Abstract
Keratinolytic microorganisms have become the subject of scientific interest due to their ability to biosynthesize specific keratinases and their prospective application in keratinic waste management. Among several bacterial classes, actinobacteria remain one of the most important sources of keratin-degrading strains, however members of the Micrococcaceae family are rarely scrutinized in regard to their applicatory keratinolytic potential. The tested Micrococcus sp. B1pz isolate from poultry feather waste was identified as M. luteus. The strain, grown in the medium with 1–2% chicken feathers and a yeast extract supplement, produced keratinases of 32 KU and lower level of proteases, 6 PU. It was capable to effectively decompose feathers or “soft” keratin of stratum corneum, in contrast to other “hard” hair-type keratins. The produced keratinolytic enzymes were mainly a combination of alkaline serine or thiol proteases, active at the optimum pH 9.4, 55 °C. Four main protease fractions of 62, 185, 139 and 229 kDa were identified in the crude culture fluid. The research on the auxiliary role of reducing factors revealed that reducing sulfur compounds could be applied in keratinolysis enhancement during enzymatic digestion of keratin, rather than in culture conditions. The presented M. luteus isolate exhibits a significant keratinolytic potential, which determines its feasible applicatory capacity towards biodegradation of poultry by-products or formulation of keratin-based feed components.
Keywords: Micrococcus luteus, keratinase, keratin, biodegradation
Introduction
The arising worldwide issue of accumulating keratinic wastes, mainly as slaughterhouse by-products, triggers scientific interest in the means of bioutilization which involves a variety of keratinolytic microorganisms. This approach offers a constructive alternative to the currently employed bioconversion methods that involve energy-consuming techniques or toxic reagents. Numerous reports relate to the subject of actinobacterial keratinolytic enzymes, however, the vast majority focuses on members of the Streptomycetaceae family, where the Streptomyces genus remains a limitless source of profound keratinase producers (Syed et al., 2009; Jaouadi et al., 2010; Jain et al., 2012). Nevertheless, several less common actinobacteria are known to demonstrate a significant keratinolytic potential. Microbacterium sp. kr10, described by Thys et al.(2004) as growing in the presence of feather meal as a sole nutrient source, was reported to produce keratinolytic proteases at mesophilic temperatures. A detailed inquiry revealed a 42 kDa, Zn2+, Mg2+-containing metalloprotease with the highest specificity towards casein, albumin and keratin (Thys and Brandelli, 2006). The purified keratinase effectively hydrolyzed native keratin in the presence of 2-mercaptoacetate. As shown by Mitsuiki et al. (2002), the alkaliphilic Nocardiopsis sp. TOA-1, grown on a skim milk/yeast extract medium, biosynthesized a highly stable keratinolytic enzyme with an optimum activity at pH 11.0–11.5 and temperature of 70–75 °C. This 20 kDa serine protease exhibited a high specific activity towards keratin and lower towards casein. From among several screened actinobacteria of poultry farm origin, Saha et al.(2012) obtained a feather-degrading Nocardiopsis sp. SD5 capable to efficiently utilize keratin at 45–50 °C in highly alkaline conditions, within 4 days of culture on a starch/casein medium. The crude keratinase extract displayed an immense activity on native feathers. Zymographic analysis revealed the presence of two proteolytic fractions of 30 kDa and 60 kDa. There are also reports on novel keratinolytic actinobacteria assigned to genera Amycolatopsis or Actinomadura (Al-Musallam et al., 2003; Puhl et al., 2009). The ubiquitous bacteria of the genus Micrococcus are known producers of extracellular serine or thiol proteases (Mohedano et al., 1997; Odu and Akujobi, 2012), as well as elastases (Clark et al., 2000). Nevertheless, the true keratinolytic potential of Micrococcus bacteria is sparsely mentioned and underestimated. While some pathogenic strains are responsible for certain skin infections (Kaminska-Winciorek and Spiewak, 2011), other can serve as a valuable source of keratinolytic enzymes or contribute to keratin waste management (El-Fadaly and Zaied, 1999; Rodziewicz and Laba, 2005). The closely related Kocuria rosea, thoroughly investigated by Bernal et al. (2006b), is an example of an eminent producer of feather-induced keratinases. The strain biosynthesized keratinolytic and caseinolytic proteases in batch cultures with 3% feathers at 40 °C, in an optimized mineral medium supplemented with yeast extract. The unique features of K. roseaallowed the authors to design a fermentation process in order to enhance the biological value of waste feathers to serve as animal feed ingredient (Bertsch and Coello, 2005).
The microbial breakdown of keratins is known to be conducted not only by specific proteolytic enzymes but also to involve reducing factors responsible for the cleavage of disulfide bonds in the substrate. Sulfur compounds like sulfite or sulfide, as well as disulfide-reductase enzymes, are known to be a part of this process (Yamamura et al., 2002; Prakash et al., 2010; Cedrola et al., 2012). Therefore, it becomes essential to investigate the presence of reducing factors in the microbial growth environment, as well as the feasibility of supplementary disulfide reducers in keratinolysis improvement.
The aim of the presented study was to estimate the keratinolytic potential of Micrococcus sp. B1pz bacterium, through evaluation of keratinase production conditions, the capability of keratin biodegradation and preliminary characterization of proteases and keratinases in crude culture fluid, followed by investigation of the role of reducing factors in the keratinolysis enhancement.
Materials and Methods
Bacterial strain and molecular phylogenetic studies
The bacterial strain was isolated in previous studies (Rodziewicz and Laba, 2005) from feather waste at a poultry-processing facility near Wroclaw, Poland, and stored at the local culture collection of the Department of Biotechnology and Food Microbiology, Wroclaw University of Environmental and Life Sciences, Poland. Genomic DNA was extracted using GeneMATRIX Bacterial & Yeast Genomic DNA Purification Kit (Eurx) from 24-h liquid cultures on nutrient broth (glucose 10 g/L; nutrient broth 8 g/L). The 16S rRNA gene was amplified by the polymerase chain reaction (PCR) with the following universal primers: (27 F) AGAGTTTGATCGTGGCTCAG and (1492L R) GGTTACCTTGTTACGACT. The PCR reaction mixtures (50 μL) contained: 25 μL Taq PCR Master Mix (2x) (Eurx); 20 pmol each primer and genomic DNA 1 μg. The PCR was carried out with initial denaturation of 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 53 °C for 30 s, extension at 72 °C for 90 s and a final extension at 72 °C for 10 min. The PCR product was purified from reaction components and sequenced using primers: 27 F, 1492L R and an additional internal primer CTCCTACGGGAGGCAGCAG (357 F). The obtained sequence was subject to Ribosomal Database Project (RDP) release 10. Sequence alignment and phylogenetic study was performed using MAFT version 6 and Archaeopteryx version 0.972+.
