Abstract
Heart disease remains a leading cause of mortality and a major worldwide healthcare burden. Recent advances in stem cell biology have made it feasible to derive large quantities of cardiomyocytes for disease modeling, drug development, and regenerative medicine. The discoveries of reprogramming and transdifferentiation as novel biological processes have significantly contributed to this paradigm. This review surveys the means by which reprogramming and transdifferentiation can be employed to generate induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) and induced cardiomyocytes (iCMs). The application of these patient-specific cardiomyocytes for both in vitro disease modeling and in vivo therapies for various cardiovascular diseases will also be discussed. We propose that, with additional refinement, human disease-specific cardiomyocytes will allow us to significantly advance the understanding of cardiovascular disease mechanisms and accelerate the development of novel therapeutic options.
Keywords: cardiomyocytes, disease modeling, genome editing, human induced pluripotent stem cells, tissue engineering
Introduction
Despite advances in medical therapy, cardiovascular disease (CVD) remains a leading cause of morbidity and mortality worldwide. Concerted efforts in fundamental and translational research are required to provide novel diagnostic tools and effective therapeutic approaches for CVD. Mechanistic modeling of CVD as well as preclinical validation of therapeutic strategies will assist in the development of next-generation medical therapies that incorporate recent discoveries in stem cell biology.
Human induced pluripotent stem cell-derived cardiomyocytes as a novel platform
Although animal models have provided indispensable insights into systemic whole-organ function in vivo as well as in vitro disease mechanisms (Fiedler et al, 2014; Houser et al, 2012; Duncker et al, 2015), not all findings from research on rodent cardiomyocytes can be translated to human cardiomyocytes at the cellular and molecular levels. Human cardiomyocytes, on the other hand, are a limited resource and cannot be indefinitely maintained in culture. These facts emphasize the need for novel human cellular and physiological models of CVD. Over the past decade, rapid technological advances have combined medical and basic sciences in the development and evaluation of novel therapeutics. One exciting advance has been the ability to generate patient-specific induced pluripotent stem cells (iPSCs; Takahashi & Yamanaka, 2006). Human iPSCs resemble human embryonic stem cells (ESCs), the “gold standard” for pluripotency, in their biological properties but without the ethical and political concerns associated with the use of human embryos. Therefore, iPSCs and their differentiated cardiomyocytes (iPSC-CMs) are considered a viable new and ethically less problematic, alternative platform for studying mechanisms of CVD and evaluating novel therapeutic avenues (Fig1). In addition, human iPSCs present the unprecedented opportunity to study disease-specific differences in a patient-specific manner, taking into account indivi-dual drug responses within a patient population. The validity of this approach is exemplified by the successful application of human iPSCs to model LEOPARD syndrome (Carvajal-Vergara et al, 2010), Timothy syndrome (Yazawa et al, 2011), long QT syndrome (Moretti et al, 2010; Itzhaki et al, 2011; Wang et al, 2014), arrhythmogenic right ventricular dysplasia (ARVD) (Kim et al, 2013; Asimaki et al, 2014), familial dilated cardiomyopathy (DCM; Sun et al, 2012), familial hypertrophic cardiomyopathy (HCM; Lan et al, 2013), viral cardiomyopathy (Sharma et al, 2014) and aldehyde dehydrogenase 2 genetic polymorphism (Ebert et al, 2014). These accomplishments demonstrate the tremendous power and versatility of iPSC-CMs in helping to develop novel therapeutic approaches for CVD and paving innovative avenues for precision medicine in the future.
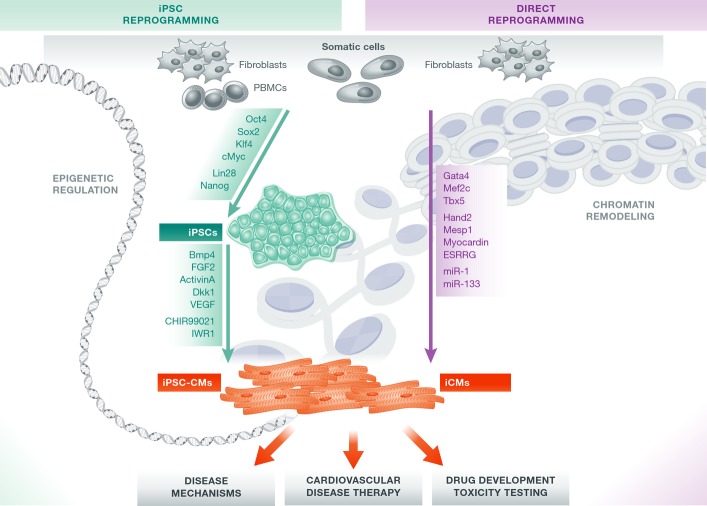
Figure 1. Generation and applications of patient-specific cardiomyocytes.
From isolated patient-specific source cells such as dermal fibroblasts or peripheral blood mononuclear cells (PBMCs), cardiomyocytes can be generated via iPSC reprogramming and subsequent differentiation to iPSC-CMs, or by transdifferentiation into iCMs. Both strategies employ a set of defined factors that cause drastic modulatory changes in the cellular epigenome. Disease-specific mutations within iPSCs can be corrected via genome editing approaches and can be employed for studying disease mechanisms, drug discovery, and regenerative medicine. While in vivo applications of iCMs are already being evaluated, the suitability of iCMs for other purposes such as disease mechanism and drug development studies remains to be ascertained.
Reprogramming of somatic cells to iPSCs
The initial proof-of-concept studies on generation of ESC-like cells were performed using retroviral transduction of mouse fibroblasts with the transcription factors Oct4, Sox2, Klf4, and c-Myc (Takahashi & Yamanaka, 2006). These first-generation iPSCs featured unlimited self-renewal, differentiation into tissues of all germ layers, and the ability to generate an entire organism. However, these earlier approaches involved random insertion of reprogramming factors into the cellular genome, with consequent risk of oncogenic transformation. Subsequently, newer and safer non-integrating approaches employing Sendai virus (Ban et al, 2011), adenovirus (Fusaki et al, 2009; Zhou & Freed, 2009), episomal plasmids (Okita et al, 2008; Yu et al, 2009), minicircle (Jia et al, 2010) or co-MIP (Diecke et al, 2015), mRNA (Warren et al, 2010) or microRNAs (Lin et al, 2011), and direct protein delivery (Kim et al, 2009) have been developed (Fig1). Based on initial observations in mouse ESCs, two states of pluripotency were defined, an earlier one occurring in normal embryonal development termed “naïve” versus a “primed” state (Nichols & Smith, 2009). Naïvety is the ground state of pluripotency. Naïve pluripotent stem cells (PSCs) can be maintained in vitro by supplying leukocyte inhibitory factor (LIF) combined with inhibition of MAPK/ERK kinase (MEK) and glycogen synthase kinase 3 (GSK3) signaling and are characterized by two active X chromosomes in female lines. Primed PSCs are dependent on fibroblast growth factor 2 (FGF2) signaling and transforming growth factor-β (TGFβ) signaling and display inactivation of one X chromosome (Nichols & Smith, 2009; Hirai et al, 2012). Human ESCs and iPSCs are consi-dered to share some properties of naïve mouse ESCs, but mainly resemble primed murine epiblast stem cells (Nichols & Smith, 2009; De Los Angeles et al, 2012). Naïve human iPSCs can be derived by reversion of primed iPSCs into a state that resembles naïve mouse ESCs (Gafni et al, 2013; Theunissen et al, 2014). Currently, it is unknown whether these naïve human ESCs and iPSCs represent distinct intermediates in embryonic development. Further research is required to elucidate whether human naïve iPSCs may be more amenable to introduction of genomic modifications (Gafni et al, 2013) or may differentiate more efficiently into somatic tissues (Honda et al, 2013; Rais et al, 2013; Theunissen et al, 2014).
It has been acknowledged that reprogrammed iPSCs can retain specific DNA methylation profiles associated with their parental source cell type (Bar-Nur et al, 2011; Kim et al, 2011; Lister et al, 2011). Variations in these signatures also appear to account for intra-line variability among different clones originating from the same iPSC line (Kim et al, 2011; Lister et al, 2011). The long-term effect of epigenetic pattern retention, such as methylation profiles from the originating somatic cell type, is not yet fully understood. However, the somatic source cell type is known to affect differentiation efficiency into iPSC-CMs. For example, cardiac progenitor cell-derived iPSC lines have shown an enhanced ability to differentiate into cardiomyocytes compared to fibroblast-derived iPSC lines (Sanchez-Freire et al, 2014). Prolonged propagation of iPSCs through many passages reduces these effects, suggesting that residual epigenetic memory is attenuated in the course of long-term culture (Ohi et al, 2011; Sanchez-Freire et al, 2014). These studies demonstrate that epigenetic memory is a key determinant of iPSC differentiation into lineages that are distinct from the parental cell type.
Pluripotent reprogramming and transdifferentiation of cells from one germ layer to another (Ladewig et al, 2013) have altered the concept of cell fate as determined by unidirectional progression (Takahashi & Yamanaka, 2006; Ieda et al, 2010; Vierbuchen et al, 2010; Huang et al, 2011) and illustrate the plasticity of differentiation and lineage specification. Epigenetic roadblocks repressing chromatin in its inactive state occur during iPSC reprogramming (Kim et al, 2010; Carey et al, 2011; Theunissen & Jaenisch, 2014), and a similar role for chromatin remodeling complexes also exists during cardiovascular development (Chang & Bruneau, 2012; Bevilacqua et al, 2014). Epigenetic modulators can alter DNA methylation and histone acetylation profiles, thereby opening or repressing chromatin in target gene loci that direct lineage fate. Clearly, epigenetic checkpoint regulators of development and lineage differentiation (Takeuchi & Bruneau, 2009; Wang, 2012) are candidates for targeted modulation during iPSC reprogramming and cardiac differentiation. In this context, small molecule compounds are a highly promising resource for further improving the efficiency of cardiovascular lineage differentiation (Efe et al, 2011).
