Abstract
Objective:
To estimate the impact and cost-effectiveness of treatment as prevention (TasP), pre-exposure prophylaxis (PrEP) and condom promotion for serodiscordant couples in Nigeria.
Design:
Mathematical and cost modelling.
Methods:
A deterministic model of HIV-1 transmission within a cohort of serodiscordant couples and to/from external partners was parameterized using data from Nigeria and other African settings. The impact and cost-effectiveness were estimated for condom promotion, PrEP and/or TasP, compared with a baseline where antiretroviral therapy (ART) was offered according to 2010 national guidelines (CD4+ <350 cells/μl) to all HIV-positive partners. The impact was additionally compared with a baseline of current ART coverage (35% of those with CD4+ <350 cells/μl). Full costs (in US $2012) of programme introduction and implementation were estimated from a provider perspective.
Results:
Substantial benefits came from scaling up ART to all HIV-positive partners according to 2010 national guidelines, with additional smaller benefits of providing TasP, PrEP or condom promotion. Compared with a baseline of offering ART to all HIV-positive partners at the 2010 national guidelines, condom promotion was the most cost-effective strategy [US $1206/disability-adjusted-life-year (DALY)], the next most cost-effective intervention was to additionally give TasP to HIV-positive partners (incremental cost-effectiveness ratio US $1607/DALY), followed by additionally giving PrEP to HIV-negative partners until their HIV-positive partners initiate ART (US $7870/DALY). When impact was measured in terms of infections averted, PrEP with condom promotion prevented double the number of infections as condom promotion alone.
Conclusions:
The first priority intervention for serodiscordant couples in Nigeria should be scaled up ART access for HIV-positive partners. Subsequent incremental benefits are greatest with condom promotion and TasP, followed by PrEP.
Keywords: antiretroviral therapy, condom, disability-adjusted life years, mathematical models, pre-exposure prophylaxis, treatment as prevention
Introduction
Although HIV incidence in Nigeria declined by more than 50% between 2001 and 2012, it remains the second-largest epidemic in the world, with 3.4 million people infected [1]. Only an estimated 35% of those eligible for antiretroviral therapy (ART) in Nigeria currently receive it [1].
Previous modelling for Nigeria using the Joint United Nations Programme on HIV and AIDS (UNAIDS) modes of transmission model estimated that a large proportion (26–46%) of new HIV infections occur among couples in stable relationships [2]. Efforts are being stepped up to identify those in serodiscordant partnerships, by testing the male partners, as well as the women attending antenatal care (ANC) for HIV [3].
Trials have demonstrated that pre-exposure prophylaxis (PrEP) [4] and treatment as prevention (TasP) [5] can substantially reduce transmission within serodiscordant partnerships. The Partners PrEP trial found that daily PrEP for HIV-negative partners in heterosexual serodiscordant couples reduced incidence by 67–75% [4]. Other studies giving PrEP to HIV-negative heterosexuals (not restricted to those in serodiscordant partnerships) have had mixed results, with PrEP effectiveness driven by medication adherence [6]. The HIV Prevention Trials Network (HTPN) 052 trial found that TasP – giving ART immediately to the HIV-positive partner, rather than waiting until they reached the recommended CD4+ threshold to initiate ART – reduced transmission within serodiscordant partnerships by 96% [5]. Prospective studies have found lower protection levels in real-world settings [7].
Several studies have found that people increase condom use once they discover they are in an HIV-serodiscordant relationship [8–10], with this increase happening soon after diagnosis [8,9]. Additional increases in condom use by serodiscordant couples have been reported following couples [9] and male-focused [10] HIV testing and counselling, with very high condom use reported by serodiscordant couples in HIV-prevention trials [4,5,11].
There is increasing interest in measuring the impact of combinations of prevention tools on HIV incidence, with mathematical modelling playing a key role in guiding policy makers by providing estimates of the combined impact of different interventions. Several models have been used to estimate the impact and cost-effectiveness of combination HIV prevention in South Africa [12,13], but these findings cannot easily be extrapolated to Nigeria, which has higher male circumcision rates, lower HIV prevalence, and lower per-capita wealth than South Africa.
The present study aimed to estimate the impact and cost-effectiveness of PrEP, TasP and condom promotion for serodiscordant couples in Nigeria.
Methods
We developed a mathematical model describing HIV-1 transmission within serodiscordant heterosexual partnerships and to/from external sexual partners. This model was parameterized with behavioural, biological and cost data from Nigeria and elsewhere, and used to estimate the impact and cost-effectiveness of the interventions among serodiscordant couples already identified through ANC.
Transmission model
The model is a deterministic, compartmental cohort model, dividing HIV-negative people by PrEP status, HIV-positive people by CD4+ cell count (>350 cells/μl, 200–350 cells/μl, <200 cells/μl) and ART status (naïve, on ART, post-ART), and couples by whether they are receiving condom promotion. PrEP and condom promotion are assumed to be initiated at the beginning, whereas ART can also be initiated later, at a rate reflecting eligibility, CD4+-testing frequency and ART acceptance. Those refusing or dropping out of ART enter the post-ART group and, assuming they only seek treatment upon becoming symptomatic, only (re-)initiate ART with CD4+ below 200 cells/μl. HIV-negative people are assumed to stop taking PrEP due to dropout, acquiring infection or (for some interventions) their partner starting ART. Couples receiving condom promotion have higher within-partnership condom use, but the same rates of condom use with external partners, as couples not receiving it. Conservatively, within-partnership condom use falls to non-intervention levels when couples drop out of condom promotion. HIV-negative partners acquire HIV either from their HIV-positive partner or from external partners, at a rate dependent upon frequency of sex, condom, ART and PrEP use, and CD4+ cell count. All newly infected individuals have an initial CD4+ cell count above 350 cells/μl. CD4+ cell counts decline amongst HIV-positive people off ART, and increase on ART. Further details and equations are in supplement 1.
Transmission model parameters
All of the parameters are defined in Table 1, with data sources in Table S1.
