Abstract
We studied whether the serum levels of glial fibrillary acidic protein (GFAP) and of antibodies against the N-methyl-d-aspartate receptor subunit NR2 (NR2 RNMDA) can discriminate between intracerebral haemorrhage (ICH) and ischaemic stroke (IS) in stroke patients. We prospectively recruited patients with suspected stroke (72 confirmed) and 52 healthy controls. The type of brain lesion (ICH or IS) was established using brain imaging. The levels of GFAP and of antibodies against NR2 RNMDA were measured in blood samples obtained within 12 hrs after stroke onset and 24, 48 and 72 hrs and 1 and 2 weeks later using ELISA immunoassay. Improvement in diagnostic performance was assessed in logistic regression models designed to predict the diagnosis and the type of stroke. GFAP peaks early during haemorrhagic brain lesions (at significantly higher levels), and late in ischaemic events, whereas antibodies against NR2 RNMDA have significantly higher levels during IS at all time-points. Neither of the two biomarkers used on its own could sufficiently discriminate patients, but when they are used in combination they can differentiate at 12 hrs after stroke, between ischaemic and haemorrhagic stroke with a sensitivity and specificity of 94% and 91%, respectively.
Keywords: ischaemic stroke, intracerebral haemorrhage, GFAP, NMDA, neuronal biomarkers
Introduction
Brain imaging is still the gold standard for differentiating the type of brain lesions in a stroke patient (ischaemia or haemorrhage) 1. Both the computerized tomography(CT) and the MRI scan of the brain require hospital admittance and lead to time-to-treatment delay. Those investigations are mandatory before specific, highly specialized therapeutic measures are taken (surgery, thrombolysis etc.). There are, however, therapeutic steps that can be performed earlier, on site, in the ambulance or in the emergency unit, such as lowering the blood pressure or the reversal of the anticoagulant therapy in case of intracerebral haemorrhage (ICH) and the pre-notification of the stroke unit for IS 2. There is clearly a need for a diagnostic test to be performed in the near-patient environment that could provide early warning as of what type of brain lesion is a stroke patient experiencing 3.
By cerebral imaging, an ICH is readily discernible; however, it is a different story with cerebral ischaemia. The therapeutic window is already closing when a diagnosis of acute ischaemic stroke (IS) is made with certainty. The question of what to do in the pre-hospital setting during the acute phase of the stroke is still unanswered: should the thrombolytic therapy be instituted immediately (without having diagnostic certainty, as ischaemia is visible with cerebral CT in the first 3 hrs after onset in only a third of the cases 4) or should the diagnostic confirmation be obtained first? Acting quickly and without diagnostic certainty could reverse the ischaemic process, but runs the risk of treating a patient who has no stroke (the neurological deficit may be caused by stroke mimics such as epileptic seizures or cerebral tumours) or who is at risk for a haemorrhagic transformation. Conversely, the certainty of an ischaemic process within the cerebral tissue leaves us with no alternative other than to take account of the damage and offer supportive treatment.
We are now able to offer comprehensive medical support for a patient after an acute stroke and to perform rehabilitation after the damage is done. Despite this, there can still be little chance for complete recovery. The sequels that exist after stroke can impose a huge financial burden on society and lead to hard-to-assess personal suffering 5. The only way of moving forward is to design new methods to diagnose IS within the therapeutic window or in the sub-acute phase. They allow the rapid identification of the correct therapeutic path. Such an opportunity seems to be offered by neuronal biomarkers 6.
Neuronal biomarkers are substances found in the neural tissue and are released into the blood stream after a neuronal injury, or more precisely after the disruption of the blood–brain barrier. If their blood levels correlate with the type and extent of the neural damage, it may be possible to supplement diagnostic capabilities, offering the best therapy as early as possible for patients in need 7.
Several neuronal proteins have been studied as would-be neuronal biomarkers and a limited number of substances are constantly cited as having significance for the diagnosis of stroke, but no consensus has been reached regarding their use in the clinic 8,9. Earlier reports state that some substances have certain specificity for ischaemic and haemorrhagic stroke. Glial fibrillary acidic protein (GFAP) is a biomarker candidate that appears to be indicative of ICH, while antibodies against N-methyl-d-aspartate receptor subunit NR2 (NR2 RNMDA) appear to be more specific for an ischaemic event 10,11. Nevertheless, neither marker alone appears to be sensitive and specific enough for clinical decision making. The GFAP has been recently investigated as a biomarker for ICH. It has been shown that it is released rapidly in acute ICH (the serum level being correlated with the size of the haematoma), whereas, a more delayed kinetic is expected in case of an ischaemic lesion. A GFAP cut-off of 0.29 μg/l provided diagnostic sensitivity of 84.2% and specificity of 96.3% for differentiating ICH from IS [Foerch 2012]. However, normal serum levels of GFAP cannot rule out ICH. There is an agreement among neurologists that a single biomarker cannot provide enough information and that a panel of substances should be used instead 12.