Cardiac differentiation of iPSCs
Today, efficient differentiation protocols allow the generation of large quantities of highly enriched cardiomyocyte populations. These achievements have been made possible by pivotal work utilizing spontaneous aggregation of iPSCs in suspension as embryoid bodies (EBs) in combination with stage-defined growth factors (Kehat et al, 2001; Mummery et al, 2007; Burrdige et al, 2011; Kattman et al, 2011). Subsequently, these protocols led to the establishment of monolayer systems that stimulate the superfamily of TGFβ receptors via growth factors such as activin A and bone morphogenetic protein 4 (BMP4; Paige et al, 2010; Zhang et al, 2012), resulting in activation and repression of the canonical Wnt signaling pathway, respectively. Most recently, specific small molecules have been employed to replace growth factors as modulators of these signaling pathways (Fig1; Lian et al, 2012).
The relative immaturity of iPSC-CMs remains a challenge because it limits their use for disease modeling, drug discovery, and regenerative medicine purposes (Karakikes et al, 2015). Attempts to bypass this limitation have demonstrated that long-term culture enhances the appearance of more mature sarcomeric structural organization in iPSC-CMs (Kamakura et al, 2013). In addition, external cues such as electrical stimulation and mechanical cyclic stretching have been reported to aid in obtaining functionally mature iPSC-CMs (Lieu et al, 2013; Hirt et al, 2014a). Improvements in iPSC-CM maturation were also achieved via novel 3D culture methods (Nunes et al, 2013; Rao et al, 2013) and genetic overexpression of distinct factors (Fu et al, 2011; Bett et al, 2013; Lieu et al, 2013), and these approaches are currently subjects of intense research.
Maturation affects specification of cardiomyocyte subtypes and is vital for accurate recapitulation of disease phenotypes (Lan et al, 2013), including fundamental functional features such as more mature ion channel currents, densities, and kinetics (Sartiani et al, 2007; Yang et al, 2014). The early developmental stage produced by state-of-the-art iPSC-CM differentiation protocols is sufficient for analysis of certain hereditary channelopathies that cause ventricular tachyarrhythmias (Sallam et al, 2015). However, depending on the exact stage of development, there are numerous differences in electrical conduction and coupling as well as the contractile rate and force, compared to human adult cardiomyocytes (Karakikes et al, 2015). Furthermore, insufficient maturation of iPSC-CMs compared to adult human cardiomyocytes has also hindered a broader application of these cells for primary drug discovery and validation. Nevertheless, their use in exploratory studies and for examination of drug toxicity is clearly justified.
A related concern regarding iPSC-CM differentiation is the varying degree of heterogeneity achieved in the generated cardiomyocyte population. Current iPSC or ESC differentiation strategies yield a heterogeneous mixture of atrial-like and ventricular-like lineages, as well as pacemaker-like lineages such as atrioventricular node-like, sinoatrial node-like, and Purkinje fiber-like cells (Burridge et al, 2012). A deeper understanding of directed lineage differentiation, followed by its modulation, would facilitate subtype-specific cardiac differentiation. This can include direct manipulations at the epigenetic level or by achieving mRNA-based delivery of lineage-specific factors (Ong et al, 2015).
The most immediate need, however, is to achieve defined culture conditions and standardized protocols that address the issue of iPSC-CM maturation. In a broader sense, reproducibility and standardization throughout the scientific community will be a key to ensuring comparable datasets, as well as strides toward a broader applicability of iPSC-CMs for disease modeling and drug development. Although still at its infancy, the field has already made significant progress toward the defined derivation and propagation of human iPSCs and iPSC-CMs (Chen et al, 2011; Burridge et al, 2014; Ribeiro et al, 2015).
Genetic engineering and personalized medicine
To understand the molecular and genetic determinants of CVD, advanced genome editing techniques are required to study genotype/phenotype relationships and to allow for the correction of patient-specific mutations in human iPSCs (Wang et al, 1995; Chen et al, 1998; Schwartz et al, 2000; Benson et al, 2003; Fig1). Initial pioneering work was performed using zinc-finger nucleases (ZFNs), a widely used technology for genomic correction that relies on the fusion of the FokI restriction endonuclease with zinc-finger proteins. These nucleases induce target site-specific double-stranded breaks, which stimulate endogenous DNA repair pathways. Due to the complexity of the required engineering steps, ZFNs have been largely supplanted by transcription activator-like effector nucleases (TALENs), and more recently by the clustered regulatory interspaced short palindromic repeats (CRISPRs)/Cas9 nuclease system. TALENs display enhanced specificity as well as reduced off-target action compared to ZFNs. Importantly, single-base pair recognition by TALENs or CRISPRs can correct single nucleotide exchange mutations (Hockemeyer et al, 2011; Ding et al, 2013; Lin et al, 2014). CRISPRs are the most accessible means to facilitate and optimize genetic engineering. Their specificity and off-target effects are currently being evaluated, as these nucleases have the potential to bind and cut sites other than the primary target site (Hendel et al, 2015). Nevertheless, in a relatively short time, CRISPRs have been demonstrated to be a cost-effective and time-efficient approach for genomic correction or introduction of site-specific mutations (Sander & Joung, 2014). Genome-corrected and disease-introduced isogenic cell lines are particularly valuable, as they share a common genotype with the exception of the disease-causing mutation, thereby eliminating confounding effects from genetic heterogeneity. Genomic modification to directly correct disease-specific point mutations in vitro is also valuable for exploring drug development in patient-specific cardiomyocytes. Human iPSC-CMs are currently being utilized as a system to evaluate novel and existing medications and to test patient-specific drug responses (Liang et al, 2013; Navarrete et al, 2013; Wang et al, 2014). For instance, iPSC-CMs from patients carrying long QT syndrome mutations (e.g., KCNQ1 G269S) and genome-edited iPSC-CMs with these disease-causing mutations were both shown to display long QT phenotypes (Liang et al, 2013; Wang et al, 2014). Furthermore, both cell types revealed comparable disease-specific responses following drug treatment (e.g., nifedipine) to rescue prolongation of action potential duration (APD) (Liang et al, 2013; Matsa et al, 2014; Wang et al, 2014). Overall, these examples illustrate the potential for using genome editing to generate accurate, reliable, and less expensive in vitro human models for understanding CVD and for accelerating drug discovery (Fig1; Ebert et al, 2012). Moreover, genome editing may accelerate the future clinical application of integration-free cell-based gene therapy, including the autologous transplantation of patient-specific, genome-corrected iPSC-CMs.
The complexity of genotype/phenotype relationships is further magnified by genetic background variation and variability among iPSC lines (Table1). Genome-wide association studies (GWAS) and subsequent data mining identify signaling pathways governing the control of disease-relevant targets. Large numbers of critical gene loci and related mutations have been described by GWAS and linked to pathogenic phenotypes. Variants occurring in these regions can influence the regulation of disease-relevant gene expression (Fig1). Moreover, late-onset or incomplete penetration of the disease phenotype can complicate further readout and genotypic correlation. In those cases, response profiling of well-established compounds and drugs in CVD might provide further insight. However, the presence of line-to-line and genetic background variation implies that additional layers of control are required to confirm genotype/phenotype relationships. Rescue of pathogenic functional features following genomic correction of the disease-related locus via TALENs or CRISPRs, the use of isogenic controls, and sufficiently powered studies are means to address these limitations. Given our ability to introduce specific disease-causing mutations into both iPSCs and ESCs, it is likely that instead of isolating primary cells from affected patients and generating disease-specific iPSCs and iPSC-CMs, the field will evolve toward standardized procedures based on introducing mutations of interest into fully sequenced and characterized reference stem cell lines (iPSCs and/or ESCs) to assess disease-specific genotypic and phenotypic relationships (Sallam et al, 2015).
Table 1.
Challenges and opportunities of de novo generated cardiomyocytes for disease modeling, drug discovery, and regenerative therapies
| Parameters | CM generation strategy | ||
|---|---|---|---|
| iPSC reprogramming and differentiation | Direct reprogramming | Human ESC differentiation | |
| Mechanism | De-differentiation to iPSCs followed by specific differentiation to CMs | Transdifferentiation | Specific differentiation to CMs |
| Timeline | 2–3 months | 2–3 weeks | 2–3 weeks |
| Efficiencies (% cTnT) | 90–95% | 9–13% | 90–95% |
| Genome editing, isogenic controls | Yes | No | Yes |
| Genetic variation | Yes | Not yet determined | No |
| Disease modeling, drug development | Yes | Current efficiencies too low | Yes |
| Patient-specific assessment of phenotypes and drug function | Yes | Currently undergoing investigation | No |
| In vivo preclinical evaluation of regenerative therapies | Yes | Yes | Yes |
| Clinical safety and efficacy | Not yet determined | Not yet determined | Currently undergoing investigation |
| Ethical concerns | No | No | Yes |
Direct conversion to induced cardiomyocytes (iCMs)
There are both advantages and disadvantages in reprogramming of somatic cells to iPSCs. The intrinsic properties of iPSCs enable the use of tools such as genome editing to facilitate our understanding of basic disease mechanisms, as well as to evaluate precision medicine approaches (Wilson & Wu, 2015). Nevertheless, despite metho-dological advances, the entire process of generating patient-specific iPSC-CMs still requires several months and presents a potential risk of teratoma formation for regenerative medicine, given that the presence of residual pluripotent cells in the final product cannot be completely excluded (Lee et al, 2013). As a result, other approaches that eliminate the need for pluripotent stem cell generation are being explored.
In recent years, proof-of-concept studies have shown that somatic cells can be directly converted to cardiomyocytes (Fig1; Ieda et al, 2010; Efe et al, 2011; Qian et al, 2012). Transgenic expression of three cardiac-specific transcription factors (Gata4, Mef2c, and Tbx5) resulted in the transdifferentiation of murine fibroblasts into contracting cardiomyocytes referred to as induced cardiomyocytes (iCMs; Ieda et al, 2010). Intriguingly, the same outcome has been observed during an epigenetic activation phase in the early stage of reprogramming. This approach employed ectopic expression of transcription factors together with growth factors (Efe et al, 2011), demonstrating elegantly how cellular complexity can be harnessed for the understanding of specific molecular processes. Other reports have shown that direct reprogramming of somatic cells to iCMs is also feasible using various small molecules and miRNAs (Jayawardena et al, 2012, 2014; Protze et al, 2012).