Keratinic materials
Keratins used in the experiments were various skin appendages like white chicken feathers, barbs and rachea of white ostrich feathers, pig bristle, lamb wool, human hair and stratum corneum of epidermis (s.c.). The substrates were prepared by washing and degreasing with a methanol-chloroform solution (1:1).
Fermentation media and culture conditions
Microbial cultures were carried out in 250 mL Erlenmayer flasks, in 50 mL of medium, at the temperature of 30–45 °C, with 170 rpm shaking, for 4–15 days. Nutrient broth culture (glucose 1%, nutrient broth 0.8%) of 0.2 absorbance at 550 nm served as the inoculum, used in 1 mL per flask. The basal medium used in the study consisted of (%): MgSO40.1, KH2PO4 0.01, CaCl2 0.01, FeSO47H2O 0.001 supplemented with a yeast extract 0.05, optionally removed or replaced by peptone or glucose/ammonium chloride. The basic carbon and nitrogen source were whole, degreased, white chicken feathers (1–6%) or other keratinic materials (1%). The medium was set to pH 7.2 and sterilized by autoclaving at 121 °C for 20 min.
In order to determine the influence of reducing agents on keratinase production and feather degradation, the basal medium containing the yeast extract 0.05% and chicken feathers 1% was used, after supplementation with 1 mM of sodium sulfite, dithioerythritol or cysteine.
The influence of reducing agents on cell growth was tested in a Bioscreen C analyzer (Labsystems) in 0.3 mL of nutrient broth, at 30 °C, with the addition of 0.5, 1.0, 2.5 or 5.0 mM of sodium sulfite, dithioerythritol, cysteine, reduced glutathione or 2-mercaptoethanol. The lag-phase duration (lag), maximum specific growth rate (μmax) and maximum biomass (ODmax) were calculated from the obtained growth curves.
Enzymatic assays
All assays were performed in collected culture fluids, after removing feather debris by filtration through Whatman grade 2 filter paper and centrifugation at 10,000 g for 10 min at 4 °C. For the determination of disulfide reductase activity, the cell sediment was collected as well.
Keratinolytic activity on soluble keratin was determined in a mixture of keratin solution 2 mg/mL(0.5 mL), Tris-HCl buffer 0.05 M, pH 9.4 (0.48 mL), culture fluid (0.02 mL) and incubated at 55 °C for 15 min. The reaction was terminated with an addition of 1 mL trichloroacetic acid (TCA) 8%. The mixture was cooled for 30 min, centrifuged at 10,000g for 10 min and the absorbance was measured at the wavelength of 280 nm. One unit of keratinolytic activity (KU) was defined as the 0.01 increase of TCA-soluble products absorbance, per 1 mL of enzyme within 1 min. The soluble keratin was prepared according to Wawrzkiewicz et al. (1987), by dissolving feathers in boiling DMSO and precipitation with acetone (1:3 ratio).
Proteolytic activity was determined as described above, using casein solution 2% as a substrate, in a reaction at 45 °C, pH 8.6. One unit of proteolytic activity (PU) represented an absorbance increase of 0.01 per 1 mL of enzyme within 1 min (Laba and Rodziewicz, 2010).
Keratinolytic activity on native chicken feathers was determined in a mixture of 100 mg of finely cut feathers, Tris-HCl buffer 0.05 M, pH 9.4, containing CaCl2 5 mM and sodium azide 0.02% (8 mL) and culture fluid (2 mL), incubated for 4 hours at 55 °C. Optionally, reducing compounds at the final concentration of 1 mM were added: cysteine, sodium sulfite or dithioerythritol. The reaction was terminated with an addition of 10 mL TCA 8%. The mixture was cooled for 30 min, filtered through Whatman grade 2 filter paper and centrifuged at 10,000 g for 10 min. Absorbance was measured at the wavelength of 280 nm. One unit of keratinolytic activity on native feathers was defined as the 0.01 increase of TCA-soluble products absorbance, per 1 mL of enzyme within 1 h.
Glutathione reductase activity was assayed according to the method of Carlberg and Mennervik (1985) in a reaction on oxidized glutathione in the presence of NADPH, at 30 °C in Tris-HCl buffer 0.05 M, pH 7.0. Supernatant from the fourth day of culture, as well as cell homogenate or supernatant after its centrifugation served as enzyme sources. The cell homogenate was obtained by sonication of a buffer-washed and chilled cell suspension for 2 min in cycles of 0.5s / 0.5s. The activity was expressed in μmol of oxidized NADPH within 1 min by 1 mL (the culture fluid fraction) of culture fluid or by 1 g of dry biomass (cell homogenate fractions).
Analytical determinations
Sulfur compounds were assayed according to following methods: reduced thiols - the method of Ellman (Riener et al., 2002) with 5,5′-dithiobis-(2-nitrobenzoic) acid (DTNB); sulfate - the method of Kolmert et al. (2000) involving barium chloride; sulfite - the method described by Kletzin (1989)with the application of fuchsin and formaldehyde; thiosulfate - the method of Sörbo (1957) using potassium cyanide.
Protein concentration in culture fluids was determined according to the method of Lowry (1951) with Folin-Ciocalteu reagent and the free amino acids were assayed with 2,4,6-trinitrobenzenesulfonic acid (TNBS) according to Snyder and Sobocinski (1975).
Residual keratin was determined after removing the substrate with Whatman grade 2 filter paper and drying at 105 °C.
Characterization of proteases and keratinases in crude culture fluid
Optimum temperature for proteases and keratinases was determined over a range 30–60 °C with 5 °C interval in 0.1 M Tris-HCl buffer, pH 7.4. The influence of pH was tested over a range of pH 5–11 using 0.1 M Britton-Robinson universal buffer, at the optimum temperature. To determine the dominating catalytic type of proteases and keratinases in crude culture fluids, the reaction on casein or soluble keratin was performed at optimum conditions, preceded by 20 min pretreatment with the following inhibitors: PMSF (phenylmethylsulfhonyl fluoride), NEM (N-ethyl maleimide), EDTA (ethylenediaminetetraacetic acid, disodium salt) and activators: cysteine, CaCl2.
Zymography
Prior to zymographic analysis, the culture fluid was concentrated at the Labscale TFF System (Millipore) using a Pellicon XL 50 casette with Ultracel-10 PLCGC membrane (10 kDa cutoff). The concentrated culture supernatant was mixed with the sample buffer (Tris-HCl 0.32 M; pH 6.8; glycerol 48%; SDS 8%; bromophenol blue 0.06%) in a proportion of 6:4. Samples were loaded onto 8% polyacrylamide gel (5% staking gel) containing casein/gelatin 0.1%. Electrophoresis was performed at 20 mA for 5.5 h at 2 °C. After the run, the gel was washed twice with Triton-X 2.5%, once with incubation buffer (Tris-HCl buffer 0.05 M, pH 8.6, containing CaCl2 5 mM and sodium azide 0.02%) and incubated for 24 h at 30 °C in the same buffer. For band detection, the gel was stained with Coomassie blue and decolorized with methanol: acetic acid: water (50:10:40).
