Abstract
Objective
The present study aimed to evaluate the accuracy of the Somnotouch-NIBP noninvasive continuous blood pressure monitor according to the European Society of Hypertension International Protocol revision 2010.
Materials and methods
Systolic and diastolic blood pressures were sequentially measured in 33 adults (11 women, mean age 63.5±11.9 years) using a mercury sphygmomanometer (two observers) and the Somnotouch-NIBP device (one supervisor). A total of 99 pairs of comparisons were obtained from 33 participants for judgments in two parts with three grading phases.
Results
All the validation requirements were fulfilled. The Somnotouch-NIBP device fulfilled the requirements of the part 1 of the validation study. The number of absolute differences between device and observers within 5, 10, and 15 mmHg was 75/99, 90/99, and 96/99, respectively, for systolic blood pressure and 90/99, 99/99, and 99/99, respectively, for diastolic blood pressure. The device also fulfilled the criteria in part 2 of the validation study. Twenty-seven and 31 participants had at least two of the three device–observers differences less than or equal to 5 mmHg for systolic and diastolic blood pressure, respectively. All three device–observer differences were greater than 5 mmHg in two participants for systolic and in one participant for diastolic blood pressure.
Conclusion
The Somnotouch-NIBP noninvasive continuous blood pressure monitor has passed the requirements of the International Protocol revision 2010, and hence can be recommended for blood pressure monitoring in adults, at least under conditions corresponding to those investigated in our study.
Keywords: continuous blood pressure monitoring, pulse transit time, validation
Introduction
Conventional 24 h ambulatory blood pressure (BP) monitoring has several important limitations including the discontinuous nature of BP measurements and poor acceptance by many patients because of discomfort associated with repeated cuff inflations. The relatively high frequency of measurements during the night (typically every 15–30 min) may significantly worsen the quality of sleep. Even if in many patients this does not affect BP levels 1, in some, an increase in nocturnal BP can be induced, leading to a loss of its prognostic significance, as reported previously for untreated hypertensive patients with significant sleep deprivation during 24 h BP monitoring 2. Somnotouch-NIBP is a novel cuffless continuous BP monitor that can derive BP levels from pulse transit time (PTT) measurement on the basis of a stretch–strain relationship model, calibrated with BP from a single initial conventional BP measurement 3,4. The aim of the present study was to assess the accuracy of Somnotouch-NIBP according to the European Society of Hypertension International Protocol (ESH-IP) revision 2010 criteria 5
Materials and methods
Device
Somnotouch-NIBP (Somnomedics GmbH, Randersacker, Germany) is an ambulatory cuffless device for continuous (beat-to-beat), noninvasive BP monitoring. The system consists of a finger photoplethysmograph (providing additional oxygen saturation measurement) and three ECG leads, connected to a watch-like control unit placed at the wrist level and equipped with a screen where beat-to-beat pulse waveform, ECG, and PTT changes are displayed. The principle of BP estimation is based on the beat-to-beat determination of PTT, calculated as the interval between R-wave on ECG and the arrival of the corresponding pulse wave (determined from finger photoplethysmography signal) at the peripheral site. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) levels are calculated on the basis of the relationship between BP levels and PTT, where the increase in BP increases arterial wall tension, thus increasing its stiffness. Consequently, pulse wave propagation velocity increases, leading to a reduction in PTT. A nonlinear model describing this relationship and based on experimental data has been published 4. Combining this model with a single initial BP measurement (performed using the traditional technique at the level of the brachial artery) used for device calibration allows to derive beat-to-beat BP values corresponding to changing PTT.
Familiarization
Two weeks before the start of the study, several training sessions were carried out for observers and supervisor following the ESH-IP revision 2010. No problems occurred in this phase.
Recruitment
A total of 41 individuals of both sexes, aged 25 years or more, in sinus rhythm, either untreated or on stable treatment, with normal arm circumference (≥22 and ≤32 cm) and no significant peripheral artery disease were included in the study. They were recruited from among inpatients and outpatients of the Cardiology Unit of San Luca Hospital (Istituto Auxologico Italiano, Milan, Italy). The study protocol was approved by the Ethics Committee of Istituto Auxologico Italiano. All participants provided informed and written consent before the start of the study.
