Abstract
Pyrethroid pesticide cypermethrin is a environmental pollutant because of its widespread use, toxicity and persistence. Biodegradation of such chemicals by microorganisms may provide an cost-effective method for their detoxification. We have investigated the degradation of cypermethrin by immobilized cells of Micrococcus sp. strain CPN 1 in various matrices such as, polyurethane foam (PUF), polyacrylamide, sodium alginate and agar. The optimum temperature and pH for the degradation of cypermethrin by immobilized cells of Micrococcus sp. were found to be 30 °C and 7.0, respectively. The rate of degradation of 10 and 20 mM of cypermethrin by freely suspended cells were compared with that of immobilized cells in batches and semi-continuous with shaken cultures. PUF-immobilized cells showed higher degradation of cypermethrin (10 mM and 20 mM) than freely suspended cells and cells immobilized in other matrices. The PUF-immobilized cells of Micrococcus sp. strain CPN 1 were retain their degradation capacity. Thus, they can be reused for more than 32 cycles, without losing their degradation capacity. Hence, the PUF-immobilized cells of Micrococcus sp. could potentially be used in the bioremediation of cypermethrin contaminated water.
Keywords: degradation, immobilization, cypermethrin, polyurethane foam, Micrococcus sp. strain CPN 1
Introduction
Cypermethrin is a synthetic pyrethroid pesticide widely used to control pests in cotton, fruits, and vegetable crops, (Tullur et al., 2008; Chen et al., 2012). In recent years, the use of cypermethrin has been increased sharply in the agricultural field for crop protection (Weston et al., 2009). As a result, cypermethrin residues were found in most of the tested samples (sediment) from urban creek (Riederer et al., 2010; Weston et al., 2009, 2011). Hence, the presence of cypermethrin in the environment is of global concern. Cypermethrin has been shown to be genotoxic, neurotoxic, immunotoxic and carcinogenic to mammals, including humans (Ansari et al., 2011; Jin et al., 2011; McKinlay et al., 2008; Wang et al., 2011; Wolansky and Harrill, 2008; Zhang et al., 2010). There are reports of degradation of cypermethrin by the free cells of microorganisms (Roberts and Standen, 1981; Jilani and Khan, 2006; Tallur et al., 2008; Zhang et al., 2010; Lin et al., 2011; Chen et al., 2012). In addition, degradation of cypermethrin by physico-chemical methods such as oxidation with ozone, photolysis, ultrasonic degradation, Fenton degradation, incineration and adsorption has also been investigated (Segal-Rosenheimer and Dubowski, 2007; Xie et al., 2011). However, these methods were found to be more expensive and less-effective than biological systems used for the remediation of toxic pollutants (Yang et al., 2011). In nature, cypermethrin may be degraded through several ways, including hydrolysis, volatilization, photolysis, and aerobic degradation by microorganisms. There is a report on the enhanced degradation of cypermethrin by mixed microbial culture (Chen et al., 2012). The use of freely suspended cells for the degradation of various toxic/hazardous compounds for industrial applications has a number of disadvantages. It is mostly because of low mechanical strength, low density of cell population and the difficulty in biomass effluent separation (Massalha et al., 2007; Wang and Hu, 2007). Immobilization techniques have now been well established to overcome these problems (Cassidy et al., 1996). Immobilized microbial cells have more advantages than freely suspended cells as well as enzymes immobilized in various matrices (Zheng et al., 2009). Moreover, the immobilizing materials like polyurethane foam (PUF), agar and sodium alginate are inert, non toxic to cells, inexpensive and practical (Mulla et al., 2012). Use of immobilized cells permit the operation of bioreactors at flow rates that are independent of the microorganisms, tolerate higher concentrations of toxic compounds than do their non immobilized counter parts (Westmeier et al., 1987; Zheng et al., 2009). The calcium alginate beads act like a slow release delivery system, where the substrate is slowly released from culture medium to the cells for microbial mineralization without a significant impact on the surrounding environment. There are several reports on the potential use of immobilized bacterial cells in different matrices for the degradation of numerous toxic/hazardous chemicals (Chen et al., 2002; Tallur et al., 2009; Ullah et al., 2010; Mulla et al., 2012; Ali et al., 2013; Mulla et al., 2013; Hoskeri et al., 2014). However, less information is available on biodegradation of cypermethrin by immobilized cells of microorganisms
Previously, we have reported the degradative pathway of cypermethrin in Micrococcus sp. strain CPN 1 (Tallur et al., 2008). In this paper, we describe the enhanced degradation of cypermethrin by the cells of Micrococcussp. strain CPN 1 immobilized in sodium alginate (SA), agar, polyacrylamide and polyurethane foam (PUF). The rate of degradation of cypermethrin by the cells immobilized in various matrices were compared with that of freely suspended cells of Micrococcus sp. strain CPN 1 in batches and semi-continuous degradation.
