Supplemental Digital Content is available in the text.
Keywords: cardiac arrhythmia, death, decision making, decision support techniques, sudden cardiac death, ventricular tachycardia, ventricular fibrillation
Background—
A conceptualized model may be useful for understanding risk stratification of primary prevention implantable cardioverter defibrillators considering the competing risks of appropriate implantable cardioverter defibrillator shock versus mortality.
Methods and Results—
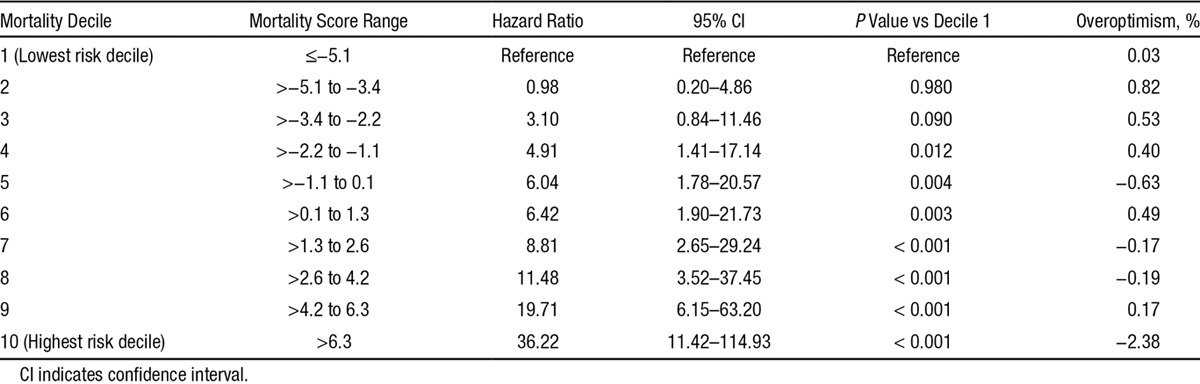
In a prospective, multicenter, population-based cohort with left ventricular ejection fraction ≤35% referred for primary prevention implantable cardioverter defibrillator, we developed dual risk stratification models to determine the competing risks of appropriate defibrillator shock versus mortality using a Fine-Gray subdistribution hazard model. Among 7020 patients referred, 3445 underwent defibrillator implant (79.7% men, median, 66 years [25th, 75th: 58–73]). During 5918 person-years of follow-up, appropriate shock occurred in 204 patients (3.6 shocks/100 person-years) and 292 died (4.9 deaths/100 person-years). Competing risk predictors of appropriate shock included nonsustained ventricular tachycardia, atrial fibrillation, serum creatinine concentration, digoxin or amiodarone use, and QRS duration near 130-ms peak. One-year cumulative incidence of appropriate shock was 0.9% in the lowest risk category, and 1.7%, 2.5%, 4.9%, and 9.3% in low, intermediate, high, and highest risk groups, respectively. Hazard ratios for appropriate shock ranged from 4.04 to 7.79 in the highest 3 deciles (all P≤0.001 versus lowest risk). Cumulative incidence of 1-year death was 0.6%, 1.9%, 3.3%, 6.2%, and 17.7% in lowest, low, intermediate, high, and highest risk groups, respectively. Mortality hazard ratios ranged from 11.48 to 36.22 in the highest 3 deciles (all P<0.001 versus lowest risk).
Conclusions—
Simultaneous estimation of risks of appropriate shock and mortality can be performed using clinical variables, providing a potential framework for identification of patients who are unlikely to benefit from prophylactic implantable cardioverter defibrillator.
Implantable cardioverter defibrillators (ICDs) reduce the risk of arrhythmic and all-cause death in patients with left ventricular systolic dysfunction, who have not previously experienced a cardiac arrest or symptomatic arrhythmia.1,2 The initial consideration for eligibility in randomized controlled trials examining the efficacy of primary prevention ICDs is a reduced left ventricular ejection fraction (LVEF). However, although reduced LVEF is an important marker signifying increased risk of cardiovascular mortality, it is a predictor of both arrhythmic and nonarrhythmic death.3,4 There is need for a conceptual framework to understand how risk stratification methods could potentially be used to improve the efficiency of ICD use.5
Editorial see p 847
Clinical Perspective on p 937
There are benefits to an enhanced ability to risk stratify patients referred for primary prevention ICD because some patients who fulfill primary prophylactic ICD criteria may not experience a benefit from the implanted defibrillator or may die of other cardiac or noncardiovascular causes despite device implantation.6 The challenge of risk stratification in ICD candidates is that occurrence of death and electric device therapies are competing events, which adds statistical complexity. Competing risk models that provide information on both ICD-delivered therapies and death could provide a framework for conceptualizing the outcomes and potential benefits of prophylactic defibrillator implantation. The absence of clinical risk algorithms to guide decisions in potential ICD candidates may lead to insufficient opportunity for a personalized discussion of the potential for benefit from device implantation.
In this study, we report the primary findings from the Ontario ICD Database—a prospective, population-based study of patients referred for an ICD at all device implantation centers in the most populous province of Canada. In this registry, mandated by the single-payer of health services, we examined predictors of the competing risks of appropriate ICD shock versus death. The aim of this study was to develop a conceptualized model for identification of patients who simultaneously demonstrate reduced likelihood of ICD benefit because of low risk of an appropriate device shock and high risk of death—the bimodal survival and implantable defibrillator shock (BaSIS) risk model.
Methods
Patient Sample
We studied patients enrolled in the Ontario ICD Database, a prospective registry of patients evaluated for ICDs in Ontario, Canada, from February 2007 to March 2011, with last follow-up on May 14, 2012. The design and methodology of the Ontario ICD Database have been described previously.7 Briefly, all patients referred for evaluation in ICD clinics were enrolled into the registry with detailed clinical data collection at baseline, at the time of device implant, and at follow-up visits in defibrillator clinics. This registry was mandated by the Ontario Ministry of Health and Long-Term Care to collect data on patients ≥18 years of age evaluated for ICDs. As a prescribed entity under Ontario’s health information privacy legislation, we were able to collect registry data without patient consent; therefore, all patients underwent data collection without participant bias. In this study, we analyzed patients with LVEF ≤35% who were ambulatory (nonhospitalized) and referred for a de novo primary prevention ICD.1,2 We excluded those with previous ventricular tachycardia (VT) or ventricular fibrillation, and those with inducible VT at electrophysiological study. Patients with paced rhythm were excluded to allow for measurement of the native QRS duration (QRSd) in all patients because of its previously recognized importance in risk stratification.8 To ensure a homogeneous primary prevention cohort, we also excluded patients with specialized indications for ICDs, including hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, long QT syndrome, Brugada syndrome, those treated with class 1C arrhythmic drugs, congenital heart disease, and infiltrative cardiomyopathies.
Data Sources
At each of the 10 ICD implantation sites in Ontario, data were collected by a trained research coordinator and electrophysiologist and entered into a real-time, password- and firewall-protected web database at the Institute for Clinical Evaluative Sciences over secure SSL-certified connections. These data included clinical characteristics, defibrillator implant–related data, and cardiac and noncardiac conditions. Occurrences of device therapies were identified at each defibrillator clinic follow-up visit and these data were entered at the 18 ICD follow-up sites in real-time into a secure, web-based database (Appendix I in the Data Supplement). Data quality was continually assessed by: (1) regular review and correspondence with study sites to ensure data accuracy, (2) automated notification of uncoded data, (3) logic and range checks, (4) independent physician review of appropriate therapy determination, and (5) random site audits for data reliability.