Table 1.
Parameters used in the model, with definitions, estimated values and ranges.
| Parameter (symbol used in equations) | Estimate | Range | Country/study for estimate |
| Number of discordant couples | 1000 | Fixed | – |
| % of couples with HIV-positive woman | 93 | 85–100 | Nigeria |
| % CD4+ at ANC testing 200–350 cells/μl | 29 | 28–29 | Nigeria ANC data |
| % CD4+ at ANC testing <200 cells/μl | 13 | 13–21 | Nigeria ANC data |
| % PrEP effectiveness (efficacy × adherence θ) | 70 | 44–90 | Partners PrEP trial in Kenya and Uganda |
| % condom efficacy (e) | 80 | 58–95 | East and southern Africa; Cochrane review |
| % of sex acts in which condom used (f) | 66 | 64–80 | Rwanda; Uganda; South Africa |
| % reduction in non-condom protected acts following condom promotion | 62 | 50–62 | Rwanda; Uganda; South Africa |
| Frequency of CD4+ testing for HIV-positive people in care but not yet on ART (ρ), per year | 2 | 1–2 | Nigeria |
| % of treatment-naive accept ART (ϕ) | 68 | 65–83 | Sub-Saharan Africa; Africa, Thailand |
| Relative prob. death per month off ART, CD4+ >350 vs. 200–350 cells/μl (α1,1 : α2,1) | 0.206 | 0.206–0.258 | Cote d’Ivoire; Zimbabwe; South Africa |
| Monthly probability of death off ART, CD4+ 200–350 cells/μl (α2,1) | 0.00272 | 0.00156 – 0.00397 | Cote d’Ivoire |
| Relative prob. death per month off ART, CD4+ <200 vs. 200–350 cells/μl (α3,1 : α2,1) | 9.08 | 3.45–9.08 | Cote d’Ivoire; Zimbabwe; South Africa |
| Relative prob. death on vs. off ART for the same CD4+ cell count (αi,2 : αi,1) | 0.19 | 0.14–0.25 | South Africa |
| Off ART, monthly probability of moving from CD4+ >350 to 200–350 cells/μl (δ1,1) | 0.0257 | 0.0119–0.0289 | eART-linc cohorts in Uganda and Cote d’Ivoire; South Africa; Ethiopia |
| Off ART, monthly probability of moving from CD4+ 200–350 to <200 cells/μl (δ2,1) | 0.0188 | 0.0186–0.0274 | eART-linc cohorts in Uganda and Cote d’Ivoire; South Africa; Ethiopia |
| On ART, monthly probability of moving from CD4+ 200–350 to >350 cells/μl (δ2,2) | 0.0569 | 0.0247–0.0888 | South Africa; Europe |
| On ART, monthly probability of moving from CD4+ <200 to CD4+ 200–350 cells/μl (δ3,2) | 0.0293 | 0.0274–0.0863 | South Africa; Europe |
| Yearly % of people who dropout of ART (σ) | 10 | 5–30 | Nigeria (multiple sites); Kenya |
| Ratio of dropout from PrEP relative to ART dropout (ω : σ) | – | 1–1.5 | PrEP trials multiple sites; allowing for higher dropout expected outside trial |
| Ratio of dropout from condom promotion relative to ART dropout (κ : σ) | – | 0.3–1 | DR Congo, Uganda |
| Ratio of dropout from pre-ART care relative to ART dropout (ν : σ) | – | 1–2 | South Africa, Malawi |
| Per vaginal sex act probability of HIV transmission from man to woman (βf) | 0.0019 | 0.0010–0.0037 | Partners HIV/HSV study, multiple sites in east and southern Africa |
| % efficacy of medical male circumcision in reducing female to male HIV transmission (υ) | 0.66 | 0.4–0.77 | South Africa, Kenya and Uganda |
| % of men circumcised (τ) | 98 | Fixed | Nigeria |
| % efficacy of ART in reducing HIV transmission (χ) | 92 | 26–99 (triangular) | Meta-analysis; east/southern Africa; HPTN 052 trial (multiple countries); China |
| Relative transmission risk from HIV-positive person with CD4+ <200 vs. >200 cells/μl (ξ) | 4.18 | 2–8 | Partners in Prevention cohort, east/southern Africa |
| DALY weight HIV-positive on ART or CD4+ >350 cells/μl | 0.947 | 0.921–0.966 | Global Burden of Disease Study 2010 |
| DALY weight HIV-positive CD4+ 200–350 cells/μl | 0.779 | 0.690–0.854 | Global Burden of Disease Study 2010 |
| DALY weight HIV-positive CD4+ <200 cells/μl | 0.453 | 0.285–0.618 | Global Burden of Disease Study 2010 |
| Sex acts per month with external partners | 3.4 | 1.3–6.4 | Nigeria |
| Sex acts per month with regular partner (c) | 5.6 | 1.4–15.3 | Nigeria |
| % of sex acts condom used with external partners (men) | 49.0 | 44.9–53.1 | Nigeria |
| % of sex acts condom used with external partners (women) | 11.8 | 6.4–17.2 | Nigeria |
| ART coverage external partners (%) | 35 | Fixed | Nigeria |
| Yearly % testing for HIV, general population | 6.5 | 6.5–11.7 | Nigeria |
| Relative infectiousness of external vs. asymptomatic regular infected partner | 2 | 1–2.5 | Uganda |
| Provider unit cost PrEP initiation (2012$) | 118 | 82.6–153.4 | Nigeria, South Africa (drug costs) |
| Provider unit cost PrEP per year (2012$) | 233 | 163.1–302.9 | Nigeria, South Africa (drug costs) |
| Provider unit cost ART initiation (2012$) | 150 | 105.0–195.0 | Nigeria costing studies |
| Provider unit cost ART per year (2012$) | 365 | 255.5–474.5 | Nigeria costing studies |
| Provider costs of condom promotion per couple per year (2012$) | 19 | 13.3–24.7 | Nigeria costs for counselling, international prices for condoms |
All sources and references are given in the supplementary material. ANC, antenatal care; ART, antiretroviral therapy.