Recently, direct reprogramming of human fibroblasts has also been achieved (Islas et al, 2012). Several studies showed that the murine direct reprogramming factors Gata4, Mef2c, and Tbx5 (GMT), or GMT plus Hand2 (GHMT), were insufficient to transform human fibroblasts into iCMs (Nam et al, 2013; Wada et al, 2013), indicating that the differences between mouse and human cardiovascular development need to be considered for optimal transdifferentiation to human iCMs. A combination of Gata4, Hand2, Tbx5, myocardin, miR-1, and miR-133 could convert human adult dermal fibroblasts into induced cardiomyocyte-like cells (iCMLs). The transduction of these factors promoted substantial cardiac troponin T expression in at least 9% of the source population (Nam et al, 2013). Shortly afterward, introduction of GMT plus Mesp1 and myocardin (GMTMM) was also shown to successfully convert human fibroblasts to iCMLs (Wada et al, 2013). Since then, alternative approaches have succeeded in generating human iCMs with gene expression profiles and functional characteristics similar to those detected in ESC-CMs (Fu et al, 2013).
Current limitations and the routes toward therapeutic application
Direct reprogramming as an alternative to deriving human iPSCs offers the advantage of a 2-week timeline, compared to 2–3 months (Table1). However, current methods for producing iCMs suffer from low efficiencies compared to iPSC differentiation (Chen et al, 2012). Depending on the combination of transcription factors used, human fibroblasts convert into iCMs with different efficiencies, ranging from 5% (GMTMM; Ieda et al, 2010) to 13% (Gata4, Hand2, Tbx5, Myocd, miRNA 1, and miRNA 133; Fu et al, 2013) based on troponin T-positive cells. Recently, polycistronic vectors have been used to express the GMT factors in appropriate stoichiometry as a single mRNA, which has significantly increased the efficiency of mouse fibroblast conversion in vitro up to 25% (Inagawa et al, 2012; Wang et al, 2015). However, these results remain to be replicated in human fibroblasts. In addition, successful generation of pure iCM populations has not yet been reported. Indeed, direct transdifferentiation has so far generated heterogeneous populations of cardiomyocyte-like cells representing various early developmental stages, which rarely display spontaneous beating and produce only sporadic action potentials (Fu et al, 2013). In general, the overall low transdifferentiation efficiency of iCMs into bona fide cardiomyocytes is the main obstacle for the required scale-up of cell production.
Like iPSC-CMs, iCMs must undergo additional maturation before they can serve as true models of adult cardiomyocytes (Bedada et al, 2014; Yang et al, 2014; Jayawardena et al, 2015). The accurate determination of the differences between these cell types requires the direct comparison of iCMs with both iPSC-CMs and human adult cardiomyocytes (Protze et al, 2012; Wada et al, 2013). While similarity of iCMs to ESC-CMs (Fu et al, 2013) has been reported, including subtype specification as a feature of mature cardiomyocyte populations (Nam et al, 2014), other studies have indicated that human iCMs generated in vitro may be even more immature than human iPSC-CMs (Wada et al, 2013). These findings imply that iCMs may reflect the early fetal stage of embryonic cardiomyocytes, and hence, co-stimulation with appropriate factors may drive maturation of iCMs in vitro. Potential approaches include mechanical stimuli or secreted molecules related to the normal myocardial environment, such as cardiac matrix scaffolds and secreted paracrine factors. Exposure to stretching forces is also thought to accelerate iCM generation and maturation (Qian & Srivastava, 2013). Thus, current limitations of efficient generation and maturation of iCMs in vitro may be addressed by advancing in vivo reprogramming instead.
miRNAs have been demonstrated to be sufficient for direct reprogramming to iCMs without addition of any transcription factors (Jayawardena et al, 2012). Current research has thus far focused on their use as powerful drivers of lineage fates (Cordes & Srivastava, 2009; Boon & Dimmeler, 2015). It is likely that miRNAs promote cardiac induction by suppressing fibroblast signatures, as for example, miRNA133-mediated inhibition of Snai1-controlled expression pathways (Muraoka et al, 2014). Therefore, an optimized cocktail to promote the generation of more mature iCMs may include specific miRNAs, as well as specific chemical epigenetic modulators. Should such strategies result in higher efficiency of iCM reprogramming and improved maturation, safety and efficacy would need to be assessed in studies similar to those required for human iPSC-CMs. For now, a direct comparison of iCMs with iPSC-CMs regarding their functional properties remains to be performed. Initial comparative evaluations should focus on functional parameters such as electrophysiology and calcium handling, and also gene and protein expression patterns. Subsequently, the beneficial effects of either cell type on improving cardiac function in preclinical models of CVD will need to be demonstrated. Likewise, the long-term stability of iPSC-CM and iCM phenotypes must be assessed to address safety and efficacy issues.
Heart disease and novel therapeutic approaches
Two of the main classes of genetically inherited heart diseases include channelopathies and cardiomyopathies. Channelopathies or arrhythmic cardiac disorders are caused by mutations in genes encoding ion channels, such as SCN5A that encodes the cardiac Na+ channel α-subunit. The functional characteristics include voltage gating and/or protein trafficking defects, which can result in gain or loss of function in the Na+ channel and subsequent ventri-cular arrhythmias, leading to diseases such as long QT syndromes (Lehnart et al, 2007). By contrast, cardiomyopathies, or defects in heart muscle contraction, are most frequently caused by mutations in cytoskeletal or contractile proteins (McNally et al, 2013). The relevant pathogenic features are transversely isotropic, consisting of irreversible ventricular dilatation and systolic dysfunction that cause severely impaired ventricular contraction. Both channelopathies and cardiomyopathies can also be caused by non-hereditary, acquired determinants such as chronic or acute ischemia (Fig2), and by drugs or autoimmune events (Kass, 2005).
Figure 2. Cell therapy and tissue engineering approaches for cardiovascular disease therapy.
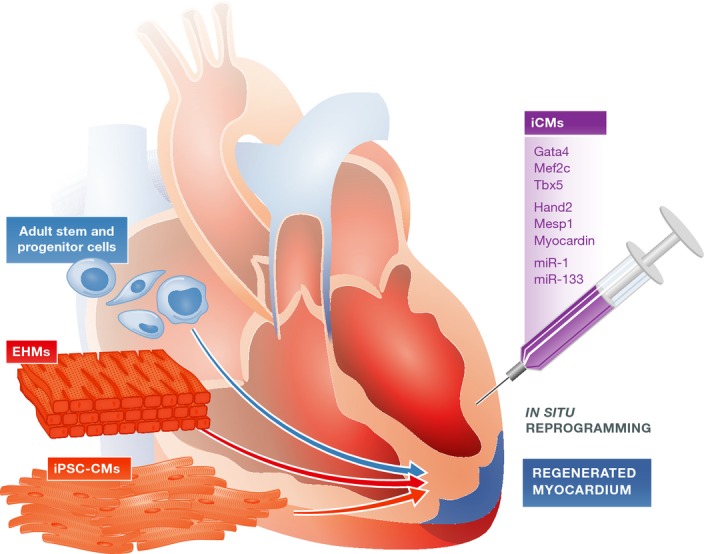
Heart failure due to ischemic heart disease or genetic disorders remains a major healthcare burden. Potential novel treatment options include transplantation of iPSC-CMs or ESC-CMs, as well as direct in vivo reprogramming of cardiac fibroblasts in the scar region to iCMs. The regenerative capacity of adult stem and progenitor cell populations is also being evaluated. Tissue engineering is a new method that aims to re-muscularize damaged myocardium via transplantation of in vitro engineered heart muscle made from iPSC-CMs or ESC-CMs.
Human models of iPSC-CMs have successfully recapitulated numerous genetically determined CVD, including long QT, DCM, HCM, and ARVD (Moretti et al, 2010; Yazawa et al, 2011; Davis et al, 2012; Sun et al, 2012; Caspi et al, 2013; Lan et al, 2013). These studies have demonstrated that iPSC-CMs display phenotypic disease features such as abnormal sarcomere alignment and striation, as well as critical functional properties such as propagation of calcium transients in amplitude, time to peak, duration, and decay (Moretti et al, 2010; Yazawa et al, 2011; Davis et al, 2012; Sun et al, 2012; Caspi et al, 2013; Lan et al, 2013). Importantly, iPSC-CMs allow for quantitative analysis of electrical properties regarding the action potential’s upstroke velocity, time to peak, and duration. For example, abnormally prolonged APD and decreased repolarization velocity are detected in long QT patient-derived iPSC-CMs (Yazawa et al, 2011; Table1).
Pathogenesis of myocardial infarction and regeneration of the heart
The early phase during and after an infarction is characterized by inflammatory, necrotic, and apoptotic cellular responses. The ensuing late or chronic phase includes an expansion of the infarcted region in the myocardial wall, recruitment of myeloid cells, tissue necrosis, and degradation of the extracellular matrix (ECM). Subsequent neoangiogenesis and remodeling of the left ventricle (LV) entails scar formation, hypertrophic expansion of cardiomyocytes, and fibrosis. The resulting dilation of the LV is accompanied by increased frequency of arrhythmias, myocardial dysfunction, and eventually heart failure. Conventional therapy of fibrosis and LV dilatation by angiotensin converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) partially counteracts these deleterious consequences and attenuates adverse remodeling (Dorn, 2009). Nevertheless, viable cardiomyocytes are lost to a large extent in the area of myocardial infarction (MI).
Recent studies suggest that the heart is capable of limited endogenous regeneration (Bergmann et al, 2009; Parmacek & Epstein, 2009; Porrello et al, 2011; van Berlo et al, 2014). While proliferation of the heart may occur to a minor extent throughout the lifetime of an organism, active cell division of cardiomyocytes is limited to the embryonic stage (Bergmann et al, 2009; Porrello et al, 2011). A different source of endogenous heart regeneration is the resident adult stem cell population, known as cardiac progenitor cells (CPCs), reported to be capable of differentiating and proliferating to replenish apoptotic cardiomyocytes (Fig2; Dimmeler et al, 2005; Leri et al, 2011). The full regenerative capacities of these cells remain controversial and are discussed in detail elsewhere (Laflamme & Murry, 2011; Anversa et al, 2013; Maillet et al, 2013, van Berlo et al, 2014). Overall, the endogenous proliferation and repair abilities of the heart are not sufficient to allow the repopulation of damaged myocardial areas with new cardiomyocytes following MI.