In this study, we wanted to determine whether GFAP and antibodies against NR2 RNMDA could together discriminate between ICH and IS. This information could be used not only to efficiently diagnose individuals to optimize the triage and management of patients, but also to identify different physiological pathways that could reveal promising targets for sub-acute therapy to improve long-term outcome.
Materials and methods
Patient selection
Between December 2010 and November 2011 we enroled consecutive stroke patients and healthy controls in a prospective manner. We included in the study, patients with symptoms suspicious for acute stroke that were evaluated in the Emergency Department of our Hospital. The informed consent was obtained from study participants or their legal designates. The inclusion criteria were: time window between symptom onset and hospital admission <12 hrs, presence of hemiparesis at hospital admission and presence of clinical signs of hemispheric involvement. We used the following exclusion criteria: stroke patients presenting later than 12 hrs after the onset of the neurologic deficit, history of tumoural or traumatic lesions of the brain and a history of stroke within the 4 weeks prior to enrolment. The type and extent of the brain lesion (ICH or IS) were established using brain CT scan and the final diagnosis was established before discharge. The healthy controls were selected among the relatives of the patients; we included men and women with no known neurological diseases. The study protocol has received approval by the ethical board of our university.
Sample collection
Samples of 5.5 ml of venous blood were taken into vacuum tubes with no additives from each patient at 12, 24, 48 and 72 hrs and 1 and 2 weeks after the onset of symptoms. For the healthy controls we assessed the blood levels of the neuronal biomarkers only once, at inclusion. Samples were centrifuged immediately at 1300 × g for 5 min. at 4°C, and the separated serum was stored at −80°C for further processing.
GFAP measurement
Serum GFAP levels were assessed using a commercial human GFAP ELISA kit (BioVendor, Heidelberg, Germany) following the manufacturer’s instructions. Samples in duplicate were diluted at 1:3 before analysis, and 100 μl was added to the microplate wells. Quality controls and increasing concentrations of standards were done for obtaining the calibration curve. After 2 hrs of incubation at room temperature, 100 μl biotin-labelled anti-GFAP-antibody solution was added to each well, followed by a 1 hr incubation period. The detection step consisted of incubation with HRP (horse radish peroxidase)-conjugate for 1 hr followed by pipetting in 100 μl 3,3′,5,5′-tetramethyl benzidine (TMB) substrate for colorimetric detection. After stopping the colour reaction, the plate was immediately read at 450 nm with a Sunrise Tecan microplate reader equipped with the acquisition software Magellan 3. The entire experiment was performed at room temperature and between each step a washing procedure was applied (4 cycles of 400 μl washing solution/well). Serum values (ng/ml) were extracted from the standard curve.
Detection of serum antibodies to NR2 subunit of NMDA receptor
The Gold Dot NR2 Antibody Assay kit (CIS Biotech, Inc., Atlanta, GA, USA) is an ELISA method designed to quantify the serum levels of antibodies directed against NR2 peptide, an NMDA receptor subunit. We analysed serum samples according to the manufacturer’s manual. 100 μl of diluted patient and control serum samples (1:50), calibrators, positive control and negative controls were added to a microtiter plate and incubated for 30 min. at 37°C on a shaker to allow the antibody to react with the NR2 peptide coated on a solid phase of the microplate. After washing for three times with 200 μl of working solution (5 min. during each wash at 37°C, on a shaker), 100 μl of protein A-HRP was added to each well and incubated for 30 min. at 37°C on a plate shaker. Another washing step was performed as described above followed by a onetime washing step with 200 μl of deionized water for 1 min. on shaker. After pipetting 100 μl of the TMB substrate, the microplate was gently shaken in darkness for 10 min. at room temperature to achieve colour development. The enzymatic reaction was stopped with acid solution and read after 10 min. using a Bioteck Synergy 2 microplate reader at 450 nm/630 nm. A standard calibration curve was generated and corresponding values in ng/ml were determined. Assays were done in duplicate.
Statistical analysis
We used Exact Wilcoxon–Mann–Whitney U-test to compare the average values of GFAP and of antibodies against NR2 RNMDA from patients who had IS or ICH. To discriminate between ischaemic and haemorrhagic stroke we used binary logit regression. We associated the area under the receiver operating characteristic (AUROC) curve to the regression.
Results
Seventy-two stroke patients were enroled (49 patients having IS, 23 having ICH). Table1 displays the baseline variables of the study population.
Table 1.