Materials and Methods
Chemicals
Cypermethrin with 98% purity was purchased from Sigma-Aldrich. Sodium alginate, acrylamide, bis-acrylamide, ammonium persulphate with 99% purity were purchased from Himedia, India. Polyurethane foam (PUF) was obtained from local suppliers. All other chemicals used in this study were of analytical grade.
Organism
Micrococcus sp. strain CPN 1 previously isolated and identified in our laboratory by cypermethrin enrichment culture technique was used in this study (Tallur et al., 2008). The organism was maintained on the slants of cypermethrin-mineral salts medium solidified with 2% agar (wt/vol).
Medium
Two different media were used in this study. The medium used for precultivation of Micrococcus sp. strain CPN 1 was a mineral salts medium (MM 1) containing (g L−1) K2HPO4, 6.30; KH2PO4, 1.82; NH4NO3, 1.00; MgSO4.7H2O, 0.20; CaCl2.2H2O, 0.10; Na2MoO4.2H2O, 0.06; MnSO4.H2O, 0.06 and FeSO4.7H2O, 0.10 (Tallur et al., 2008). The pH of the medium was adjusted to 7.0. One hundred mL aliquots of this medium were transferred into 500 mL Erlenmeyer flask and sterilized by autoclaving at 121 °C for 15 min. Cypermethrin (10 mM) dissolved in ethyl acetate (solvent) was sterilized by membrane filtration, added to the medium and kept on a rotary shaker for solvent evaporation. After two days, the organism was inoculated into the sterilized medium containing cypermethrin (10 mM) and this process was followed for all experiments.
The medium (MM 2) used for cypermethrin degradation studies contained (gL−1) K2HPO4, 6.30; MgSO4.7H2O, 0.20; CaCl2.2H2O, 0.20; NH4NO3, 1.0 and FeCl3, 0.05 (Tallur et al., 2009). The pH of the medium was adjusted to 7.0 and sterilized by autoclaving. Cypermethrin (10 mM and 20 mM) dissolved in ethyl acetate was added after sterilization of the medium. The cultures were incubated on a rotary shaker (150 rpm) at 30 °C. The bacterial cell concentrations were measured by the plate-count method (Mulla et al., 2013).
Immobilization of whole cells in various matrices
The cypermethrin-degrading organism (Micrococcus sp. strain CPN 1) was harvested during the mid-logarithmic growth phase from 4 L of culture medium. The cell suspension (3 × 1012 cfu/mL) was obtained by centrifugation at 8,000 × g for 10 min at 4 °C and washed thrice with 50 mM phosphate buffer pH 7.0. The washed cells were immobilized in different matrices namely; Polyurethane foam (PUF), polyacrylamide, sodium alginate (SA) and agar (Mulla et al., 2013).
Preparation of PUF-entrapment: The PUF was cut into approximately 5 mm cubes, washed thrice with sterile double distilled water and dried. 100 mL of bacterial cell suspensions (3 × 1012 cfu/mL) were added to 500 mL conical flasks containing sterilized foam cubes (5 g). The content of the flasks were mixed by stirring on magnetic stirrer for 2 h and then the flasks were kept on a rotary shaker for 1 h at 150 rpm. The flasks were then allowed to stand undisturbed for additional 4 h. The medium was removed and foam cubes containing the immobilized bacteria were washed with saline. The decanted bacterial suspension and the saline wash were combined and the bacterial population in the mixture was counted by the plate-count method.
Preparation of sodium alginate (SA) entrapment: Sodium alginate (4%, wt/vol) was dissolved in boiling water and autoclaved at 121 °C for 15 min. 50 mL of bacterial cell suspension (18 g wet weight/ 50 mL sterilized SA solution) was added to 200 mL sterilized sodium alginate solution (4%, wt/vol) and mixed by stirring on a magnetic stirrer. This sodium alginate cell mixture was extruded drop by drop cold, sterile CaCl2 (0.2 M) solution. Gel beads of approximately 4 mm diameter were obtained. The beads were hardened by resuspending in a fresh CaCl2 solution for 7 h with gentle agitation and then frozen at −18 °C for 24 h. Finally, these beads were washed several times with sterile double distilled water and stored at 4 °C for further investigations.