Death rates and CD4+ transition probabilities by CD4+ cell count and ART status were estimated from data from other African countries, with additional data from Europe informing on ART estimates. Age and sex-specific non-HIV-related death rates were estimated from WHO life-tables. All rates were converted to monthly probabilities. Data on sexual behaviour, CD4+ cell counts at diagnosis, discordant couple characteristics and HIV testing came from Nigerian studies [14,15].
Condom use by serodiscordant couples, with and without condom promotion, and ART acceptance rates were estimated from studies conducted elsewhere in Africa. Rates of ART dropout were taken from Nigerian studies [16,17], dropout rates from condom promotion and pre-ART care were informed by data from elsewhere in Africa, and PrEP dropout was informed by dropout rates in PrEP trials. The efficacy of PrEP, ART, male circumcision and condoms in reducing HIV transmission came from published trials and meta-analyses.
Cost model
Full costs (in US $2012) of programme introduction and implementation were estimated from a provider perspective and computed from costing studies conducted in Nigeria [18,19] and elsewhere. For PrEP and ART, total costs include start-up costs (training and mass media campaigns), drug, laboratory tests and logistics, and facility delivery costs. Facility delivery costs take into account the number of visits patients make to healthcare facilities (including additional visits early on), and the duration and staff salary costs for each visit. ART costs include the costs of treating opportunistic infections. The costs of identifying serodiscordant couples are not included. Costs were calculated per person per year for PrEP and ART, and per couple per year for condom promotion. For further details see supplement 1.
Intervention scenarios
The main baseline scenario was offering ART at the 2010 national guidelines (CD4+ <350 cells/μl) to all HIV-positive partners in serodiscordant couples. An additional baseline used for the impact analysis only assumed current ART coverage levels (35%) amongst eligible HIV-positive partners.
The intervention scenarios considered (in addition to the baseline offering ART at CD4+ <350 cells/μl to all HIV-positive partners) were: TasP (offering ART to all HIV-positive partners); short-term PrEP (offering PrEP to HIV-negative partners whose HIV-positive partner is not on ART, ceasing when their HIV-positive partner initiates ART or they contract HIV); long-term PrEP (offering PrEP to all HIV-negative partners, ceasing when they contract HIV); condom promotion and all combinations of these. For PrEP scenarios, we assumed 60% of HIV-negative partners accept and start using PrEP. We assumed 80% of couples begin the condom promotion programme when offered.
Model calibration and analysis
Latin hypercube sampling [20] was used to sample all biological, behavioural and cost parameters from their ranges (Table 1) 2000 times. All parameters were uniformly distributed apart from ART efficacy (triangular distribution).
The model was run for 2 years without ART using each of these 2000 parameter combinations in turn; those giving an average incidence among HIV-negative partners between 2 and 9 new infections per 100 person-years were retained for subsequent analyses. This range reflects low HIV incidence in serodiscordant couples in clinical trials [5] up to higher rates seen in cohorts in Zambia and Uganda [21,22]. All retained parameter sets were run for 20 years, for each baseline scenario and each combination of interventions.
The impact of each intervention scenario was estimated in comparison with each of the baseline scenarios in terms of infections averted, percentage infections averted or disability-adjusted life-years (DALYs) averted, amongst members of both the serodiscordant couple cohort and the pool of external partners, over 20 years. DALYs were calculated by summing up person-years spent in different CD4+ and ART categories, weighted with values from the 2010 Global Burden of Disease study. Incremental costs associated with each intervention were estimated in comparison to the baseline scenario (with ART offered to all eligible HIV-positive partners). Cost-effectiveness was calculated as the incremental cost per infection or DALY averted over 20 years. Both impacts and costs were discounted into the future at a rate of 10% per year in the main analysis, accounting for the high preference for the present observed in Nigeria [23–26]. Uncertainty in predicted impacts were summarized using 95% credible intervals (CrIs), which bound the central 95% of impacts obtained across all parameter combinations. Analysis of co-variance (ANCOVA) was used to identify which parameters most influenced intervention impact and cost.
The median incremental cost and impact of each intervention combination were plotted to identify the efficiency frontier, which joins the incrementally most cost-effective interventions as resources increase. The cost-effectiveness thresholds used were 1× gross domestic product (GDP) (US $2742) [27] and 3× GDP (US $8226) per DALY averted for highly cost-effective and cost-effective interventions, respectively.
In sensitivity analyses, alternative discount rates (3%, 15%), time horizons (10 years, full cohort lifetime), acceptance rates for PrEP (40%, 80%) and condom promotion (40%, 60%), and higher rates of ART re-initiation for HIV-positive members of serodiscordant couples (same treatment CD4+ criteria and 50%,100% rate of ART-naive) were also investigated.
Results
Selected parameter sets
Of 2000 parameter combinations investigated, 1103 gave HIV incidence amongst HIV-negative partners between 2 and 9 per 100 person-years over the first 2 years (median incidence 5/100 person-years). These parameter combinations were used for all subsequent analyses. A median 0.2% of infections came from external partners (range 0.02–2.4%).
Impact
Compared with current antiretroviral therapy coverage
Compared with the present ART coverage (35% of those eligible), offering ART to all eligible (CD4+ <350 cells/μl) HIV-positive partners in serodiscordant couples averted 15% of all infections over 20 years. This equated to 35% of the infections averted under the strategy with the greatest impact (TasP, long-term PrEP and condom promotion), and 73% of the DALYs (Fig. 1).
Fig. 1.
Impact compared with current ART coverage (35% of those with CD4+ below 350 cells/μl), for all intervention combinations considered.
