Abstract
Insight into the maintenance of naive T cells is essential to understand defective immune responses in the context of aging and other immune compromised states. In humans, naive CD4+ T cells, in contrast to CD8+ T cells, are remarkably well retained with aging. Here, we show that low-affinity TCR engagement is the main driving force behind the emergence and accumulation of naive-like CD4+ T cells with enhanced sensitivity to IL-2 in aged humans. In vitro, we show that these CD45RA+CD25dimCD4+ T cells can develop from conventional naive CD25−CD4+ T cells upon CD3 cross-linking alone, in the absence of costimulation, rather than via stimulation by the homeostatic cytokines IL-2, IL-7, or IL-15. In vivo, TCR engagement likely occurs in secondary lymphoid organs as these cells were detected in lymph nodes and spleen where they showed signs of recent activation. CD45RA+CD25dimCD4+ T cells expressed a broad TCRVβ repertoire and could readily differentiate into functional T helper cells. Strikingly, no expansion of CD45RA+CD25dimCD8+ T cells was detected with aging, thereby implying that maintenance of naive CD4+ T cells is uniquely regulated. Our data provide novel insight into the homeostasis of naive T cells and may guide the development of therapies to preserve or restore immunity in the elderly.
Keywords: aging, homeostasis, interleukin-2, interleukin-7, TCR engagement, T lymphocytes
Introduction
A broad naive T cell repertoire is essential for optimal immunity against novel antigenic challenges (Nikolich-Zugich et al., 2004; Goronzy et al., 2007; Blackman & Woodland, 2011). Contractions of the T cell repertoire have been linked to poor immunity in the context of aging, stem cell transplantation, cancer, and HIV infection (Gorochov et al., 1998; Maury et al., 2001; Saurwein-Teissl et al., 2002; Baum et al., 2012; Manuel et al., 2012). To develop strategies for preserving and restoring the naive T cell repertoire, insight into the basic mechanisms driving naive T cell homeostasis is critical.
So far, most knowledge on the homeostasis of naive T cells is derived from mouse studies (Surh & Sprent, 2008). Mechanisms of naive Tcell maintenance, however, differ substantially in mice and men (den Braber et al., 2012). Whereas thymic output in mice is sustained on to high age, thymic involution drastically reduces T cell replenishment in adult humans (den Braber et al., 2012). Maintenance of the naive T cell pool in humans therefore relies on low-grade proliferation and long-term survival of already existing naive T cells in the periphery (Bains et al., 2009; den Braber et al., 2012). Furthermore, a clear difference has been noted between the maintenance of naive CD4+ and CD8+ T cells in humans. (Goronzy et al., 2007). Whereas circulating numbers of naive CD8+ T cells decline with age, naive CD4+ T cells are remarkably well retained (Wertheimer et al., 2014). So far, little is known about the mechanisms underlying this difference.
Two types of signals are thought to drive the peripheral homeostasis of naive CD4+ T cells in humans (Kohler & Thiel, 2009). The first are T-cell receptor (TCR)-derived signals, as evidenced by an increase in naive CD4+ T cells lacking CD31 in aged humans (Kimmig et al., 2002). CD31 (PECAM-1) is expressed by recent thymic emigrant naive CD4+ T cells (CD31+ naive CD4+ T cells) but is lost upon TCR-derived signaling only (Kohler et al., 2005; Azevedo et al., 2009). In addition, homeostatic cytokines can promote the maintenance of naive CD4+ T cells. IL-7 promotes the expansion and survival of human, naive CD4+ T cells (Sportes et al., 2008). Other cytokines such as IL-2 and IL-15 have long been thought less important for the homeostasis of naive CD4+ T cells (Rochman et al., 2009).
Recently, a novel population of naive CD4+ T cells expressing the IL-2Rα chain (CD25) was observed in peripheral blood of aged humans (Pekalski et al., 2013). These naive CD4+ T cells were characterized by an enhanced response to IL-2 and were thought to develop after IL-7 stimulation (Pekalski et al., 2013). Yet, important questions regarding the origin and functional relevance of these cells remain to be answered. Here, we performed an extensive phenotypical and functional analysis of aging-associated CD25-expressing naive CD4+ T cells. Furthermore, the effects of TCR stimulation and homeostatic cytokines on the in vitro development of CD25-expressing naive CD4+ T cells were evaluated. To elucidate where CD25-expressing naive CD4+ T cells may develop in vivo, different types of human, lymphoid tissues were analyzed. Finally, we assessed the functionality of CD25-expressing naive CD4+ T cells in aged humans.
Results
Maintenance of naive CD4+ T cells but not naive CD8+ T cells in aged humans
We first assessed the numbers of naive CD4+ and CD8+ T cells in peripheral blood of healthy adults of different ages. Donors were divided according to CMV serostatus, as CMV infection may influence naive T-cell numbers (Wertheimer et al., 2014). Naive CD4+ and CD8+ T cells were identified as CD45RO-CCR7+ cells (Fig.1A), co-expressing CD45RA and CD27 (data not shown). As recently reported (Wertheimer et al., 2014), the proportions and absolute numbers of naive CD8+ T cells correlated inversely with age in both CMV-seropositive and CMV-seronegative individuals (Figs1B and S1). In contrast, the proportions and absolute numbers of naive CD4+ T cells only demonstrated an aging-associated decrease in CMV-seropositive, but not in CMV-seronegative, individuals. Thus, aging by itself is associated with a loss of naive CD8+ T cells, but not naive CD4+ T cells.
Fig 1.
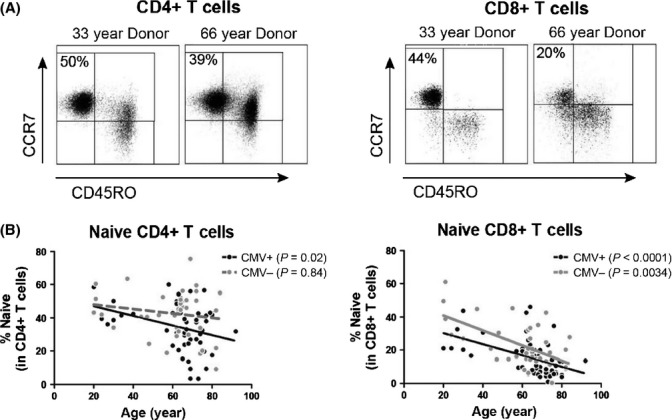
Maintenance of naive CD4+ T cells and loss of naive CD8+ T cells in aged humans. (A) Representative flow cytometric staining of naive (CD45RO-CCR7+) CD4+ and CD8+ T cells in a young and aged individual. The percentage of naive cells among CD4+ and CD8+ T cells is shown. (B) Proportions of naive CD4+ and CD8+ T cells as a function of age in 52 CMV-seropositive and 39 CMV-seronegative healthy, adult humans. Donor ages ranged between 20 and 92. Correlations were tested with Spearman’s rank correlation coefficient. P values are shown in the graph.