Baseline characteristics of the study population
| Ischemic stroke | Intracerebral haemorrhage | Controls | |
|---|---|---|---|
| N | 49 | 23 | 52 |
| Mean age (years) (SD, interval) | 71.8 (4.38, 54–80) | 63.3 (4.51, 59–74) | 68.9 (5.91, 53–81) |
| Men, % | 79.5% | 73.9% | 75% |
| NHISS at admission | 13.3 (10, 1–31) | 18 (5.9, 6–28) | |
| NHISS at 2 weeks (SD, interval) | 13.7 (11.7, 1–36) | 15.8 (6.3, 4–26) | |
| Aetiology of IS (N, %) | |||
| Large artery disease | 9 (18.4%) | ||
| Embolic stroke | 28 (57.1%) | ||
| Small artery disease | 7 (14.3%) | ||
| Unknown | 5 (10.2%) | ||
| Size of the ischemic brain lesion (N, %) | |||
| Less than one-third of middle cerebral artery territory | 19 (38.8%) | ||
| One-third to two-thirds of middle cerebral artery territory | 7 (14.3%) | ||
| More than two-thirds of middle cerebral artery territory | 13 (26.5%) | ||
| Vertebro-basilar artery territory | 10 (20.4%) | ||
| Size of the haematoma (N, %) | |||
| 1–20 ml | 9 (39.1%) | ||
| 21–40 ml | 8 (34.8%) | ||
| 41–60 ml | 4 (17.4%) | ||
| >60 ml | 2 (8.7%) |
The two neuronal biomarkers were assessed in serum samples taken at the above-mentioned time-points and the results are presented in Figures1 and 2. The serum levels of the antibodies against NR2 RNMDA were significantly higher in cases of IS compared to ICH. They peaked at 12 hrs (IS) and 24 hrs (ICH) after the onset of the neurological deficit and then slowly declined; this behaviour is similar in cases of IS and ICH, but the absolute values significantly differed, as seen in Figure3.
Figure 1.
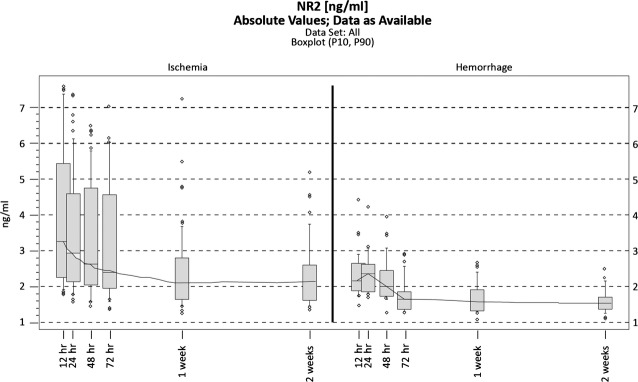
Antibodies against NR2 RNMDA over time in ischemic and haemorrhagic stroke patients (box plots). The boundaries of the box indicate the 25th and 75th percentile, and the line within the box marks the median. Whiskers above and below the box indicate the 90th and 10th percentiles.
Figure 2.
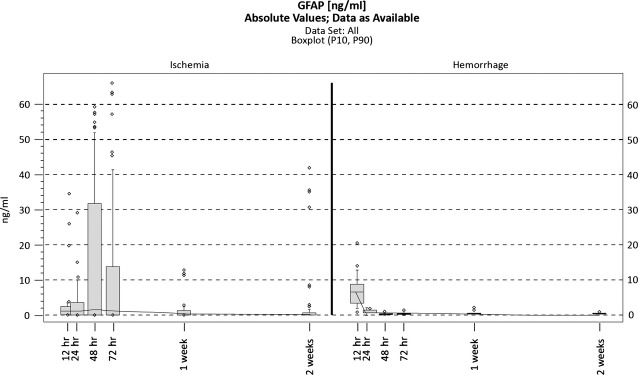
Antibodies against NR2 RNMDA levels may be indicative of IS at all time-points.
Figure 3.
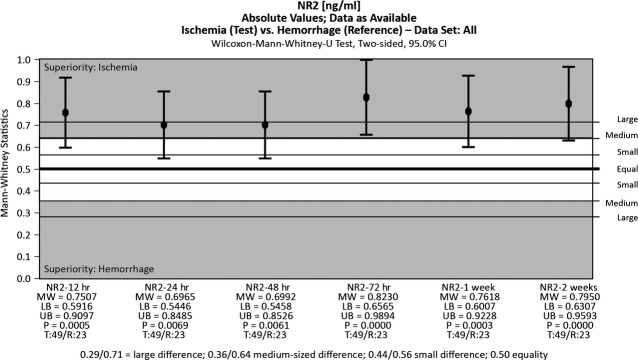
Glial fibrillary acidic protein (GFAP) over time in ischemic and haemorrhagic stroke patients (box plots).
