Abstract
Vaccine potency testing is necessary to evaluate the immunogenicity of inactivated rabies virus (RABV) vaccine preparations before human or veterinary application. Currently, the NIH test is recommended by the WHO expert committee to evaluate RABV vaccine potency. However, numerous disadvantages are inherent concerning cost, number of animals and biosafety requirements. As such, several in vitro methods have been proposed for the evaluation of vaccines based on RABV glycoprotein (G) quality and quantity, which is expected to correlate with vaccine potency. In this study an antigen-capture electrochemiluminescent (ECL) assay was developed utilizing anti-RABV G monoclonal antibodies (MAb) to quantify RABV G. One MAb 2-21-14 was specific for a conformational epitope so that only immunogenic, natively-folded G was captured in the assay. A second MAb (62-80-6) that binds a linear epitope or MAb 2-21-14 was used for detection of RABV G. Vaccine efficacy was also assessed in vivo using pre-exposure vaccination of mice. Purified native RABV G induced a RABV neutralizing antibody (rVNA) response with a geometric mean titer of 4.2 IU/ml and protected 100% of immunized mice against RABV challenge, while an experimental vaccine with a lower quality and quantity of G induced a rVNA titer <0.05 IU/ml and protected <50% of immunized mice. These preliminary results support the hypothesis that in vivo immunogenicity may be predicted from the in vitro measurement of RABV G using an ECL assay. Based upon these results, the ECL assay may have utility in replacement of the NIH test.
Keywords: Rabies virus, Vaccine, Glycoprotein, Electrochemiluminescent assay, Antigenicity, Immunogenicity
Introduction
Rabies virus (RABV) continues to present challenges for vaccine development related to its neurotropism, immune evasion, and antigenic diversity, as well as the access to affordable modern biologics [1-3]. Given the necessity for highly effective pre- and post-exposure prophylaxis (PEP), potency issues are paramount to ensure released lots of RABV vaccine can elicit an adequate level of RABV- neutralizing antibodies (rVNA) in humans and other animals. Rabies vaccines that are applied in human PEP regimes should have a potency of at least 2.5 international units (IU) per dose, as measured by the NIH test [4, 5]. The NIH potency test has been used for over 50 years as a measure of RABV vaccine potency. In this test, groups of 4-week old mice are inoculated intraperitoneally over a two week period. Then RABV is administered intracerebrally in parallel for vaccinated and control mice, to calculate an effective dose (ED50). The ED50 of a standard NIH reference vaccine is divided by the ED50 of the test vaccine to yield its relative potency to permit comparison between different reference vaccine preparations [6].
Shortcomings of the NIH test are numerous and include unnatural routes of vaccination and challenge, the requirement of hundreds of mice per test, the wide confidence interval of results, and the associated time and costs of in vivo testing [7]. Refinement and replacement of this historical measure continues to be an issue facing vaccine manufacturers [8]. Alternative methods will require highly robust, reproducible and flexible characteristics to accommodate future development in the field including: greater consideration of rabies as a vaccine-preventable disease for non-occupational, pre-exposure immunization of those at greatest risk; further dose-sparing schedules; development of additional non-inactivated recombinant biologics for both veterinary (including wildlife) and public health applications; and inclusion of other viral antigens towards the production of broader, less expensive pan-lyssavirus vaccines [9].
Various in vitro methods have been suggested to replace the NIH test. Single radial immunodiffusion was originally proposed as a measure of RABV glycoprotein (G) content in vaccine preparations [10, 11]. In addition, ELISA techniques were developed [12-15]. Of these, an immune-capture (i.e. antigen-capture or sandwich) ELISA using a RABV neutralizing monoclonal antibody (MAb) emerged as a preferred method [16, 17]. Using a MAb that only recognizes the native, trimeric and immunogenic form of RABV G prevents detection of non-immunogenic, soluble, G in vaccines [18-21]. Recently, this method has been further refined using a single-chain variable fragment antibody [22] or a diabody, to replace MAbs for antigen capture [23].
In the current study, an antigen-capture assay was selected to assess the antigen content of various RABV vaccine preparations. The Meso Scale Discovery (MSD) platform was used to quantify the electrochemiluminescence (ECL). The ECL assay is based on the proximity of a sulfo-tag-label to an electrical current on the plate surface, resulting in the release of light which can be measured. An arbitrary unit of counts is assigned to the intensity of the light by the MSD imager. The ECL counts were expressed in terms of the total protein concentration in the test vaccine. This value was compared to the immunogenicity of the vaccine in mice 30 days after a single intramuscular (IM) immunization.