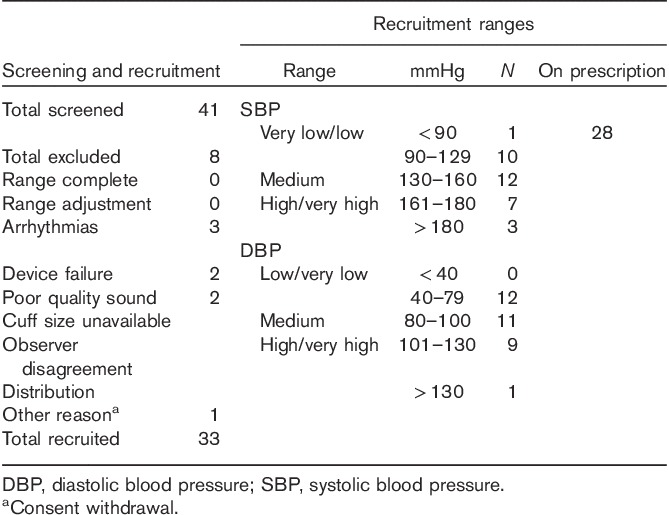
Eight individuals were excluded after enrollment, leaving 33 participants for analyses. The details on screening and recruitment as well as on the distribution in BP categories are shown in Table 1.
Table 1.
Screening and recruitment details
Procedure
The validation procedure was organized in accordance with the ESH-IP revision 2010 for the validation of BP measuring devices in adults 5, although because of the particular characteristics of the tested device, several adaptations were necessary. Overseen by a supervisor, auscultatory measurements were always recorded by two independent observers who were unaware of each other’s readings and of the tested device readings using a mercury sphygmomanometer and a binaural stethoscope. These measurements were performed in the sitting position with the forearm at the level of the heart. Phases I and V Korotkoff sounds were used to identify SBP and DBP levels, respectively. All auscultatory BP measurements considered for the present analysis were performed on the nondominant upper arm (corresponding to the left arm in all participants).
After performing entry BP measurements required to classify participants into BP categories, two tested devices were placed on both wrists and an additional auscultatory measurement, used later for signal calibration, was performed. To ensure short-term stability of device calibration, a 15 min rest was allowed before the start of validation measurements. Four auscultatory measurements were then performed while the Somnotouch-NIBP devices registered the values continuously throughout the duration of the procedure and the BP values for the tested device were only obtained by offline analysis (see below). A pause of 2 min was allowed between measurements. The supervisor registered the timing of each auscultatory BP measurement using the marker function of the tested device. The quality of ECG and photoplethysmography signals was checked periodically. No measurement failure for the device occurred during the study measurements, although in a few cases, the placement of the sensors (ECG electrodes or finger probe) had to be adjusted to obtain an adequate signal.
After completing the validation session, the tested devices were downloaded using the dedicated software (Domino Light; Somnomedics GmbH). To comply with the ESH-IP, the tested device placed on the nondominant arm was used for validation. During cuff inflation, the photoplethysmography signal is lost and direct calibration on the same arm is not possible. Therefore, the recording from the device on the dominant arm was first calibrated with auscultatory BP. Then, a BP value obtained with the dominant arm device was obtained at a different point and used to calibrate the device on the nondominant arm.
Validation BP values from the tested device were obtained by calculating the average SBP and DBP levels over good-quality segments of 20–30 s, beginning not less than 30 s after a complete cuff deflation. The values obtained were then compared with the adjacent auscultatory BP measurements as described in the ESH-IP.
Results
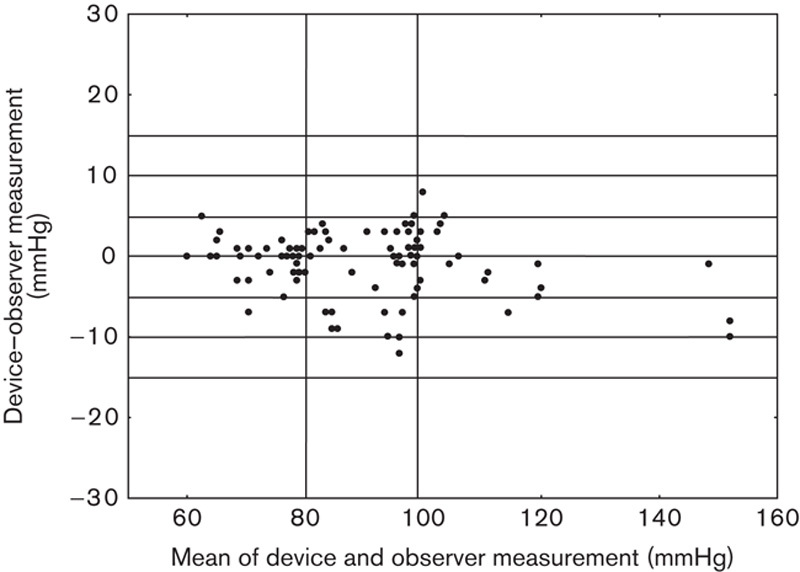
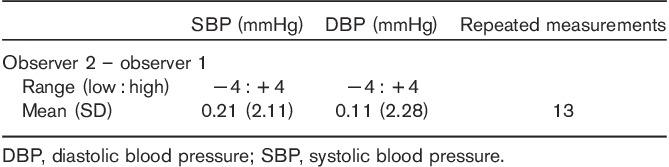
The results are presented in Tables 2–5, and in Figs 1 and 2. The average observer differences were less than 1 mmHg. The device–observer disagreement was −0.44±6.1 for SBP and −0.33±3.4 mmHg for DBP. Disagreement in SBP tended to increase at higher SBP levels, whereas at higher DBP levels, the tested device tended to underestimate DBP compared with the reference method (correlation between DBP discrepancy mean device and observer BP level r=−0.20, P=0.051; Fig. 1). A strong correlation was observed between Somnotouch-NIBP and auscultatory method for SBP (r=0.973) and for DBP (r=0.976).