Glial fibrillary acidic protein levels have markedly different patterns of variation in IS and ICH. During IS the blood levels were low in the first hours after onset and then rose and peaked at 48 hrs. In cases of ICH, they peaked within the first 12 hrs, and then the serum concentration fell rapidly to normal levels, as seen in Figure4.
Figure 4.
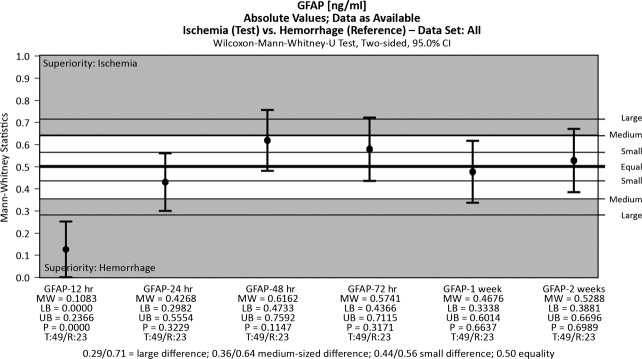
Glial fibrillary acidic protein (GFAP) may be indicative of intracerebral haemorrhage (ICH) at 12 hrs after onset.
The levels of GFAP and of antibodies against NR2 RNMDA assessed within 12 hrs after the onset of symptoms could be used to identify the type of stroke (IS or ICH) with a discriminant function – binary logit regression (see Table2). By using binary logit regression we can estimate the probability of ischaemia or haemorrhage in the case of a stroke patient with the formula presented below, knowing that Prob(ICH = 1) = 1 − Prob(IS = 1).
Table 2.
Binary Logit Regression – discriminant function between ischemic and haemorrhagic stroke
| 12 hrs | 24 hrs | 48 hrs | ||||
|---|---|---|---|---|---|---|
| Coef. | P-value | Coef. | P-value | Coef. | P-value | |
| GFAP_12 hrs | −0.236 | 0.001 | −0.002 | 0.981 | 0.303 | 0.280 |
| NR2_12 hrs | 1.954 | 0.005 | 0.833 | 0.020 | 0.195 | 0.682 |
| Constant | −3.353 | 0.023 | −1.564 | 0.081 | −0.318 | 0.756 |
The Receiver operating characteristic (ROC) analysis based on the values derived from the presented formula indicates that together, GFAP and NR2 RNMDA can discriminate between ischaemic and haemorrhagic stroke at 12 hrs after onset with a sensitivity and specificity of 94% and 91%, respectively, as shown in Figure5.
Figure 5.
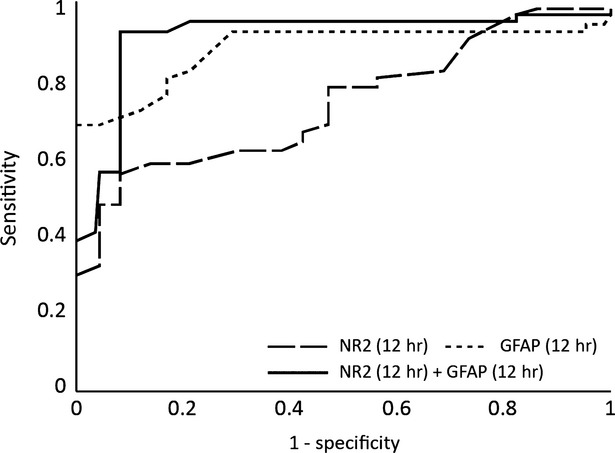
The ROC analysis after Binary Logit Model for glial fibrillary acidic protein (GFAP) and antibodies against NR2 RNMDA at 12 hrs after the onset.
At 24 hrs the same biomarkers are no longer relevant, as the percentage of correct predictions is insufficient (Fig.6). Furthermore, the AUROC for both markers is almost identical as the one for NR2, so GFAP has no significant impact after this time-point (Fig.7).
Figure 6.
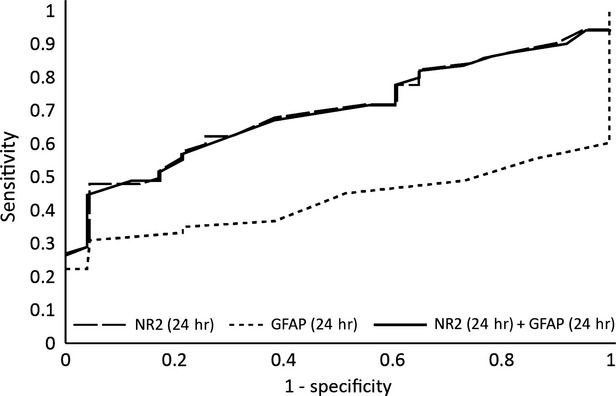
The ROC analysis after Binary Logit Model for glial fibrillary acidic protein (GFAP) and antibodies against NR2 RNMDA at 24 hrs after the onset.