Results
Bacterial identification and molecular phylogenetic studies
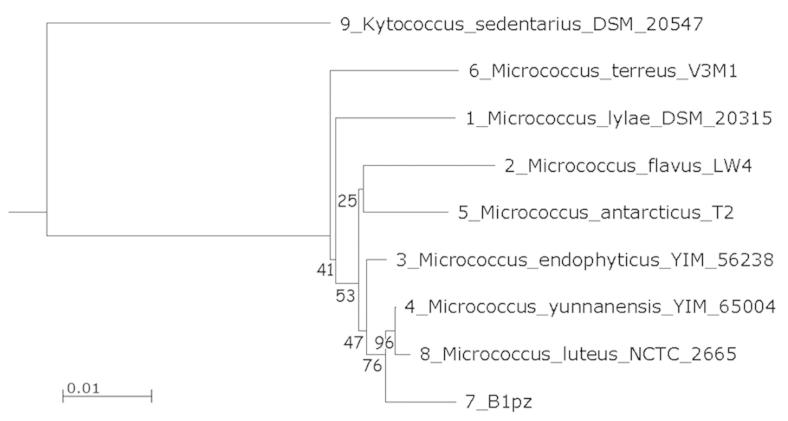
The isolate designated as B1pz in the previous studies was reidentified on molecular basis. Initial comparison of the 16S rDNA partial sequence against the RDP revealed a close relation with members of the genus Micrococcus, confirmed by submission to GenBank database showing high similarity of 100% with the strain M. luteus NCTC 2665. In the neighbor joining phylogenetic tree, the strain B1pz was closely related to a sub-branch of M. luteus NCTC 2665 and M. yunnanensis YIM 65004, with a high bootstrap value (Figure 1). The isolated strain was identified as Micrococcus luteus, a gram-positive, aerobic, non-sporeforming coccus, arranging into cell tetrads and producing yellow pigment.
Figure 1. Phylogenetic tree built with the neighbor-joining method based on 16S rRNA gene sequence. Bootstrap values are indicated at the relevant branching points (percent values from 1000 replicate bootstrap samplings). The bar representing evolutionary distance of 0.01. Kytococcus sedentarius DSM 20547 was used as an outgroup.
Keratinase production in feather broth medium
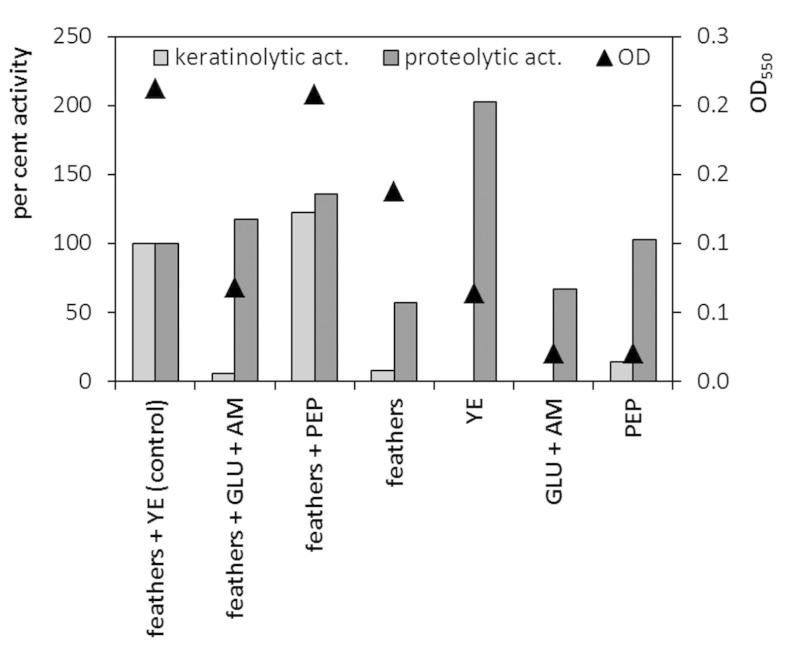
During the 15-day culture period, the M. luteus B1pz strain utilized the feather substrate as the main nutrient source in the medium, which was accompanied by the production of extracellular proteases. The production peak for both keratinolytic and caseinolytic activity, occurred between the fourth and fifth day of culture, with the maximum of 32.3 KU and 6.0 PU, respectively (Figure 2). The feather content in the culture medium posed an effect on the biosynthesis of the enzymes of interest, affecting substrate utilization. The level of keratinolytic activity was comparable for the substrate concentration of 1–2%, and a significant decrease in biosynthesis was observed above this level (Table 1). Nevertheless, slight stimulation of caseinolytic protease production occurred at 2% feather content. The presence of additional supplements to the feather medium was also a crucial factor for keratinase production. The addition of peptone resulted in 22% stimulation of the maximum keratinolytic activity, as compared to the control medium with feathers and yeast extract (Figure 3). Glucose caused nearly complete inhibition of keratinase production, but did not affect the proteolytic activity. The presence of the feather inducer appeared to be crucial for the biosynthesis of keratinases, but not for the caseinolytic proteases. The tested strain was, however, unable to produce a significant keratinase level in the absence of any supplement to the feather medium.
Figure 2. Production of keratinases, proteases and pH changes during culture of M. luteus B1pz in the chicken feather medium.
Table 1. Maximum keratinase and protease activity and the concentration of keratin hydrolysis products in cultures of M. luteus grown in the presence of 1–4% feathers.
| Feather content [%] | Keratinolytic activity [KU] | Proteolytic activity [PU] | pH | Protein [mg/mL] | Amino acid [mM] |
|---|---|---|---|---|---|
| 1 | 32.3 ±1.2 | 6.0 ± 0.3 | 9.25 | 1.24 | 1.70 |
| 2 | 34.0 ±7.2 | 19.3 ± 2.1 | 9.08 | 1.47 | 1.68 |
| 4 | 20.4 ±6.6 | 7.1 ± 3.2 | 9.05 | 0.38 | 0.17 |
Figure 3. Maximum keratinase and protease production and growth (as OD at 550 nm), in media with different supplements (0.05%), in the presence and absence of chicken feathers (1%): YE - yeast extract, GLU - glucose, AM - ammonium chloride, PEP - peptone. Medium with feathers and yeast extract served as a control (100% value).