Materials and Methods
Monoclonal antibodies
The MAbs against RABV G were purified from existing hybridomas (CDC, Atlanta GA, USA). MAb, 2-21-14, was generated in two cloning steps from B-cells isolated from a BALB/c mouse immunized with Ethiopian street RABV fused with Sp2/0-Ag 14 derivative of the BALB/c myeloma line P3-X63-AG8 in 1984. Another MAb, 62-80-6, was generated in two cloning steps from B-cells isolated from a BALB/c mouse immunized with the RABV ERA strain fused with Sp2/0-Ag 14 derivative of the BALB/c myeloma line P3-X63-AG8 in 1982. Both MAbs are subtype IgG2a/IgG2b. The epitope for each MAb was determined by selecting escape mutants according to the method of Marissen, et al. [24]. The coding region of the G gene was sequenced following Ellison, et al. [25].
Vaccines
Commercially available RABV vaccines Imovax (lot: G1076) and RabAvert (lot: 464011A) were purchased from Sanofi Pasteur (Swiftwater, PA, USA) and Novartis Pharmaceuticals (Dorval, Quebec, Canada), respectively, and reconstituted according to the manufacturer’s instructions. Depleted vaccines were generated by reconstituting the same vaccines and incubating for 100 days at 37°C. An experimental RABV vaccine (lot: CMB-0300-007) not commercially available was provided by Fraunhofer (Newark, DE). Adjuvanted vaccine was generated by mixing the same vaccine 50% (v/v) with Alhydrogel (Accurate Chemical, Westbury, NY). A second experimental RABV vaccine (lot: 475-03-020) was provided by Medicago (Quebec, Quebec, Canada).
Purified RABV G was produced from ERA and CVS-11 RABV strains. These RABV strains were propagated with cultured BHK cells (Invitrogen, Carlsbad, CA, USA) in 150 cm2 flasks (Corning Life Sciences, Tewksbury, MA, USA) to 1×109 ffu/ml and inactivated with 0.1% (v/v) β-propiolactone (Sigma-Aldrich, St. Louis, MO, USA) for 5 h at 0°C. The RABV was concentrated from the cell culture supernatant by centrifugation for 2 h at 50,000×g and envelope G was purified as previously described [26]. Denatured G was prepared by sonication of concentrated virus during purification, or by heating native purified G for 10 min at 98°C. The total protein concentration of the test vaccines was determined using the BCA Protein Assay (Thermo Scientific, Rockford, IL, USA) according to manufacturer’s instructions.
Conjugation of secondary antibody
Zeba Spin Desalting Columns, 7K MWCO (Thermo Scientific, Rockford, IL, USA) were equilibrated with 0.01M PBS pH 7.9 according to manufacturer’s instructions before eluting the secondary MAb. The protein concentration was adjusted to 2 mg/ml using the same buffer, and the antibody was conjugated with 3 nmol/μl SULFO-TAG NHS-Ester (MSD, Gaithersburg, MD, USA) according to manufacturer’s instructions for 2 h at room temperature in the dark with shaking. Excess SULFO-TAG NHS-Ester was removed using the Zeba Spin Desalting Columns following MSD instructions. Protein concentration of the conjugated antibody was determined using the BCA Protein Assay (Thermo Scientific, Rockford, IL, USA) according to manufacturer’s instructions.
Direct ECL assay
Standard, bare 96-well carbon-electrode plates (MSD, Gaithersburg, MD, USA) were coated with 10 μg/ml of antigen from RABV ERA, CVS, or Flury (from RabAvert® vaccine) strains either in the native form or heat denatured and incubated overnight at 4°C. Plates were washed three times with 0.01M PBS-0.05% Tween20 (Sigma-Aldrich, St. Louis, MO, USA), 150 μl/well of casein (Vector Laboratories, Burlingame, CA, USA) was added, and plates were incubated 60 min at room temperature with shaking. Casein was removed, and 1 μg/ml conjugated MAb was added to the wells. Plates were incubated 60 m at room temperature with shaking, then washed three times with 0.01M PBS-0.05% Tween20, and 150 μl/well of Reading Buffer T (MSD, Gaithersburg, MD, USA) was added. The ECL values for each well were measured using a SECTOR Imager 6000 (MSD, Gaithersburg, MD, USA).