Figure 7.
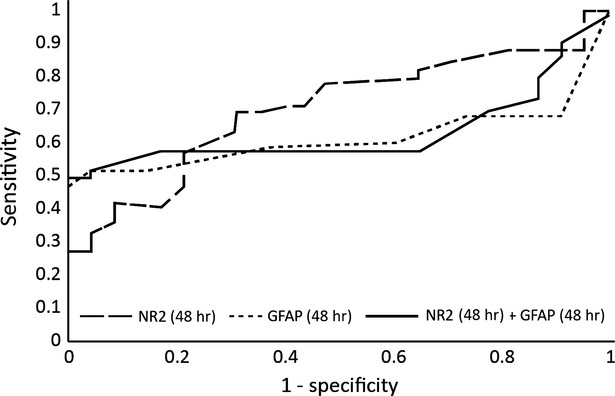
The ROC analysis after Binary Logit Model for glial fibrillary acidic protein (GFAP) and antibodies against NR2 RNMDA at 48 hrs after the onset.
Discussion
A diagnostic approach based on biomarker testing has potential advantages, including cost-effectiveness and widespread availability. Performing those tests in the primary care units would facilitate rapid patient transport to a hospital with an integrated stroke team. In this study, we found that the neuronal biomarkers GFAP and NR2 RNMDA are able to distinguish IS from ICH with 94% sensitivity and 91% specificity at 12 hrs after onset of symptoms.
The role that neuronal biomarkers should play in the diagnostic workup of a stroke patient is still not well-established. The occurrence of neuronal proteins in the blood stream implies two necessary conditions: a source and an avenue, i.e. neuronal death, disorganization of the extracellular matrix and increased permeability of the blood–brain barrier. Both ischaemic and the haemorrhagic stroke lead to brain cell death and barrier dysfunction. However, there are subtle differences between them, both in the way they cause cell death and in the way they increase barrier permeability, and those differences could provide the opportunity for a differential diagnosis.
During ischaemic episodes, sudden cerebral tissue hypoperfusion initiates a series of events known as the ischaemic cascade. These events include cellular energetic failure, excitotoxicity, oxidative stress, erratic enzymatic activation, microvascular injury and cell death 13. Disruption of the blood–brain barrier in IS is a biphasic event and is influenced by the reperfusion that occurs after injury. Within the first 24 hrs, there is increased permeability of the blood–brain barrier as a result of extracellular enzymatic activation, and further damage occurs 48–72 hrs after the infarction 14. In the case of an ICH, the neural damage is caused initially by the compressive effect (thus the damage depends greatly on the volume and expansion rate of the haematoma), and the blood–brain barrier remains intact 15. Within the first hours after onset, varying degrees of oedema appear in the surrounding tissue and osmotically active substances are released. The systemic inflammatory response and activation of thrombolysis within the cerebral clot, coupled with the neuronal toxicity of haemoglobin (events occurring 2–3 days after the hemorrhagic event), complete the spectrum of factors that cause neuronal death and irreversible brain damage. The brain–blood barrier remains intact longer during an IS, thus brain-specific proteins appear in the blood stream 8–12 hrs after onset. Other authors have reported the reverse order, with the barrier being destroyed sooner during a haemorrhagic stroke than during an IS 16. Despite these contradictions, knowing that the brain tissue damage is caused through different mechanisms in the two types of stroke offers the potential to discriminate between types of stroke through the use of circulating neuronal biomarkers.
Many molecular biomarkers that have been implicated in the neurovascular injury cascade after stroke have been studied in recent years with regard to their ability to predict the type of stroke (ischaemic or haemorrhagic), including inflammatory mediators such as monocyte chemotactic protein-1, matrix metalloproteinase-9, tissue inhibitor of matrix metalloproteinase-1, interleukin-6, C-reactive protein, ficolin-3 and the brain damage markers S100B and proenkephalin neuropeptide 17. Other studied markers include B-type neurotrophic growth factor, von Willebrand factor, GFAP and NMDA-R fragments 18–20. The diagnostic significance and potential clinical utility of these biomarkers remains to be validated.