All-cause mortality was determined using the Registered Persons Database, and hospitalizations were identified via the Canadian Institute for Health Information Discharge Abstract Database using the International Classification of Diseases Tenth Revision (ICD-10-CA) coding system. Ethical approval was obtained from Sunnybrook Health Sciences Center.
Outcome Events
The coprimary outcomes were an appropriate shock delivered by the ICD for VT/ventricular fibrillation and the occurrence of death, which was treated as a competing event. ICD electrograms were examined on-site by an experienced cardiac electrophysiologist, and independently reviewed by ≤2 electrophysiology experts who were blinded to patient and provider information. Any discrepancies in the classification of appropriate shocks between the site and the first review panel electrophysiologist were arbitrated by a second reviewing electrophysiologist. The κ-statistic for agreement between the on-site electrophysiologist and external review panel was 0.928 for appropriate shock, as detailed previously.9 Standardized programming was not mandatory, but strategies for strategically delayed programming were generally adopted after publication of the Primary Prevention Parameters Evaluation study results.10 Deaths were determined at clinic visits and via linkages with the Registered Persons vital statistics database using the patient’s unique, encrypted health card number. Vital status information was available for all study patients.
Predictor Variables
Two separate models were developed for predicting the outcomes of appropriate shock or death. Potential predictors for these models included demographics (age and sex), ventricular arrhythmia history (nonsustained VT, and syncope), disease pathogenesis (eg, ischemic versus cardiomyopathy versus other), coronary revascularization procedures (eg, percutaneous coronary intervention or coronary artery bypass graft surgery), previous heart failure (HF) hospitalizations, Canadian Cardiovascular Society angina class, New York Heart Association classification, pre-existing pacemaker system, and previous or current atrial fibrillation. We also examined the following noncardiac factors: diabetes mellitus, stroke or transient ischemic attack, cigarette smoking (current or past), peripheral vascular disease, chronic obstructive lung disease, cognitive impairment, and home oxygen use. We examined the use of cardiac medications (eg, β-adrenoreceptor antagonists, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker [ARB], loop diuretic, digoxin, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor, and amiodarone), and laboratory investigations, including QRSd, LVEF, left ventricular end-systolic dimension, left atrial size, serum creatinine, serum sodium, hemoglobin concentration, body weight, and systolic blood pressure. We also examined the association of ICD type (cardiac resynchronization therapy defibrillator versus dual-chamber device versus single-chamber device) with competing risks of appropriate shock and death.
Statistical Analysis
We reported continuous variables using median (25th, 75th percentile) values, although categorical variables were reported as frequencies. Time-to-event outcomes were reported as events per 100 person-years follow-up. Because patients who die early are no longer at risk for an appropriate therapy or shock, our analyses were conducted using a competing risk framework.11 Predictors were identified using the Fine-Gray subdistribution hazard model because our focus was on incidence and prognosis rather than on pathogenesis.12 Univariate predictors significant at a P<0.25 level were considered for inclusion in the multivariable model. We performed stepwise modeling, retaining variables that were significant at P<0.05 or that were deemed a priori to be clinically important in the multivariable model. Continuous variables were examined using cubic spline analysis to determine the strength and shape of the association with appropriate shocks, and upper and lower thresholds were identified. Using the fitted competing risk regression models, age-standardized regression coefficient-based risk scores for each of the 2 outcomes were developed using an approach similar to that used in the Framingham Risk Score.13 As performed previously, risk scores were corrected by shifting all scores upward or downward such that the final ICD shock and death scores had median values near zero.14 As we have done previously, after initially stratifying subjects into risk score quintiles, the quintiles corresponding to highest risk of death and lowest probabilities of appropriate shock were further subdivided into deciles, while less-informative intermediate risk quintiles were aggregated.14 The incidence of events was estimated in each risk stratum using cumulative incidence functions that accounted for the presence of the competing event.