Antigen capture ECL assay
Standard, bare 96-well carbon-electrode plates were coated with 0.5 μg/ml of primary MAb diluted in 0.01M PBS pH 7.4 - 7.6 and incubated overnight at 4°C. Plates were washed three times with 0.01M PBS-0.05% Tween20, 150 μl/well of casein was added, and plates were incubated 60 min at room temperature with shaking. Casein was removed, antigens were added with a minimum 24 μl/well, and plates were incubated 60 min at room temperature with shaking. Antigens were serially diluted 1:5 in casein. Plates were washed three times with 0.01M PBS-0.05% Tween20, 30 μl/well of 1 μg/ml conjugated secondary MAb was added, and plates were incubated 60 m at room temperature with shaking. Plates were washed three times with 0.01M PBS-0.05% Tween20, 150 μl/well of Reading Buffer T was added, and ECL values for each well were measured using a SECTOR Imager 6000. The mean of at least four statistical replicates from at least two biological replicates was graphed.
In vivo immunogenicity testing
An approved animal use protocol was established with CDC’s Institutional Animal Care and Use Committee (protocol #2332SMIMOUC-A3). Female, 4-week-old, CD-1 mice were purchased from Charles River Laboratory (Wilmington, MA, USA). For each vaccine tested, mice were divided randomly into groups (n=10) on day 0, and vaccinated intramuscular (IM) with 50 μl of test vaccine in the quadriceps muscle. The humoral immune response was assessed in all mice by taking approximately 200 μl of blood from the submandibular region using a Goldenrod Lancet (MEDIpoint, Mineola, NY, USA) on days 0, 14, and 30 for determination of rVNA using a rapid fluorescent focus inhibition test (RFFIT) or a modified RABV neutralization test for small volumes of serum [27, 28]. On day 30, all animals were challenged IM in the quadriceps muscle with 50 μl of street canine RABV (RV3374R). All IM injections were performed with a tuberculin syringe with pre-measured inoculum and 31 gauge needle (Becton Dickinson, Franklin Lakes, NJ, USA). Post challenge (p.i.) mice were observed once daily, and from days 7 to 21 p.i. mice were observed twice daily by investigators for clinical signs of RABV infection. Upon clinical manifestation of signs associated with RABV infection (e.g. paresis, paralysis, aggression), mice were euthanized by carbon dioxide asphyxiation, according to approved euthanasia criteria, or as directed by the attending veterinarian. Cervical dislocation was used as a secondary mode of euthanasia. During necropsy, the brain stem was harvested to perform a RABV diagnosis by the direct fluorescent antibody test [29]. All mice surviving up to day 45 p.i. were euthanized, and randomly selected animals were necropsied for RABV diagnosis.
Results
Characterization of monoclonal antibodies
Two available RABV neutralizing MAbs, 2-21-14 (10 IU/ml) and 62-80-6 (3 IU/ml), were tested. The binding epitope for MAb 62-80-6 was mapped to antigenic site I by sequencing escape mutants. Attempts to isolate escape mutants of MAb 2-21-14 were not successful. However, analysis of natural escape RABV variants from red bats (Lasiurus borealis) and hoary bats (Lasiurus cinereus) suggests binding to antigenic site III. Using a direct ECL assay, MAb 2-21-14 could bind to RABV antigens in the native form but could not bind to heat denatured antigens; while, MAb 62-80-6 could bind to RABV antigens in the native or heat denatured form (data not shown). From these results the binding epitope is conformational for MAb 2-21-14 and linear for 62-80-6. To ensure that only native RABV G was captured in the ECL assay, MAb 2-21-14 was selected as the primary antibody. An alternative assay was evaluated using MAb 62-80-6 as the primary antibody and either MAb 62-80-6 or 2-21-14 for detection to measure total G and native G. However, due to differences in the antibodies, results from this assay were difficult to interpret.