CD45RA+CD25dimCD4+ T cells accumulate in the circulation of aged humans
Next, we sought to confirm that aging is associated with an increase in CD25-expressing naive CD4+ T cells (Pekalski et al., 2013). Therefore, a CD45RA/CD25-based gating strategy allowing delineation of functionally distinct populations of naive and memory CD4+ T cells was applied (Miyara et al., 2009). Naive CD25-CD4+ T cells and naive CD25int regulatory T (Treg) cells were readily detected in peripheral blood of adult humans (Fig.2A) (Miyara et al., 2009). The proportions of both cell populations, however, gradually decreased with age (Fig.2B and C). Interestingly, the aging-associated increase in CD25-expressing naive CD4+ T cells could be entirely attributed to the development of CD45RA+CD25dimCD4+ T cells. Not only the proportions but also the absolute numbers of CD45RA+CD25dimCD4+ T cells increased with age (Fig.2D and E). CD45RA+CD25dimCD4+ T cells accumulated in aged humans irrespective of gender and CMV serostatus (Fig. S2). Although some dim expression of CD25 was observed among CD45RA+CD8+ T cells (Fig.2F), CD45RA+CD25dimCD8+ T cells did not accumulate in the peripheral blood of aged humans (Fig.2G). Thus, the expansion of CD45RA+CD25dimcells with aging was restricted to the CD4+ T cell compartment.
Fig 2.
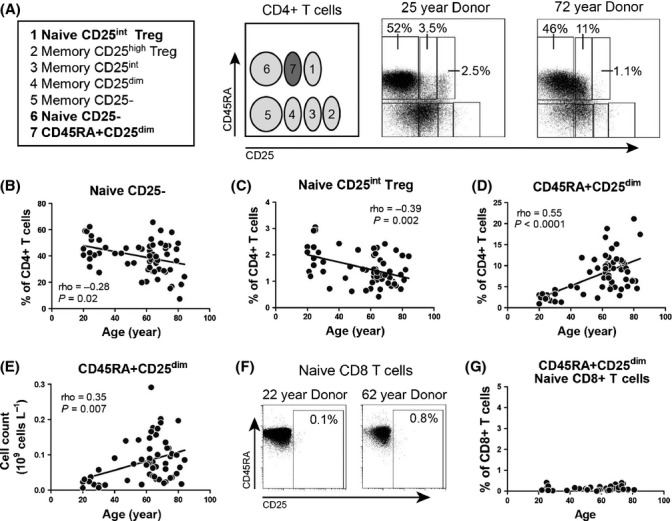
Accumulation of CD45RA+CD25dimCD4+ T cells in peripheral blood of aged humans. (A) Flow cytometric gating strategy for analysis of CD45RA and CD25 defined subsets in peripheral blood, as reported by Miyara et al. (Miyara et al., 2009). Representative flow cytometry plots are shown for a young and an aged individual. (B) Proportions of circulating naive CD25-CD4 T cells, (C) naive CD25int regulatory T (Treg) cells, and (D) CD45RA+CD25dimCD4+ T cells in 63 healthy, adult humans of different ages. (E) Absolute numbers of CD45RA+CD25dimCD4+ T cells in 58 individuals of different ages. (F) Representative staining for CD45RA and CD25 in naive CD45RO-CD27+CD8+ T cells of a young and aged individual. (G) Proportions of circulating naive CD45RA+CD25dimCD8+ T cells in 44 healthy, adult humans of different ages. Correlations were assessed with Spearman’s rank correlation coefficient.
CD45RA+CD25dimCD4+ T cells display a naive phenotype
Additional phenotypical analysis showed that CD45RA+CD25dimCD4+ T cells were not late-stage memory T cells that re-express CD45RA and lack CD27 and CD28 (Fig. S3A). Like naive CD25- cells, CD45RA+CD25dimCD4+ T cells showed high expression of CCR7 and limited expression of CXCR3 and CCR6 (Fig. S3B). These findings indicated that CD45RA+CD25dimCD4+ T cells are indeed naive CD4+ T cells. Furthermore, CD45RA+CD25dimCD4+ T cells could produce some IL-2, but not IFN-γ, IL-4, or IL-17, upon short-term stimulation with PMA and calcium ionophore (Fig. S3C). This cytokine profile was similar to that of naive CD25-CD4+ T cells. CD45RA+CD25dimCD4+ T cells also lacked expression of Treg cell markers FOXP3, Helios, and IL-10, as well as the activation marker CD69 (Fig. S3D and E). Thus, CD45RA+CD25dimCD4+ T cells represented naive CD4+ T cells rather than Treg cells or recently activated T cells.
CD45RA+CD25dimCD4+ T cells show signs of prior TCR engagement
Although peripheral blood CD45RA+CD25dimCD4+ T cells lacked expression of CD69, we found evidence for prior in vivo activation of these cells. Already in our first analysis (Fig.2A), a somewhat lower per-cell expression level of CD45RA was noted on CD45RA+CD25dimCD4+ T cells than on naive CD25-CD4+ T cells. CD45RA to CD45RO transgression typically occurs upon TCR stimulation of naive T cells (Kristensson et al., 1992; Geginat et al., 2001). Analysis of CD45 isoforms confirmed the reduced per-cell expression of CD45RA, and slightly enhanced expression of CD45RO, on CD45RA+CD25dimCD4+ T cells when compared to naive CD25-CD4+ T cells (Fig.3A and data not shown). Although the CD45RO expression on CD45RA+CD25dimCD4+ T cells was markedly lower than on memory CD4+ T cells, we also observed that around 20% of CD45RA+CD25dimCD4+ T cells demonstrated a CD45RAintCD45ROint phenotype vs. only 10% of naive CD25-CD4+ T cells (Fig.3B). These data imply prior in vivo TCR engagement of CD45RA+CD25dimCD4+ T cells.
Fig 3.
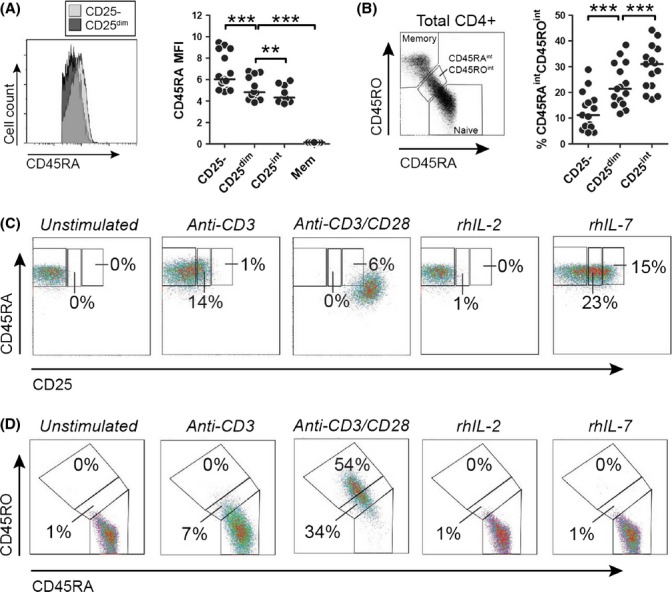
CD45RA+CD25dimCD4+ T cells show signs of prior TCR engagement. (A) Flow cytometric staining for CD45RA in CD45RA+CD25dim and naive CD25- CD4+ T cells (left panel) and mean fluorescence intensity (MFI) of CD45RA in naive CD25-CD4+ T cells, CD45RA+CD25dim CD4+ T cells, naive CD25int regulatory T cells, and memory (Mem) CD4+ T cells of 15 aged individuals. (B) Gating for CD45RAint CD45ROint CD4+ T cells (left panel) and proportions of these cells in the 3 CD45RA+CD4+ T-cell subsets of aged individuals. (C) Development of CD45RA+CD25dim cells from naive CD25-CD4+ T cells and (D) expression of CD45 isoforms upon 6 days of culture with plate-bound anti-CD3 antibodies (plate coated at 1 μg mL−1), plate-bound anti-CD3 antibodies/soluble anti-CD28 antibodies (0.1 μg mL−1), recombinant human (rh) IL-2 (100 U mL−1), or rhIL-7 (10 ng mL−1). Data are representative for experiments with three different donors. Statistical significance is indicated as ** P < 0.01, and *** P < 0.001 by Wilcoxon signed-rank test.