Table 2.
Characteristics of the study population
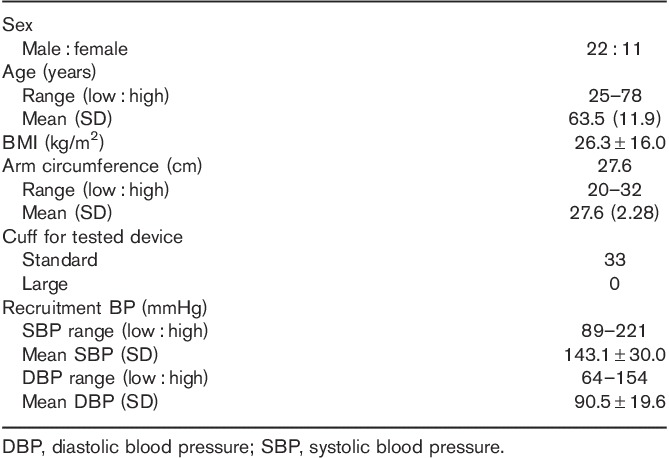
Table 5.
Validation results
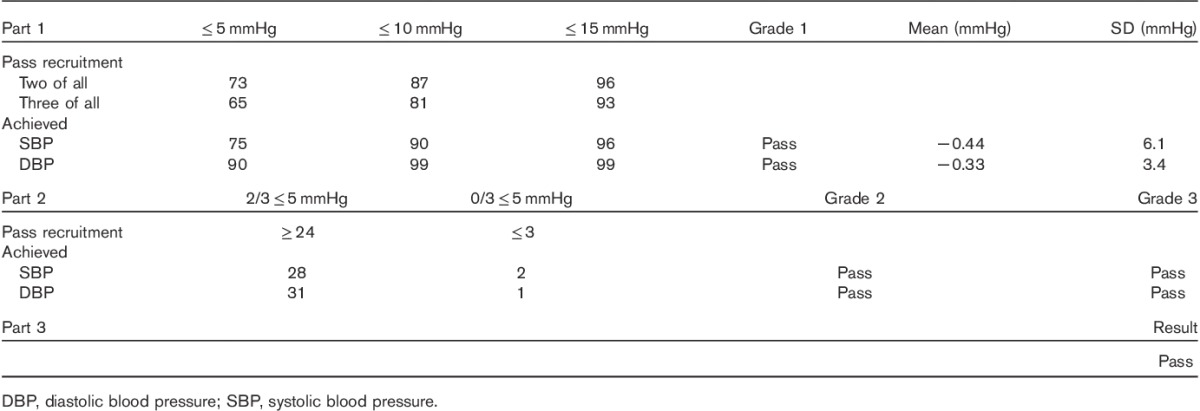
Fig. 1.
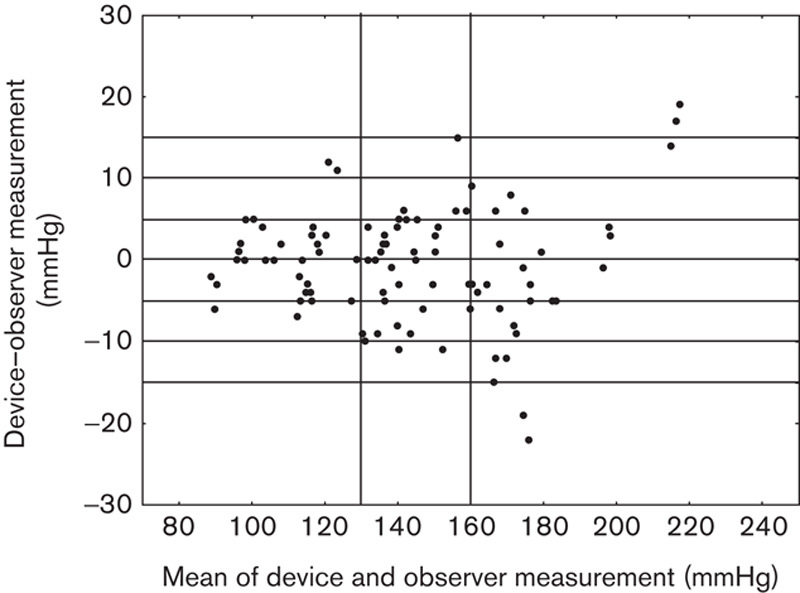
Scatter plot of the systolic blood pressure differences between the Somnotouch-NIBP auscultatory method (y-axis) against the average of device and auscultatory pressure values (x-axis).