Stem cell-derived cardiomyocytes for heart disease therapy
Previous clinical trials have employed various adult stem cell and progenitor cell populations to test their efficacy for therapeutic applications (Fig2; Assmus et al, 2002; Schachinger et al, 2004; Losordo et al, 2007; Chugh et al, 2012; Hare et al, 2012; Makkar et al, 2012; Traverse et al, 2012; Vrtovec et al, 2013; Karantalis et al, 2014). Safety and feasibility of these cells have been demonstrated in these scenarios, and extensive efforts have been spent on exploring the therapeutic potential of these cells. Overall, the results have shown varying degrees of clinical benefit in MI patients (Sanganalmath & Bolli, 2013). Currently, additional approaches are being explored, including transplantation of new cell types (e.g., human ESC-cardiac progenitor cells (Menasche et al, 2015) or iPSC-CMs), or application of alternative delivery approaches such as implantation of in vitro constructed cell sheets of engineered heart muscles (EHMs) (Fig2; Zimmermann, 2013; Emmert et al, 2014; Hirt et al, 2014b). One mechanism by which cell therapy (e.g., ESC-CMs or iPSC-CMs) may improve outcomes is via engraftment of transplanted cells within the host environment, which in theory would lead to the replacement of damaged cardiomyocytes and fibrotic tissue, and restore structural support of the ventricular walls. Extensive studies have focused on grafts within the non-infarcted versus infarcted myocardium in small and large animal models (Laflamme et al, 2007; van Laake et al, 2008). Human grafts express cardiac markers and displayed sarcomere alignment as well as integration with the host’s vasculature. In this context, coupling of transplanted cardiomyocytes with the host myocardium is considered a vital factor contributing to improved cardiac function. Stem cell-derived cardiomyocytes couple to a limited extent with the host myocardial cells in small animal models (Kehat et al, 2004). Large animal models such as pigs and non-human primates are more valuable due to the greater resemblance of their heart rates with the beating frequency of transplanted cardiomyocytes (Chong et al, 2014). However, to a large extent, functional improvement in left ventri-cular ejection fraction (LVEF) in some of these transplantation models has been suggested to result through the release of paracrine factors (Gnecchi et al, 2005; Gu et al, 2012; Huber et al, 2013). Transplanted cells may secrete signaling molecules that exert beneficial functions directly or by altering gene expression patterns in the surrounding myocardium. Such paracrine mechanisms have been frequently proposed to contribute to the recovery of cardiac function (Gnecchi et al, 2005). Well-studied factors such as vascular endothelial growth factor (VEFG; Zangi et al, 2013) and thymosin β-4 (TB4; Smart et al, 2010, 2011) have been selectively characterized in murine infarct models for their capacity to mediate cardiac repair. Recently, targeted approaches to identify specific paracrine factors revealed novel paracrine-acting proteins that could improve tissue and heart function following MI (Korf-Klingebiel et al, 2015). In the future, cell-based therapies may benefit from these findings by integrating delivery of specific factors into the transplanted therapeutic composite. Moreover, complementing the cell mixture with iPSC-derived endothelial cells may lead to beneficial effects from the developed vasculature (Ye et al, 2014). Together, these synergistic approaches may help promote engraftment, vascularization, and structural integrity of the ventricular walls.
To date, three fundamental issues have slowed the clinical translation of iPSC-CMs or ESC-CMs: the risk of tumor formation, poor survival of transplanted cells, and the need for immunosuppression for allogeneic ESC and iPSC derivatives. The first obstacle includes both tumors potentially arising from random insertion of integrating reprogramming vectors, and the risk of teratomas arising from residual undifferentiated stem cells (Lee et al, 2009, 2013). Novel non-integrating iPSC reprogramming strategies may decrease risk, while increasing the efficiency of differentiation or the purity of the final cell product could minimize teratoma risk (Tang et al, 2011). The second major obstacle is acute donor cell death due to hypoxia, anoikis, and inflammation, as well as lack of blood supply (Li et al, 2009a,b; Liu et al, 2012; Nguyen et al, 2014). Hence, the majority of current cardiac cell therapies (both basic and clinical) appear to achieve beneficial effects without long-term persistence of the cells, presumably through the release of paracrine factors to the host heart prior to transplanted cell loss as described earlier (Gnecchi et al, 2005). Relating practical issues include generation of sufficient infarct sizes in the chosen species to induce a measureable decline in heart function without killing the animal. In some cases (e.g., guinea pigs and dogs), the collateral circulation is so high that meaningful infarcts cannot be generated by coronary artery ligation (Verdouw et al, 1998). These concerns are critical for investigational new drug (IND)-enabling large animal studies in the evaluation of the safety and efficacy of stem cell-derived therapies.
The third obstacle toward clinical applications of iPSC-CMs or ESC-CMs is the need for effective immunosuppression to reduce rejection in allogeneic settings, which can be daunting (Pearl et al, 2011, 2012). The use of more sophisticated immunosuppressive or tolerance induction strategies (Huber et al, 2013) as well as combinations of iPSC-CMs with potentially immunotolerant iPSC-derived mesenchymal stem cells (iPSC-MSCs) is being investigated (Lian et al, 2010). An alternative approach is the creation of human leukocyte antigen (immunosuppression HLA)-matched cell banks from healthy donors that contain selected iPSC lines with maximized HLA genotype overlap, which may minimize the need for immunosuppression (Taylor et al, 2012; Neofytou et al, 2015). However, an important caveat to this approach was recently found in the heterogeneity of human mitochondria and, specifically, mismatched mitochondrial antigens, which by themselves can trigger rejection in transplant models (Deuse et al, 2015).
In vivo applications of iCMs
Direct application of iCM reprogramming in vivo may promote patient-specific precision therapy by reducing the accompanying costs and efforts, which are considerable with in vitro generation of patient-specific iPSC-CMs. Induced cardiac regeneration in vivo via iCMs might circumvent current unresolved issues in iPSC-CM therapy, such as poor survival and engraftment of transplanted cells. However, the degree of functional cardiac improvement resulting from in situ transdifferentiated iCMs is unknown, as is the extent of their coupling and integration within the host myocardium (Table1). Safety and potential off-target effects of iCM reprogramming cocktails have yet to be studied in detail, and the consequences of in vivo transfection of “off-target” cells such as endothelial, smooth muscle, or cardiac cells in the heart are also unknown and can be problematic. Finally, another consideration is the reproducibility of iCM generation using viral delivery approaches, which can lead to host immune response, as compared to non-viral or small molecule approaches, which may have poor pharmacokinetics in vivo (Chen et al, 2012). In summary, many challenges remain to be resolved before therapeutic application of iCMs in the clinic can even begin.
Tissue engineering
Currently, heart transplantation is the only viable therapy for end-stage heart failure but remains problematic due to a chronic shortage of organ supply, as well as the persistent risk of immune rejection. An alternative strategy for regeneration of damaged myocardium is to exploit therapeutic cells such as iPSC-CMs for the construction of 3D structures in vitro, and subsequent transplantation of these engineered cardiac patches (Caspi et al, 2007; Tulloch et al, 2011; Kawamura et al, 2012). This technology is known as “tissue engineering” or generation of engineered heart muscle (EHM). Transplantation of a tissue patch/EHM ensures increased precision of delivery onto damaged myocardial areas, as well as full retention of transplanted material. EHM transplants may also allow direct substitution of scar tissue in the infarcted area with new, healthy cardiac muscle, minimizing long-term damage resulting from scar growth and ultimately reducing adverse remodeling and improving cardiac function (Fig2). Moreover, it is expected that 3D cardiac tissues may mature into more adult-like structures compared to single cardiomyocytes, which is considered essential for optimal integration into the host environment. Several key features of maturation, such as alignment, orientation, and binucleation of cardiomyocytes (particularly their sarcomeric structural organization), were found to be improved in engineered tissues (Zimmermann et al, 2002; Tiburcy et al, 2011; Zhang et al, 2013). The beneficial outcomes of tissue engineering-based therapy have been extensively demonstrated in small animal models (Naito et al, 2006; Zimmermann et al, 2006; Sekine et al, 2008; Tulloch et al, 2011) and are currently being tested in large animal models. Eventually, EHMs may facilitate patient-specific organ transplantation via in vitro generation of partial or whole-organ structures.
Significant potential problems, such as poor survival of transplanted EHMs and their problematic integration into the host myocardium, need to be resolved before human trials can commence. A recent study using mouse cardiac sliced tissue as a benchmark to validate and model tissue-engineered patches showed poor survival with > 400 μ?m thickness (Riegler et al, 2014). Detailed understanding of the complex molecular mechanisms that determine the engraftment of transplanted EHMs within the host will lead to better strategies to address these issues. Possible solutions include co-delivery of supporting scaffold matrices, pro-survival cocktails, and stimulation of host myocardium via specific chemical molecules. Standardization of protocols for EHM generation, performance, and maturation will be a crucial step before moving forward to clinical trials. The substantial progress made during the past decade holds promise for a future clinical translation of EHM technology (Tee et al, 2012; Sekine et al, 2013; Ye et al, 2013; Hirt et al, 2014b).
Conclusions
Pluripotent stem cell-derived cardiomyocytes, induced cardiomyocytes, and engineered heart muscle present exciting new opportunities for the development of novel CVD treatments. While iCM production is currently being optimized, iPSC-CMs provide a state-of-the-art patient-specific model system to study disease mechanisms and develop new drugs. Future studies will have to ascertain whether ESC-CM-, iPSC-CM-, iCM-, or EHM-based transplantation can achieve sustained improvement of cardiac function. These synergistic, multidisciplinary approaches should improve understanding of the mechanisms governing cardiovascular health and disease at the molecular, cellular, and organ levels. Transformation of this knowledge into therapeutic strategies is the key to achieve the full potential of regenerative medicine and open a new era of advances in cardiovascular therapy.
Pending issues.
Maturation of pluripotent stem cell-derived cardiomyocytes (e.g., iPSC-CMs or ESC-CMs) and iCMs, as well as defined populations of cardiomyocyte subtypes (e.g., atrial-, nodal-, and ventricular-like cells).