Both MAbs were conjugated with SULFO-NHS-Ester to be used as secondary antibodies for detection in the ECL assay. A gradient from 0.5 μg/ml to 4 μg/ml of both primary and secondary antibody was used to determine the best antibody concentrations for the ECL assay. When using MAb 2-21-14 for both capture and detection, the highest signal to background ratio was at a low primary MAb concentration and high secondary MAb concentration. The opposite was true when using MAb 62-80-6 for detection: the highest signal to background ratio was at a high primary MAb concentration and low secondary MAb concentration. Using 0.5 μg/ml of MAb 2-21-14 for capture and 1 μg/ml of MAb 2-21-14 for detection resulted in a signal to background ratio of approximately 70, and using 1 μg/ml of MAb 2-21-14 for capture and 0.5 μg/ml of MAb 62-80-6 for detection resulted in a signal to background ratio of approximately 10,000. The difference between MAb 62-80-6 and 2-21-14 may result from differences in the SULFO tag labeling or availability of the binding epitopes.
Positive and negative samples assigned based on their in vivo immunogenicity, as described below, were run in six replicates per plate. When MAb 2-21-14 was used for capture (0.5 μg/ml) and detection (1 μg/ml), the average coefficient of variance was 14% intraplate, 17% interplate, and 29% day-to-day over all samples. For a single positive sample the coefficient of variance was 2% intraplate, 13% interplate, and 20% day-to-day. The specificity was 100%, and sensitivity was 100%. When MAb 62-80-6 was used for detection, the average coefficient of variance was 5% intraplate and 6% interplate over all samples, the specificity was 80%, and sensitivity was 100%. Despite higher variance, MAb 2-21-14 was selected for both capture and detection based on better specificity.
Characterization of vaccine lots
Using the MAb 2-21-14 for antigen capture, the ECL counts of tested vaccines were determined using eight 5-fold dilutions (Fig. 1). The antigen content or antigenicity of the vaccines was defined as the quality and quantity of RABV G detected by the antigen capture ECL assay. Based on the linear regression of the ECL counts and total protein concentration, antigenicity was calculated at 25 μg/ml for each vaccine (Table 1, Fig. 2). This protein concentration was chosen to maximize the differences between the vaccines. The purified RABV ERA G (2005) had the highest antigenicity of any vaccine tested. When this vaccine was diluted, the counts μg−1 ml−1 actually increased. Purified RABV G was prepared at different times, such that antigenicity increased with each subsequent lot. Purified RABV ERA G (2012) had the next highest antigenicity followed by CVS G (2011) followed by ERA G (2010).
Figure 1. ECL counts over total protein concentration.

Antigen was captured and detected using the 2-21-14 αRABV G MAb, and ECL was measured for eight 5-fold serial dilutions of different rabies vaccine preparations. ECL counts were plotted against the total protein concentration on logarithmic scales. Means of at least four statistical replicates from at least two biological replicates are shown. Shown in decreasing order are purified RABV G ERA G lot 2005 (■), Medicago® (✲), ERA G lot 2012 (◆), RabAvert® (■), RabAvert® depleted (•), Imovax® (−), CVS G lot 2011 (×), ERA G lot 2010 (▲), Fraunhofer® (+), Imovax® depleted (◆), and Fraunhofer® Adjuvant (•).
Table 1.
Calculation of antigen content in counts μg−1 ml−1.
| Vaccine (lot) | Protein concentration (μg/ml) |
ma | bb | yc | counts μg−1 ml−1d |
|---|---|---|---|---|---|
| ERA G (2005) | 1 200 | 1215800 | −9157 | 30385843 | 1 000 000 |
| ERA G (2005) Diluted | 200 | 1397800 | −7185 | 34937815 | 1 000 000 |
| ERA G (2012) | 2 500 | 2949 | 70297 | 144022 | 6 000 |
| ERA G (2012) Diluted | 200 | 2798 | 15637 | 85587 | 3 000 |
| CVS G (2011) | 120 | 253 | 3048 | 9373 | 400 |
| ERA G (2010) | 200 | 31 | 142 | 917 | 40 |
| RabAvert® (464011A) | 6 000 | 4015 | 14553 | 114928 | 4 000 |
| RabAvert® Diluted | 2 000 | 3214 | 2576 | 82926 | 3 000 |
| RabAvert® Depleted | 4 500 | 1217 | 2702 | 33127 | 1 000 |
| Imovax® (G1076) | 20 000 | 473 | 4742 | 16567 | 700 |
| Imovax® Depleted | 20 000 | 0.023 | 247 | 248 | 10 |
| Fraunhofer® | 150 000 | 0.177 | 606 | 610 | 20 |
| Fraunhofer® Adjuvant | 75 000 | 0.001 | 119 | 119 | 5 |
| Medicago® | 150 | 20482 | 7320 | 519370 | 20 000 |
Slope of the linear regression based on average ECL counts for eight 5-fold dilutions plotted against protein concentration
y-intercept of the linear regression
ECL counts (y) at25 μg/ml (x) based on the linear regression
ECL counts divided by 25 μg/ml
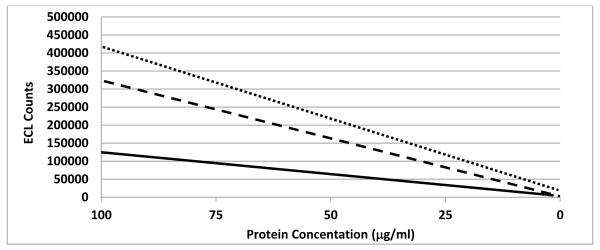
Figure 2. Linear regressions of ECL counts over total protein concentration.