For validation, we used a bootstrap-based method to correct for optimism in our estimates of incidence in each of the risk strata. To do so, we resampled with replacement, creating 100 bootstrap samples drawn from the original study cohort. In each of these 100 bootstrap samples, we repeated all modeling steps described above for deriving the initial model (including the univariate analyses, subsequent variable selection, and graphical examination of continuous variables for potential transformation and imposition of thresholds) to derive a new risk score and estimates of outcome incidence in each stratum.15 Iteratively, a predictive model and corresponding point-based scoring system was developed in each bootstrap sample and then divided into 10 risk strata using point score deciles. Using cumulative incidence functions, incident outcomes were determined in each risk stratum. We calculated the difference in incidence in a given risk stratum between the bootstrap sample and the overall cohort as an estimate of optimism (or bias), which was then averaged over the 100 bootstrap repetitions.
Analyses were performed using SAS version 9.3 (Cary, NC) and the R statistical programming language version X. The subdistribution hazard models were fit using the crr function in the cmprsk package for R. Two-sided P<0.05 were considered statistically significant.
Results
Patient Characteristics
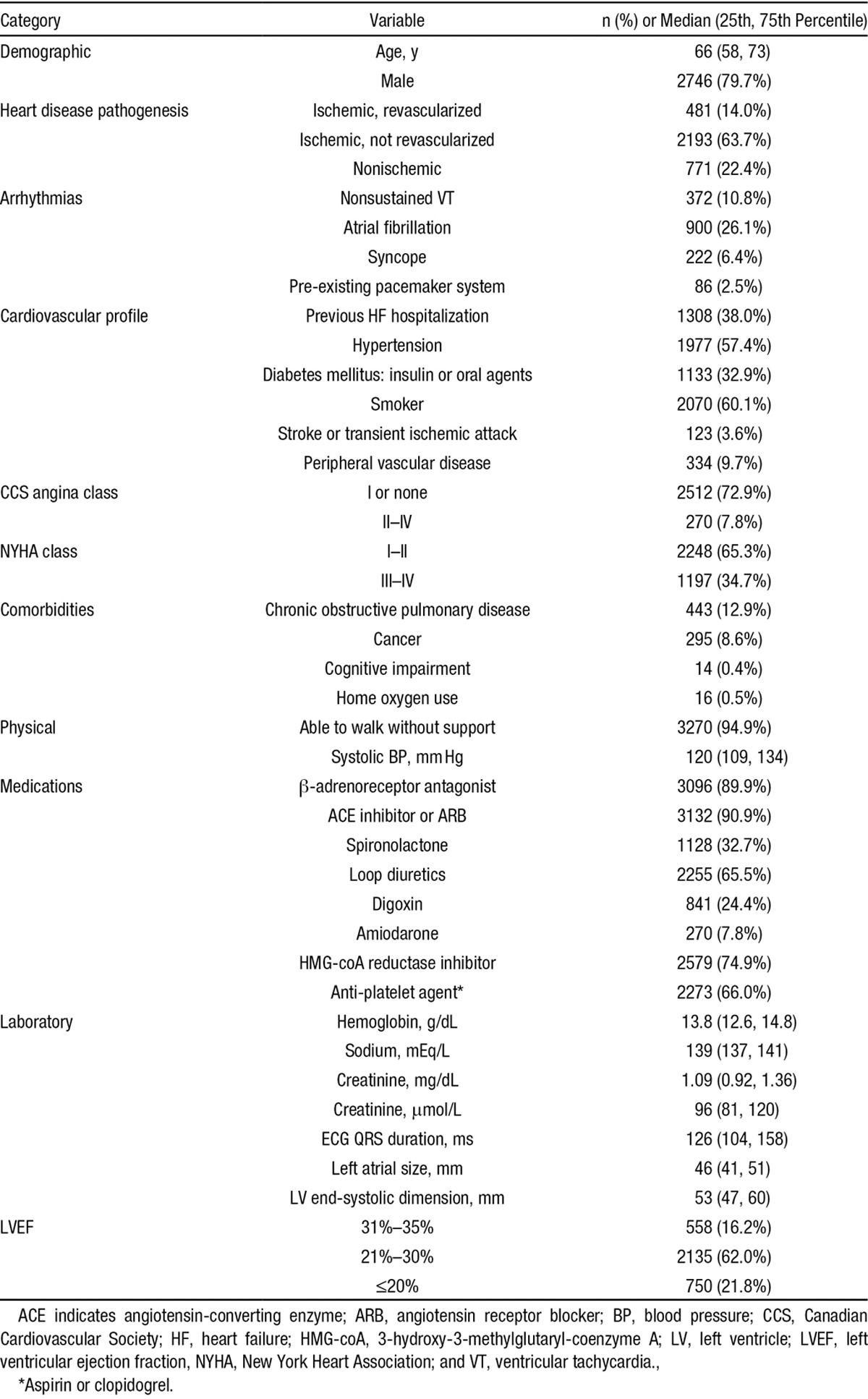
Among 7020 referrals for primary prevention ICD with LVEF ≤35%, 1794 patients refused the device, whereas 717 primary prevention ICD patients were excluded because they were unlinkable to administrative databases, had missing data, or because native QRSd was unmeasurable (Figure 1). There were no differences in age and sex between primary prevention patients in the included versus excluded study cohorts (Table I in the Data Supplement). The final study cohort was 3445 patients (median age, 66 [58, 73] years) comprised of 2746 (79.7%) men. There were 1552 single-chamber (45.1%), 726 dual-chamber (21.1%), and 1165 cardiac resynchronization therapy defibrillator (CRT-D; 33.8%) devices implanted. Cohort characteristics are shown in Table 1.
Figure 1.
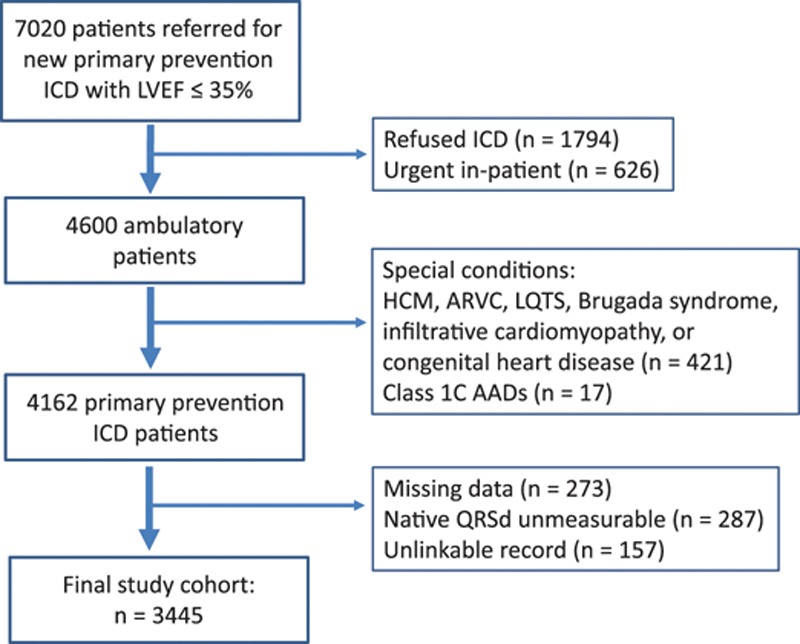
Patient flow diagram. AAD indicates antiarrhythmic drug; ARVC, arrythmogenic right ventricular cardiomyopathy; HCM, hypertrophic cardiomyopathy; ICD, implantable cardioverter defibrillator; LQTS, long QT syndrome; LVEF, left ventricular ejection fraction; and QRSD, QRS duration.
Table 1.
Baseline Cohort Characteristics (n=3445)
Outcome Events
A total of 5918 person-years of follow-up were examined during which 292 deaths (4.9 deaths/100 person-years) occurred. Median follow-up was 2.0 (1.5, 2.0) years. Appropriate shock occurred in 204 patients (3.6 shocks/100 person-years). The proportion of patients surviving ≥30 days after appropriate shock was 98.5%, suggesting that an ICD shock was not associated with high absolute rate of short-term mortality. There were no losses to follow-up for mortality, and 2917 (84.7%) of the study cohort had follow-up in the defibrillator clinic at least 365 days after ICD implant.
Competing Risk Regression Models
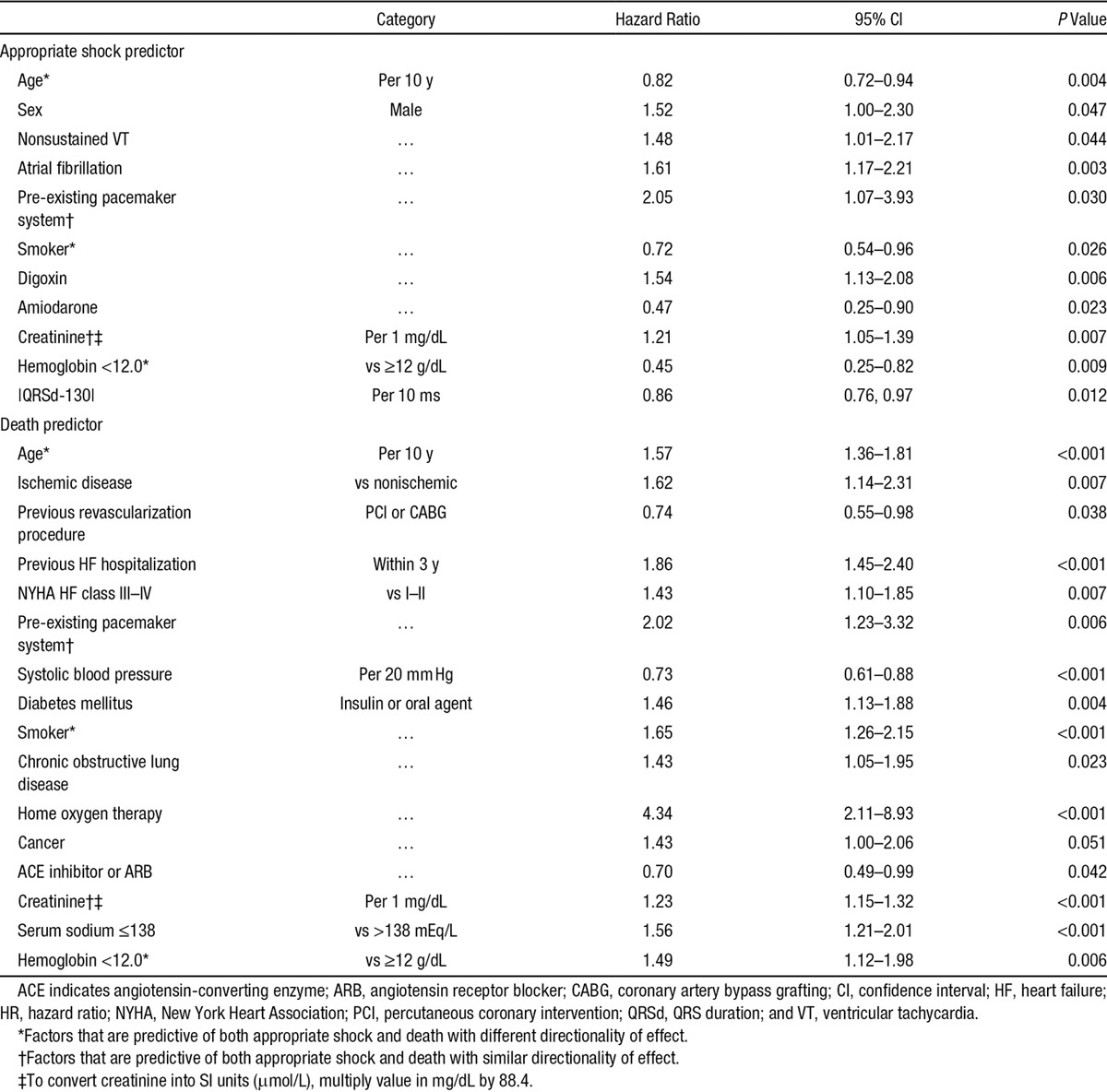
Univariate predictors of appropriate shock are shown in Table II in the Data Supplement. Multivariable predictors of appropriate shock from the competing risk models (Table 2) included previously documented nonsustained VT, atrial fibrillation, higher serum creatinine, and the absolute difference of QRSd from a high risk value of 130 ms (ie, |QRSd=130|). The QRSd risk function was based on cubic spline analyses demonstrating that risk of appropriate shock was maximal at 130 ms and decreased with both shorter and longer QRSd values. Predictors of death competing with occurrence of ICD shock included lower systolic blood pressure, which demonstrated a plateau at values exceeding 130 mm Hg (Table 2).
