Publications; 1970. [Google Scholar]