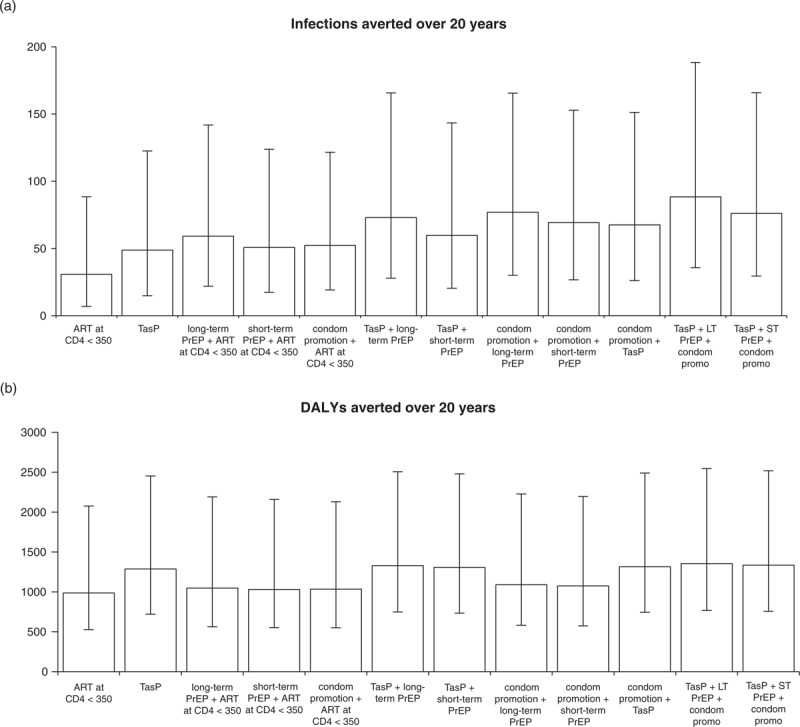
Impact is measured in terms of (a) infections averted, (b) DALYs averted. Error bars are 95% credible intervals from 1103 simulations. ART, antiretroviral therapy; DALYs, disability-adjusted life-years; PrEP, pre-exposure prophylaxis.
Compared with 2010 national treatment guidelines baseline
Compared with a baseline scenario where all HIV-positive partners in serodiscordant couples were offered ART once they reached a CD4+ cell count below 350 cells/μl, the relative impact of each intervention differed by impact measure (Fig. 2). Long-term PrEP averted the greatest proportion of remaining infections (15%), followed by condom promotion (11%), short-term PrEP (10%) and TasP (10%) (Fig. 2a). In terms of DALYs averted, TasP had the greatest impact, followed by long-term PrEP, short-term PrEP and condom promotion (Fig. 2b). The greatest impact using either measure was achieved with a combination of TasP, long-term PrEP and condom promotion, which averted 30% of infections (95% CrI 20–50%), and 356 DALYs (95% CrI 213–565).
Fig. 2.
Impact compared with baseline scenario (ART offered to all HIV-positive partners within discordant couples once their CD4+ <350 cells/μl), for all intervention combinations considered.
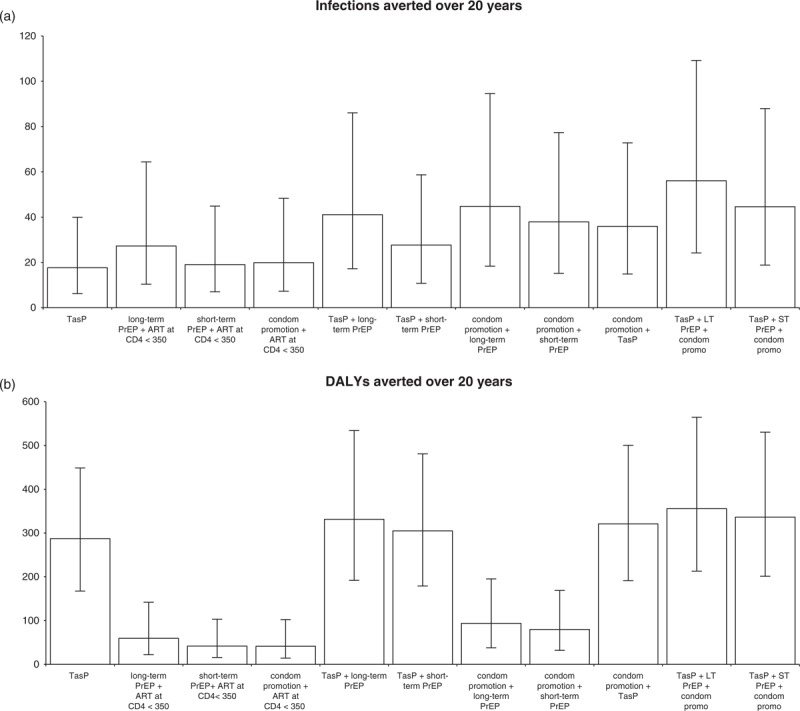
Impact is measured in terms of (a) infections averted, (b) DALYs averted. Error bars are 95% credible intervals from 1103 simulations. ART, antiretroviral therapy; DALYs, disability-adjusted life-years; PrEP, pre-exposure prophylaxis.
Influential parameters
The parameters most influencing the impact were frequency of sex within serodiscordant partnerships, per-sex-act transmission probability, intervention efficacy and dropout rates (supplement 1, ). Cost estimates were most influenced by intervention dropout rates and yearly per-person intervention costs.
Cost-effectiveness
Cost-effectiveness was only estimated in comparison with a baseline scenario where all HIV-positive partners in serodiscordant couples were offered ART at CD4+ cell counts below 350 cells/μl. For impact on infections averted, Fig. 3a suggests that as more resources become available, after giving ART to HIV-positive partners with CD4+ cell counts below 350 cells/μl, the most cost-effective interventions were: condom promotion, then additionally giving short-term PrEP to HIV-negative partners (almost doubling the number of infections averted compared with condom promotion alone), then switching to long-term PrEP alongside condom promotion, and finally, additionally giving TasP to HIV-positive partners. This order was well conserved across the different parameter combinations; 61% (668/1103) gave this same sequence. All parameter combinations suggested condom promotion as the initial intervention, and 90% (997/1103) suggested adding short-term PrEP next.