Glial fibrillary acidic protein peaks early during haemorrhagic brain lesions and late (at significantly higher levels) in ischaemic events. Glial fibrillary acidic protein was recently identified as a biomarker that is indicative of ICH in the acute phase of stroke. In a prospective study that included 93 patients who had an IS and 42 patients who had an ICH, within 6 hrs after the onset of symptoms, GFAP was detectable in the serum of 81% of ICH patients, but only in the serum of 5% of IS patients 10. The average GFAP serum concentration was significantly higher in patients who had an ICH. A cut-off point of 2.9 ng/ml was found to provide a sensitivity of 79% and a specificity of 98% for the differentiation of ICH from IS. In our study, GFAP was a good marker for identifying haemorrhagic stroke within 12 hrs after the onset of symptoms, a high blood level being indicative of a haemorrhagic event. The maximum level was seen at 12 hrs after an ICH and at 48 hrs after an IS. At 12 hrs after the onset of symptoms the sensitivity and specificity for discriminating ischaemic and haemorrhagic events were 94% and 69%, respectively.
The fragmentation of the NR2 subunit into the peptides NR2A and NR2B is caused by ischaemia or neurotoxicity. The NMDA receptor peptide is thus an indicator of glutamate excitotoxicity associated with acute stroke pathophysiology and can be used for the diagnosis of acute IS (in preliminary clinical studies, the biomarker had a sensitivity of 97% and a specificity of 98%) 21. In our study, the antibodies against NR2 RNMDA had different patterns of variation during IS and ICH, as has been previously described 22. This biomarker was very good at discriminating between stroke patients and healthy controls at any time-point (specificity 96% at 12 hrs and 100% at 24 and 48 hrs after the onset of symptoms). When used to differentiate IS from ICH, the results did not produce the same significance at 12 hrs after stroke.
Used independently, the above-mentioned biomarkers are insufficient to differentiate between ischaemic and haemorrhagic stroke with any accuracy. However, we demonstrated that the sensitivity for GFAP alone is 94%. The additional predictive ability comes from an increase in sensitivity when adding in NR2, so that by using the levels of GFAP and of NR2 RNMDA antibodies, at 12 hrs after onset, we can identify with a very good sensitivity and specificity (94% and 91%, respectively) the type of stroke. This time-point is significant for a number of reasons. First, the anatomical brain lesion is usually not fully established prior to 12–24 hrs after the onset of symptoms 23. Within this period, the penumbral tissue is potentially salvageable by therapeutic intervention, but only if driven by thorough knowledge of the nature of the brain lesion (ischaemic or haemorrhagic). Unfortunately, the type of stroke is not always identified by early brain imaging (the limitations of CT to show acute ischaemia in the posterior fossa are well known,), and access to brain CT scan is not always available 24. Wardlaw demonstrated that access to a brain CT scan was distributed unevenly in Scotland, with only 65% of patients benefitting from a CT scan within the first 48 hrs after a stroke. This figure rises to 100% only after 7 days for hospital-admitted patients 25. However, an important conclusion of his study was that the best strategy for approaching stroke patients (both in terms of limiting sequelae and long-term costs) was to invest more in the acute phase (i.e. scan all patients at the earliest moment). Given this line of reasoning, any other tests that would increase our diagnostic capabilities are welcome.
There is a rigid 3-hr limit imposed for the initiation of thrombolytic therapy in IS imposed by the limitations of CT brain imaging. Several reports have suggested that a preselection of patients using other diagnostic tools, such as multiparametric MRI protocols that use diffusion-weighted and perfusion imaging can identify patients who have had a stroke and who may benefit from intravenous thrombolysis within and beyond 3 hrs after the onset of stroke symptoms. Thrombolysis therapy based on MRI seems safer and potentially more efficacious than standard CT-based thrombolysis 26. Therefore, another reason for trying to establish the aetiology of stroke beyond this limit is to extend the time interval in which thrombolytic therapy could be used. The neuronal biomarkers would be very valuable for increasing clinical confidence and diagnostic accuracy for therapeutic decision making.
Vascular cognitive impairment (VCI) is now the preferred term to describe the spectrum of cognitive impairments that are associated with frank stroke, vascular brain injury or subclinical disease. This impairment imposes a huge burden upon both the healthcare system and the family of the patients. The prevalence of post-stroke dementia varies in relation to the interval after stroke, location and size of the brain lesion. The prevalence of dementia is 30% immediately after stroke, and the incidence of new-onset dementia increases from 7% after 1 year to 48% after 25 years 27. To date, there is no specific treatment for VCI approved by regulatory institutions worldwide. These figures underline the need for accurate diagnosis in the acute and subacute phase of stroke because aggressive treatment in this phase with control of other vascular risk factors could lower the prevalence of VCI 28.
Targeted therapies for stroke are a new, promising field of research. Using nanotechnology, researchers have recently proved in experimental models that certain drugs can be delivered precisely to the injured areas of the brain, thus maximizing their therapeutic effect and minimizing their side effects 29. Tissue factor is a membrane glycoprotein and the primary initiator of coagulation. It is over-expressed in areas of brain microvascular endothelial cells injury and triggers a cascade of molecular events that leads to coagulation. Expression of its gene can be reduced by an RNA-induced silencing complex based on small interfering RNA. It has been recently proven that it can be delivered precisely to the area of the brain endothelial lesion by modified poly(lactic-co-glycolic acid) (PLGA) nanoparticles coupled with the Enhanced Green Fluorescent Protein-Epidermal Growth Factor (EGFP-EGF) fusion protein 30.