Preparation of polyacrylamide entrapment: About 12 g wet cells were suspended in 10 mL distilled water and chilled in ice. To 10 mL of 0.2 M potassium phosphate buffer, (pH 7.0), 2.85 g acrylamide, 0.15 g bisacrylamide and 10 mg ammonium persulphate were added this buffer solution was mixed with the chilled cell suspension followed by the addition of 10 μL of TEMED and poured into 2 or 3 glass petri dishes. It was then allowed for polymerization for 1 h. The sieved gels were suspended in 100 mL of 0.2 M potassium phosphate buffer pH 7.0 and allowed to settle.
Preparation of agar entrapment: 100 mg agar was dissolved in 4.5 mL of 0.9% (wt/vol) sodium chloride by heating at 100 °C and then cooled to 40 °C. Cell slurry was suspended in 0.9% (wt/vol) sodium chloride solution. 0.5 mL of the cell slurry was added to 4.5 mL of the agar solution and mixed. Immediately, the mixture was poured on nylon net placed on a glass plate and cooled to 5 °C. The membrane was stored in 0.1 M phosphate buffer, pH 7.0.
Degradation conditions
Batch degradation experiments
The batch degradation experiments were performed to evaluate the degradation of cypermethrin by both freely suspended cells and immobilized cells in various matrices.
For freely suspended cell cultures, 10 mL of exponentially growing cells were inoculated into 90 mL of MM 2 medium in 500 mL Erlenmeyer flasks along with the same amount of heat-killed cells as controls. The cell concentration was adjusted (3 × 1012 cfu/mL) and different amounts of cypermethrin (10 and 20 mM) were added. For immobilized cells, 12 g wet beads/foam cubes of the various matrices were added to a 500 mL Erlenmeyer flask containing 100 mL of mineral salts medium (MM 2) with 10 and 20 mM of cypermethrin. The cell counts in the immobilized matrices of the PUF, SA, polyacrylamide and agar were found to be 1.7 × 1012 cfu/g foam cubes, 1.6 × 1012 cfu/g, 1.8 × 1012 cfu/g and 1.6 × 1012 cfu/g beads, respectively. Both the freely suspended cell culture and the immobilized cells of Micrococcus sp. strain CPN 1 in various matrices were incubated at 30 °C on a rotary shaker (150 rpm) under identical conditions along with controls. The samples from the culture broth were withdrawn under sterile conditions at different incubation periods and analyzed for residual cypermethrin by HPLC (Metwally et al., 1997). The rate of degradation of cypermethrin by freely suspended and PUF-immobilized cells of Micrococcus sp. strain CPN 1 at different pH (4.0–10.0) and temperature (25–45 °C) were measured after 96 h of incubation. The storage stability of both freely suspended cells and PUF immobilized cells were tested up to 60 days at 4 °C.
Semi-continuous degradation
For establishing the longevity of degrading activity of immobilized cells in various matrices, repeated batch degradations were performed. After each cycle of incubation (96 h/cycle), the spent medium was decanted and beads/foam cubes were washed with sterile water and transferred into fresh MM2 containing cypermethrin. The degradation process was performed under identical conditions as described above and the residual cypermethrin in the spent medium was analyzed (Metwally et al., 1997).
Analytical methods
Cypermethrin concentration in the spent medium was determined by High Performance Liquid Chromatography (HPLC) as described in Metwally et al. (1997). At regular intervals, 5 mL samples were withdrawn and centrifuged at 8,000 × g for 10 min. The supernatants were extracted with ethyl acetate and the residue obtained after solvent evaporation was dissolved in methanol and used for HPLC analysis. 10 μL of each residual sample of cypermethrin was analyzed by HPLC (Shimadzu, Japan) equipped with SPD-10AVP UV-Detector using shim-pack CLC-C8 column (4.6 × 150 mm) of particle size (5 μm) (Phenomenex) and methanol-water (90:10, vol/vol) as mobile phase at the flow rate of 1 mL min−1.
Statistical analysis
All Experiments were carried out in triplicate and their results are presented as mean ± standard deviations (SD).