Next, we sought to obtain in vitro evidence that TCR-derived signals drive the development of CD45RA+CD25dimCD4+ T cells. Indeed, CD45RA+CD25dim cells developed from naive CD25-CD4+ T cells upon stimulation by anti-CD3 antibodies only (Fig.3C). These in vitro CD45RA+CD25dimCD4+ T cells also demonstrated slightly modulated expression of CD45 isoforms (Fig.3D). In contrast, combined CD3/CD28 cross-linking largely resulted in complete differentiation of naive CD25-CD4+ T cells into CD45RA-CD45RO+ memory cells and high CD25 expression (Fig.3C and D). Neither IL-2 (Fig.3C) nor IL-15 (data not shown) induced CD25 expression on CD25- naive CD4+ T cells. IL-7 readily induced CD25 expression on naive CD25-CD4+ T cells (Fig.3C), as previously reported (Cimbro et al., 2012; Pekalski et al., 2013). However, the per-cell CD25 expression of IL-7 stimulated cells was higher than typically observed for ex vivo analyzed CD45RA+CD25dimCD4+ T cells. Furthermore, IL-7 did not modulate CD45 isoform expression (Fig.3D). These combined data indicate that CD45RA+CD25dimCD4+ T cells develop from naive CD25-CD4+ T cells upon TCR stimulation only.
CD45RA+CD25dimCD4+ T cells also reside in the CD31+ naive CD4+ T cell pool
Next, we assessed the relation between CD45RA+CD25dimCD4+ T cells and the previously reported population of CD31- central naive CD4+ T cells (Kohler et al., 2005). In accordance with the notion of prior TCR engagement, CD45RA+CD25dimCD4+ T cells demonstrated less CD31 expression than naive CD25-CD4+ T cells (Fig. S4A and B). Consequently, a substantial proportion of CD45RA+CD25dimCD4+ T cells were found in the CD31- central naive CD4+ T cell population (Fig. S4C). However, a significant proportion of CD45RA+CD25dimCD4+ T cells were also found among the CD31+ recent thymic emigrant naive CD4+ T cell population. The presence of these cells within the CD31+ naive CD4+ T cell fraction was not surprising, as we found that CD31 is only gradually lost upon CD3/CD28 cross-linking of CD31+ naive CD4+ T cells (Fig. S4D). Thus, the expression of CD25 reveals further heterogeneity among naive human CD4+ T cells.
CD45RA+CD25dimCD4+ T cells develop in secondary lymphoid tissues
As naive CD4+ T cells continuously circulate through lymphoid organs to encounter antigens, we hypothesized that CD45RA+CD25dimCD4+ T cells would develop in these organs. Analysis of mononuclear cells from human bone marrow, spleen, liver-draining lymph nodes, and inguinal lymph nodes obtained from young and intermediate age donors (median age 42, range 14–62) showed that naive CD25-CD4+ T cells, CD45RA+CD25dimCD4+ T cells, and memory CD4+ T cells were all present in these tissues (Fig.4A). No correlation between tissue CD45RA+CD25dimCD4+ T cells and age was observed, possibly due to the limited number of samples available. Next, we analyzed the expression of CD69, an activation marker that is induced on naive CD4+ T cells after TCR stimulation rather than cytokine stimulation (Simms & Ellis, 1996; Cimbro et al., 2012). Whereas T cells hardly expressed CD69 in peripheral blood and bone marrow, we observed prominent expression of CD69 on T cells in secondary lymphoid organs (Fig.4B). The percentage of CD69-expressing cells was highest among memory CD4+ T cells in lymph nodes and spleen, whereas only few naive CD25-CD4+ T cells expressed CD69 in these secondary lymphoid organs. Interestingly, a substantial part of CD45RA+CD25dimCD4+ T cells in lymph nodes and spleen showed expression of CD69. This suggests that TCR-derived signals drive the development of CD45RA+CD25dimCD4+ T cells in secondary lymphoid organs.
Fig 4.
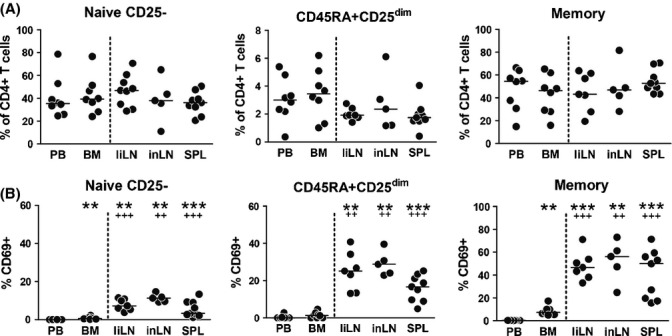
CD45RA+CD25dimCD4+ T cells develop in secondary lymphoid tissues. (A) Naive CD25-, CD45RA+CD25dim, and memory CD4+ T cells were enumerated by flow cytometry in mononuclear cell fractions isolated from peripheral blood (PB) and paired bone marrow (BM) samples, and from nonpaired liver-draining lymph node (liLN), inguinal lymph node (inLN), and spleen (SPL) samples.. (B) Expression of the activation marker CD69 by naive CD25-, CD45RA+CD25dim, and memory CD4+ T cells in peripheral blood and lymphoid tissues. Statistical significance was tested with Wilcoxon signed-rank test or Mann–Whitney U-test. Statistical significance vs. peripheral blood is indicated as **P < 0.01, ***P < 0.001. Statistical significance vs. bone marrow is indicated as ++ P < 0.01, +++ P < 0.001.
Evidence for low-affinity TCR engagement of CD45RA+CD25dimCD4+ T cells
Current evidence from animal studies implies that low-affinity TCR engagement by peptide/MHC complexes promotes the homeostatic maintenance of naive T cells (Surh & Sprent, 2008). Therefore, we next hypothesized that CD45RA+CD25dimCD4+ T cells have undergone low-affinity TCR engagement in vivo. An accepted method to study the strength of TCR and peptide/MHC interactions is measurement of CD3 Ϛ chain phosphorylation levels (Mandl et al., 2013). As this procedure requires immediate analysis of T cell samples, it is incompatible with the currently employed T cell sorting strategy. Therefore, we decided to analyze expression levels of the inhibitory receptor CD5, which reflects prior TCR signaling strength (Mandl et al., 2013). In line with the notion of low-affinity TCR stimulation, CD45RA+CD25dimCD4+ T cells expressed low levels of CD5 (median MFI 54, range 48-98) which were substantially lower than in memory CD4+ T cells (median MFI 67, range 55-121) and comparable to that of naive CD25-CD4+ T cells (median MFI 54, range 12-101), as shown in Fig. S5. The combined data suggest that CD45RA+CD25dimCD4+ T cells develop upon low-affinity TCR–peptide/MHC interaction in vivo.