Table 2.
Multivariable Predictors of Appropriate Shock Competing With Death
Scoring System for ICD Outcomes
The ICD appropriate shock score is shown in Table 3 and the mortality score is shown in Table 4. Among the 22 variables in the competing risk models, 17 (77.3%) were predictive of only 1 outcome—either shock or death. The ICD shock score was normally distributed with near-zero median value −0.08 (−3.66, 3.72). Similarly, the mortality score was normally distributed with median 0.11 (−2.80, 3.33).
Table 3.
Point Score for Appropriate Shock
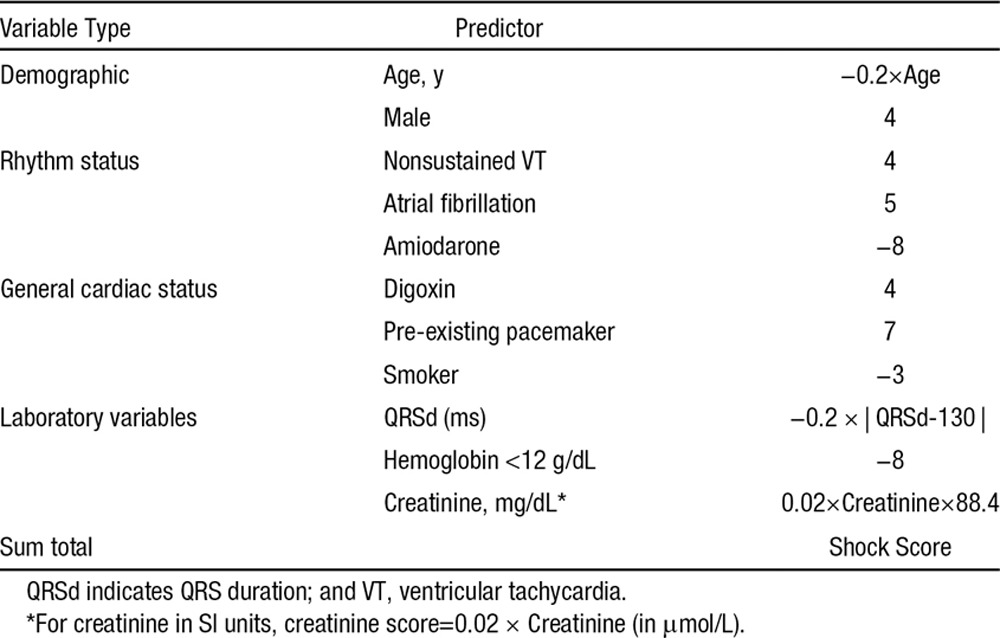
Table 4.
Point Score for Death
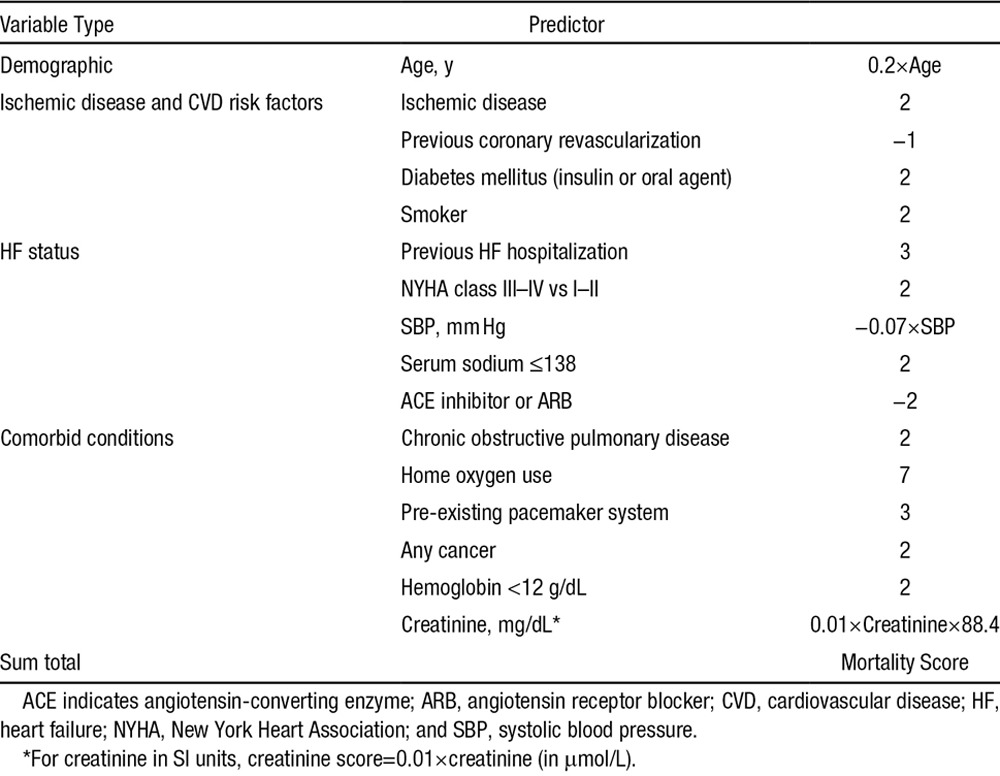
Outcomes by Shock, Therapy, and Death Scores
Cumulative incidence of appropriate shock at 1 year postimplantation was 0.9% in the lowest (decile 1), 1.7% in low, 2.5% in intermediate, 4.9% in high, and 9.3% in the highest risk groups (Figure 2). The cumulative incidence of death at 1 year was 0.6% in the lowest, 1.9% in low, 3.3% in intermediate, 6.2% in high, and 17.7% in the highest (decile 10) risk groups at 1-year follow-up (Figure 3). There was a graded increase in risk with higher score categories, with an adjusted hazard of appropriate shock that was 7.8-fold and mortality risks that were 36-fold higher in the highest risk decile versus the first decile (Tables 5 and 6). In the validation analysis, the score-predicted risks of appropriate shock and death in each decile were comparable with bootstrap-corrected estimates confirming that the model was not overoptimistic (Tables 5 and 6).