Standardization of efficient, reproducible, and defined culture conditions to generate comparable data.
Improving the conclusiveness of patient-specific iPSC-CM models with approaches to address line-to-line variability and genetic background variations via CRISPR-generated isogenic controls, or fully sequenced and characterized reference stem cell lines (either iPSCs or ESCs).
Optimizing efficiencies in direct reprogramming to iCMs in vitro and in vivo.
Assessing efficacy of ESC-CM-, iPSC-CM-, iCM-, and EHM-based therapies.
Acknowledgments
We thank Joseph Gold and Blake Wu for the critical reading of the manuscript. This work is supported by research grants from the National Institute of Health T32 training grant (IYC), American Heart Association 13EIA14420025, NIH R01 HL123968, and NIH R01 HL126527, CIRM DR2A-05394, and CIRM TR3-05556 (JCW).
Glossary
- Cell therapy
Transplantation of therapeutic cell populations (e.g., adult stem cells or PSC-CMs) to ameliorate damage in the infarcted heart and improve cardiac function
- Disease modeling
Understanding the functional and molecular causes of diseases by recapitulating their respective phenotypes in organisms (e.g., mouse, zebrafish) or tissue-specific cell models (e.g., human iPSC-CMs)
- Drug discovery
Identifying new therapeutics by employing a wide range of scientific methods and model systems
- Genomewide association study (GWAS)
A whole-genome association study that assesses common genetic variants (e.g., single nucleotide polymorphisms) in defined populations of individuals
- Genome editing
Modifying the DNA of a given genome in specific sites with genetically engineered nucleases
- Investigational new drug (IND) application
Filing admission of research on a new drug, treatment, or patient population in human subjects with a regulatory agency (e.g., the US Food and Drug Administration)
- Non-integrating reprogramming
De-differentiation of somatic cells to induced pluripotent stem cells (iPSCs) using reprogramming factors that do not integrate into the host cell genome
- Paracrine mechanisms
Chemical signals secreted from one cell (e.g., growth factors or chemokines) to induce functional changes in nearby cells
- Tissue engineering
A strategy to replace damaged myocardium via generation of engineered heart muscle (EHM), which is a functional tissue-like structure constructed in vitro from adult stem cells or PSC derivatives
- Transdifferentiation
Direct somatic cell reprogramming from one germ layer of origin to another
Conflict of interest
JCW is a cofounder of Stem Cell Theranostics. The other authors declare that they have no conflict of interest.
References
- Anversa P, Kajstura J, Rota M, Leri A. Regenerating new heart with stem cells. J Clin Invest. 2013;123:62–70. doi: 10.1172/JCI63068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Asimaki A, Kapoor S, Plovie E, Karin ArndtA, Adams E, Liu Z, James CA, Judge DP, Calkins H, Churko J, et al. Identification of a new modulator of the intercalated disc in a zebrafish model of arrhythmogenic cardiomyopathy. Sci Transl Med. 2014;6:240ra274. doi: 10.1126/scitranslmed.3008008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, Grunwald F, Aicher A, Urbich C, Martin H, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- Ban H, Nishishita N, Fusaki N, Tabata T, Saeki K, Shikamura M, Takada N, Inoue M, Hasegawa M, Kawamata S, et al. Efficient generation of transgene-free human induced pluripotent stem cells (iPSCs) by temperature-sensitive Sendai virus vectors. Proc Natl Acad Sci USA. 2011;108:14234–14239. doi: 10.1073/pnas.1103509108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Nur O, Russ HA, Efrat S, Benvenisty N. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell. 2011;9:17–23. doi: 10.1016/j.stem.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Bedada FB, Chan SS, Metzger SK, Zhang L, Zhang J, Garry DJ, Kamp TJ, Kyba M, Metzger JM. Acquisition of a quantitative, stoichiometrically conserved ratiometric marker of maturation status in stem cell-derived cardiac myocytes. Stem Cell Reports. 2014;3:594–605. doi: 10.1016/j.stemcr.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berlo JH, Molkentin JD. An emerging consensus on cardiac regeneration. Nat Med. 2014;20:1386–1393. doi: 10.1038/nm.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DW, Wang DW, Dyment M, Knilans TK, Fish FA, Strieper MJ, Rhodes TH, George AL., Jr Congenital sick sinus syndrome caused by recessive mutations in the cardiac sodium channel gene (SCN5A) J Clin Invest. 2003;112:1019–1028. doi: 10.1172/JCI18062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bett GC, Kaplan AD, Lis A, Cimato TR, Tzanakakis ES, Zhou Q, Morales MJ, Rasmusson RL. Electronic “expression” of the inward rectifier in cardiocytes derived from human-induced pluripotent stem cells. Heart Rhythm. 2013;10:1903–1910. doi: 10.1016/j.hrthm.2013.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua A, Willis MS, Bultman SJ. SWI/SNF chromatin-remodeling complexes in cardiovascular development and disease. Cardiovasc Pathol. 2014;23:85–91. doi: 10.1016/j.carpath.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon RA, Dimmeler S. MicroRNAs in myocardial infarction. Nat Rev Cardiol. 2015;12:135–142. doi: 10.1038/nrcardio.2014.207. [DOI] [PubMed] [Google Scholar]
- Burridge PW, Keller G, Gold JD, Wu JC. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 2012;10:16–28. doi: 10.1016/j.stem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, Lan F, Diecke S, Huber B, Mordwinkin NM, et al. Chemically defined generation of human cardiomyocytes. Nat Methods. 2014;11:855–860. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey BW, Markoulaki S, Hanna JH, Faddah DA, Buganim Y, Kim J, Ganz K, Steine EJ, Cassady JP, Creyghton MP, et al. Reprogramming factor stoichiometry influences the epigenetic state and biological properties of induced pluripotent stem cells. Cell Stem Cell. 2011;9:588–598. doi: 10.1016/j.stem.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Carvajal-Vergara X, Sevilla A, D’Souza SL, Ang YS, Schaniel C, Lee DF, Yang L, Kaplan AD, Adler ED, Rozov R, et al. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature. 2010;465:808–812. doi: 10.1038/nature09005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi O, Huber I, Gepstein A, Arbel G, Maizels L, Boulos M, Gepstein L. Modeling of arrhythmogenic right ventricular cardiomyopathy with human induced pluripotent stem cells. Circ Cardiovasc Genet. 2013;6:557–568. doi: 10.1161/CIRCGENETICS.113.000188. [DOI] [PubMed] [Google Scholar]
- Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R, Gepstein L. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50:1884–1893. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- Chang CP, Bruneau BG. Epigenetics and cardiovascular development. Annu Rev Physiol. 2012;74:41–68. doi: 10.1146/annurev-physiol-020911-153242. [DOI] [PubMed] [Google Scholar]
- Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JX, Krane M, Deutsch MA, Wang L, Rav-Acha M, Gregoire S, Engels MC, Rajarajan K, Karra R, Abel ED, et al. Inefficient reprogramming of fibroblasts into cardiomyocytes using Gata4, Mef2c, and Tbx5. Circ Res. 2012;111:50–55. doi: 10.1161/CIRCRESAHA.112.270264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Kirsch GE, Zhang D, Brugada R, Brugada J, Brugada P, Potenza D, Moya A, Borggrefe M, Breithardt G, et al. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature. 1998;392:293–296. doi: 10.1038/32675. [DOI] [PubMed] [Google Scholar]
- Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54–S64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes KR, Srivastava D. MicroRNA regulation of cardiovascular development. Circ Res. 2009;104:724–732. doi: 10.1161/CIRCRESAHA.108.192872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RP, Casini S, van den Berg CW, Hoekstra M, Remme CA, Dambrot C, Salvatori D, Oostwaard DW, Wilde AA, Bezzina CR, et al. Cardiomyocytes derived from pluripotent stem cells recapitulate electrophysiological characteristics of an overlap syndrome of cardiac sodium channel disease. Circulation. 2012;125:3079–3091. doi: 10.1161/CIRCULATIONAHA.111.066092. [DOI] [PubMed] [Google Scholar]
- De Los Angeles A, Loh YH, Tesar PJ, Daley GQ. Accessing naive human pluripotency. Curr Opin Genet Dev. 2012;22:272–282. doi: 10.1016/j.gde.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuse T, Wang D, Stubbendorff M, Itagaki R, Grabosch A, Greaves LC, Alawi M, Grunewald A, Hu X, Hua X, et al. SCNT-derived ESCs with mismatched mitochondria trigger an immune response in allogeneic hosts. Cell Stem Cell. 2015;16:33–38. doi: 10.1016/j.stem.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Diecke S, Lu J, Lee J, Termglinchan V, Kooreman NG, Burridge PW, Ebert AD, Churko JM, Sharma A, Kay MA, et al. Novel codon-optimized mini-intronic plasmid for efficient, inexpensive, and xeno-free induction of pluripotency. Sci Rep. 2015;5:8081. doi: 10.1038/srep08081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest. 2005;115:572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Lee YK, Schaefer EA, Peters DT, Veres A, Kim K, Kuperwasser N, Motola DL, Meissner TB, Hendriks WT, et al. A TALEN genome-editing system for generating human stem cell-based disease models. Cell Stem Cell. 2013;12:238–251. doi: 10.1016/j.stem.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn GW., 2nd Novel pharmacotherapies to abrogate postinfarction ventricular remodeling. Nat Rev Cardiol. 2009;6:283–291. doi: 10.1038/nrcardio.2009.12. [DOI] [PubMed] [Google Scholar]