The main limitation we see in using biomarkers in stroke patients (and the one that precluded their use outside clinical trials) is the lack of commercially available rapid diagnostic tests that could be easily performed in the Emergency Department or even in a pre-hospital setting. However, once their importance is convincingly established, it is likely that the technology will follow. The use of biomarkers is routine nowadays in other fields of emergency medicine, such as in the workup of chest pain (for instance B-type natriuretic peptide).
We feel that there are several limitations with regard to the study design, such as the small number of individuals, the potential for lack of generalizability and the lack of an earlier time-point, within the first 3 hrs after onset. The latter was not included in the protocol because of technical and logistical issues, as the clinical path assigned to a patient with suspected stroke did not always allow the study team to perform the study-related tests. A 3 hrs time-point could do an even better job of discriminating between IS and ICH by placing the diagnosis during the therapeutic window. This can be an area of future study, prior to establishing a biomarker-based diagnostic protocol. Nevertheless, by describing the kinetics of both GFAP and antibodies against NR2 RNMDA in the following 2 weeks after stroke we demonstrated that used together, the biomarkers can discriminate between the two types of lesions even after the first 12 hrs. This time limit after stroke was established earlier for the GFAP use and is important mostly for the cases in which we do not know the time-point for the onset of the symptoms (‘wake-up strokes’) 31.
The patients who had other neurological factors (e.g. traumatic brain injury, epileptic seizures or cerebral tumours) were not included as controls; thus, no statement can be made about the ability of these biomarkers to differentiate between stroke and these other neurological disorders with similar clinical presentation.
We conclude that the levels of GFAP and of antibodies against NR2 RNMDA can discriminate between ICH and IS in acute stroke patients. When used together, these two biomarkers offer a very good sensitivity and specificity in differentiating between the two types of stroke at 12 hrs after onset. If the model described in this paper were to be adopted in the pre-hospital setting, it could potentially improve patient outcome after stroke by optimizing the triage and thus allowing clinicians to rapidly determine the appropriate clinical care path. Nevertheless, we consider that this model should be validated prospectively in an independent cohort.
Acknowledgments
This work was supported by CNCSIS –UEFISCSU research grant of the Romanian Government, project number PNII – IDEI 2621/2008.
Conflicts of interest
The authors confirm that there are no conflicts of interest.
References
- Adams HP, Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke. Stroke. 2007;38:1655–711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update. Stroke. 2007;38:2001–23. doi: 10.1161/STROKEAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293–8. doi: 10.1016/S0140-6736(07)60151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics - 2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- Whiteley W, Tseng MC, Sandercock P. Blood biomarkers in the diagnosis of ischemic stroke: a systematic review. Stroke. 2008;39:2902–9. doi: 10.1161/STROKEAHA.107.511261. [DOI] [PubMed] [Google Scholar]
- Marginean IC, Stanca DM, Vacaras V, et al. Plasmatic markers in hemorrhagic stroke. J Med Life. 2011;4:148–50. [PMC free article] [PubMed] [Google Scholar]
- Undén J, Strandberg K, Malm J, et al. Explorative investigation of biomarkers of brain damage and coagulation system activation in clinical stroke differentiation. J Neurol. 2009;256:72–7. doi: 10.1007/s00415-009-0054-8. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Yáñez M, Sobrino T, Arias S, et al. Early biomarkers of clinical-diffusion mismatch in acute ischemic stroke. Stroke. 2011;42:2813–8. doi: 10.1161/STROKEAHA.111.614503. [DOI] [PubMed] [Google Scholar]
- Foerch C, Niessner M, Back T, et al. Diagnostic accuracy of plasma glial fibrillary acidic protein for differentiating intracerebral hemorrhage and cerebral ischemia in patients with symptoms of acute stroke. Clin Chem. 2012;58:237–45. doi: 10.1373/clinchem.2011.172676. [DOI] [PubMed] [Google Scholar]
- Dambinova SA, Skoromets AA, Skoromets AP, et al. Role and place of biochemical markers in diagnosis of cerebral ischemic stroke. Zh Nevrol Psikhiatr Im S S Korsakova. 2010;110:24–9. [PubMed] [Google Scholar]