CD45RA+CD25dimCD4+ T cells display increased sensitivity to IL-2
Common γ-chain cytokines such as IL-2, IL-7, and IL-15 may support the maintenance of T cells by promoting STAT5-dependent proliferation and survival (Rochman et al., 2009). In addition to the IL-2Rα chain (CD25), CD45RA+CD25dimCD4+ T cells also expressed slightly more IL-2/IL-15Rβ chain (CD122) than naive CD25-CD4+ T cells (Figs5A and S6A). In contrast, expression of the common γ-chain (CD132), IL-7Rα chain (CD127), and IL-15Rα chain was similar in CD45RA+CD25dimCD4+ T cells and naive CD25-CD4+ T cells. Based on these findings, we predicted that CD45RA+CD25dimCD4+ T cells would be more responsive to IL-2 and perhaps IL-15 when compared to naive CD25-CD4+ T cells. CD45RA+CD25dimCD4+ T cells indeed were more sensitive to IL-2 than naive CD25-CD4+ T cells, as shown by more IL-2 induced STAT5 phosphorylation. (Figs5B and S6B). CD45RA+CD25dimCD4+ T cells were also slightly more sensitive to IL-15 than naive CD25-CD4+ T cells. In contrast, IL-7 induced similar STAT5 phosphorylation in CD45RA+CD25dimCD4+ T cells and naive CD25-CD4+ T cells. As additional sensitivity to IL-2 and IL-15 may provide extra signals to undergo proliferation (Rochman et al., 2009), we next compared the expression of the proliferation marker Ki-67 in CD45RA+CD25dimCD4+ T cells and naive CD25-CD4+ T cells. As expected, the percentage of proliferating cells was slightly higher among directly analyzed ex vivo CD45RA+CD25dimCD4+ T cells than naive CD25-CD4+ T cells (Fig.5B).
Fig 5.

Increased sensitivity for IL-2 in CD45RA+CD25dim CD4+ T cells. (A) Percentages of cells expressing CD122 (IL-2Rβ chain, n = 9), CD127 (n = 7), and the IL-15Rα chain (n = 9), and the mean fluorescence intensity (MFI) for CD132 (n = 13) in naive CD25-, CD45RA+CD25dim, and memory CD4+ T cells. For CD132, the MFI is shown, as all CD4+ T cells expressed CD132. (B) MFI for pSTAT5 in naive CD25-, CD45RA+CD25dim, and memory CD4+ T cells in response to increasing concentrations of recombinant human IL-2 (rhIL-2), IL-7 (rhIL-7), and IL-15 (rhIL-15). Mean values from experiments with cells from three donors are shown. Percentage of Ki-67-expressing cells (right panel) among naive CD25-, CD45RA+CD25dim and memory CD4+ T cells of 29 healthy aged individuals. Bars and whiskers represent mean with SEM. Statistical significance is indicated as **P < 0.01, ***P < 0.001, by Wilcoxon signed-rank test. ns = nonsignificant.
CD45RA+CD25dimCD4+ T cells represent a broad and functional reservoir of naive T cells
As a broad TCR repertoire is essential for optimal immunity, we next studied the TCR repertoire of CD45RA+CD25dimCD4+ T cells. Although CD45RA+CD25dimCD4+ T cells showed subtle differences in TCR Vβ usage when compared to naive CD25-CD4+ T cells, CD45RA+CD25dimCD4+ T cells still demonstrated a broad TCR Vβ repertoire (Fig.6A).
Fig 6.
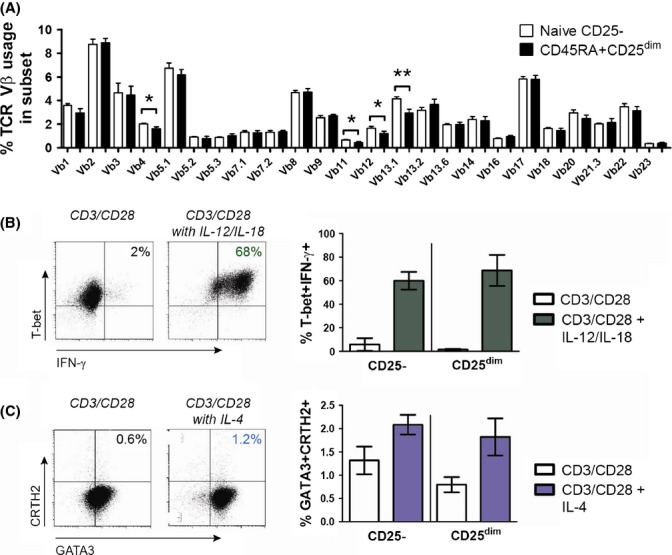
CD45RA+CD25dim CD4+ T cells constitute a broad and functional reservoir of naive T cells. (A) Analysis of TCR Vβ usage in naive CD25- and CD45RA+CD25 dim CD4+ T cells of 9 aged individuals. (B) Representative T-bet and IFN-γ staining in CD45RA+CD25dim CD4+ T cells (left panel) and percentages of T-bet+IFN-γ+ Th1 cells among naive CD25- and CD45RA+CD25dim CD4+ T cells of 3 aged individuals (right panel) after 6 days of culture with anti-CD3/CD28-coated beads with/without IL-12+IL-18 (each 10 ng mL−1) and neutralizing anti-IL-4 antibodies. Cells were restimulated with PMA and Ca2+ ionophore in the presence of brefeldin A for 4 h on the final day. (C) Representative GATA-3 and CRTH2 staining in CD45RA+CD25dim CD4+ T cells (left panel) and percentages of GATA-3+CRTH2+ Th2 cells among naive CD25- and CD45RA+CD25dim CD4+ T cells of 3 aged individuals (right panel) after 6 day culture with anti-CD3/CD28-coated beads with/without IL-4 (25 ng mL−1) and anti-IFN-γ antibodies. Statistical significance is indicated as * p < 0.05 and ** p < 0.01, by Wilcoxon signed rank test.
Subsequently, we tested the ability of CD45RA+CD25dimCD4+ T cells to differentiate into memory T cells. CD45RA+CD25dimCD4+ T cells readily differentiated into CD45RO+ memory cells upon in vitro CD3/CD28 stimulation (Fig. S7). As CD45RA+CD25dimCD4+ T cells were not blocked in their development, we assessed whether CD45RA+CD25dimCD4+ T cells were capable of acquiring T helper (Th) cell effector functions. When cultured under Th1-polarizing conditions, CD45RA+CD25dimCD4+ T cells differentiated into IFN-γ+T-bet+ T helper 1 (Th1) cells (Fig.6B). The Th1-polarizing potential of CD45RA+CD25dimCD4+ T cells was similar to that of naive CD25-CD4+ T cells. CD45RA+CD25dimCD4+ T cells and naive CD25-CD4+ T cells also showed a similar ability to differentiate into GATA3+CRTH2+ T helper 2 (Th2) cells (De Fanis et al., 2007) under Th2-polarizing conditions (Fig.6C). In aggregate, our data show that CD45RA+CD25dimCD4+ T cells constitute a broad and functional reservoir of naive-like CD4+ T cells in aged humans.
Discussion
Here, we report on a unique mechanism of post-thymic maintenance of human naive CD4 T cells, which was not observed for naive CD8 T cells. We found that a robust population of naive CD4 T cells with evidence of prior in vivo TCR engagement develops as a function of age in healthy individuals. We show that this subset, defined by increased CD25 expression, likely develops in secondary lymphoid organs as a result of low-affinity TCR engagement and is further maintained by IL-2. Thus, as thymic output wanes, IL-2 becomes an important homeostatic cytokine for the peripheral maintenance of naive CD4+ T cells in aged humans. We conclude that these CD25 expressing cells represent an important reservoir of naive-like cells that contributes to immunity in the elderly.