Keratinolytic potential on different keratinic substrates
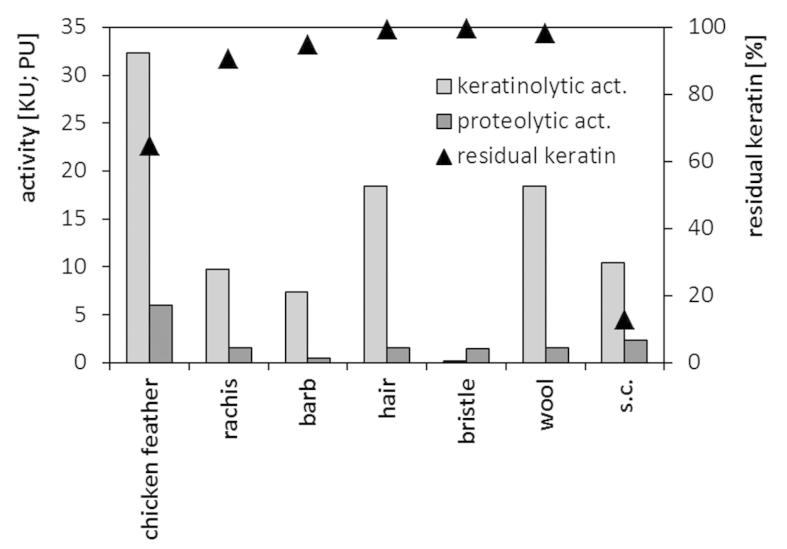
The M. luteus B1pz strain, besides its ability to effectively decompose chicken feather substrate, was also capable of growth and keratinase production in the presence of various keratinic materials. The maximum level of keratinolytic activity observed in cultures on human hair or lamb wool was approximately 50% lower, as compared to the chicken feather medium (Figure 4). The separated rachea and barbs of ostrich feathers proved to be much less effective keratinase inducers, while no keratinase activity was detected in the medium with pig bristle. The tested strains preferentially produced keratinolytic enzymes during the growth on chicken feathers than on other highly resilient “hard keratins”, which also reflected the extent of substrate decomposition reaching 35.3% within the short 4-day culture. The stratum corneum of epidermis, an example of “soft” cytokeratin-rich component, despite its high susceptibility to bacterial biodegradation, remained a moderate keratinase inducer for the tested strain.
Figure 4. Maximum keratinase and protease production and residual keratin during 4-day cultures in media containing different keratinic substrates (1.0%) and yeast extract (0.05%).
Preliminary characterization of keratinases and proteases in crude culture broth
Keratinases and proteases in crude culture fluids demonstrated overall optimum activity at pH 9.4, 55 °C and pH 8.6, 45 °C, respectively. The analysis with specific protease inhibitors revealed the dominating presence of serine proteases, due to the high sensitivity to PMSF, referring to both, activity on casein or soluble keratin. Nevertheless, the significant constituent of thiol-dependent proteases is highly probable, as verified by cysteine activation and sensitivity to NEM. Also the decrease of keratinolytic activity in the presence of EDTA, together with an immense activation with Ca2+ might suggest the presence of metalloproteases or other metal-dependent proteases in the tested culture fluid (Table 2). Keratinases in the cell-free crude culture fluid exhibited an activity towards native feather keratin, largely influenced by an addition of CaCl2 (data not shown). The measured activity was 3.5 ± 1.9 KU/h, while in the presence of CaCl2 0.5 mM it was 9.8 ± 0.2 KU/h (corresponding activity on soluble keratin without CaCl2: 32.3 KU).
Table 2. The effect of inhibitors and activators on keratinase and protease activity in the crude culture fluid of M. luteus B1pz.
| Inhibitor / activator | Concentration [mM] | Residual activity [%] | |
|---|---|---|---|
| Keratinolytic activity | PMSF | 10 | 28 |
| EDTA | 5 | 100 | |
| 10 | 30 | ||
| NEM | 10 | 0 | |
| Cys | 1 | 164 | |
| Ca2+ | 5 | 1606 | |
| Proteolytic activity | PMSF | 10 | 0 |
| EDTA | 5 | 100 | |
| 10 | 100 | ||
| NEM | 5 | 86 | |
| 10 | 23 | ||
| Cys | 1 | 586 | |
| Ca2+ | 10 | 814 |
Zymography
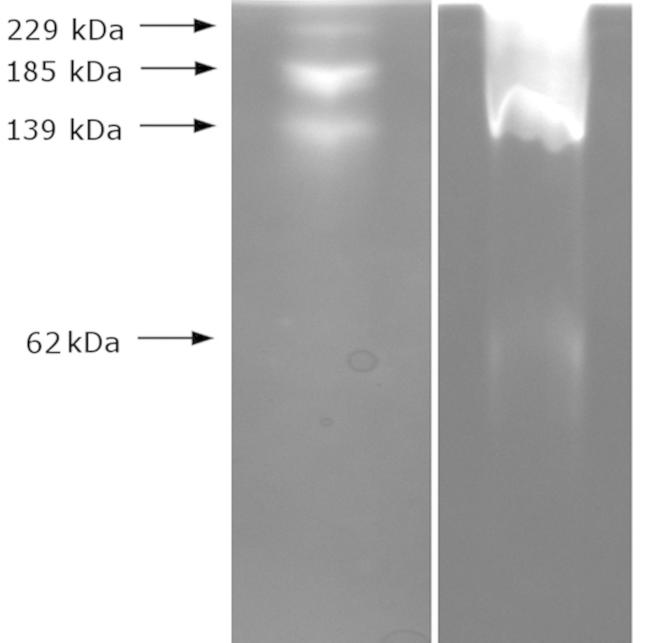
The zymographic analysis of the concentrated culture fluid of M. luteusB1pz using 8% SDS-polyacrylamide gel with copolymerized casein revealed the presence of three activity bands (Figure 5). Each of the peptidases exhibited high molecular weight of 229 kDa, 185 kDa and 139 kDa. Gelatin zymography exposed an additional activity band at 62 kDa.
Figure 5. Zymography of proteases in concentrated culture fluid of M. luteus B1pz on casein (left) and gelatin (right).