- Duncker DJ, Bakkers J, Brundel BJ, Robbins J, Tardiff JC, Carrier L. Animal and in silico models for the study of sarcomeric cardiomyopathies. Cardiovasc Res. 2015;105:439–448. doi: 10.1093/cvr/cvv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert AD, Kodo K, Liang P, Wu H, Huber BC, Riegler J, Churko J, Lee J, de Almeida P, Lan F, et al. Characterization of the molecular mechanisms underlying increased ischemic damage in the aldehyde dehydrogenase 2 genetic polymorphism using a human induced pluripotent stem cell model system. Sci Transl Med. 2014;6:255ra130. doi: 10.1126/scitranslmed.3009027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert AD, Liang P, Wu JC. Induced pluripotent stem cells as a disease modeling and drug screening platform. J Cardiovasc Pharmacol. 2012;60:408–416. doi: 10.1097/FJC.0b013e318247f642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, Chen J, Ding S. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol. 2011;13:215–222. doi: 10.1038/ncb2164. [DOI] [PubMed] [Google Scholar]
- Emmert MY, Hitchcock RW, Hoerstrup SP. Cell therapy, 3D culture systems and tissue engineering for cardiac regeneration. Adv Drug Deliv Rev. 2014;69–70:254–269. doi: 10.1016/j.addr.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Fiedler LR, Maifoshie E, Schneider MD. Mouse models of heart failure: cell signaling and cell survival. Curr Top Dev Biol. 2014;109:171–247. doi: 10.1016/B978-0-12-397920-9.00002-0. [DOI] [PubMed] [Google Scholar]
- Fu JD, Rushing SN, Lieu DK, Chan CW, Kong CW, Geng L, Wilson KD, Chiamvimonvat N, Boheler KR, Wu JC, et al. Distinct roles of microRNA-1 and -499 in ventricular specification and functional maturation of human embryonic stem cell-derived cardiomyocytes. PLoS ONE. 2011;6:e27417. doi: 10.1371/journal.pone.0027417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu JD, Stone NR, Liu L, Spencer CI, Qian L, Hayashi Y, Delgado-Olguin P, Ding S, Bruneau BG, Srivastava D. Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Reports. 2013;1:235–247. doi: 10.1016/j.stemcr.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85:348–362. doi: 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafni O, Weinberger L, Mansour AA, Manor YS, Chomsky E, Ben-Yosef D, Kalma Y, Viukov S, Maza I, Zviran A, et al. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282–286. doi: 10.1038/nature12745. [DOI] [PubMed] [Google Scholar]
- Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- Gu M, Nguyen PK, Lee AS, Xu D, Hu S, Plews JR, Han L, Huber BC, Lee WH, Gong Y, et al. Microfluidic single-cell analysis shows that porcine induced pluripotent stem cell-derived endothelial cells improve myocardial function by paracrine activation. Circ Res. 2012;111:882–893. doi: 10.1161/CIRCRESAHA.112.269001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendel A, Fine EJ, Bao G, Porteus MH. Quantifying on- and off-target genome editing. Trends Biotechnol. 2015;33:132–140. doi: 10.1016/j.tibtech.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H, Firpo M, Kikyo N. Establishment of LIF-dependent human iPS cells closely related to basic FGF-dependent authentic iPS cells. PLoS ONE. 2012;7:e39022. doi: 10.1371/journal.pone.0039022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt MN, Boeddinghaus J, Mitchell A, Schaaf S, Bornchen C, Muller C, Schulz H, Hubner N, Stenzig J, Stoehr A, et al. Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation. J Mol Cell Cardiol. 2014a;74:151–161. doi: 10.1016/j.yjmcc.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Hirt MN, Hansen A, Eschenhagen T. Cardiac tissue engineering: state of the art. Circ Res. 2014b;114:354–367. doi: 10.1161/CIRCRESAHA.114.300522. [DOI] [PubMed] [Google Scholar]
- Hockemeyer D, Wang H, Kiani S, Lai CS, Gao Q, Cassady JP, Cost GJ, Zhang L, Santiago Y, Miller JC, et al. Genetic engineering of human pluripotent cells using TALE nucleases. Nat Biotechnol. 2011;29:731–734. doi: 10.1038/nbt.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda A, Hatori M, Hirose M, Honda C, Izu H, Inoue K, Hirasawa R, Matoba S, Togayachi S, Miyoshi H, et al. Naive-like conversion overcomes the limited differentiation capacity of induced pluripotent stem cells. J Biol Chem. 2013;288:26157–26166. doi: 10.1074/jbc.M113.502492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser SR, Margulies KB, Murphy AM, Spinale FG, Francis GS, Prabhu SD, Rockman HA, Kass DA, Molkentin JD, Sussman MA, et al. Animal models of heart failure: a scientific statement from the American Heart Association. Circ Res. 2012;111:131–150. doi: 10.1161/RES.0b013e3182582523. [DOI] [PubMed] [Google Scholar]
- Huang P, He Z, Ji S, Sun H, Xiang D, Liu C, Hu Y, Wang X, Hui L. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475:386–389. doi: 10.1038/nature10116. [DOI] [PubMed] [Google Scholar]
- Huber BC, Ransohoff JD, Ransohoff KJ, Riegler J, Ebert A, Kodo K, Gong Y, Sanchez-Freire V, Dey D, Kooreman NG, et al. Costimulation-adhesion blockade is superior to cyclosporine A and prednisone immunosuppressive therapy for preventing rejection of differentiated human embryonic stem cells following transplantation. Stem Cells. 2013;31:2354–2363. doi: 10.1002/stem.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagawa K, Miyamoto K, Yamakawa H, Muraoka N, Sadahiro T, Umei T, Wada R, Katsumata Y, Kaneda R, Nakade K, et al. Induction of cardiomyocyte-like cells in infarct hearts by gene transfer of Gata4, Mef2c, and Tbx5. Circ Res. 2012;111:1147–1156. doi: 10.1161/CIRCRESAHA.112.271148. [DOI] [PubMed] [Google Scholar]
- Islas JF, Liu Y, Weng KC, Robertson MJ, Zhang S, Prejusa A, Harger J, Tikhomirova D, Chopra M, Iyer D, et al. Transcription factors ETS2 and MESP1 transdifferentiate human dermal fibroblasts into cardiac progenitors. Proc Natl Acad Sci USA. 2012;109:13016–13021. doi: 10.1073/pnas.1120299109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhaki I, Maizels L, Huber I, Zwi-Dantsis L, Caspi O, Winterstern A, Feldman O, Gepstein A, Arbel G, Hammerman H, et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature. 2011;471:225–229. doi: 10.1038/nature09747. [DOI] [PubMed] [Google Scholar]
- Jayawardena T, Mirotsou M, Dzau VJ. Direct reprogramming of cardiac fibroblasts to cardiomyocytes using microRNAs. Methods Mol Biol. 2014;1150:263–272. doi: 10.1007/978-1-4939-0512-6_18. [DOI] [PubMed] [Google Scholar]
- Jayawardena TM, Egemnazarov B, Finch EA, Zhang L, Payne JA, Pandya K, Zhang Z, Rosenberg P, Mirotsou M, Dzau VJ. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res. 2012;110:1465–1473. doi: 10.1161/CIRCRESAHA.112.269035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayawardena TM, Finch EA, Zhang L, Zhang H, Hodgkinson CP, Pratt RE, Rosenberg PB, Mirotsou M, Dzau VJ. MicroRNA induced cardiac reprogramming in vivo: evidence for mature cardiac myocytes and improved cardiac function. Circ Res. 2015;116:418–424. doi: 10.1161/CIRCRESAHA.116.304510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Wilson KD, Sun N, Gupta DM, Huang M, Li Z, Panetta NJ, Chen ZY, Robbins RC, Kay MA, et al. A nonviral minicircle vector for deriving human iPS cells. Nat Methods. 2010;7:197–199. doi: 10.1038/nmeth.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamakura T, Makiyama T, Sasaki K, Yoshida Y, Wuriyanghai Y, Chen J, Hattori T, Ohno S, Kita T, Horie M, et al. Ultrastructural maturation of human-induced pluripotent stem cell-derived cardiomyocytes in a long-term culture. Circ J. 2013;77:1307–1314. doi: 10.1253/circj.cj-12-0987. [DOI] [PubMed] [Google Scholar]
- Karakikes I, Ameen M, Termglinchan V, Wu JC. Human induced pluripotent stem cell-derived cardiomyocytes: insights into molecular, cellular, and functional phenotypes. Circ Res. 2015;117:80–88. doi: 10.1161/CIRCRESAHA.117.305365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karantalis V, DiFede DL, Gerstenblith G, Pham S, Symes J, Zambrano JP, Fishman J, Pattany P, McNiece I, Conte J, et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: the Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ Res. 2014;114:1302–1310. doi: 10.1161/CIRCRESAHA.114.303180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass RS. The channelopathies: novel insights into molecular and genetic mechanisms of human disease. J Clin Invest. 2005;115:1986–1989. doi: 10.1172/JCI26011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T, Kawamura T, Kuratani T, Daimon T, Shimizu T, et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation. 2012;126:S29–S37. doi: 10.1161/CIRCULATIONAHA.111.084343. [DOI] [PubMed] [Google Scholar]
- Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, Huber I, Satin J, Itskovitz-Eldor J, Gepstein L. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22:1282–1289. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- Kim C, Wong J, Wen J, Wang S, Wang C, Spiering S, Kan NG, Forcales S, Puri PL, Leone TC, et al. Studying arrhythmogenic right ventricular dysplasia with patient-specific iPSCs. Nature. 2013;494:105–110. doi: 10.1038/nature11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Zhao R, Doi A, Ng K, Unternaehrer J, Cahan P, Huo H, Loh YH, Aryee MJ, Lensch MW, et al. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat Biotechnol. 2011;29:1117–1119. doi: 10.1038/nbt.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf-Klingebiel M, Reboll MR, Klede S, Brod T, Pich A, Polten F, Napp LC, Bauersachs J, Ganser A, Brinkmann E, et al. Myeloid-derived growth factor (C19orf10) mediates cardiac repair following myocardial infarction. Nat Med. 2015;21:140–149. doi: 10.1038/nm.3778. [DOI] [PubMed] [Google Scholar]
- van Laake LW, Passier R, Doevendans PA, Mummery CL. Human embryonic stem cell-derived cardiomyocytes and cardiac repair in rodents. Circ Res. 2008;102:1008–1010. doi: 10.1161/CIRCRESAHA.108.175505. [DOI] [PubMed] [Google Scholar]
- Ladewig J, Koch P, Brustle O. Leveling Waddington: the emergence of direct programming and the loss of cell fate hierarchies. Nat Rev Mol Cell Biol. 2013;14:225–236. doi: 10.1038/nrm3543. [DOI] [PubMed] [Google Scholar]
- Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan F, Lee AS, Liang P, Sanchez-Freire V, Nguyen PK, Wang L, Han L, Yen M, Wang Y, Sun N, et al. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell. 2013;12:101–113. doi: 10.1016/j.stem.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AS, Tang C, Cao F, Xie X, van der Bogt K, Hwang A, Connolly AJ, Robbins RC, Wu JC. Effects of cell number on teratoma formation by human embryonic stem cells. Cell Cycle. 2009;8:2608–2612. doi: 10.4161/cc.8.16.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AS, Tang C, Rao MS, Weissman IL, Wu JC. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med. 2013;19:998–1004. doi: 10.1038/nm.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnart SE, Ackerman MJ, Benson DW, Jr, Brugada R, Clancy CE, Donahue JK, George AL, Jr, Grant AO, Groft SC, January CT, et al. Inherited arrhythmias: a National Heart, Lung, and Blood Institute and Office of Rare Diseases workshop consensus report about the diagnosis, phenotyping, molecular mechanisms, and therapeutic approaches for primary cardiomyopathies of gene mutations affecting ion channel function. Circulation. 2007;116:2325–2345. doi: 10.1161/CIRCULATIONAHA.107.711689. [DOI] [PubMed] [Google Scholar]
- Leri A, Kajstura J, Anversa P. Role of cardiac stem cells in cardiac pathophysiology: a paradigm shift in human myocardial biology. Circ Res. 2011;109:941–961. doi: 10.1161/CIRCRESAHA.111.243154. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Li Z, Han Z, Wu JC. Transplantation of human embryonic stem cell-derived endothelial cells for vascular diseases. J Cell Biochem. 2009a;106:194–199. doi: 10.1002/jcb.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Lee A, Huang M, Chun H, Chung J, Chu P, Hoyt G, Yang P, Rosenberg J, Robbins RC, et al. Imaging survival and function of transplanted cardiac resident stem cells. J Am Coll Cardiol. 2009b;53:1229–1240. doi: 10.1016/j.jacc.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Zhang Y, Lam FF, Kang S, Xia JC, Lai WH, et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113–1123. doi: 10.1161/CIRCULATIONAHA.109.898312. [DOI] [PubMed] [Google Scholar]
- Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ, Palecek SP. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci USA. 2012;109:E1848–E1857. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P, Lan F, Lee AS, Gong T, Sanchez-Freire V, Wang Y, Diecke S, Sallam K, Knowles JW, Wang PJ, et al. Drug screening using a library of human induced pluripotent stem cell-derived cardiomyocytes reveals disease-specific patterns of cardiotoxicity. Circulation. 2013;127:1677–1691. doi: 10.1161/CIRCULATIONAHA.113.001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu DK, Fu JD, Chiamvimonvat N, Tung KC, McNerney GP, Huser T, Keller G, Kong CW, Li RA. Mechanism-based facilitated maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Arrhythm Electrophysiol. 2013;6:191–201. doi: 10.1161/CIRCEP.111.973420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SL, Chang DC, Lin CH, Ying SY, Leu D, Wu DT. Regulation of somatic cell reprogramming through inducible mir-302 expression. Nucleic Acids Res. 2011;39:1054–1065. doi: 10.1093/nar/gkq850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Fine EJ, Zheng Z, Antico CJ, Voit RA, Porteus MH, Cradick TJ, Bao G. SAPTA: a new design tool for improving TALE nuclease activity. Nucleic Acids Res. 2014;42:e47. doi: 10.1093/nar/gkt1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R, Pelizzola M, Kida YS, Hawkins RD, Nery JR, Hon G, Antosiewicz-Bourget J, O’Malley R, Castanon R, Klugman S, et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature. 2011;471:68–73. doi: 10.1038/nature09798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Narsinh KH, Lan F, Wang L, Nguyen PK, Hu S, Lee A, Han L, Gong Y, Huang M, et al. Early stem cell engraftment predicts late cardiac functional recovery: preclinical insights from molecular imaging. Circ Cardiovasc Imaging. 2012;5:481–490. doi: 10.1161/CIRCIMAGING.111.969329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losordo DW, Schatz RA, White CJ, Udelson JE, Veereshwarayya V, Durgin M, Poh KK, Weinstein R, Kearney M, Chaudhry M, et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115:3165–3172. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- Maillet M, van Berlo JH, Molkentin JD. Molecular basis of physiological heart growth: fundamental concepts and new players. Nat Rev Mol Cell Biol. 2013;14:38–48. doi: 10.1038/nrm3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsa E, Burridge PW, Wu JC. Human stem cells for modeling heart disease and for drug discovery. Sci Transl Med. 2014;6:239 ps236. doi: 10.1126/scitranslmed.3008921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally EM, Golbus JR, Puckelwartz MJ. Genetic mutations and mechanisms in dilated cardiomyopathy. J Clin Invest. 2013;123:19–26. doi: 10.1172/JCI62862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Cacciapuoti I, Parouchev A, Benhamouda N, Tachdjian G, Tosca L, et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J. 2015;36:2011–2017. doi: 10.1093/eurheartj/ehv189. [DOI] [PubMed] [Google Scholar]
- Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott-Flugel L, Dorn T, Goedel A, Hohnke C, Hofmann F, et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med. 2010;363:1397–1409. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- Mummery C, van der Heyden MA, de Boer TP, Passier R, Ward D, van den Brink S, van Rooijen M, van de Stolpe A. Cardiomyocytes from human and mouse embryonic stem cells. Methods Mol Med. 2007;140:249–272. doi: 10.1007/978-1-59745-443-8_14. [DOI] [PubMed] [Google Scholar]
- Muraoka N, Yamakawa H, Miyamoto K, Sadahiro T, Umei T, Isomi M, Nakashima H, Akiyama M, Wada R, Inagawa K, et al. MiR-133 promotes cardiac reprogramming by directly repressing Snai1 and silencing fibroblast signatures. EMBO J. 2014;33:1565–1581. doi: 10.15252/embj.201387605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito H, Melnychenko I, Didie M, Schneiderbanger K, Schubert P, Rosenkranz S, Eschenhagen T, Zimmermann WH. Optimizing engineered heart tissue for therapeutic applications as surrogate heart muscle. Circulation. 2006;114:I72–I78. doi: 10.1161/CIRCULATIONAHA.105.001560. [DOI] [PubMed] [Google Scholar]
- Nam YJ, Lubczyk C, Bhakta M, Zang T, Fernandez-Perez A, McAnally J, Bassel-Duby R, Olson EN, Munshi NV. Induction of diverse cardiac cell types by reprogramming fibroblasts with cardiac transcription factors. Development. 2014;141:4267–4278. doi: 10.1242/dev.114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam YJ, Song K, Luo X, Daniel E, Lambeth K, West K, Hill JA, DiMaio JM, Baker LA, Bassel-Duby R, et al. Reprogramming of human fibroblasts toward a cardiac fate. Proc Natl Acad Sci USA. 2013;110:5588–5593. doi: 10.1073/pnas.1301019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete EG, Liang P, Lan F, Sanchez-Freire V, Simmons C, Gong T, Sharma A, Burridge PW, Patlolla B, Lee AS, et al. Screening drug-induced arrhythmia using human induced pluripotent stem cell-derived cardiomyocytes and low-impedance microelectrode arrays. Circulation. 2013;128:S3–S13. doi: 10.1161/CIRCULATIONAHA.112.000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neofytou E, O’Brien CG, Couture LA, Wu JC. Hurdles to clinical translation of human induced pluripotent stem cells. J Clin Invest. 2015;125:2551–2557. doi: 10.1172/JCI80575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PK, Riegler J, Wu JC. Stem cell imaging: from bench to bedside. Cell Stem Cell. 2014;14:431–444. doi: 10.1016/j.stem.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J, Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4:487–492. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Nunes SS, Miklas JW, Liu J, Aschar-Sobbi R, Xiao Y, Zhang B, Jiang J, Masse S, Gagliardi M, Hsieh A, et al. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods. 2013;10:781–787. doi: 10.1038/nmeth.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi Y, Qin H, Hong C, Blouin L, Polo JM, Guo T, Qi Z, Downey SL, Manos PD, Rossi DJ, et al. Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells. Nat Cell Biol. 2011;13:541–549. doi: 10.1038/ncb2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- Ong SG, Lee WH, Kodo K, Wu JC. MicroRNA-mediated regulation of differentiation and trans-differentiation in stem cells. Adv Drug Deliv Rev. 2015;88:3–15. doi: 10.1016/j.addr.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige SL, Osugi T, Afanasiev OK, Pabon L, Reinecke H, Murry CE. Endogenous Wnt/beta-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PLoS ONE. 2010;5:e11134. doi: 10.1371/journal.pone.0011134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmacek MS, Epstein JA. Cardiomyocyte renewal. N Engl J Med. 2009;361:86–88. doi: 10.1056/NEJMcibr0903347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl JI, Kean LS, Davis MM, Wu JC. Pluripotent stem cells: immune to the immune system? Sci Transl Med. 2012;4:164ps125. doi: 10.1126/scitranslmed.3005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl JI, Lee AS, Leveson-Gower DB, Sun N, Ghosh Z, Lan F, Ransohoff J, Negrin RS, Davis MM, Wu JC. Short-term immunosuppression promotes engraftment of embryonic and induced pluripotent stem cells. Cell Stem Cell. 2011;8:309–317. doi: 10.1016/j.stem.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protze S, Khattak S, Poulet C, Lindemann D, Tanaka EM, Ravens U. A new approach to transcription factor screening for reprogramming of fibroblasts to cardiomyocyte-like cells. J Mol Cell Cardiol. 2012;53:323–332. doi: 10.1016/j.yjmcc.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Srivastava D. Direct cardiac reprogramming: from developmental biology to cardiac regeneration. Circ Res. 2013;113:915–921. doi: 10.1161/CIRCRESAHA.112.300625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rais Y, Zviran A, Geula S, Gafni O, Chomsky E, Viukov S, Mansour AA, Caspi I, Krupalnik V, Zerbib M, et al. Deterministic direct reprogramming of somatic cells to pluripotency. Nature. 2013;502:65–70. doi: 10.1038/nature12587. [DOI] [PubMed] [Google Scholar]
- Rao C, Prodromakis T, Kolker L, Chaudhry UA, Trantidou T, Sridhar A, Weekes C, Camelliti P, Harding SE, Darzi A, et al. The effect of microgrooved culture substrates on calcium cycling of cardiac myocytes derived from human induced pluripotent stem cells. Biomaterials. 2013;34:2399–2411. doi: 10.1016/j.biomaterials.2012.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro MC, Tertoolen LG, Guadix JA, Bellin M, Kosmidis G, D’Aniello C, Monshouwer-Kloots J, Goumans MJ, Wang YL, Feinberg AW, et al. Functional maturation of human pluripotent stem cell derived cardiomyocytes in vitro - Correlation between contraction force and electrophysiology. Biomaterials. 2015;51:138–150. doi: 10.1016/j.biomaterials.2015.01.067. [DOI] [PubMed] [Google Scholar]