Fig. 3.
Incremental impact and cost compared to baseline scenario (ART offered to all HIV-positive partners within discordant couples once their CD4+ <350 cells/μl), for all intervention combinations.
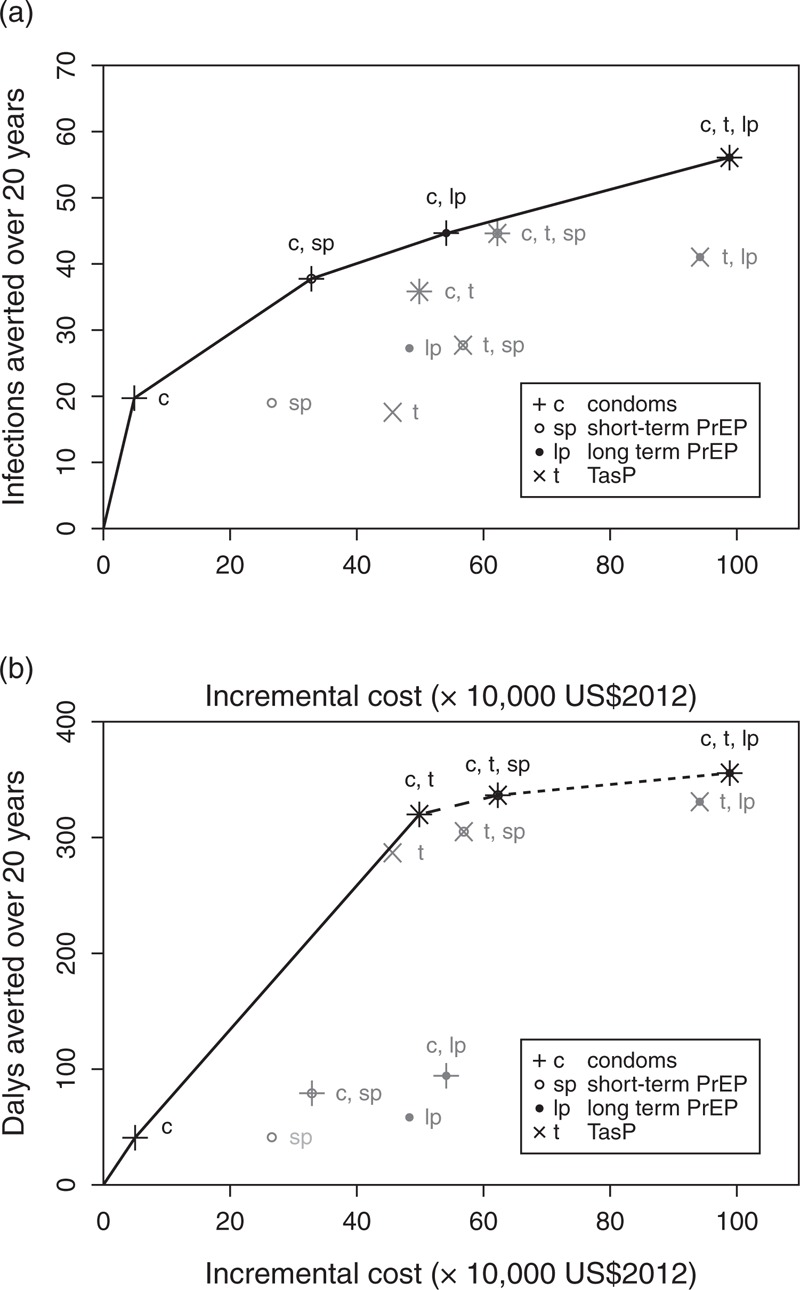
Impact is measured in terms of (a) infections averted and (b) DALYs averted. Plotted points are medians for each intervention from 1103 parameter sets. Intervention combinations are shown by letters and symbols combining individual intervention elements shown in the key (e.g. open circle with a cross through it, labelled ‘c, sp’, indicates condom promotion + short-term PrEP). Interventions appearing on the efficiency frontier are shown in black, with those interventions not appearing on the frontier in grey. The solid straight lines join the medians for the incrementally most cost-effective interventions as budget increases. In (b) incremental additions which are highly cost-effective (incremental cost per DALY averted <1× GDP) are shown by solid lines, additions which are cost-effective (incremental cost per DALY averted <3× GDP) are shown by dashed lines, and additions which are not cost-effective (incremental cost per DALY averted >3× GDP) are shown with a dotted line. ART, antiretroviral therapy; DALYs, disability-adjusted life-years; PrEP, pre-exposure prophylaxis.
For impact on DALYs, the most cost-effective initial intervention was condom promotion, but as more resources become available, the next most cost-effective intervention was additionally giving TasP to HIV-positive partners, followed by additionally giving short-term PrEP (Fig. 3b). For the median values, condom promotion and the addition of TasP were incrementally highly cost-effective at a threshold of 1× GDP [incremental cost-effectiveness ratio (ICER) US $1206/DALY and US $1607/DALY, respectively]. The median incremental benefit of adding short-term PrEP to the condom promotion and TasP interventions was cost-effective at a threshold of 3× GDP (ICER US $7870/DALY), but not at 1× GDP. With more resources, a slight additional benefit came from switching to long-term PrEP, but this was not cost-effective (ICER US $19054/DALY). There was substantial agreement across parameter sets: 57% gave this same order for prioritizing interventions, with a further 32% suggesting TasP as the most cost-effective initial intervention, followed by condom promotion with TasP; 99% of parameter combinations had condom promotion with TasP on the efficiency frontier as the second or third intervention. Using a cost-effectiveness threshold of 1× GDP, when condom promotion was the first indicated strategy, this was cost-effective for 741 of 742 (99%) of these parameter sets, and progressing to an intervention with TasP and condom promotion was incrementally cost-effective for 96% (712/742) of parameter combinations. When TasP was the first indicated strategy, this was cost-effective (at 1× GDP) for 356 of 361 (99%) of parameter sets, and adding condom promotion to TasP was cost-effective for 151/352 (43%). Adding short-term PrEP to a condom use with TasP strategy was only cost-effective for 1% of parameter combinations using a 1× GDP threshold. Using a cost-effectiveness threshold of 3× GDP, condoms with TasP (via TasP or condoms alone) was incrementally cost-effective for 99% of parameter combinations, the addition of short-term PrEP was cost-effective for 73% and the subsequent switch to long-term PrEP for 6%.