Accumulation of sulfur compounds during growth in feather broth medium
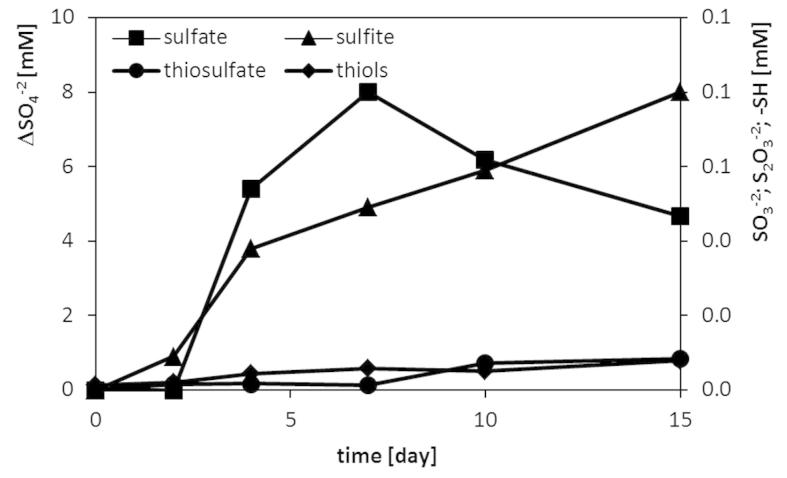
The microbial utilization of keratinic substrates is known to be supported by a reducing potential of chemical or enzymatic factors. Degradation of feathers in culture conditions and hydrolysis of cysteine-rich feather keratins resulted in the accumulation of sulfur compounds at different oxidation levels (Figure 6). The dominating sulfur form was sulfate, reaching the level of 7.8 mM above the concentration initially present in the medium composition, with its peak on the 7th day of culture, following the period of maximum keratinase production. The significant presence of sulfite at one fold lower concentration was also detected, and its liberation was of constantly increasing trend. The concentration of reduced thiols and thiosulfate remained at a relatively minor level, however mounting to the level of nearly 0.1 mM throughout the culture course.
Figure 6. Accumulation of sulfur compounds in culture of M. luteus B1pz in medium with feathers (1%) and yeast extract (0.05%).
Disulfide reductase activity
Disulfide reductase activity might serve as a factor supporting proteolytic cleavage of disulfide bridges in the keratinic substrate during bacterial growth. In the presented experiment, disulfide reductase activity could not be detected in the supernatant from the 4-day culture, where the maximum level of accumulated thiols and keratinolytic activity occurred. Nonetheless, the activity of 5.40 U was determined in the fraction of cell homogenate. After centrifugation of the supernatant, the activity declined to merely 0.32 U, implying the membrane-bound nature of the tested enzymes (data not shown).
Bacterial growth and keratinase production in the presence of reducing sulfur compounds
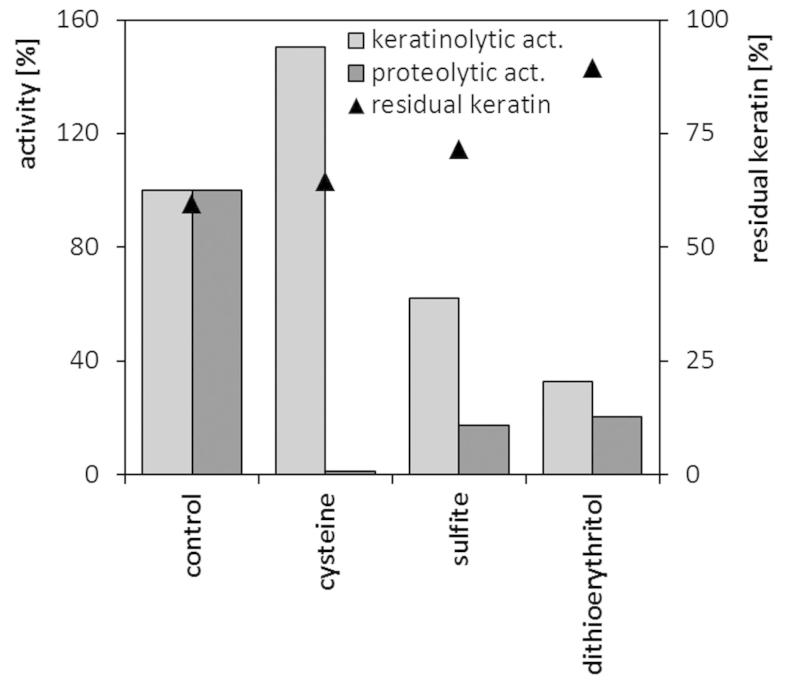
Reducing agents undoubtedly play a role in microbial keratinolysis, thus, the feasibility of introducing additional reducers into the culture environment or into a reaction mixture was analyzed. In microcultures in standard nutrient broth, cysteine at the concentration of 0.5–1 mM posed little effect on the maximum specific growth rate, as well as on the maximum biomass yield. On the contrary, 2-mercaptoethanol 1–5 mM or each compound at 5 mM concentration significantly impaired cell growth. (data not shown). Sulfite 1 mM and dithioerythritol 1 mM, despite elongation of lag phase and minor deterioration of growth rate, did not severely reduce strain biomass production (Table 3). The analysis of keratinase and protease production in flask cultures in the feather medium supplemented with 1 mM of selected reducing agents, revealed diverse effects (Figure 7). The level of proteolytic activity was significantly diminished in the case of each, sulfite, dithioerythritol and mainly cysteine. Production of keratinases was also negatively affected by sulfite, dithioerythritol, yet, the addition of cysteine resulted in 50% increase of keratinolytic activity. Nevertheless, none of the compounds at given concentration resulted in the enhancement of feather degradation.
Table 3. Growth of M. luteus B1pz on nutrient broth in the presence of reducing agents (1 mM).
| Reducing agent | Lag phase [h] | μmax [h−1] | ODmax |
|---|---|---|---|
| control | 2 | 0.198 | 1.868 |
| cysteine | 2.5 | 0.132 | 1.764 |
| sulfite | 3 | 0.084 | 1.619 |
| dithioerythritol | 6 | 0.097 | 1.694 |
| glutathione | 3 | 0.180 | 1.533 |
| 2-mercaptoeth. | 3 | 0.095 | 1.648 |
Figure 7. The effect of reducing agents (1 mM) on keratinase and protease production and keratin utilization during 4-day cultures of M. luteus B1pz in in medium with feathers (1.0%) and yeast extract (0.05%).