Recently, increased proportions of CD25-expressing naive CD4+ T cells were observed in aged individuals (Pekalski et al., 2013). We here extend these findings using a state-of-the-art flowcytometry strategy (Miyara et al., 2009, 2011) and identified these cells as CD45RA+CD25dimCD4+ T cells in the peripheral blood of aged humans. A major advantage of our strategy is the possibility to delineate the aging-associated CD45RA+CD25dimCD4+ T cells from conventional naive CD25-CD4+ T cells and naive CD25int Treg cells. We precluded that CD45RA+CD25dimCD4+ T cells are late-stage memory CD4+ T cells that re-express CD45RA (Akbar & Henson, 2011; Di Mitri et al., 2011) and also confirmed their naive phenotype by analyzing differentiation markers, homing receptors, and intracellular cytokine production. Although the proportional increase in CD45RA+CD25dimCD4+ T cells could merely result from the decrease in the other naive CD4+ T cell fractions, we also found that the absolute number of CD45RA+CD25dimCD4+ T cells increased with age. Our study is therefore the first to show that aging is associated with a genuine increase in CD25-expressing naive CD4+ T cells.
Recently, the accumulation of CD25-expressing naive CD4+ T cells was explained by IL-7-mediated expansion of these cells (Pekalski et al., 2013). We now provide evidence that CD45RA+CD25dimCD4+ T cells primarily develop from naive CD25-CD4+ T cells upon low-affinity TCR engagement. CD45RA+CD25dimCD4+ T cells showed a slight shift in CD45 isoform expression (i.e., from CD5RA to CD45RO) and less CD31 expression in comparison with naive CD25-CD4+ T cells. Changes in expression of CD45 isoforms and CD31 on naive CD4+ T cells occur after TCR engagement rather than IL-7 stimulation (Kohler et al., 2005; Cimbro et al., 2012). Furthermore, typical CD45RA+CD25dimCD4+ T cells developed in vitro from naive CD25-CD4+ T cells after stimulation with anti-CD3 antibodies only. Although we confirmed that IL-7 can induce CD25 expression on naive CD25-CD4+ T cells, the per-cell expression of CD25 was much higher than typically observed on directly ex vivo analyzed CD45RA+CD25dimCD4+ T cells. Furthermore, IL-7 did not modulate CD45 isoform expression. An important role for IL-7 in the development of CD45RA+CD25dimCD4+ T cells in aged humans also seems unlikely, as IL-7 levels decline with age (Kang et al., 2004; Banerjee et al., 2011). Taken together, our data imply that TCR-derived signals rather than IL-7 drive the development of CD45RA+CD25dimCD4+ T cells in humans.
CD45RA+CD25dimCD4+ T cells likely receive TCR-derived signals in secondary lymphoid tissues. CD45RA+CD25dimCD4+ T cells showed substantial expression of the activation marker CD69 in human lymph nodes and spleen, but not in the peripheral blood and bone marrow. Importantly, CD69 is induced on naive T cells after TCR stimulation, but not cytokine stimulation (Simms & Ellis, 1996; Cimbro et al., 2012). Recently, regulatory T cells and conventional memory T cells were reported to express CD69 in secondary lymphoid tissues (Peters et al., 2013; Sathaliyawala et al., 2013). Although commonly used as an activation marker, CD69 may actually play a role in retention of activated T cells in lymphoid tissues via downmodulation of the sphingosine-1-phosphate receptor-1 (Shiow et al., 2006). This could explain the absence of CD69-expressing CD45RA+CD25dimCD4+ T cells in the circulation.
The exact nature of the peptide/MHC complexes involved in the development of human CD45RA+CD25dimCD4+ T cells remains unclear. Animal studies indicate that endogenous peptides may be involved in this process. Depriving mouse naive T cells of self-peptide/MHC complexes typically leads to naive T cell apoptosis (Sprent & Surh, 2011). Non-self (i.e., microbial)-peptides are unlikely to play an important role in the maintenance of the naive CD4+ T cell repertoire, as such peptides are usually offered to T cells in the presence of costimulatory signals. TCR triggering in the presence of costimulation typically leads to full memory T cell differentiation, as also demonstrated in the current study. Although we confirmed that CMV may have a profound impact on the maintenance of the naive CD4+ T cell pool (Wertheimer et al., 2014), we here precluded CMV as a driving force behind the accumulation of CD45RA+CD25dimCD4+ T cells.
Our study reveals further heterogeneity within the human naive CD4+ T cell pool. Previously, the naive CD4+ T cell compartment was mainly classified into nonprimed CD31+ thymic naive cells and primed CD31- central naive cells (Kimmig et al., 2002). Although CD45RA+CD25dimCD4+ T cells were abundant in the CD31- naive population, we also observed CD45RA+CD25dimCD4+ T cells in the CD31+ naive population. The presence of CD45RA+CD25dimCD4+ T cells within the supposedly non-TCR-engaged CD31+ naive population was not surprising, as our data demonstrated that CD31 is only gradually lost upon CD3/CD28 stimulation. The latter finding is in accordance with Demeure et al., showing that T cells loose CD31 only after multiple rounds of CD3/IL-2 stimulation (Demeure et al., 1996). Thus, in addition to the surface marker CD31 (Kohler & Thiel, 2009), the expression of CD25 allows for the identification of naive-like CD4+ T cells with a history of prior TCR engagement.
Whereas primarily IL-7 is considered important for the homeostasis of naive T cells (Rochman et al., 2009), CD45RA+CD25dimCD4+ T cells showed enhanced sensitivity to IL-2, and to some extent IL-15. IL-2 and IL-15 are also involved in the homeostasis of memory T cells and regulatory T cells (Rochman et al., 2009). Cytokines such as IL-2 and IL-15 can induce STAT5-dependent proliferation in T cells (Rochman et al., 2009). Interestingly, circulating CD45RA+CD25dimCD4+ T cells demonstrated a slightly higher proliferation rate than naive CD25-CD4+ T cells. The latter finding may be relevant as naive CD4+ T cells are long-lived cells that may undergo slow but substantial homeostatic proliferation over a longer period of time (den Braber et al., 2012). As serum levels of IL-2 and IL-15 are retained in the elderly (Kang et al., 2004; Gangemi et al., 2005; Banerjee et al., 2011; Kim et al., 2011), the enhanced sensitivity for IL-2 (and IL-15) may be an important adaptation of naive CD4+ T cells to the changing cytokine milieu in aged humans (Fig. S8).
CD45RA+CD25dim cells did not accumulate within the naive CD8+ T-cell compartment of aged humans. The absence of naive CD45RA+CD25dimCD8+ T cells could suggest that IL-2 has no role in the peripheral maintenance of naive CD8+ T cells. However, several reports have shown that IL-2 imposes strong stimulatory effects on naive CD8+ T cells (Cho et al., 2007, 2013; Kamimura & Bevan, 2007). IL-2 not only promotes the proliferation of naive CD8+ T cells, but can also drive the differentiation into memory cells (Cho et al., 2007; Kamimura & Bevan, 2007). Therefore, the absence of naive CD45RA+CD25dim CD8+ T cells and the pronounced decline in naive CD8+ T cells in aged humans could be related to the relatively low threshold of naive CD8+ T cells to convert into memory cells. Indeed, aging is associated with a substantial shift from naive CD8+ T cells toward central and effector memory cells (Wertheimer et al., 2014).