Results
Degradation of cypermethrin of free and immobilized cells of Micrococcus sp. strain CPN 1 in batch cultures
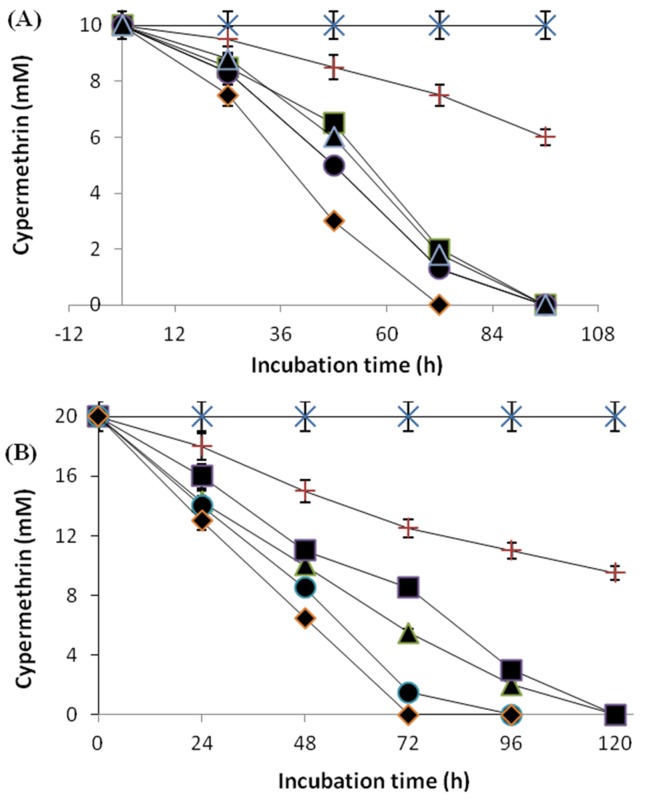
The results in the enhanced degradation of 10 and 20 mM of cypermethrin in batch cultures, both by freely suspended cells and immobilized cells of Micrococcus sp. strain CPN 1 in polyurethane foam (PUF), sodium alginate, polyacrylamide and agar are shown in Figure 1A and B. With the initial concentration of 10 mM cypertmethrin, the freely suspended cells degraded 9 mM of cypermethrin after 168 h incubation period, whereas immobilized cells degraded the same concentration within 96 h of incubation (Figure 1A). But with the increased initial concentration to 20 mM, the rate of degradation freely suspended cells was decreased, whereas immobilized cells degraded the same concentration within 120 h (Figure 1B). These results suggest that higher concentration of cypermethrin was better tolerated and more rapidly degraded by immobilized cells than freely suspend cells.
Figure 1. Degradation of cypermethrin at 10 mM (A) and 20 mM (B) in batch cultures by cells of Micrococcus sp. strain CPN 1 immobilized on PUF (-◆-), polyacrylamide (-●-), sodium alginate (-▲-), agar (-■-), freely suspended cells (-+-) and uninoculated control (-x-). Data values represent means of triplicate and error bars indicate 95% confidence intervals.
Semi-continuous degradation of cypermethrin by immobilized cells of strain CPN 1
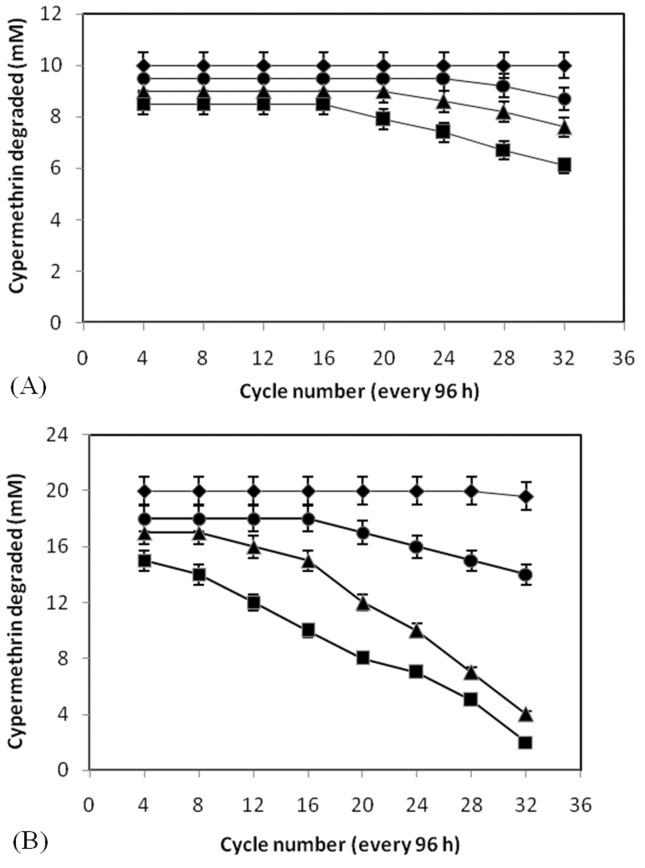
The results of the semi-continuous degradation of 10 and 20 mM cypermethrin by cells of Micrococcus sp. strain CPN 1 immobilized in PUF, alginate, polyacrylamide and agar are shown in Figure 2A and B. The PUF-immobilized cells can be reused for up to 32 cycles without losing their ability to degrade cypermethrin at the initial concentration of 10 and 20 mM. In contrast, agar, sodium alginate and polyacrylamide immobilized cells could be reused for 16, 20 and 24 cycles, respectively (Figure 2A). However, when the initial concentration of cypermethrin was increased to 20 mM, these immobilized cells could be reused with a decreased rate of degradation of cypermethrin (Figure 2B). These observations suggest that lower concentration of cypermethrin (10 mM) could be fed at much higher frequency than higher concentration of cypermethrin (20 mM).