Influence of reducing sulfur compounds on the activity of keratinases on native feathers
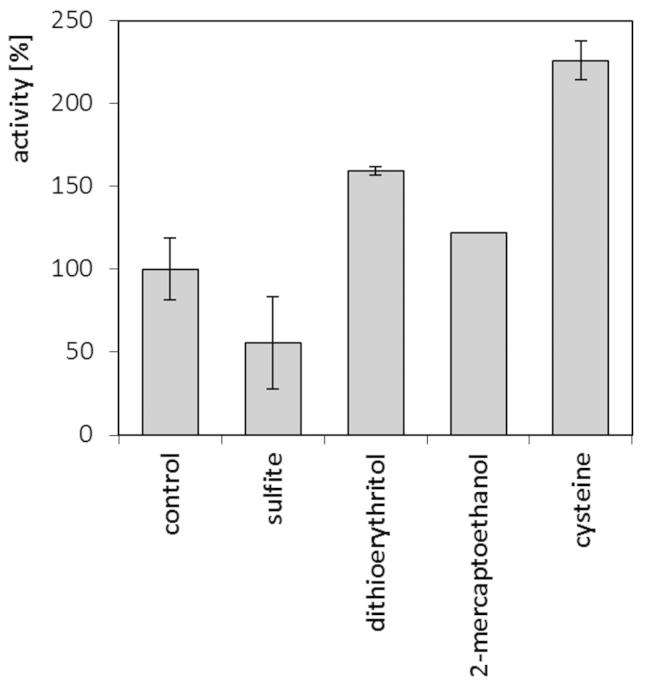
During the reaction on native feathers, involving cell-free crude culture fluid of M. luteus B1pz, tangible positive effects of additional reducing factors were observed (Figure 8). The presence of 1 mM cysteine resulted in the 125% stimulation of keratinolytic activity, followed by dithioerythritol and 2-mercaptoethanol, unlike sulfite, which negatively influenced keratin hydrolysis.
Figure 8. Activity of keratinases in crude culture fluid against native feathers in the presence of reducing agents (1 mM).
Discussion
A protease-producing bacterium, isolated from poultry feather waste, was phylogenetically characterized as a member of the Micrococcus luteus species and inquired for its keratinolytic potential. Its capability for effective feather keratin biodegradation was confirmed and the rare feature of higher overall activity of produced keratinases, as compared to caseinolytic proteases, was revealed. The highest keratinase production occurred in the presence of 1–2% feather keratin and the addition of a medium supplement like yeast extract or peptone was essential. This corresponds with the majority of reports concerning a variety of keratin-degrading actinobacteria and other bacteria, including Bacillus sp. Often, low amount of a proteinaceous supplement is required for initial growth support on hardly degradable keratin and increased concentration of the keratinous substrate generates unfavorable conditions for growth and keratinase production (Bernal et al., 2006b; Ni et al., 2011). It is, however, significant that M. luteus B1pz was capable of moderate growth and enzyme biosynthesis even in the medium with feathers as a sole nutrient source, which verifies its keratinolytic nature. Also, the protease production dynamics was comparable to that of Kocuria rosea (Vidal et al., 2000). The keratinolytic potential of the strain was also tested during brief 4-day cultures on various keratinic appendages. The hydrolytic action of M. luteus was directed against poultry feathers or the “soft” keratin of stratum corneum, rather than other “hard” keratins. The hair-type appendages, due to their extremely resilient structure, remained less prone to biodegradation, nevertheless still acted as effective keratinase inducers.
The keratinolytic and proteolytic enzymes of M. luteus B1pz appeared to be a combination of mainly serine and thiol proteases, of overall alkaline optimum. This resembles the case of K. rosea, from Micrococcaceaefamily, producing keratinases less sensitive to EDTA (Bernal et al., 2006a). The zymographic analysis revealed the presence of two major activity bands, 90 kDa and over 200 kDa, that correlate with the results obtained for M. luteus B1pz, revealing low m.w. protease fraction of 62 kDa and additional three proteases above 139 kDa. Further purification of a K. rosea keratinase allowed demonstrating a 240 kDa enzyme with an optimum activity at 40 °C and pH 10. The vast majority of bacterial keratinases belong to low m.w. serine proteinases (Brandelli, 2008; Talebi et al., 2013). Most often, high m.w. of proteolytic enzymes is restricted to homomultimeric structures, like in the case of Fervidobacterium islandicum > 200 kDa keratinase complex of 97 kDa subunits (Nam et al., 2002) or > 669 kDa of 31 kDa subunits in hyperthermophilic Thermotoga maritima (Hicks et al., 1998), however the keratinase of K. rosea remains a single protein fraction.
Different mechanisms were proposed to explain microbial decomposition of keratins, where cleavage of disulfide bonds prior to proteolytic breakdown is inherent. One mode is based on the sulfitolytic cleavage of cystine by means of sulfite, excreted by cells as an excess sulfur derived from keratin. It was described mainly in keratinolytic filamentous fungi and actinomycetes, but it is also possible in some bacteria (Kunert, 1989; Cedrola et al., 2012; Laba et al., 2013). The other mode involves direct reduction with specific reductase-like enzymes, leading to the accumulation of reduced thiols, which was confirmed for several bacterial strains (Yamamura et al., 2002; Ramnani et al., 2005; Kumar et al., 2008).
Keratinolytic potential of microbial isolates is usually considered in regard to both, keratinolysis in the growth environment and the activity of keratinases against keratinic substrates. The presence of reducing factors in the growth environment is often discussed as an influential factor in keratin utilization. For the tested M. luteus, grown in feather medium, the presence of disulfide reductase activity was confirmed, however mainly in the cell homogenate fraction, but not in the culture fluid. The membrane-bound location of reductases is most typical, as highlighted by Böckle and Möller (1997) for S. pactum or Ramnani et al. (2005) for B. licheniformis. Nevertheless, reports of Yamamura et al. (2002) and Prakash et al. (2010) for Stenotrophomonas sp. and B. halodurans, respectively, demonstrated extracellular disulfide reductases present during feather-broth cultivations. The cultures of M. luteus in feather medium were also tested for the level of sulfur compounds like sulfate, sulfite, thiosulfate and thiols. Sulfate, as the most oxidized form of sulfur excretion was produced at the highest concentration, however this compound does not play a role in sulfitolysis. In contrast sulfite, the metabolic precursor of sulfate, mounting up to a level of 0.8 mM throughout a 15-day culture, is known to be an important support for disulfide bonds reduction. A slightly reduced level of sulfite (0.15 mM) was reported by Cedrola et al. (2012) for 8-day cultures of B. subtilis SLC in a feather medium, whereas Ramnani et al. (2005) confirmed the presence of sulfite in cultures of B. licheniformis RG1.
Reducing sulfur compounds even at low concentrations may be harmful for cell metabolism, therefore the influence of these components supplementation on both, the cell growth and keratinase production, was tested. The analysis of microcultures in nutrient broth without feathers using the Bioscreen C, confirmed inhibitory effect of most sulfur compounds, however at the 1 mM concentration the level of inhibition was moderate, therefore acceptable for further inquiries. Consequently, selected reducing compounds at 1 mM: sulfite, cysteine and dithioerythritol were introduced into culture medium with feathers. Only cysteine stimulated keratinase production, which could however partially result from enzyme activation at the expense of lower proteolytic activity. The remaining agents caused significant impairment of keratinase and protease biosynthesis, along with feather utilization, leading to a conclusion that the degree of sulfitolysis in the substrate was ineffective, as compared with the harmful effect of reducing agents on microbial cells. Likewise, Cedrola et al. (2012)through an addition of 0.1–1% sulfite into cultures of B. subtilis achieved stimulation of gelatinase, but not keratinase production and a slight increase in feather degradation.