Figure 2.
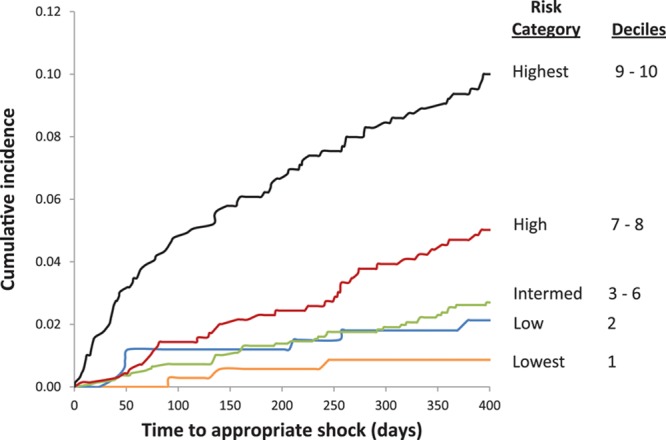
Cumulative incidence of appropriate shock.
Figure 3.
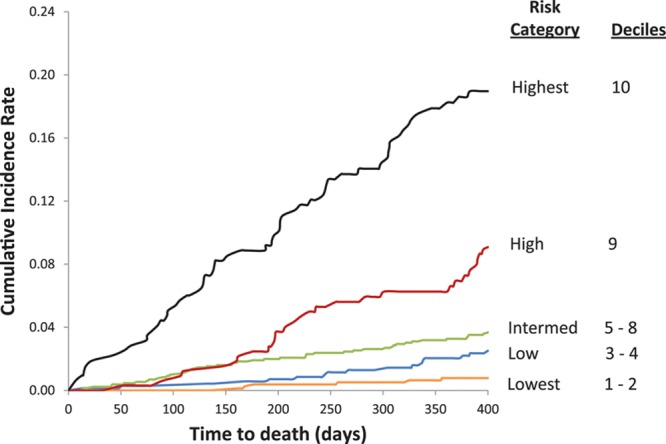
Cumulative incidence of death.
Table 5.
Relative Hazards and Validation Analysis for Appropriate Shock
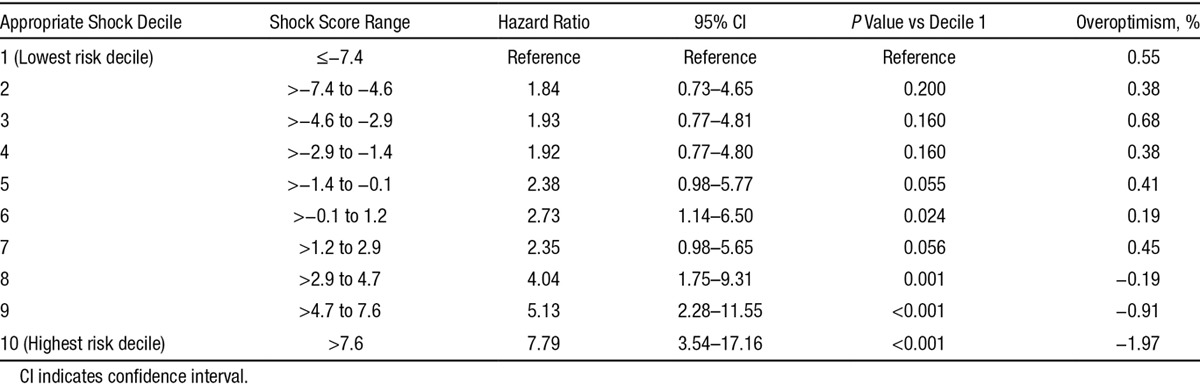
Table 6.
Relative Hazards and Validation Analysis for Death
CRT-D Subanalysis
Although ICD device type was not a significant predictor of the competing risks of appropriate shock or death, we tested for all 1-way interactions with CRT-D device type. There were no significant interactions between CRT-D with any of the predictors of mortality. For the competing risk of appropriate shock, none of the variables interacted with CRT-D except serum creatinine (P interaction=0.011). Inclusion of CRT-D and CRT-D×creatinine interaction terms into the model reduced the cumulative incidence of appropriate shock in the lowest risk decile to 0.6%, and increased the cumulative incidence of death marginally in the highest mortality decile to 18.1% at 1 year.
Bimodal Risk Scores
A scatterplot of predicted risk scores for estimation of cumulative incidence of appropriate shock (x axis) versus death (y axis) at 1-year follow-up is shown in Figure 4. The vertical dashed line demarcates the lowest decile of shock scores to the left of the line, whereas the horizontal dashed line identifies the highest decile of mortality scores above. Patients were divided into 4 groups: (1) quadrant I, lowest risk of appropriate shock and highest risk of death (blue diamonds); (2) quadrant II, lowest risk of appropriate shock (red squares); (3) quadrant III, increased risk of both death and appropriate shock (green triangles); and (4) quadrant IV, all remaining patients with high potential to benefit from primary prevention ICD. Compared with the 2847 (82.6%) patients in quadrant IV (high benefit), the 86 (2.5%) patients in quadrant I (lowest benefit) exhibited features consistent with worse HF status. They were more often in New York Heart Association class III to IV (29.0 versus 67.4%), previously hospitalized for HF (33.1 versus 81.4%), and more often on loop diuretics (62.2 versus 89.5%) than the high benefit group (all P<0.001).
Figure 4.
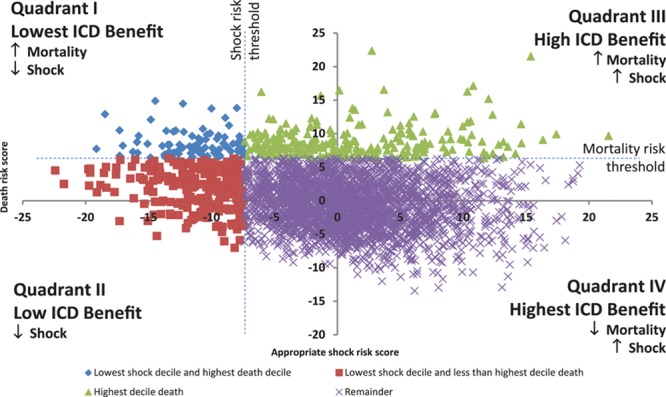
Depiction of conceptual model of predicted risks of appropriate shock vs death using quadrants; number of patients in quadrants I (n=86), II (n=262), III (n=250), and IV (2847). ICD indicates implantable cardioverter defibrillator.