- Whiteley W, Wardlaw J, Dennis M, et al. Blood biomarkers for the diagnosis of acute cerebrovascular diseases: a prospective cohort study. Cerebrovasc Dis. 2011;32:141–7. doi: 10.1159/000328517. [DOI] [PubMed] [Google Scholar]
- Brouns R, De Deyn PP. The complexity of neurobiological processes in acute ischemic stroke. Clin Neurol Neurosurg. 2009;111:483–95. doi: 10.1016/j.clineuro.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Saenger KA, Christenson RH. Stroke biomarkers: progress and challenges for diagnosis, prognosis, differentiation, and treatment. Clin Chem. 2010;56:21–33. doi: 10.1373/clinchem.2009.133801. [DOI] [PubMed] [Google Scholar]
- Testai FD, Aiyagari V. Acute hemorrhagic stroke pathophysiology and medical interventions: blood pressure control, management of anticoagulant-associated brain hemorrhage and general management principles. Neurol Clin. 2008;26:963–85. doi: 10.1016/j.ncl.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Foerch C, Curdt I, Yan B, et al. Serum glial fibrillary acidic protein as a biomarker for intracerebral haemorrhage in patients with acute stroke. J Neurol Neurosurg Psychiatry. 2006;77:181–4. doi: 10.1136/jnnp.2005.074823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JR, Blessing R, White WD, et al. Novel diagnostic test for acute stroke. Stroke. 2004;35:57–63. doi: 10.1161/01.STR.0000105927.62344.4C. [DOI] [PubMed] [Google Scholar]
- Worthmann H, Tryc AB, Goldbecker A, et al. The temporal profile of inflammatory markers and mediators in blood after acute ischemic stroke differs depending on stroke outcome. Cerebrovasc Dis. 2010;30:85–92. doi: 10.1159/000314624. [DOI] [PubMed] [Google Scholar]
- Füst G, Munthe-Fog L, Illes Z, et al. Low ficolin-3 levels in early follow-up serum samples are associated with the severity and unfavorable outcome of acute ischemic stroke. J Neuroinflammation. 2011;8:185. doi: 10.1186/1742-2094-8-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehner W, von Haehling S, Suhr J, et al. Elevated plasma levels of neuropeptide proenkephalin a predict mortality and functional outcome in ischemic stroke. J Am Coll Cardiol. 2012;60:346–54. doi: 10.1016/j.jacc.2012.04.024. [DOI] [PubMed] [Google Scholar]
- Bettermann K. Biomarkers for stroke: in search of fingerprints. J Stroke Cerebrovasc Dis. 2011;20:173–6. doi: 10.1016/j.jstrokecerebrovasdis.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Dambinova SA, Khounteev GA, Izykenova GA, et al. Blood test detecting autoantibodies to N-methyl-d-aspartate neuroreceptors for evaluation of patients with transient ischemic attack and stroke. Clin Chem. 2003;49:1752–62. doi: 10.1373/49.10.1752. [DOI] [PubMed] [Google Scholar]
- Boysen G, Christensen H. Early stroke: a dynamic process. Stroke. 2001;32:2423–5. doi: 10.1161/hs1001.098155. [DOI] [PubMed] [Google Scholar]
- Kerber KA, Burke JF, Brown DL, et al. Does intracerebral haemorrhage mimic benign dizziness presentations? A population based study. Emerg Med J. 2012;29:43–6. doi: 10.1136/emj.2010.104844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Keir SL, Seymour J, et al. What is the best imaging strategy for acute stroke? Health Technol Assess. 2004;8:1–180. doi: 10.3310/hta8010. iii, ix–x, [DOI] [PubMed] [Google Scholar]
- Schellinger PD, Thomalla G, Fiehler J, et al. MRI-based and CT-based thrombolytic therapy in acute stroke within and beyond established time windows: an analysis of 1210 patients. Stroke. 2007;38:2640–5. doi: 10.1161/STROKEAHA.107.483255. [DOI] [PubMed] [Google Scholar]
- Leys D, Hénon H, Mackowiak-Cordoliani MA, et al. Poststroke dementia. Lancet Neurol. 2005;4:752–9. doi: 10.1016/S1474-4422(05)70221-0. [DOI] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar S, Tian F, Stoeger T, et al. Multifunctional Nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: perspectives on tracking and neuroimaging. Part Fibre Toxicol. 2010;7:3. doi: 10.1186/1743-8977-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Mei H, Shi W, et al. EGFP-EGF1-conjugated PLGA nanoparticles for targeted delivery of siRNA into injured brain microvascularendothelial cells for efficient RNA interference. PLoS ONE. 2013;8:e60860. doi: 10.1371/journal.pone.0060860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak F, Haberer I, Sitzer M, et al. Characterisation of the diagnostic window of serum glial fibrillary acidic protein for the differentiation of intracerebral haemorrhage and ischaemic stroke. Cerebrovasc Dis. 2009;27:37–41. doi: 10.1159/000172632. [DOI] [PubMed] [Google Scholar]