At higher incidence, more infections and DALYs were averted by each intervention (P < 0.001 for all), and the cost per infection or DALY averted decreased (P < 0.001 for all). With higher incidence, condom promotion (rather than TasP) was more likely to be the initial intervention on the DALY efficiency frontier, but no difference was seen in the order of the efficiency frontier for infections averted.
Sensitivity analyses
Higher discount rates reduced cost-effectiveness (Fig. S2, ). With the more standard 3% annual discount rate (vs. 10%), the order of incrementally cost-effective interventions remained the same, but ICERs were improved (condom promotion: US $590/DALY, adding TasP: US $1054/DALY, adding short-term PrEP: US $3536/DALY, switching to long-term PrEP: US $9259/DALY).
Increased impact and cost-effectiveness were seen over longer time-scales (Fig. S3, ). Assessing the impact over 10 rather than 20 years changed the order of incrementally cost-effective interventions, with TasP becoming the most cost-effective initial intervention, followed by condom promotion with TasP (Fig. S3, ).
Assuming different initial PrEP coverage (40 or 80% rather than 60%) made little difference to the number of DALYs averted, but higher coverage increased total costs (results not shown). Lower initial condom promotion coverage (40 or 60% rather than 80%) slightly reduced both costs and DALYs averted, and slightly reduced the cost per DALY (results not shown). The order of incrementally cost-effective interventions remained the same.
Higher rates of ART re-initiation gave increased impact for TasP, but decreased impact for PrEP and condom promotion, and all interventions cost more per DALY averted. With increasing ART re-initiation, TasP was more likely to be the most cost-effective initial intervention, followed by condom promotion, and the addition of PrEP was less likely to be cost-effective.
Discussion
We found that offering ART to all HIV-positive partners in serodiscordant couples in line with national treatment guidelines would have a large effect on survival and HIV transmission. Condom promotion for serodiscordant couples was predicted to be highly cost-effective and effective in reducing transmission within partnerships. In addition, treatment as prevention – already recommended by WHO for serodiscordant partnerships [28] – was predicted to bring about substantial and highly cost-effective additional gains in DALYs averted. Additionally offering HIV-negative partners PrEP until their HIV-positive partner initiated ART was also predicted to be cost-effective (US $7870/DALY). After offering ART to HIV-positive partners at CD4+ cell count below 350 cells/μl, offering HIV-negative partners PrEP until their HIV-positive partner began ART was predicted to avert 10% of remaining new HIV infections.
Despite considerable uncertainty in several model parameters, we found consistent results for the efficiency frontier, with condom promotion and TasP for HIV-positive partners almost always recommended as the first two interventions to initiate to avert DALYs, followed by short-term PrEP for HIV-negative partners, and condom promotion with short-term PrEP for HIV-negative partners recommended to prevent new infections efficiently.
The present analysis highlights the importance of condom promotion as part of combination prevention for serodiscordant couples – this costs the healthcare provider relatively little, but can substantially increase condom use, reducing the likelihood of within-couple transmission.
We allowed transmission rates amongst serodiscordant couples to vary between two and nine new infections per 100 person-years, in the absence of Nigeria-specific incidence estimates. We expect transmission rates amongst serodiscordant couples in Nigeria to be somewhat lower than the higher transmission estimates (up to 9 per 100 person-years) from studies in Zambia and Uganda, since Nigeria has higher circumcision rates [29], and these studies were conducted in populations with very low condom use [21], or amongst couples unaware of their HIV status [22]. We predicted that PrEP, TasP and condom promotion would be more cost-effective at higher incidence, and so data on behaviour and incidence from demonstration projects will be crucial for improving the accuracy of cost-effectiveness estimates for Nigeria.
We predicted that very few infections (<3%) amongst HIV-negative people in serodiscordant partnerships would come from external partners, lower than the estimated 20–30% in clinical trials [5,30]. This is in agreement with previous modelling for sub-Saharan Africa, which predicted that less than 3% of HIV infections among serodiscordant couples would come from external partners in countries with low (≤3%) HIV prevalence [31].
In comparison with previous African cost-effectiveness studies [12,13,32], there is less difference in our study between the estimated annual costs of PrEP and ART; this is likely to be because our cost estimates for PrEP and ART include all of the same components, whereas previous studies often took into account more components for ART than for PrEP. A previous modelling study of serodiscordant couples in South Africa [13] suggested that PrEP may be more cost-effective than early ART for the relative ratio of PrEP to ART costs and PrEP efficacy used in our study, in couples with ‘typical’ levels of risk. This was not found in our study, perhaps because we assumed lower levels of unprotected sex with external partners and a lower ratio of risk of infection from external partners relative to stable partners, leading to fewer infections coming from external partners. In agreement with our findings that condom promotion and TasP were more cost-effective than PrEP, a modelling study of a hyper-endemic southern African setting found that it was more cost-effective to first give early ART before introducing PrEP [12]. Another study of combination prevention in Kenya found that it was usually most cost-effective to first implement behaviour change interventions, followed by early ART, and then PrEP [33].
We assumed a discount rate of 10% for both impacts and costs in our baseline analysis, which led to lower estimated cost-effectiveness than for the more standard 3% discount rate. Although the 3% discount rate is usually recommended [34], it may not reflect the willingness to trade-off future for present consumption in Nigeria. Although the preference for present is in theory well captured by the interest rate, the presence of market imperfections, as well as the inability of the interest rate to account for the interest of future generations [35], justifies our decision not to use the interest rate as a proxy for the societal discount rate. Instead, we found evidence that preference for present is particularly high in Nigeria since discount rates used in Nigeria project reports varied between 8.0 and 13.7% [23–26].