Fig. 2.
Scatter plot of the diastolic blood pressure differences between the Somnotouch-NIBP auscultatory method (y-axis) against the average of device and auscultatory pressure values (x-axis).
Table 3.
Observer measurements in each recruitment range
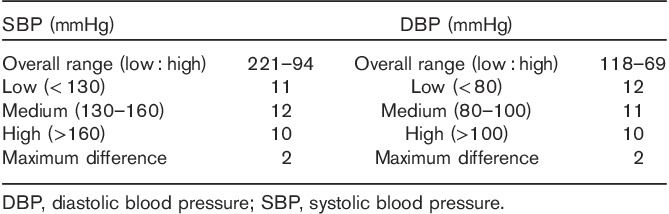
Table 4.
Observer differences
The tested device fulfilled all the ESH-IP 2010 criteria and passed all validation grades. The BP discrepancies did not vary according to sex (P>0.15). No correlation was found between the SBP or DBP discrepancies and age, body weight, arm circumference, and the mean device and observer BP level (all P>0.10), but a significant correlation was found between SBP discrepancy and height (r=0.40) also after adjusting for sex.
The tested device was well accepted by all participants. Except for two patients who were excluded because the quality of the ECG signal did not enable an accurate BP computation, no difficulties were found either in relation to the device performance or during the phase of data analysis.
Discussion
The results of the present study showed that the Somnotouch-NIBP noninvasive continuous BP monitor fulfills all validation criteria of ESH-IP 2010 both for SBP and for DBP levels 5. The accuracy of the device may be reduced in patients with very high BP and some degree of systematic underestimation of DBP may occur in such patients. We also found a correlation between SBP discrepancy and height, meaning that in taller patients, the device tended to overestimate SBP and in shorter patients, it tended to underestimate SBP. This might be a chance finding, but may also depend on the fact that the pulse wave travel distance used for calculating pulse wave velocity from PTT is estimated on the basis of the patient’s height.
Arguably, ESH-IP only tests device validity in the laboratory setting and, therefore, does not guarantee its performance under dynamic conditions over 24 h. This issue may be particularly relevant for a device whose operation principle relies on a one-point initial calibration. However, the accuracy of the device under dynamic conditions was previously shown in studies following ad-hoc protocols comparing Somnotouch-NIBP with the auscultatory method during continuous positive airway pressure application 3 and during exercise testing 4.
Conclusion
Somnotouch-NIBP complies with the ESH-IP standards and may be recommended as a method for BP monitoring, at least for the time window after calibration explored in our study. The cuffless and thus less interfering nature of the device may render it particularly useful in patients who poorly tolerate traditional ambulatory BP monitoring and whenever undisturbed assessment of sleep BP is of importance, for example, in patients with sleep disordered breathing.
Acknowledgements
Somnomedics GmbH provided support (funding, devices, and training in device use) for this study.
Conflicts of interest
G.P. received speaker’s fee from Somnomedics GmbH. For the remaining authors there are no conflicts of interest.
References
- 1.Parati G, Pomidossi G, Casadei R, Malaspina D, Colombo A, Ravogli A, Mancia G. Ambulatory blood pressure monitoring does not interfere with the haemodynamic effects of sleep. J Hypertens Suppl 1985; 3:S107–S109. [PubMed] [Google Scholar]
- 2.Verdecchia P, Angeli F, Borgioni C, Gattobigio R, Reboldi G. Ambulatory blood pressure and cardiovascular outcome in relation to perceived sleep deprivation. Hypertension 2007; 49:777–783. [DOI] [PubMed] [Google Scholar]
- 3.Schmalgemeier H, Bitter T, Bartsch S, Bullert K, Fischbach T, Eckert S, et al. Pulse transit time: validation of blood pressure measurement under positive airway pressure ventilation. Sleep Breath 2012; 16:1105–1112. [DOI] [PubMed] [Google Scholar]
- 4.Gesche H, Grosskurth D, Kuchler G, Patzak A. Continuous blood pressure measurement by using the pulse transit time: comparison to a cuff-based method. Eur J Appl Physiol 2012; 112:309–315. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien E, Atkins N, Stergiou G, Karpettas N, Parati G, Asmar R, et al. European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit 2010; 15:23–38. [DOI] [PubMed] [Google Scholar]