- Riegler J, Gillich A, Shen Q, Gold JD, Wu JC. Cardiac tissue slice transplantation as a model to assess tissue-engineered graft thickness, survival, and function. Circulation. 2014;130:S77–S86. doi: 10.1161/CIRCULATIONAHA.113.007920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallam K, Li Y, Sager PT, Houser SR, Wu JC. Finding the rhythm of sudden cardiac death: new opportunities using induced pluripotent stem cell–derived cardiomyocytes. Circ Res. 2015;116:1989–2004. doi: 10.1161/CIRCRESAHA.116.304494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Freire V, Lee AS, Hu S, Abilez OJ, Liang P, Lan F, Huber BC, Ong SG, Hong WX, Huang M, et al. Effect of human donor cell source on differentiation and function of cardiac induced pluripotent stem cells. J Am Coll Cardiol. 2014;64:436–448. doi: 10.1016/j.jacc.2014.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014;32:347–355. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–834. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartiani L, Bettiol E, Stillitano F, Mugelli A, Cerbai E, Jaconi ME. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: a molecular and electrophysiological approach. Stem Cells. 2007;25:1136–1144. doi: 10.1634/stemcells.2006-0466. [DOI] [PubMed] [Google Scholar]
- Schachinger V, Assmus B, Britten MB, Honold J, Lehmann R, Teupe C, Abolmaali ND, Vogl TJ, Hofmann WK, Martin H, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol. 2004;44:1690–1699. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ, Priori SG, Dumaine R, Napolitano C, Antzelevitch C, Stramba-Badiale M, Richard TA, Berti MR, Bloise R. A molecular link between the sudden infant death syndrome and the long-QT syndrome. N Engl J Med. 2000;343:262–267. doi: 10.1056/NEJM200007273430405. [DOI] [PubMed] [Google Scholar]
- Sekine H, Shimizu T, Hobo K, Sekiya S, Yang J, Yamato M, Kurosawa H, Kobayashi E, Okano T. Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. Circulation. 2008;118:S145–S152. doi: 10.1161/CIRCULATIONAHA.107.757286. [DOI] [PubMed] [Google Scholar]
- Sekine H, Shimizu T, Sakaguchi K, Dobashi I, Wada M, Yamato M, Kobayashi E, Umezu M, Okano T. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat Commun. 2013;4:1399. doi: 10.1038/ncomms2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Marceau C, Hamaguchi R, Burridge PW, Rajarajan K, Churko JM, Wu H, Sallam KI, Matsa E, Sturzu AC, et al. Human induced pluripotent stem cell-derived cardiomyocytes as an in vitro model for coxsackievirus B3-induced myocarditis and antiviral drug screening platform. Circ Res. 2014;115:556–566. doi: 10.1161/CIRCRESAHA.115.303810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart N, Bollini S, Dube KN, Vieira JM, Zhou B, Davidson S, Yellon D, Riegler J, Price AN, Lythgoe MF, et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474:640–644. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart N, Dube KN, Riley PR. Identification of Thymosin beta4 as an effector of Hand1-mediated vascular development. Nat Commun. 2010;1:46. doi: 10.1038/ncomms1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Yazawa M, Liu J, Han L, Sanchez-Freire V, Abilez OJ, Navarrete EG, Hu S, Wang L, Lee A, et al. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci Transl Med. 2012;4:130ra147. doi: 10.1126/scitranslmed.3003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takeuchi JK, Bruneau BG. Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature. 2009;459:708–711. doi: 10.1038/nature08039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C, Lee AS, Volkmer JP, Sahoo D, Nag D, Mosley AR, Inlay MA, Ardehali R, Chavez SL, Pera RR, et al. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat Biotechnol. 2011;29:829–834. doi: 10.1038/nbt.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CJ, Peacock S, Chaudhry AN, Bradley JA, Bolton EM. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell. 2012;11:147–152. doi: 10.1016/j.stem.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Tee R, Morrison WA, Dusting GJ, Liu GS, Choi YS, Hsiao ST, Dilley RJ. Transplantation of engineered cardiac muscle flaps in syngeneic rats. Tissue Eng Part A. 2012;18:1992–1999. doi: 10.1089/ten.tea.2012.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen TW, Jaenisch R. Molecular control of induced pluripotency. Cell Stem Cell. 2014;14:720–734. doi: 10.1016/j.stem.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen TW, Powell BE, Wang H, Mitalipova M, Faddah DA, Reddy J, Fan ZP, Maetzel D, Ganz K, Shi L, et al. Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell. 2014;15:471–487. doi: 10.1016/j.stem.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiburcy M, Didie M, Boy O, Christalla P, Doker S, Naito H, Karikkineth BC, El-Armouche A, Grimm M, Nose M, et al. Terminal differentiation, advanced organotypic maturation, and modeling of hypertrophic growth in engineered heart tissue. Circ Res. 2011;109:1105–1114. doi: 10.1161/CIRCRESAHA.111.251843. [DOI] [PubMed] [Google Scholar]
- Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DX, Ellis SG, Forder JR, Anderson RD, Hatzopoulos AK, Penn MS, et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. JAMA. 2012;308:2380–2389. doi: 10.1001/jama.2012.28726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulloch NL, Muskheli V, Razumova MV, Korte FS, Regnier M, Hauch KD, Pabon L, Reinecke H, Murry CE. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res. 2011;109:47–59. doi: 10.1161/CIRCRESAHA.110.237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdouw PD, van den Doel MA, de Zeeuw S, Duncker DJ. Animal models in the study of myocardial ischaemia and ischaemic syndromes. Cardiovasc Res. 1998;39:121–135. doi: 10.1016/s0008-6363(98)00069-8. [DOI] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrtovec B, Poglajen G, Lezaic L, Sever M, Socan A, Domanovic D, Cernelc P, Torre-Amione G, Haddad F, Wu JC. Comparison of transendocardial and intracoronary CD34+ cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation. 2013;128:S42–S49. doi: 10.1161/CIRCULATIONAHA.112.000230. [DOI] [PubMed] [Google Scholar]
- Wada R, Muraoka N, Inagawa K, Yamakawa H, Miyamoto K, Sadahiro T, Umei T, Kaneda R, Suzuki T, Kamiya K, et al. Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. Proc Natl Acad Sci USA. 2013;110:12667–12672. doi: 10.1073/pnas.1304053110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu Z, Yin C, Asfour H, Chen O, Li Y, Bursac N, Liu J, Qian L. Stoichiometry of Gata4, Mef2c, and Tbx5 influences the efficiency and quality of induced cardiac myocyte reprogramming. Circ Res. 2015;116:237–244. doi: 10.1161/CIRCRESAHA.116.305547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Shen J, Splawski I, Atkinson D, Li Z, Robinson JL, Moss AJ, Towbin JA, Keating MT. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80:805–811. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- Wang QT. Epigenetic regulation of cardiac development and function by polycomb group and trithorax group proteins. Dev Dyn. 2012;241:1021–1033. doi: 10.1002/dvdy.23796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liang P, Lan F, Wu H, Lisowski L, Gu M, Hu S, Kay MA, Urnov FD, Shinnawi R, et al. Genome editing of isogenic human induced pluripotent stem cells recapitulates long QT phenotype for drug testing. J Am Coll Cardiol. 2014;64:451–459. doi: 10.1016/j.jacc.2014.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KD, Wu JC. Induced pluripotent stem cells. JAMA. 2015;313:1613–1614. doi: 10.1001/jama.2015.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Pabon L, Murry CE. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Res. 2014;114:511–523. doi: 10.1161/CIRCRESAHA.114.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa M, Hsueh B, Jia X, Pasca AM, Bernstein JA, Hallmayer J, Dolmetsch RE. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471:230–234. doi: 10.1038/nature09855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Chang YH, Xiong Q, Zhang P, Zhang L, Somasundaram P, Lepley M, Swingen C, Su L, Wendel JS, et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 2014;15:750–761. doi: 10.1016/j.stem.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Zimmermann WH, Garry DJ, Zhang J. Patching the heart: cardiac repair from within and outside. Circ Res. 2013;113:922–932. doi: 10.1161/CIRCRESAHA.113.300216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangi L, Lui KO, von Gise A, Ma Q, Ebina W, Ptaszek LM, Spater D, Xu H, Tabebordbar M, Gorbatov R, et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat Biotechnol. 2013;31:898–907. doi: 10.1038/nbt.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Shadrin IY, Lam J, Xian HQ, Snodgrass HR, Bursac N. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials. 2013;34:5813–5820. doi: 10.1016/j.biomaterials.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Klos M, Wilson GF, Herman AM, Lian X, Raval KK, Barron MR, Hou L, Soerens AG, Yu J, et al. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circ Res. 2012;111:1125–1136. doi: 10.1161/CIRCRESAHA.112.273144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Freed CR. Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem Cells. 2009;27:2667–2674. doi: 10.1002/stem.201. [DOI] [PubMed] [Google Scholar]
- Zimmermann WH. Biomechanical regulation of in vitro cardiogenesis for tissue-engineered heart repair. Stem Cell Res Ther. 2013;4:137. doi: 10.1186/scrt348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- Zimmermann WH, Schneiderbanger K, Schubert P, Didie M, Munzel F, Heubach JF, Kostin S, Neuhuber WL, Eschenhagen T. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223–230. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]