Our data imply that CD45RA+CD25dimCD4+ T cells may contribute to immunity in aged humans. CD45RA+CD25dimCD4+ T cells represent a broad and functional reservoir of naive CD4+ T cells in aged humans. CD45RA+CD25dimCD4+ T cells showed broad TCR Vβ usage and a similar ability to differentiate into Th1 and Th2 cells as naive CD25-CD4+ T cells. Importantly, our in vitro experiments show that CD45RA+CD25dimCD4+ T cells can be generated from naive CD25-CD4+ T cells. Furthermore, prior studies with IL-2 therapy have shown that nonregulatory CD25-expressing naive CD4+ T cells can be expanded by intermittent IL-2 therapy (Natarajan et al., 2002; Sereti et al., 2002, 2004). Enhancing circulating numbers of CD45RA+CD25dimCD4+ T cells could therefore be an interesting strategy for preserving or restoring immunity in aged humans.
In conclusion, our study shows that TCR engagement drives the emergence and accumulation of CD25-expressing naive CD4+ T cells in healthy aged humans. These cells, which likely develop in secondary lymphoid tissues, represent a broad and functional reservoir of naive CD4+ T cells in aged humans. Our study provides new insight into the homeostasis of human, naive CD4+ T cells and justifies further studies into CD4+ T cell expanding treatments to promote immunity in aged and immune compromised humans.
Experimental procedures
Donor samples and study approval
In a cross-sectional study, blood samples were obtained from 91 healthy individuals (age 20–92). Health was assessed by a health assessment questionnaire, a physical examination, and blood tests. Only a slight elevation in blood pressure and the use of antihypertensive treatment were accepted. Also, clinical and laboratory data attesting to the donors’ overall health were assessed. In addition, blood/bone marrow samples were obtained from 8 healthy stem cell donors, before mobilization with G-CSF, spleen samples from 9 deceased kidney donors, liver-draining lymph nodes from 7 deceased liver donors, and inguinal lymph nodes from 5 kidney transplant recipients at the time of transplantation (not treated with immunosuppressive drugs prior to lymph node excision). Single cell suspensions were obtained from tissues as previously described (Peters et al., 2013). Mononuclear cell fractions were isolated by density gradient centrifugation with Lymphoprep (Axis-Shield and Nycomed Pharma). Written informed consent was obtained from all study participants or their representatives, and the study was approved by medical ethical committees at the participating study centers.
Flow cytometry
Isolated mononuclear cells or whole blood samples were stained with the following fluorochrome-conjugated monoclonal antibodies: CD4-PcP, CD8-APC-H7, CD31-PE, CD45RA-FITC, CD25-PE, CD45RO-PE-Cy7, CD45RO-FITC, CCR7-PcP-Cy5.5, TCRγδ-BV421, pSTAT5-PE-Cy7, Ki-67-PcP-Cy5.5, Tbet-PE, CXCR3-PE-Cy5, CD69-APC-Cy7, CCR4-PE-Cy7, CD5-APC, CRTH2-PE, GATA3-APC, CD5-APC (all BD), CD4-ECD, CD4-PC7, CD69-PC5, CD69-ECD, Beta Mark TCR V β kit (all Beckman Coulter, Woerden, The Netherlands), CD122-PE, CD132-PE, CCR6-PcP-Cy5.5, IL-2-AF700, IL-4-PE, IFN-γ-PcP-Cy5.5, FOXP3-AF647, Helios-AF488 (all Biolegend, Uithoorn, The Netherlands), CD4-ef450, CD25-APC, CD25-PE, CD45RA-PE, CD45RA-ef605, HLA-DR-APC-ef780, IL-17-AF488, CD27-AF700, CD28-PcP-cy5.5 (all eBioscience, Vienna, Austria). In case of whole blood staining, samples were lysed with BD FACS lysing solution. Samples were measured on a LSR-II (BD) or FC500 (Beckman Coulter) and analyzed with Kaluza Analysis Software (Beckman Coulter). Absolute numbers of CD4+ and CD8+ T cells were determined according to the BD MultiTest TruCount method, as described by the manufacturer. TruCount measurements were taken on a FACS Canto-II (BD) and analyzed with FACSCanto Clinical Software (BD).
Intracellular cytokine and transcription factor staining
Whole blood samples were diluted 1:1 with RPMI and stimulated with PMA and Ca2+ ionophore A23187 in the presence of brefeldin A (Sigma-Aldrich, Zwijndrecht, The Netherlands) for 4 h. After red blood cell lysis with ammonium chloride, cells were fixed and permeabilized with a Foxp3 Staining Buffer Set (eBioscience) followed by intracellular staining.
Phosphorylated STAT5 staining
Peripheral blood mononuclear cells (PBMCs) were stimulated with indicated concentrations of recombinant human (rh) IL-2, rhIL-7, or rhIL-15 (all Peprotech) during 15 min, directly followed by fixation with Cytofix Buffer (BD) for 10 min at 37 °C. Subsequently, the cells were treated with Perm Buffer III (BD) for 30 min on ice and stained for pSTAT5.
Cell culture
Naive T cells fractions of interest were sorted on a MoFlo Astrios sorter (Beckman Coulter). For assessment of CD31 loss, sorted CD31+ naive CD4+ T cells were stained with 5 μm proliferation dye CFSE. The cells were cultured for 6 days in 96-well flat-bottomed plates in RPMI1640 with 10% human pooled serum (HPS) and gentamycin. Cells were stimulated with CD3/CD28-coated beads (Life Technologies, Paisley, UK) at a cell to bead ratio of 1:1. For induction of CD45RA+CD25dimCD4+ T cells, sorted naive CD45RO-CCR7+CD25- CD4+ T cells were cultured for 6 days in the presence of plate-bound anti-CD3 antibodies (wells coated at concentration of 1 μg mL−1) with/without soluble anti-CD28 antibodies (0.1 μg mL−1), or with indicated concentrations of rhIL-2 or rhIL-7. For polarizing experiments, sorted CD45RA+CD25- and CD45RA+CD25dim CD4+ T cells were cultured with CD3/CD28-coated beads at a cell to bead ratio of 1:1 in the presence or absence of rhIL-12 (10 ng mL−1), rhIL-18 (10 ng mL−1), and anti-IL-4 mAb (10 μg mL−1) for Th1 skewing; or rhIL-4 (25 ng mL−1) and anti-IFN-γ mAb (10 ng mL−1) for Th2 skewing. The recombinant human cytokines were purchased from Peprotech and blocking antibodies from BD. After 4 days, the medium was refreshed and all cytokines and blocking antibodies were added again. After 6 days, the cells were restimulated for 4 h with PMA and calcium ionophore in the presence of brefeldin A.
CMV-specific antibodies
Serum levels of CMV-specific IgG were determined with an in-house ELISA. 96-well ELISA plates (Greiner) were coated with lysates of CMV-infected fibroblasts overnight. Lysates of noninfected fibroblasts were used as negative controls. Following the coating, dilutions of serum samples were incubated for 1 h. Goat anti-human IgG was added and incubated for 1 h. Samples were incubated with phosphatase for 15 min, and the reaction was stopped with NaOH. The plates were scanned on a Versamax reader (Molecular Devices). A pool of sera from 3 CMV-seropositive individuals with known concentrations of CMV-specific IgG was used to quantify CMV IgG in the tested samples.
Acknowledgments
We are grateful to Jaap Kwekkeboom (Erasmus MC, Netherlands) and Michel Schaap (Radboud UMC, Netherlands) for their previous support in tissue sampling. We thank Anna Richt Miedema for assessing the health status of the healthy volunteers, Suzanne Arends for her statistical advice, and Johan Bijzet for his technical assistance with the anti-CMV ELISA. We also thank Henk Moes, Geert Mesander, and Roelof Jan van der Lei of the flow cytometry unit for their technical assistance with sorting of cells.