Discussion
The rise of ICD implant rates has generated numerous considerations for healthcare providers and policy makers because they grapple with translation of evidence from clinical trials to the population. Risk stratification methods may allow treatment recommendations to be customized according to patient characteristics. The need for risk stratification methods for primary prevention ICDs was the impetus for the array of cardiac imaging and electrodiagnostic modalities, which were proposed even before the development of clinical risk models. Consequently, novel cardiac imaging and electrophysiological techniques for sudden death risk stratification have not been compared with multivariate clinical risk models.16,17 In the absence of an adequate risk stratification method, patients who are at lower risk of arrhythmia and greater risk of nonarrhythmic death may be implanted, with the potential for reduced benefit resulting from mismatch between risk and treatment.
In this population-based prospective study of ICD recipients, we used a competing risk framework to derive an exemplar for simultaneous prediction of low incidence of defibrillator shock and high risk of death. We used competing risk methodology to construct this conceptualized model because patients who survive longer remain at risk for an appropriate ICD shock, whereas those who die are no longer at risk for an arrhythmic event. Factors that conferred increased risk of appropriate shock included a history of nonsustained VT, atrial fibrillation, pacemaker system in situ, and higher creatinine concentration. Patients taking amiodarone were at decreased risk while those requiring digoxin were at increased risk of an appropriate shock. Interestingly, values of QRSd near 130 ms exhibited the highest risk of appropriate shock, and shorter or longer values from this peak demonstrated lower hazards. The covariates included in the risk model have been identified as potential predictors of sudden cardiac death or appropriate device shock for isolated outcomes,9,18–21 confirming the importance of these variables. However, previous studies were unable to risk stratify patients with low potential benefit from an ICD using a competing risks analytic framework, and have not developed a method to simultaneously predict the risk of both defibrillator shocks and death.
Previous studies examined only the isolated outcome of death among ICD candidates, and the risk factors overlap with the known prognostic factors for HF death.22–24 The occurrence of and number of previous HF hospitalizations, and worse New York Heart Association class have been associated with higher mortality.23,25,26 Furthermore, other prognostic factors, such as renal dysfunction, diabetes mellitus, not taking an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, and with lower systolic blood pressure or serum sodium concentration have been associated with higher mortality risk.23,25 Among the mortality predictors, a notable indicator of increased risk was ischemic heart disease,27 whereas those who were revascularized exhibited reduced mortality risk. The above factors, in conjunction with the noncardiac predictors of mortality reflect greater comorbidity burden and associated higher levels of frailty that often coexist with older age.28 Although it is important to know that the mortality of ICD candidates will be high, it is only one component of the decision-making process because some patients will not experience therapeutic intervention from the device.
Few published studies have described clinical approaches to risk stratification with the intent to identify patients who are more or less likely to benefit from ICD implantation. The Multicenter Automatic Defibrillator Implantation Trial (MADIT-2) risk score was designed as a simple method to predict ICD benefit, and a biphasic pattern of ICD benefit was reported.29 It is interesting, however, that among the 5 predictive factors in the MADIT-2 risk score, 2 variables (ie, atrial fibrillation and QRSd) were predictors of appropriate shock, whereas other characteristics (ie, New York Heart Association class and older age) were associated with death in our competing risk analysis. The intermixing of these variables, which were differently associated with death or appropriate shock, may partly explain the biphasic pattern in the MADIT-2 risk score. It is interesting to note that low hemoglobin and smoking were associated with higher mortality but lower likelihood of appropriate shock, possibly indicating that the increased risk of death with these factors was not mediated by ventricular arrhythmia. The Seattle Heart Failure Model was extended to estimate mean survival among advanced HF patients with versus without a defibrillator implanted, however, appropriate ICD therapy was not examined as an outcome.30 Concomitant knowledge of the risk of appropriate shocks and death may inform risk stratification, by dividing patients into 4 competing risk groups with differing potential ICD benefit. There may be a continuum of benefit from a prophylactic ICD, being lowest in quadrant I, low in quadrant II, high in quadrant III, and of highest benefit in quadrant IV with highest probability of appropriate ICD shock and lowest mortality (Figure 4).
There are several potential applications of a clinical risk algorithm for primary prevention ICD candidacy. The decision to implant an ICD must be considered carefully because it commits the patient to an invasive treatment strategy, which includes repeat device-related procedures, potential complications, and reduced quality of life from shock-related pain.31,32 Cardiac specialists could use decision support algorithms before electrophysiology referral to enable more informed, shared decisions about potential risk-benefit tradeoffs from ICD implantation. In our conceptual model, patients who are at low risk of appropriate shock and high risk of death (quadrant I, Figure 4), could engage in discussions with their caregivers to potentially obviate ICD implantation.33 Among those who are at low risk for an appropriate shock, but not at high risk of death (quadrant II), there may be an opportunity for shared decision making to optimize medical therapy, reassess the degree of LVEF recovery, and re-evaluate the decision to implant an ICD at a subsequent annual visit. Finally, risk models could provide a clinical comparator for determining the incremental prognostic value or net reclassification improvement of electrophysiological tests and advanced imaging modalities (eg, cardiac magnetic resonance imaging), which have been proposed for risk stratification of ICD candidates.
There are some notable limitations of our study. First, the predictive model was not used to decide on implantation of the ICD. However, independence of the decision to implant an ICD from a predictive model is required to obtain unbiased estimates of effect to better reflect the broad patient cohort in whom the decision algorithm may be applied. Second, our model was not validated in an independent external data set or using a split-sample approach. We validated our model internally using bootstrap resampling, which has been demonstrated to be superior to traditional split-sample derivation–validation, provides greater certainty of model performance, and results in estimates with lower mean squared error that those obtained using split-sample validation.34 At the current time, without external validation, BaSIS cannot be actioned into policy change, but it does provide a method by which several important predictors can be combined to conceptualize the potential benefits of ICD implantation. The gains in life expectancy from assigning patients to an ICD cannot be determined from the BaSIS risk score because the study did not randomly assign treatment intervention. The BaSIS model was derived in ambulatory patients in Canada, and generalizability to those in other jurisdictions and those hospitalized in the acute care setting is unknown. These limitations were outweighed by the unique strengths of our study, including its prospective design, completeness and careful ascertainment of device outcomes, and its population-based nature where all patients were recruited without the need to obtain informed consent and the attendant risks of selection bias.
In an exploration of patients with left ventricular systolic dysfunction, we found that the risks of appropriate ICD shock and mortality can be determined simultaneously using clinical variables alone. The competing events framework allows for a conceptual model of dual risk stratification, which could potentially assist shared decisions to defer or not implant an ICD when the anticipated benefits of prophylactic defibrillator implantation are low. The BaSIS risk scores also provide a potential clinical comparator for examining the incremental prognostic value of advanced cardiac imaging or electrophysiological procedures for risk stratification.
Sources of Funding
The Institute for Clinical Evaluative Sciences (ICES) is supported, in part, by a grant from the Ontario Ministry of Health and Long Term Care. The opinions, results and conclusions are those of the authors and no endorsement by the Ministry of Health and Long-Term Care or by the ICES is intended or should be inferred. This research was supported by an operating grant from the Canadian Institutes of Health Research (CIHR MOP 111150) and the Ontario Ministry of Health and Long-Term Care. Dr Lee is a clinician-scientist of the CIHR. Dr Austin is a career investigator of the Heart and Stroke Foundation of Ontario. Dr Tu is a career investigator of the Heart and Stroke Foundation of Ontario and a Canada Research Chair in health services research.