Figure 2. Semi-continuous degradation of cypermethrin at 10 mM (A) and 20 mM (B) by cells of Micrococcus sp. strain CPN 1 immobilized on PUF (-◆-), polyacrylamide (-■-), sodium alginate (-▲-) and agar (-●-). Data values represent means of triplicate and error bars indicate 95% confidence intervals.
Effect of pH, temperature and storage stability on degradation capacity of PUF-immobilized cells of Micrococcus sp. strain CPN 1
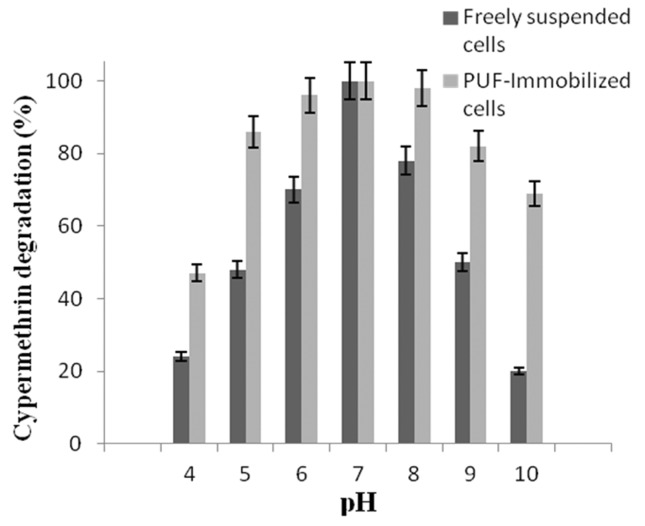
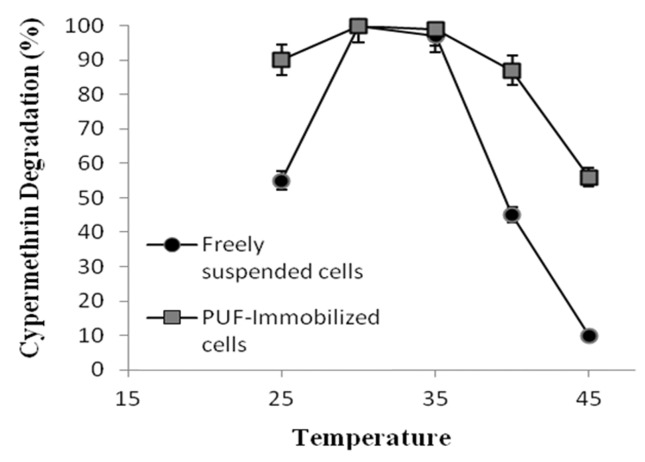
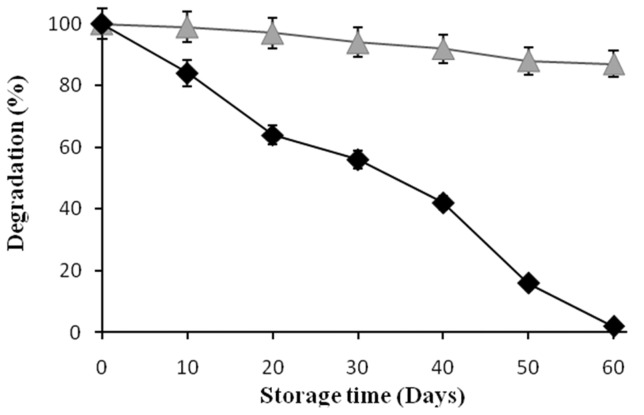
The effect of pH on the degradation of cypermethrin by freely suspended cells and PUF immobilized cells shows that variations of the initial pH between 5.0 and 9.0 (Figure 3) had no effect on cypermethrin degradation (Figure 3). In contrast, the freely suspended cells were able to degrade cypermethrin at narrow range pH between 6.5 to 7.5. The effect of temperatures on the degradation of cypermethrin by PUF-immobilized cells showed higher activity at the temperature between 25 and 40 °C (Figure 4). Whereas the freely suspended cells showed activity at the temperature between 30 and 35 °C (Figure 4). However, the optimal pH and temperature were found to be 7.0 and 30 °C both for PUF-immobilized cells and freely suspended cells in culture medium. The PUF-immobilized cells can be stored for 60 days at 4 °C without loss of its degradation capacity while the freely suspended cells lost their degrading capacity after 60 days at 4 °C (Figure 5).
Figure 3. Effect of pH on the degradation of cypermethrin (10 mM) by freely suspended cells and PUF-immobilized cells of Micrococcus sp. strain CPN 1. Data values represent means of triplicate and error bars indicate 95% confidence intervals.
Figure 4. Effect of Temperature on the degradation of cypermethrin (10 mM) by freely suspended cells and PUF-immobilized cells of Micrococcus sp. strain CPN 1. Data values represent averages of three replicate determinations.
Figure 5. Storage stability of freely suspended cells (-◆-) and PUF-immobilized (-▲-) cells of Micrococcus sp. strain CPN 1 grown on cypermethrin (10 mM). Data values represent means of triplicate and error bars indicate 95% confidence intervals.
Discussion