Author contributions
KSMvdG, WA, ST, JHP, NAB, BJK, HJPMK, IJ, EB, and AMHB planned and designed experiments. KSMvdG, NT, ST, JHP, GH, PGL, AL, and CR executed experiments. KSMvdG, WA, NT, ST, JHP, GH, PGL, NAB, AL, CR, HJPMK, IJ, EB, and AMHB analyzed the data. KSMvdG, WA, BJK, IJ, EB, and AMHB wrote the manuscript.
Funding info
No funding information provided. This work was supported by grants from MSD, Foundation Jan Kornelis de Cock, and Foundation De Drie Lichten.
Conflict of interest
AMH Boots was previously (until 2011) employed by MSD. We received an unrestricted grant from MSD.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site.
Fig. S1 Maintenance of naive CD4+ T cells and loss of naive CD8+ T cells in aged humans.
Fig. S2 Accumulation of CD45RA+CD25dimCD4+ T cells in peripheral blood of aged humans is not driven by sex or CMV serostatus.
Fig. S3 Circulating CD45RA+CD25dimCD4+ T cells demonstrate a naive-like phenotype.
Fig. S4 CD45RA+CD25dimCD4+ T cells show signs of prior TCR engagement.
Fig. S5 Per-cell expression of CD5 by CD45RA+CD25dimCD4+ T cells.
Fig. S6 Increased sensitivity for IL-2 in CD45RA+ CD25dimCD4+ T cells.
Fig. S7 Acquisition of CD45RO by anti-CD3/CD28 stimulated CD45RA+CD25dimCD4+ T cells.
Fig. S8 Schematic overview of naive CD4+ T cell maintenance in young and aged humans.
References
- Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat. Rev. Immunol. 2011;11:289–295. doi: 10.1038/nri2959. [DOI] [PubMed] [Google Scholar]
- Azevedo RI, Soares MV, Barata JT, Tendeiro R, Serra-Caetano A, Victorino RM, Sousa AE. IL-7 sustains CD31 expression in human naive CD4+ T cells and preferentially expands the CD31+ subset in a PI3K-dependent manner. Blood. 2009;113:2999–3007. doi: 10.1182/blood-2008-07-166223. [DOI] [PubMed] [Google Scholar]
- Bains I, Thiebaut R, Yates AJ, Callard R. Quantifying thymic export: combining models of naive T cell proliferation and TCR excision circle dynamics gives an explicit measure of thymic output. J. Immunol. 2009;183:4329–4336. doi: 10.4049/jimmunol.0900743. [DOI] [PubMed] [Google Scholar]
- Banerjee C, Ulloor J, Dillon EL, Dahodwala Q, Franklin B, Storer T, Sebastiani P, Sheffield-Moore M, Urban RJ, Bhasin S, Montano M. Identification of serum biomarkers for aging and anabolic response. Immun. Ageing. 2011;8:5–4933. doi: 10.1186/1742-4933-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum PD, Young JJ, Schmidt D, Zhang Q, Hoh R, Busch M, Martin J, Deeks S, McCune JM. Blood T cell receptor diversity decreases during the course of HIV infection, but the potential for a diverse repertoire persists. Blood. 2012;119:3469–3477. doi: 10.1182/blood-2011-11-395384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman MA, Woodland DL. The narrowing of the CD8 T cell repertoire in old age. Curr. Opin. Immunol. 2011;23:537–542. doi: 10.1016/j.coi.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mögling R, de Boer AB, Willems N, Schrijver EH, Spierenburg G, Gaiser K, Mul E, Otto SA, Ruiter AF, Ackermans MT, Miedema F, Borghans JA, de Boer RJ, Tesselaar K. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity. 2012;36:288–297. doi: 10.1016/j.immuni.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Cho JH, Boyman O, Kim HO, Hahm B, Rubinstein MP, Ramsey C, Kim DM, Surh CD, Sprent J. An intense form of homeostatic proliferation of naive CD8+ cells driven by IL-2. J. Exp. Med. 2007;204:1787–1801. doi: 10.1084/jem.20070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Kim HO, Kim KS, Yang DH, Surh CD, Sprent J. Unique features of naive CD8+ T cell activation by IL-2. J. Immunol. 2013;191:5559–5573. doi: 10.4049/jimmunol.1302293. [DOI] [PubMed] [Google Scholar]
- Cimbro R, Vassena L, Arthos J, Cicala C, Kehrl JH, Park C, Sereti I, Lederman MM, Fauci AS, Lusso P. IL-7 induces expression and activation of integrin alpha4beta7 promoting naive T cell homing to the intestinal mucosa. Blood. 2012;120:2610–2619. doi: 10.1182/blood-2012-06-434779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Fanis U, Mori F, Kurnat RJ, Lee WK, Bova M, Adkinson NF, Casolaro V. GATA3 up-regulation associated with surface expression of CD294/CRTH2: a unique feature of human Th cells. Blood. 2007;109:4343–4350. doi: 10.1182/blood-2006-05-025940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeure CE, Byun DG, Yang LP, Vezzio N, Delespesse G. CD31 (PECAM-1) is a differentiation antigen lost during human CD4 T cell maturation into Th1 or Th2 effector cells. Immunology. 1996;88:110–115. doi: 10.1046/j.1365-2567.1996.d01-652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mitri D, Azevedo RI, Henson SM, Libri V, Riddell NE, Macaulay R, Kipling D, Soares MV, Battistini L, Akbar AN. Reversible senescence in human CD4+CD45RA+CD27- memory T cells. J. Immunol. 2011;187:2093–2100. doi: 10.4049/jimmunol.1100978. [DOI] [PubMed] [Google Scholar]
- Gangemi S, Basile G, Monti D, Merendino RA, Di Pasquale G, Bisignano U, Nicita-Mauro V, Franceschi C. Age-related modifications in circulating IL-15 levels in humans. Mediators Inflamm. 2005;2005:245–247. doi: 10.1155/MI.2005.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J. Exp. Med. 2001;194:1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorochov G, Neumann AU, Kereveur A, Parizot C, Li T, Katlama C, Karmochkine M, Raguin G, Autran B, Debré P. Perturbation of CD4+ and CD8+ T cell repertoires during progression to AIDS and regulation of the CD4+ repertoire during antiviral therapy. Nat. Med. 1998;4:215–221. doi: 10.1038/nm0298-215. [DOI] [PubMed] [Google Scholar]
- Goronzy JJ, Lee WW, Weyand CM. Aging and T cell diversity. Exp. Gerontol. 2007;42:400–406. doi: 10.1016/j.exger.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura D, Bevan MJ. Naive CD8+ T cells differentiate into protective memory-like cells after IL-2 anti IL-2 complex treatment in vivo. J. Exp. Med. 2007;204:1803–1812. doi: 10.1084/jem.20070543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang I, Hong MS, Nolasco H, Park SH, Dan JM, Choi JY, Craft J. Age-associated change in the frequency of memory CD4+ T cells impairs long term CD4+ T cell responses to influenza vaccine. J. Immunol. 2004;173:673–681. doi: 10.4049/jimmunol.173.1.673. [DOI] [PubMed] [Google Scholar]
- Kim HO, Kim HS, Youn JC, Shin EC, Park S. Serum cytokine profiles in healthy young and elderly population assessed using multiplexed bead-based immunoassays. J. Transl. Med. 2011;9:113. doi: 10.1186/1479-5876-9-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmig S, Przybylski GK, Schmidt CA, Laurisch K, Möwes B, Radbruch A, Thiel A. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J. Exp. Med. 2002;195:789–794. doi: 10.1084/jem.20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S, Thiel A. Life after the thymus: CD31+ and CD31- human naive CD4+ T cell subsets. Blood. 2009;113:769–774. doi: 10.1182/blood-2008-02-139154. [DOI] [PubMed] [Google Scholar]