Disclosures
Dr Yee is a consultant and has received speaker’s fees from Medtronic. The other authors report no conflicts.
Supplementary Material
Footnotes
The Data Supplement is available at http://circheartfailure.ahajournals.org/lookup/suppl/doi:10.1161/CIRCHEARTFAILURE.115.002414/-/DC1.
CLINICAL PERSPECTIVE
Implantable cardioverter defibrillators (ICDs) for primary prevention of sudden cardiac death are considered among patients with reduced left ventricular ejection fraction. However, although left ventricular ejection fraction is an important marker signifying increased risk of cardiovascular mortality, it is a predictor of both arrhythmic and nonarrhythmic death. Several potential predictors of death have been identified among ICD candidates. However, few methods have been developed that can predict arrhythmic risk and mortality simultaneously. A conceptual model may be useful for understanding risk stratification of primary prevention ICDs considering the competing risks of appropriate ICD shock versus mortality. We studied 3445 ambulatory patients in the Ontario ICD Database, a prospective, population-based study of those undergoing defibrillator implantation. Using a Fine-Gray subdistribution hazard model, we developed dual risk stratification models for the competing risks of appropriate ICD shock versus mortality. We propose a conceptual framework where concomitant knowledge of the risk of appropriate shocks and death may inform risk stratification, by dividing patients into 4 competing risk groups with differing potential for ICD benefit. Simultaneous estimation of risks of appropriate shock and mortality can be performed using clinical variables, providing a potential framework for identification of patients who are unlikely to benefit from prophylactic ICD.
References
- 1.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 2.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 3.Lee DS, Gona P, Albano I, Larson MG, Benjamin EJ, Levy D, Kannel WB, Vasan RS. A systematic assessment of causes of death after heart failure onset in the community: impact of age at death, time period, and left ventricular systolic dysfunction. Circ Heart Fail. 2011;4:36–43. doi: 10.1161/CIRCHEARTFAILURE.110.957480. doi: 10.1161/CIRCHEARTFAILURE.110.957480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Priori SG, Aliot E, Blomstrom-Lundqvist C, Bossaert L, Breithardt G, Brugada P, Camm AJ, Cappato R, Cobbe SM, Di Mario C, Maron BJ, McKenna WJ, Pedersen AK, Ravens U, Schwartz PJ, Trusz-Gluza M, Vardas P, Wellens HJ, Zipes DP. Task force on sudden cardiac death of the European Society of Cardiology. Eur Heart J. 2001;22:1374–1450. doi: 10.1053/euhj.2001.2824. doi: 10.1053/euhj.2001.2824. [DOI] [PubMed] [Google Scholar]
- 5.Fishman GI, Chugh SS, Dimarco JP, Albert CM, Anderson ME, Bonow RO, Buxton AE, Chen PS, Estes M, Jouven X, Kwong R, Lathrop DA, Mascette AM, Nerbonne JM, O’Rourke B, Page RL, Roden DM, Rosenbaum DS, Sotoodehnia N, Trayanova NA, Zheng ZJ. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation. 2010;122:2335–2348. doi: 10.1161/CIRCULATIONAHA.110.976092. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koller MT, Schaer B, Wolbers M, Sticherling C, Bucher HC, Osswald S. Death without prior appropriate implantable cardioverter-defibrillator therapy: a competing risk study. Circulation. 2008;117:1918–1926. doi: 10.1161/CIRCULATIONAHA.107.742155. doi: 10.1161/CIRCULATIONAHA.107.742155. [DOI] [PubMed] [Google Scholar]
- 7.Lee DS, Birnie D, Cameron D, Crystal E, Dorian P, Gula LJ, Healey JS, Janmohammed A, Khaykin Y, Krahn AD, LeFeuvre C, Simpson CS, Yee R, Hardy J, Slaughter PM, Chen Z, Alter DA, Laupacis A, Tu JV Population-Based Registry of Implantable Cardioverter Defibrillators. Design and implementation of a population-based registry of Implantable Cardioverter Defibrillators (ICDs) in Ontario. Heart Rhythm. 2008;5:1250–1256. doi: 10.1016/j.hrthm.2008.05.015. doi: 10.1016/j.hrthm.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Saxon LA, Bristow MR, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, Feldman AM, Galle E, Ecklund F. Predictors of sudden cardiac death and appropriate shock in the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Trial. Circulation. 2006;114:2766–2772. doi: 10.1161/CIRCULATIONAHA.106.642892. doi: 10.1161/CIRCULATIONAHA.106.642892. [DOI] [PubMed] [Google Scholar]
- 9.MacFadden DR, Crystal E, Krahn AD, Mangat I, Healey JS, Dorian P, Birnie D, Simpson CS, Khaykin Y, Pinter A, Nanthakumar K, Calzavara AJ, Austin PC, Tu JV, Lee DS. Sex differences in implantable cardioverter-defibrillator outcomes: findings from a prospective defibrillator database. Ann Intern Med. 2012;156:195–203. doi: 10.7326/0003-4819-156-3-201202070-00007. doi: 10.7326/0003-4819-156-3-201202070-00007. [DOI] [PubMed] [Google Scholar]
- 10.Wilkoff BL, Williamson BD, Stern RS, Moore SL, Lu F, Lee SW, Birgersdotter-Green UM, Wathen MS, Van Gelder IC, Heubner BM, Brown ML, Holloman KK PREPARE Study Investigators. Strategic programming of detection and therapy parameters in implantable cardioverter-defibrillators reduces shocks in primary prevention patients: results from the PREPARE (Primary Prevention Parameters Evaluation) study. J Am Coll Cardiol. 2008;52:541–550. doi: 10.1016/j.jacc.2008.05.011. doi: 10.1016/j.jacc.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Dorian P, Hohnloser SH, Thorpe KE, Roberts RS, Kuck KH, Gent M, Connolly SJ. Mechanisms underlying the lack of effect of implantable cardioverter-defibrillator therapy on mortality in high-risk patients with recent myocardial infarction: insights from the Defibrillation in Acute Myocardial Infarction Trial (DINAMIT). Circulation. 2010;122:2645–2652. doi: 10.1161/CIRCULATIONAHA.109.924225. doi: 10.1161/CIRCULATIONAHA.109.924225. [DOI] [PubMed] [Google Scholar]
- 12.Fine JPG, R. J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 13.Sullivan LM, Massaro JM, D’Agostino RB., Sr Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004;23:1631–1660. doi: 10.1002/sim.1742. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 14.Lee DS, Stitt A, Austin PC, Stukel TA, Schull MJ, Chong A, Newton GE, Lee JS, Tu JV. Prediction of heart failure mortality in emergent care: a cohort study. Ann Intern Med. 2012;156:767–75, W. doi: 10.7326/0003-4819-156-11-201206050-00003. doi: 10.7326/0003-4819-156-11-201206050-00003. [DOI] [PubMed] [Google Scholar]
- 15.Steyerberg EW. Clinical Prediction Models. New York, NY: Springer Science; 2009. [Google Scholar]