The degradation of cypermethrin by immobilized cells of Micrococcussp. strain CPN 1 in various matrices such as PUF, sodium alginate, polyacrylamide and agar were compared with that of freely suspended cells in batches and semi-continuous shaken cultures. The results obtained from cells immobilized in various matrices with batch cultures suggested that the rate of degradation of cypermethrin, even at a higher concentration (20 mM), was much higher than that with freely suspended cells. The enhanced degradation of cypertmethrin by immobilized cells may be due to availability of a high density of cells in or on immobilized matrices. Immobilization of cells may also lead to a stabilization of membrane permeability and protect against the toxicity of high substrate concentration, thus leading to enhanced degradation rate (Cassidy et al., 1996).
The results of semi-continuous degradation suggests that the PUF and polyacrylamide immobilized cells retained the cypermethrin degradation capacity for a longer period and they could be reused for 32 and 24 cycles, respectively. When the initial concentration of cypermethrin (10 mM) was increased to 20 mM, the PUF immobilized cells could be reused without losing their degrading capacity. The immobilized cells in other matrices could also be reused but with decreased rate of degradation at higher concentration (20 mM). The storage stability and activity of cells entrapped in PUF were better than those cells entrapped in other matrices. The alginate and agar-immobilized cells showed lower degradability of cypermethrin with increased cycle numbers. The mechanical instability and gradual cell leakage from these beads decreased the degradation rate with an increasing cycle number (Trevors et al., 1992; Tallur et al., 2009; Mulla et al., 2012). The PUF-immobilized cells showed more tolerance to pH and temperature changes than freely suspended cells. The advantages of PUF in chemical and physical properties compared to other matrices are its high porosity, mechanical strength, stability and adsorbing capacity. PUF is an ideal support of cell growth (Romaskevic et al., 2006). The cells immobilized in PUF showed a better and faster degradation rate even at higher initial concentration of substrate. These immobilized cells could be stored for longer periods without losing their degradation ability. Furthermore, the longevity of cells immobilized in PUF and their operational stability is better than those of other matrices determined.