- Kohler S, Wagner U, Pierer M, Kimmig S, Oppmann B, Möwes B, Jülke K, Romagnani C, Thiel A. Post-thymic in vivo proliferation of naive CD4+ T cells constrains the TCR repertoire in healthy human adults. Eur. J. Immunol. 2005;35:1987–1994. doi: 10.1002/eji.200526181. [DOI] [PubMed] [Google Scholar]
- Kristensson K, Borrebaeck CA, Carlsson R. Human CD4+ T cells expressing CD45RA acquire the lymphokine gene expression of CD45RO+ T-helper cells after activation in vitro. Immunology. 1992;76:103–109. [PMC free article] [PubMed] [Google Scholar]
- Mandl JN, Monteiro JP, Vrisekoop N, Germain RN. T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity. 2013;38:263–274. doi: 10.1016/j.immuni.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel M, Tredan O, Bachelot T, Clapisson G, Courtier A, Parmentier G, Rabeony T, Grives A, Perez S, Mouret JF, Perol D, Chabaud S, Ray-Coquard I, Labidi-Galy I, Heudel P, Pierga JY, Caux C, Blay JY, Pasqual N, Ménétrier-Caux C. Lymphopenia combined with low TCR diversity (divpenia) predicts poor overall survival in metastatic breast cancer patients. Oncoimmunology. 2012;1:432–440. doi: 10.4161/onci.19545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury S, Mary JY, Rabian C, Schwarzinger M, Toubert A, Scieux C, Carmagnat M, Esperou H, Ribaud P, Devergie A, Guardiola P, Vexiau P, Charron D, Gluckman E, Socié G. Prolonged immune deficiency following allogeneic stem cell transplantation: risk factors and complications in adult patients. Br. J. Haematol. 2001;115:630–641. doi: 10.1046/j.1365-2141.2001.03135.x. [DOI] [PubMed] [Google Scholar]
- Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, Sakaguchi S. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Miyara M, Gorochov G, Ehrenstein M, Musset L, Sakaguchi S, Amoura Z. Human FoxP3+ regulatory T cells in systemic autoimmune diseases. Autoimmun. Rev. 2011;10:744–755. doi: 10.1016/j.autrev.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Natarajan V, Lempicki RA, Sereti I, Badralmaa Y, Adelsberger JW, Metcalf JA, Prieto DA, Stevens R, Baseler MW, Kovacs JA, Lane HC. Increased peripheral expansion of naive CD4+ T cells in vivo after IL-2 treatment of patients with HIV infection. Proc. Natl Acad. Sci. USA. 2002;99:10712–10717. doi: 10.1073/pnas.162352399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat. Rev. Immunol. 2004;4:123–132. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- Pekalski ML, Ferreira RC, Coulson RM, Cutler AJ, Guo H, Smyth DJ, Downes K, Dendrou CA, Castro Dopico X, Esposito L, Coleman G, Stevens HE, Nutland S, Walker NM, Guy C, Dunger DB, Wallace C, Tree TI, Todd JA, Wicker LS. Postthymic Expansion in Human CD4 Naive T Cells Defined by Expression of Functional High-Affinity IL-2 Receptors. J. Immunol. 2013;190:2554–2566. doi: 10.4049/jimmunol.1202914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JH, Koenen HJ, Fasse E, Tijssen HJ, Ijzermans JN, Groenen PJ, Schaap NP, Kwekkeboom J, Joosten I. Human secondary lymphoid organs typically contain polyclonally-activated proliferating regulatory T cells. Blood. 2013;122:2213–2223. doi: 10.1182/blood-2013-03-489443. [DOI] [PubMed] [Google Scholar]
- Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat. Rev. Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, Bickham KL, Lerner H, Goldstein M, Sykes M, Kato T, Farber DL. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–197. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurwein-Teissl M, Lung TL, Marx F, Gschösser C, Asch E, Blasko I, Parson W, Böck G, Schönitzer D, Trannoy E, Grubeck-Loebenstein B. Lack of antibody production following immunization in old age: association with CD8(+)CD28(-) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J. Immunol. 2002;168:5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- Sereti I, Martinez-Wilson H, Metcalf JA, Baseler MW, Hallahan CW, Hahn B, Hengel RL, Davey RT, Kovacs JA, Lane HC. Long-term effects of intermittent interleukin 2 therapy in patients with HIV infection: characterization of a novel subset of CD4(+)/CD25(+) T cells. Blood. 2002;100:2159–2167. [PubMed] [Google Scholar]
- Sereti I, Anthony KB, Martinez-Wilson H, Lempicki R, Adelsberger J, Metcalf JA, Hallahan CW, Follmann D, Davey RT, Kovacs JA, Lane HC. IL-2-induced CD4+ T cell expansion in HIV-infected patients is associated with long-term decreases in T-cell proliferation. Blood. 2004;104:775–780. doi: 10.1182/blood-2003-12-4355. [DOI] [PubMed] [Google Scholar]
- Shiow LR, Rosen DB, Brdicková N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- Simms PE, Ellis TM. Utility of flow cytometric detection of CD69 expression as a rapid method for determining poly- and oligoclonal lymphocyte activation. Clin. Diagn. Lab. Immunol. 1996;3:301–304. doi: 10.1128/cdli.3.3.301-304.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, Fleisher TA, Krumlauf MC, Babb RR, Chow CK, Fry TJ, Engels J, Buffet R, Morre M, Amato RJ, Venzon DJ, Korngold R, Pecora A, Gress RE, Mackall CL. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J. Exp. Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J, Surh CD. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat. Immunol. 2011;12:478–484. doi: 10.1038/ni.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Wertheimer AM, Bennett MS, Park B, Uhrlaub JL, Martinez C, Pulko V, Currier NL, Nikolich-Žugich D, Kaye J, Nikolich-Žugich J. Aging and cytomegalovirus infection differentially and jointly affect distinct circulating T cell subsets in humans. J. Immunol. 2014;192:2143–2155. doi: 10.4049/jimmunol.1301721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Maintenance of naive CD4+ T cells and loss of naive CD8+ T cells in aged humans.
Fig. S2 Accumulation of CD45RA+CD25dimCD4+ T cells in peripheral blood of aged humans is not driven by sex or CMV serostatus.
Fig. S3 Circulating CD45RA+CD25dimCD4+ T cells demonstrate a naive-like phenotype.
Fig. S4 CD45RA+CD25dimCD4+ T cells show signs of prior TCR engagement.
Fig. S5 Per-cell expression of CD5 by CD45RA+CD25dimCD4+ T cells.
Fig. S6 Increased sensitivity for IL-2 in CD45RA+ CD25dimCD4+ T cells.
Fig. S7 Acquisition of CD45RO by anti-CD3/CD28 stimulated CD45RA+CD25dimCD4+ T cells.
Fig. S8 Schematic overview of naive CD4+ T cell maintenance in young and aged humans.