We permitted a wide range of ART efficacy in our analysis, with a preference for higher efficacies, reflecting the fact that some observational studies have found lower levels of transmission reduction than clinical trials [7,36]. High rates of viral suppression, between 77 and 90% [17,37], have been reported for ART patients in Nigeria, which compares well with the levels of viral suppression (70% [38], 89% [5]) measured in transmission studies which found high levels of reduction in transmission (92 and 96%, respectively). Therefore, assuming a large effect in this setting seems reasonable.
Formative research looking at perceptions of PrEP use in Nigeria found broad acceptability, although concerns were raised about the impact of stigma and sustainability of PrEP interventions [39]. The current PrEP demonstration project occurring among Nigerian serodiscordant couples will give a clearer picture of PrEP feasibility, as well as levels of condom use and ART uptake among serodiscordant couples.
Limitations
Much of the data used to inform this analysis came from outside Nigeria, and while HIV progression and mortality estimates are not expected to differ greatly between countries, there could be strain-related differences in survival and progression [40]. Most of the behavioural data came from married people of unknown HIV status, which may underestimate the risk behaviour of serodiscordant couples. We did not consider second-line ART which may have affected our ART cost estimates. We assumed that the infectiousness of HIV-positive individuals decreased to low levels as soon as they started ART, with partners immediately stopping short-term PrEP. We therefore did not evaluate the cost-effectiveness of the more realistic scenario where HIV-negative partners continue to take PrEP for several months after ART initiation, while viral loads decline. Previous modelling suggests only a small impact is achieved over this period [13].
In conclusion, these results suggest that the best first intervention strategy for serodiscordant couples in Nigeria would be ensuring that all HIV-positive partners are offered ART at current national guidelines. Additional reduction in new infections could be achieved by promoting condom use amongst serodiscordant couples, and offering PrEP to HIV-negative partners until their HIV-positive partner initiates ART. Additional DALYs could be averted through condom promotion for couples and TasP for HIV-positive partners, which would both be incrementally highly cost-effective, followed by offering PrEP to HIV-negative partners until their HIV-positive partner initiates ART.
Acknowledgements
The authors would like to thank Patrick Ikani, Godwin Etim and Samuel Udemezue for help with collecting data to inform the cost model, and Simon Cartier, Edward Oladele and Ignatius Mogaba for help with providing the data on discordant couples in Nigeria.
Author contributions: K.M.M. and P.V. designed the modelling study; F.T.P. and A.L. designed the costing study; M.O.F. and J.I. co-ordinated the study and advised on the overall direction; J.I. oversaw the project; K.M.M. constructed and analysed the dynamic model; A.L. collected cost data and constructed the cost model; K.T., H.K., A.S., J.M., J.A. and E.A. provided data for the models; K.M.M. wrote the first draft of the manuscript; A.L. wrote the cost sections of the manuscript; P.V. and F.T.P. critically revised the manuscript; M.O.F. and K.T. made additions to the manuscript; all authors approved the final version of the manuscript.
Source of funding: Bill and Melinda Gates Foundation.
Conflicts of interest
The authors have no conflicts of interest to declare.
Supplementary Material
References
- 1.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2013. Geneva: UNAIDS; 2013. [Google Scholar]
- 2.UNAIDS. New HIV infections by mode of transmission in West Africa: a multicountry analysis. Geneva: UNAIDS; 2010. [Google Scholar]
- 3.Ezeama C, Eleje G, Okonkwo T, Ikechebelu J. Partner human immunodeficiency virus sero-discordance in Nnewi, Nigeria. J HIV Human Reprod 2014; 2:2–7. [Google Scholar]
- 4.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendrix Craig W. Exploring concentration response in HIV preexposure prophylaxis to optimize clinical care and trial design. Cell 2013; 155:515–518. [DOI] [PubMed] [Google Scholar]
- 7.Anglemyer A, Horvath T, Rutherford G. Antiretroviral therapy for prevention of HIV transmission in HIV-discordant couples. JAMA 2013; 310:1619–1620. [DOI] [PubMed] [Google Scholar]
- 8.Kamenga M, Ryder RW, Jingu M, Mbuyi N, Mbu L, Behets F, et al. Evidence of marked sexual-behavior change associated with low HIV-1 seroconversion in 149 married couples with discordant HIV-1 serostatus: experience at an HIV counseling-center in Zaire. AIDS 1991; 5:61–67. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg NE, Pettifor AE, De Bruyn G, Westreich D, Delany-Moretlwe S, Behets F, et al. HIV testing and counseling leads to immediate consistent condom use among South African stable HIV-discordant couples. J Acquir Immune Defic Syndr 2013; 62:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roth DL, Stewart KE, Clay OJ, van der Straten A, Karita E, Allen S. Sexual practices of HIV discordant and concordant couples in Rwanda: effects of a testing and counselling programme for men. Int J STD AIDS 2001; 12:181–188. [DOI] [PubMed] [Google Scholar]
- 11.Hughes JP, Baeten JM, Lingappa JR, Magaret AS, Wald A, de Bruyn G, et al. Determinants of per-coital-act HIV-1 infectivity among African HIV-1–serodiscordant couples. J Infect Dis 2012; 205:358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cremin Í, Alsallaq R, Dybul M, Piot P, Garnett G, Hallett TB. The new role of antiretrovirals in combination HIV prevention: a mathematical modelling analysis. AIDS 2013; 27:447–458. [DOI] [PubMed] [Google Scholar]