- 16.Gold MR, Ip JH, Costantini O, Poole JE, McNulty S, Mark DB, Lee KL, Bardy GH. Role of microvolt T-wave alternans in assessment of arrhythmia vulnerability among patients with heart failure and systolic dysfunction: primary results from the T-wave alternans sudden cardiac death in heart failure trial substudy. Circulation. 2008;118:2022–2028. doi: 10.1161/CIRCULATIONAHA.107.748962. doi: 10.1161/CIRCULATIONAHA.107.748962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, Morarji K, Brown TD, Ismail NA, Dweck MR, Di Pietro E, Roughton M, Wage R, Daryani Y, O’Hanlon R, Sheppard MN, Alpendurada F, Lyon AR, Cook SA, Cowie MR, Assomull RG, Pennell DJ, Prasad SK. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309:896–908. doi: 10.1001/jama.2013.1363. doi: 10.1001/jama.2013.1363. [DOI] [PubMed] [Google Scholar]
- 18.Chen LY, Sotoodehnia N, Bůžková P, Lopez FL, Yee LM, Heckbert SR, Prineas R, Soliman EZ, Adabag S, Konety S, Folsom AR, Siscovick D, Alonso A. Atrial fibrillation and the risk of sudden cardiac death: the atherosclerosis risk in communities study and cardiovascular health study. JAMA Intern Med. 2013;173:29–35. doi: 10.1001/2013.jamainternmed.744. doi: 10.1001/2013.jamainternmed.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurl S, Mäkikallio TH, Rautaharju P, Kiviniemi V, Laukkanen JA. Duration of QRS complex in resting electrocardiogram is a predictor of sudden cardiac death in men. Circulation. 2012;125:2588–2594. doi: 10.1161/CIRCULATIONAHA.111.025577. doi: 10.1161/CIRCULATIONAHA.111.025577. [DOI] [PubMed] [Google Scholar]
- 20.Yung D, Birnie D, Dorian P, Healey JS, Simpson CS, Crystal E, Krahn AD, Khaykin Y, Cameron D, Chen Z, Lee DS. Survival after implantable cardioverter-defibrillator implantation in the elderly. Circulation. 2013;127:2383–2392. doi: 10.1161/CIRCULATIONAHA.113.001442. doi: 10.1161/CIRCULATIONAHA.113.001442. [DOI] [PubMed] [Google Scholar]
- 21.Zehender M, Büchner C, Meinertz T, Just H. Prevalence, circumstances, mechanisms, and risk stratification of sudden cardiac death in unipolar single-chamber ventricular pacing. Circulation. 1992;85:596–605. doi: 10.1161/01.cir.85.2.596. [DOI] [PubMed] [Google Scholar]
- 22.van Rees JB, Borleffs CJ, van Welsenes GH, van der Velde ET, Bax JJ, van Erven L, Putter H, van der Bom JG, Schalij MJ. Clinical prediction model for death prior to appropriate therapy in primary prevention implantable cardioverter defibrillator patients with ischaemic heart disease: the FADES risk score. Heart. 2012;98:872–877. doi: 10.1136/heartjnl-2011-300632. doi: 10.1136/heartjnl-2011-300632. [DOI] [PubMed] [Google Scholar]
- 23.Parkash R, Stevenson WG, Epstein LM, Maisel WH. Predicting early mortality after implantable defibrillator implantation: a clinical risk score for optimal patient selection. Am Heart J. 2006;151:397–403. doi: 10.1016/j.ahj.2005.04.009. doi: 10.1016/j.ahj.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Lee DS, Tu JV, Austin PC, Dorian P, Yee R, Chong A, Alter DA, Laupacis A. Effect of cardiac and noncardiac conditions on survival after defibrillator implantation. J Am Coll Cardiol. 2007;49:2408–2415. doi: 10.1016/j.jacc.2007.02.058. doi: 10.1016/j.jacc.2007.02.058. [DOI] [PubMed] [Google Scholar]
- 25.Borleffs CJ, van Welsenes GH, van Bommel RJ, van der Velde ET, Bax JJ, van Erven L, Putter H, van der Bom JG, Rosendaal FR, Schalij MJ. Mortality risk score in primary prevention implantable cardioverter defibrillator recipients with non-ischaemic or ischaemic heart disease. Eur Heart J. 2010;31:712–718. doi: 10.1093/eurheartj/ehp497. doi: 10.1093/eurheartj/ehp497. [DOI] [PubMed] [Google Scholar]
- 26.Chen CY, Stevenson LW, Stewart GC, Seeger JD, Williams L, Jalbert JJ, Setoguchi S. Impact of baseline heart failure burden on post-implantable cardioverter-defibrillator mortality among medicare beneficiaries. J Am Coll Cardiol. 2013;61:2142–2150. doi: 10.1016/j.jacc.2013.02.043. doi: 10.1016/j.jacc.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV, Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119:3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Setoguchi S, Warner Stevenson L, Stewart GC, Bhatt DL, Epstein AE, Desai M, Williams LA, Chen CY. Influence of healthy candidate bias in assessing clinical effectiveness for implantable cardioverter-defibrillators: cohort study of older patients with heart failure. BMJ. 2014;348:g2866. doi: 10.1136/bmj.g2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H, Zareba W, McNitt S, Andrews ML MADIT-II Investigators. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008;51:288–296. doi: 10.1016/j.jacc.2007.08.058. doi: 10.1016/j.jacc.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 30.Kalogeropoulos AP, Georgiopoulou VV, Giamouzis G, Smith AL, Agha SA, Waheed S, Laskar S, Puskas J, Dunbar S, Vega D, Levy WC, Butler J. Utility of the Seattle Heart Failure Model in patients with advanced heart failure. J Am Coll Cardiol. 2009;53:334–342. doi: 10.1016/j.jacc.2008.10.023. doi: 10.1016/j.jacc.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 31.Lee DS, Krahn AD, Healey JS, Birnie D, Crystal E, Dorian P, Simpson CS, Khaykin Y, Cameron D, Janmohamed A, Yee R, Austin PC, Chen Z, Hardy J, Tu JV Investigators of the Ontario ICD Database. Evaluation of early complications related to De Novo cardioverter defibrillator implantation insights from the Ontario ICD database. J Am Coll Cardiol. 2010;55:774–782. doi: 10.1016/j.jacc.2009.11.029. doi: 10.1016/j.jacc.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 32.Kinch Westerdahl A, Sjöblom J, Mattiasson AC, Rosenqvist M, Frykman V. Implantable cardioverter-defibrillator therapy before death: high risk for painful shocks at end of life. Circulation. 2014;129:422–429. doi: 10.1161/CIRCULATIONAHA.113.002648. doi: 10.1161/CIRCULATIONAHA.113.002648. [DOI] [PubMed] [Google Scholar]
- 33.Goldberger JJ, Basu A, Boineau R, Buxton AE, Cain ME, Canty JM, Jr, Chen PS, Chugh SS, Costantini O, Exner DV, Kadish AH, Lee B, Lloyd-Jones D, Moss AJ, Myerburg RJ, Olgin JE, Passman R, Stevenson WG, Tomaselli GF, Zareba W, Zipes DP, Zoloth L. Risk stratification for sudden cardiac death: a plan for the future. Circulation. 2014;129:516–526. doi: 10.1161/CIRCULATIONAHA.113.007149. doi: 10.1161/CIRCULATIONAHA.113.007149. [DOI] [PubMed] [Google Scholar]
- 34.Austin PC, Steyerberg EW. Events per variable (EPV) and the relative performance of different strategies for estimating the out-of-sample validity of logistic regression models [published online ahead of print November 19, 2014]. Stat Methods Med Res. doi: 10.1177/0962280214558972. doi: 10.1177/0962280214558972. [DOI] [PMC free article] [PubMed] [Google Scholar]