Additionally, an effect of reducing compounds was tested in enzymatic reactions on native feathers with crude, cell-free culture fluid. Significant enhancement of keratinolytic activity was achieved mainly in the presence of cysteine, but also 2-mercaptoethanol and dithioerythritol, due to the sulfitolytic effect or protease activation. Similar stimulation of keratin hydrolysis by cell-free keratinases is found in several reports, often considered as a compulsory condition for the complete substrate degradation (Suh and Lee, 2001; Cai et al., 2008; Cai and Zheng, 2009). In order to achieve effective keratin hydrolysis in the absence of microbial cells red-ox system, proteolytic action of keratinases requires a support of disulfide-reducing compounds (Ramnani et al., 2005; Rahayu et al., 2012). Reducing environment creates favorable conditions for keratin hydrolysis even with conventional, non-keratinolytic proteases, like subtilisin, Savinase, chymotrypsin or papain (Ramnani and Gupta, 2007).
Since the initial characterization of the M. luteus B1pz strain confirms its keratinolytic potential, mainly against raw feather, further studies are required to investigate specific proteases and detailed conditions of enzyme production. Nonetheless, the presented isolate poses a feasible applicatory capacity either towards biodegradation of poultry by-products or modification of quality value of keratins as proteinaceous feed ingredients.
Acknowledgments
Project supported by Wroclaw Centre of Biotechnology, programme The Leading National Research Centre (KNOW) for years 2014–2018.
References
- Al-Musallam AA, Al-Zarban SS, Fasasi YA, et al. Amycolatopsis keratiniphila sp. nov., a novel keratinolytic soil actinomycete from Kuwait. Int J Syst Evol Micr. 2003;53:871–874. doi: 10.1099/ijs.0.02515-0. [DOI] [PubMed] [Google Scholar]
- Bernal C, Cairó J, Coello N. Purification and characterization of a novel exocellular keratinase from Kocuria rosea . Enzyme Microb Tech. 2006;38:49–54. [Google Scholar]
- Bernal C, Diaz I, Coello N. Response surface methodology for the optimization of keratinase production in culture medium containing feathers produced by Kocuria rosea . Can J Microbiol. 2006;52:445–450. doi: 10.1139/w05-139. [DOI] [PubMed] [Google Scholar]
- Bertsch A, Coello N. A biotechnological process for treatment and recycling poultry feathers as a feed ingredient. Bioresour Technol. 2005;96:1703–1708. doi: 10.1016/j.biortech.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Böckle B, Möller R. Reduction of disulfide bonds by Streptomyces pactum during growth on chicken feathers. Appl Environ Microbiol. 1997;63:790–792. doi: 10.1128/aem.63.2.790-792.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandelli A. Bacterial keratinases: useful enzymes for bioprocessing agroindustrial wastes and beyond. Food Bioprocess Technol. 2008;1:105–116. [Google Scholar]
- Cai C, Chen J, Qi J, et al. Purification and characterization of keratinase from a new Bacillus subtilis strain. J Zhejiand Univ Sci B. 2008;9:713–720. doi: 10.1631/jzus.B0820128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C, Zheng X. Medium optimization for keratinase production in hair substrate by a new Bacillus subtilis KD-N2 using response surface methodology. J Ind Microbiol Biotechnol. 2009;36:875–883. doi: 10.1007/s10295-009-0565-4. [DOI] [PubMed] [Google Scholar]
- Carlberg I, Mannervik B. Glutathione reductase. Meth Enzymol. 1985;113:484–490. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- Cedrola SML, de Melo ACN, Mazotto AM, et al. Keratinases and sulfide from Bacillus subtilis SLC to recycle feather waste. World J Microbiol Biotechnol. 2012;28:1259–1269. doi: 10.1007/s11274-011-0930-0. [DOI] [PubMed] [Google Scholar]
- Clark DJ, Hawrylik SJ, Kavanagh E, et al. Purification and characterization of a unique alkaline elastase from Micrococcus luteus . Protein Expres Purif. 2000;18:46–55. doi: 10.1006/prep.1999.1166. [DOI] [PubMed] [Google Scholar]
- El-Fadaly H, Zaied KA. Microbial degradation of native keratin in batch fermentation. Pak J Biol Sci. 1999;2:627–634. [Google Scholar]
- Hicks PM, Rinker KD, Baker JR, et al. Homomultimeric protease in the hyperthermophilic bacterium Thermotoga maritima has structural and amino acid sequence homology to bacteriocins in mesophilic bacteria. FEBS Lett. 1998;440:393–398. doi: 10.1016/s0014-5793(98)01451-3. [DOI] [PubMed] [Google Scholar]
- Jain R, Jain PC, Agrawal SC. Feather degradation by Strptomyces exfoliates CFS 1068. Ann Microbiol. 2012;62:973–978. [Google Scholar]
- Jaouadi B, Abdelmalek B, Fodil D, et al. Purification and characterization of a thermostable keratinolytic serine alkaline proteinase from Streptomyces sp. strain AB1 with high stability in organic solvents. Bioresour Technol. 2010;101:8361–8369. doi: 10.1016/j.biortech.2010.05.066. [DOI] [PubMed] [Google Scholar]
- Kaminska-Winciorek G, Spiewak R. Pitted keratolysis - how to treat? Pol Merk Lek. 2011;182:127–129. [in polish] [PubMed] [Google Scholar]
- Kletzin A. Coupled enzymatic production of sulfite, thiosulfate and hydrogen sulfide from sulfur: purification and properties of a sulfur oxygenase reductase from the facultatively anaerobic archaebacterium Desulfurolobus ambivalens . J Bacteriol. 1989;171:1638–1643. doi: 10.1128/jb.171.3.1638-1643.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolmert A, Wikström P, Hallberg KB. A fast and simple turbidimetric method for the determination of sulfate in sulfate-reducing bacterial cultures. J Microbiol Meth. 2000;41:179–184. doi: 10.1016/s0167-7012(00)00154-8. [DOI] [PubMed] [Google Scholar]