The present study have revealed that the more effective degradation of cypermethrin at higher concentration could be achieved by immobilized cells of Micrococcus sp. strain CPN 1 than freely suspend cells. The immobilized microbial system has an advantage of enhanced rate of degradation, tolerance to higher substrate concentrations and their reuseability. Thus, the immobilized microbial technology provides a highly versatile and cost-effective approach that can be used for degradation of pesticide contaminated wastewater.
References
- Ali O, Namane A, Hellal A. Use and recycling of Ca-alginate biocatalyst for removal of phenol from wastewater. J Ind Eng Chem. 2013;19:1384–1390. [Google Scholar]
- Ansari AR, Rahman S, Kaur M, et al. In vivo cytogenetic and oxidative stress-inducing effects of cypermethrin in freshwater fish, Channa punctata Bloch. Ecotoxicol Environ Saf. 2011;74:150–156. doi: 10.1016/j.ecoenv.2010.08.036. [DOI] [PubMed] [Google Scholar]
- Cassidy MB, Lee H, Trevors JT. Environmental applications of immobilized microbial cells: a review. J Ind Microbiol. 1996;16:79–101. [Google Scholar]
- Chen KC, Lin YH, Chen WH, et al. Degradation of phenol by immobilized Candida tropicalis . Enzyme Microbiol Technol. 2002;31:490–497. [Google Scholar]
- Chen S, Luo J, Hu M, et al. Enhancement of cypermethrin degradation by a coculture of Bacillus cereus ZH-3 and Streptomyces aureus HP-S-01. Bioresource Technology. 2012;110:97–104. doi: 10.1016/j.biortech.2012.01.106. [DOI] [PubMed] [Google Scholar]
- Hall DO, Rao KK. Immobilized photosynthetic membranes and cells for the production of fuels and chemicals. Chemicaoggi. 1989;1:41–47. doi: 10.1007/978-1-4684-7908-9_18. [DOI] [PubMed] [Google Scholar]
- Hoskeri RS, Mulla SI, Ninnekar HZ. Biodegradation of chloroaromatic pollutants by bacterial consortium immobilized in polyurethene foam and other matrices. Biocatal Agric Biotechnol. 2014;3(4):390–396. http://dx.doi.org/10.1016/j.bcab.2014.03.001i. [Google Scholar]
- Jilani S, Khan A. Biodegradation of Cypermethrin by Pseudomonas in a batch activated sludge process. Int J Environ Sci Tech. 2006;3:371–380. Bottom of Form. [Google Scholar]
- Jin YX, Zheng SS, Fu ZW. Embryonic exposure to cypermethrin induces apoptosis and immunotoxicity in zebrafish (Danio rerio) Fish Shellfish Immunol. 2011;30:1049–1054. doi: 10.1016/j.fsi.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Lin QS, Chen SH, Hu MY, et al. Biodegradation of cypermethrin by a newly isolated actinomycetes HU-S-01from wastewater sludge. Int J Environ Sci Technol. 2011;8:45–56. [Google Scholar]
- McKinlay R, Plant JA, Bell JN, et al. Endocrine disrupting pesticides: implications for risk assessment. Environ Int. 2008;34:168–183. doi: 10.1016/j.envint.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Massalha N, Basheer S, Sabbah I. Effect of adsorption and bead size of immobilized biomass on the rate of biodegradation of phenol at high concentration levels. Ind Eng Chem Res. 2007;46:6820–6824. [Google Scholar]
- Metwally ME-S, Osman MS, Al-Rushaid R. A high-performance liquid chromatographic method for the determination of cypermethrin in vegetables and its application to kinetic studies after greenhouse treatment. Food Chemistry. 1997;59:283–290. [Google Scholar]
- Mulla SI, Talwar MP, Hoskeri RS, et al. Enhanced Degradation of 3-Nitrobenzoate by Immobilized Cells of Bacillus flexus strain XJU-4. Biotech Bioprocess Eng. 2012;17:1294–1299. [Google Scholar]
- Mulla SI, Talwar MP, Bagewadi ZK, et al. Enhanced degradation of 2-nitrotoluene by immobilized cells of Micrococcus sp. strain SMN-1. Chemosphere. 2013;90:1920–1924. doi: 10.1016/j.chemosphere.2012.10.030. [DOI] [PubMed] [Google Scholar]