- 13.Hallett TB, Baeten JM, Heffron R, Barnabas R, de Bruyn G, Cremin Í, et al. Optimal uses of antiretrovirals for prevention in HIV-1 serodiscordant heterosexual couples in South Africa: a modelling study. PLoS Med 2011; 8:e1001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Population Commission (NPC) [Nigeria] and ICF Macro. Nigeria demographic and health survey 2008. Abuja, Nigeria; ICF Macro; 2009. [Google Scholar]
- 15.National Agency for the Control of AIDS (Nigeria). United Nations general assembly special session (UNGASS) country progress report 2008–2009. 2010. [Google Scholar]
- 16.Charurat M, Oyegunle M, Benjamin R, Habib A, Eze E, Ele P, et al. Patient retention and adherence to antiretrovirals in a large antiretroviral therapy program in Nigeria: a longitudinal analysis for risk factors. PLoS One 2010; 5:e10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anude CJ, Eze E, Onyegbutulem HC, Charurat M, Etiebet MA, Ajayi S, et al. Immuno-virologic outcomes and immuno-virologic discordance among adults alive and on antiretroviral therapy at 12 months in Nigeria. BMC Infect Dis 2013; 13:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawizza HE, Chaplin B, Meloni ST, Eisen G, Rao T, Sankalé J-L, et al. Immunologic criteria are poor predictors of virologic outcome: implications for HIV treatment monitoring in resource-limited settings. Clin Infect Dis 2011; 53:1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aliyu HB, Chuku NN, Kola-Jebutu A, Abubakar Z, Torpey K, Chabikuli ON. What is the cost of providing outpatient HIV counseling and testing and antiretroviral therapy services in selected public health facilities in Nigeria?. J Acquir Immune Defic Syndr 2012; 61:221–225. [DOI] [PubMed] [Google Scholar]
- 20.Blower SM, Dowlatabadi H. Sensitivity and uncertainty analysis of complex models of disease transmission: an HIV model, as an example. Int Stat Rev 1994; 62:229–243. [Google Scholar]
- 21.Fideli US, Allen SA, Musonda R, Trask S, Hahn BH, Weiss H, et al. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS Res Hum Retroviruses 2001; 17:901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynolds SJ, Makumbi F, Nakigozi G, Kagaayi J, Gray RH, Wawer M, et al. HIV-1 transmission among HIV-1 discordant couples before and after the introduction of antiretroviral therapy. AIDS 2011; 25:473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennedy-Darling J, Hoyt N, Murao K, Ross A. The energy crisis of Nigeria: an overview and implications for the future. The University of Chicago, Chicago 2008. [Google Scholar]
- 24.Economides MJ, Fasina A, Oloyede B. Nigeria natural gas: a transition from waste to resource. World Energy 2004; 7:134–140. [Google Scholar]
- 25.Centre for People and Environment and Tryby Energy Minerals and Environmental Corporation. Landfill recovery and use in Nigeria; 2010. [Google Scholar]
- 26.Egheosa O. What are the major risks that affect the viability of gas power plants in Nigeria and how can they be mitigated? University of Dundee; 2004. [Google Scholar]
- 27.World Bank. Data indicators: GDP per capita (current US$). 2013. http://data.worldbank.org/indicator/NY.GDP.PCAP.CD [Accessed 20 May 2015] [Google Scholar]
- 28.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: WHO; 2013. [PubMed] [Google Scholar]
- 29.de Bruyn G, Martinson NA, Gray GE. Male circumcision for HIV prevention: developments from sub-Saharan Africa. Expert Rev Anti Infect Ther 2010; 8:23–31. [DOI] [PubMed] [Google Scholar]
- 30.Celum C, Wald A, Lingappa JR, Magaret AS, Wang RS, Mugo N, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med 2010; 362:427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chemaitelly H, Abu-Raddad LJ. External infections contribute minimally to HIV incidence among HIV sero-discordant couples in sub-Saharan Africa. Sex Transm Infect 2013; 89:138–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pretorius C, Stover J, Bollinger L, Bacaer N, Williams B. Evaluating the cost-effectiveness of preexposure prophylaxis (PrEP) and its impact on HIV-1 transmission in South Africa. PLoS One 2010; 5:e13646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson SJ, Cherutich P, Kilonzo N, Cremin I, Fecht D, Kimanga D, et al. Maximising the effect of combination HIV prevention through prioritisation of the people and places in greatest need: a modelling study. Lancet 2014; 384:249–256. [DOI] [PubMed] [Google Scholar]
- 34.Edejer TT-T, Baltussen R, Adam T, Hutubessy R, Acharya A, Evans D, et al. , WHO guide to cost-effectiveness analysis. 2012. [Google Scholar]
- 35.Sen A. Approaches to the choice of discount rates for social benefit-cost analysis. In Lind R, editor. Discounting for time and risk in energy policy. Baltimore: Johns Hopkins University Press; 1982. pp. 325–350. [Google Scholar]
- 36.Jia Z, Ruan Y, Li Q, Xie P, Li P, Wang X, et al. Antiretroviral therapy to prevent HIV transmission in serodiscordant couples in China (2003-11): a national observational cohort study. Lancet 2013; 382:1195–1203. [DOI] [PubMed] [Google Scholar]
- 37.Ugbena R, Aberle-Grasse J, Diallo K, Bassey O, Jelpe T, Rottinghaus E, et al. Virological response and HIV drug resistance 12 months after antiretroviral therapy initiation at 2 clinics in Nigeria. Clin Infect Dis 2012; 54 Suppl 4:S375–S380. [DOI] [PubMed] [Google Scholar]
- 38.Donnell D, Baeten JM, Kiarie J, Thomas KK, Stevens W, Cohen CR, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet 2010; 375:2092–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Idoko J, Folayan MO, Dadem NY, Kolawole GO, Anenih J, Alhassan E. ‘Why should I take drugs for your infection?’: outcomes of formative research on the use of HIV preexposure prophylaxis in Nigeria. BMC Public Health 2015; 15:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lodi S, Phillips A, Touloumi G, Geskus R, Meyer L, Thiébaut R, et al. Time from human immunodeficiency virus seroconversion to reaching CD4+ cell count thresholds <200, <350, and <500 cells/mm3: assessment of need following changes in treatment guidelines. Clin Infect Dis 2011; 53:817–825. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