- Kumar AG, Swarnalatha S, Gayathri S, et al. Characterization of an alkaline active - thiol forming extracellular serine keratinase by the newly isolated Bacillus pumilus . J Appl Microbiol. 2008;104:411–419. doi: 10.1111/j.1365-2672.2007.03564.x. [DOI] [PubMed] [Google Scholar]
- Kunert J. Biochemical mechanism of keratin degradation by the actinomycete Streptomyces fradiae and the fungus Microsporum gypseum: A comparison. J Basic Microbiol. 1989;29:597–604. [Google Scholar]
- Laba W, Choinska A, Rodziewicz A. The release of sulfur compounds during degradation of feather keratin by two Bacillus strains. Acta Sci Pol Biotechnol. 2013;12:29–40. [Google Scholar]
- Laba W, Rodziewicz A. Keratinolytic potential of feather-degrading Bacillus polymyxa and Bacillus cereus . Pol J Environ Stud. 2010;19:371–378. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–273. [PubMed] [Google Scholar]
- Mitsuiki S, Sakai M, Moriyama Y, et al. Purification and some properties of a keratinolytic enzyme from an alkaliphilic Nocardiopsis sp. TOA-1. Biosci Biotechnol Biochem. 2002;66:164–167. doi: 10.1271/bbb.66.164. [DOI] [PubMed] [Google Scholar]
- Mohedano AF, Fernandez J, Gaya P, et al. Effect of pH, temperature and culture medium composition on the production of an extracellular cysteine proteinase by Micrococcus sp. INlA 528. J Appl Microbiol. 1997;82:81–86. doi: 10.1111/j.1365-2672.1997.tb03300.x. [DOI] [PubMed] [Google Scholar]
- Nam GW, Lee DW, Lee HS, et al. Native-feather degradation by Fervidobacterium islandicumAW-1, a newly isolated keratinase-producing thermophilic anaerobe. Arch Microbiol. 2002;178:538–547. doi: 10.1007/s00203-002-0489-0. [DOI] [PubMed] [Google Scholar]
- Ni H, Chen Q, Chen F, et al. Improved keratinase production for feather degradation by Bacillus licheniformis ZJUEL31410 in submerged cultivation. Afr J Biotechnol. 2011;10:7236–7244. [Google Scholar]
- Odu NN, Akujobi CO. Protease production capabilities of Micrococcus luteus and Bacillus species isolated from abattoir environment. J Microbiol Res. 2012;2:127–132. [Google Scholar]
- Prakash P, Jayalakshmi SK, Sreeramulu K. Purification and characterization of extreme alkaline, thermostable keratinase, and keratin disulfide reductase produced by Bacillus halodurans PPKS-2. Appl Microbiol Biotechnol. 2010;87:625–633. doi: 10.1007/s00253-010-2499-1. [DOI] [PubMed] [Google Scholar]
- Puhl AA, Selinger LB, McAllister TA, et al. Actinomadura keratinilytica sp. nov., a keratin-degrading actinobacterium isolated from bovine manure compost. Int J Syst Evol Micr. 2009;59:828–834. doi: 10.1099/ijs.0.003640-0. [DOI] [PubMed] [Google Scholar]
- Rahayu S, Syah D, Suhartono MT. Degradation of keratin by keratinase and disulfide reductase from Bacillus sp. MTS of Indonesian origin. Biocatal Agric Biotechnol. 2012;1:152–158. [Google Scholar]
- Ramnani P, Gupta R. Keratinases vis-à-vis conventional proteases and feather degradation. World J Microbiol Biotechnol. 2007;23:1537–1540. [Google Scholar]
- Ramnani P, Singh R, Gupta R. Keratinolytic potential of Bacillus licheniformis RG1: structural and biochemical mechanism of feather degradation. Can J Microbiol. 2005;51:191–196. doi: 10.1139/w04-123. [DOI] [PubMed] [Google Scholar]
- Riener CR, Kada G, Gruber HJ. Quick measurement of protein sulfhydryls with Ellman’s reagent and with 4,4′-dithiodipyridine. Anal Bioanal Chem. 2002;373:266–276. doi: 10.1007/s00216-002-1347-2. [DOI] [PubMed] [Google Scholar]
- Rodziewicz A, Laba W. Biological degradation of feather keratin by saprophytic bacteria. Pol J Chem Technol. 2005;7:46–49. [Google Scholar]
- Saha S, Dhanasekaran D, Shanmugapriya S, et al. Nocardiopsis sp. SD5: A potent feather degrading rare actinobacterium isolated from feather waste in Tamil Nadu, India. J Basic Microbiol. 2012;52:1–10. doi: 10.1002/jobm.201100105. [DOI] [PubMed] [Google Scholar]
- Snyder SL, Sobocinski PZ. An improved 2,4,6-trinitrobenzenesulfonic acid method for the determination of amines. Anal Biochem. 1975;64:284–288. doi: 10.1016/0003-2697(75)90431-5. [DOI] [PubMed] [Google Scholar]
- Sörbo B. A colorimetric method for the determination of thiosulfate. Biochim Biophys Acta. 1957;23:412–416. doi: 10.1016/0006-3002(57)90346-3. [DOI] [PubMed] [Google Scholar]
- Suh HJ, Lee HK. Characterization of a keratinolytic serine protease from Bacillus subtilis KS-1. J Prot Chem. 2001;20:165–169. doi: 10.1023/a:1011075707553. [DOI] [PubMed] [Google Scholar]
- Syed DG, Lee JC, Li WJ, et al. Production, characterization and application of keratinase from Streptomyces gulbargensis . Bioresour Technol. 2009;100:1868–1871. doi: 10.1016/j.biortech.2008.09.047. [DOI] [PubMed] [Google Scholar]
- Talebi M, Emtiazi G, Sepahy AA, et al. Zymogram analysis of alkaline keratinase produced by nitrogen fixing Bacillus pumilus ZED17 exhibiting multiprotease enzyme activities. Jundishapur J Microbiol. 2013;6:e7974. [Google Scholar]
- Thys RCS, Brandelli A. Purification and properties of a keratinolytic metalloprotease from Microbacterium sp. J Appl Microbiol. 2006;101:1259–1268. doi: 10.1111/j.1365-2672.2006.03050.x. [DOI] [PubMed] [Google Scholar]
- Thys RCS, Lucas FS, Riffel A, et al. Characterization of a protease of a feather-degrading Microbacterium species. Lett Appl Microbiol. 2004;39:181–186. doi: 10.1111/j.1472-765X.2004.01558.x. [DOI] [PubMed] [Google Scholar]
- Vidal L, Christen P, Coello MN. Feather degradation by Kocuria rosea in submerged culture. World J Microbiol Biotechnol. 2000;16:551–554. [Google Scholar]
- Wawrzkiewicz K, Lobarzewski J, Wolski T. Intracellular keratinase of Trichophyton gallinae . J Med Vet Mycol. 1987;25:261–268. [PubMed] [Google Scholar]
- Yamamura S, Morita Y, Hasan Q, et al. Keratin degradation: a cooperative action of two enzymes from Stenotrophomonas sp. Biochem Biophys Res Com. 2002;294:1138–114. doi: 10.1016/S0006-291X(02)00580-6. [DOI] [PubMed] [Google Scholar]