- Romaskevic T, Budriene S, Pielichowski K, et al. Application of polyurethane-based materials for immobilization of enzymes and cells: A review. CHEMIJA. 2006;17:74–89. [Google Scholar]
- Riederer AM, Hunter RE, Hayden SW, et al. Pyrethroid and organophosphorus pesticides in composite diet samples from Atlanta, USA adults. Environ Sci Technol. 2010;44:483–490. doi: 10.1021/es902479h. [DOI] [PubMed] [Google Scholar]
- Roberts TR, Standen ME. Further studies of the degradation of the pyrethroid insecticide cypermethrin in soils. Pest Science. 1981;12:285–296. [Google Scholar]
- Segal-Rosenheimer M, Dubowski Y. Heterogeneous ozonolysis of cypermethrin using real-time monitoring FTIR techniques. J Phys Chem C. 2007;111:11682–11691. [Google Scholar]
- Tallur PN, Megadi VB, Ninnekar HZ. Biodegradation of cypermethrin by Micrococcussp. strain CPN1. Biodegradation. 2008;19:77–82. doi: 10.1007/s10532-007-9116-8. [DOI] [PubMed] [Google Scholar]
- Tallur PN, Megadi VB, Ninnekar HZ. Biodegradation of p-cresol by immobilized cells of Bacillus sp. strain PHN 1. Biodegradation. 2009;20:79–83. doi: 10.1007/s10532-008-9201-7. [DOI] [PubMed] [Google Scholar]
- Trevors JT, VanElsa JD, Lee H, et al. Use of alginate and other carriers for encapsulation of microbial cells for use in soil. Microb Release. 1992;1:61–69. [Google Scholar]
- Ullah H, Shah AA, Hasan F, et al. Biodegradation of trinitrotoluene by immobilized Bacillus sp. YRE1. Pak J Bot. 2010;42:3357–3367. [Google Scholar]
- Wang BE, Hu YY. Comparison of four supports for adsorption of reactive dyes by immobilized Aspergillus fumigates beads. J Environ Sci. 2007;19:451–457. doi: 10.1016/s1001-0742(07)60075-8. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang SF, Ning H, et al. Maternal cypermethrin exposure during lactation impairs testicular development and spermatogenesis in male mouse offspring. Inc Environ Toxicol. 2011;26:382–394. doi: 10.1002/tox.20566. [DOI] [PubMed] [Google Scholar]
- Westmeier F, Rehm HJ. Degradation of 4-chlorophenol in municipal wastewater by adsorptive immobilized Alcaligenes sp. A7-2. Appl Microbiol Biotechnol. 1987;26:78–83. doi: 10.1007/BF00169634. [DOI] [PubMed] [Google Scholar]
- Weston DP, Holmes RW, Lydy MJ. Residential runoff as a source of pyrethroid pesticides to urban creeks. Environ Pollut. 2009;157:287–294. doi: 10.1016/j.envpol.2008.06.037. [DOI] [PubMed] [Google Scholar]
- Weston DP, Asbell AM, Hecht SA, et al. Pyrethroid insecticides in urban salmon streams of the Pacific Northwest. Environ Pollut. 2011;159:3051–3056. doi: 10.1016/j.envpol.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Wolansky MJ, Harrill JA. Neurobehavioral toxicology of pyrethroid insecticides in adult animals: a critical review. Neurotoxicol Teratol. 2008;30:55–78. doi: 10.1016/j.ntt.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Xie JM, Wang PL, Liu J, et al. Photodegradation of lambda-cyhalothrin and cypermethrin in aqueous solution as affected by humic acid and/or copper: intermediates and degradation pathways. Environ Toxicol Chem. 2011;30:2440–2448. doi: 10.1002/etc.655. [DOI] [PubMed] [Google Scholar]
- Yang L, Chen SH, Hu MY, et al. Biodegradation of carbofuran by Pichia anomalastrain HQ-C-01 and its application for bioremediation of contaminated soils. Biol Fertil Soils. 2011;47:917–923. [Google Scholar]
- Zhang C, Jia L, Wang S, et al. Biodegradation of Beta-cypermethrin by two Serratia spp. with different cell surface hydrophobicity. Bioresour Technol. 2010;101:3423–3429. doi: 10.1016/j.biortech.2009.12.083. [DOI] [PubMed] [Google Scholar]
- Zheng C, Zhou J, Wang J, et al. Aerobic degradation of nitrobenzene by immobilized of Rhodotorula mucilaginosa in polyurethane foam. J Haz Mat. 2009;168:298–303. doi: 10.1016/j.jhazmat.2009.02.029. [DOI] [PubMed] [Google Scholar]