Antigen was captured and detected using the 2-21-14 αRABV G MAb, and ECL was measured for eight 5-fold serial dilutions of different rabies vaccine preparations. The linear regression of the ECL counts was plotted against the total protein concentration on a linear scale. ECL counts μg−1 ml−1 were calculated based on the linear regression at 25 μg/ml. As an example, linear regressions are shown for RabAvert®, 4000 counts μg−1 ml−1 (dotted line); RabAvert® diluted, 3000 counts μg−1 ml−1 (dashed line); and RabAvert® depleted, 1000 counts μg−1 ml−1 (solid line).
Available commercial vaccines Imovax® and RabAvert® were reconstituted and tested. Both vaccines had similar total ECL counts. However, RabAvert® had a lower protein concentration resulting in higher counts μg−1 ml−1. As expected, when RabAvert® was diluted the antigenicity decreased. Depletion of these vaccines was attempted by incubating at increased temperature. While the antigen content of both depleted vaccines decreased, the antigenicity of RabAvert® remained high, but that of Imovax® was diminished (Table 1).
An experimental rabies vaccine produced by Fraunhofer® had a very high total protein concentration but low antigen content. When the same vaccine was mixed with adjuvant (Fraunhofer® adjuvant), both the total protein concentration and antigenicity were reduced. Fraunhofer® with adjuvant had the lowest counts μg−1 ml−1 of any vaccine tested. A second experimental rabies vaccine produced by Medicago® had a low total protein concentration but high antigenicity (Table 1).
Selected vaccines were given pre-exposure as a single dose to mice, and rVNA titers were measured 30 days later (Table 2). Of the vaccines tested, ERA G (2005 lot diluted), Imovax®, RabAvert® and RabAvert® depleted induced VNA titers >0.5 IU/ml and protected 100% of mice from peripheral RABV challenge. In contrast, ERA G (2010), CVS G, Imovax® depleted, Fraunhofer®, and Fraunhofer® with adjuvant induced VNA titers <0.5 IU/ml and provided disparate levels of protection. The in vivo test was designed to test the efficacy of selected rabies vaccines to compare with the ECL results. A peripheral challenge was chosen because it more accurately models natural infection [30], but peripheral challenge introduces greater variability in survivorship.
Table 2.
Pre-exposure Vaccination in Mice
| Vaccine (lot) | Dose (total protein) | rVNA | Survivorship |
|---|---|---|---|
| ERA G (2005) Diluted | 10 μg | 4.2 IU/ml | 10/10 (100%) |
| ERA G (2010) | 10 μg | 0.17 IU/ml | 6/10 (60%) |
| CVS G (2011) | 6 μg | 0.07 IU/ml | 7/9 (77%) |
| Imovax® (G1076) | 1000 μg | 2.9 IU/ml | 10/10 (100%) |
| Imovax® Depleted | 1000 μg | <0.05 IU/ml | 7/10 (70%) |
| RabAvert® (464011A) Diluted | 100 μg | 0.6 IU/ml | 9/9 (100%) |
| RabAvert® Depleted | 225 μg | 4.7 IU/ml | 10/10 (100%) |
| Fraunhofer® (0300-007) | 7500 μg | 0.06 IU/ml | 5/9 (55%) |
| Fraunhofer® Diluted | 1000 μg | <0.05 IU/ml | 1/8 (12%) |
| Fraunhofer® Adjuvant | 3750 μg | 0.07 IU/ml | 7/10 (70%) |
| PBS Negative Control | ND | <0.05 IU/ml | 3/16 (19%) |
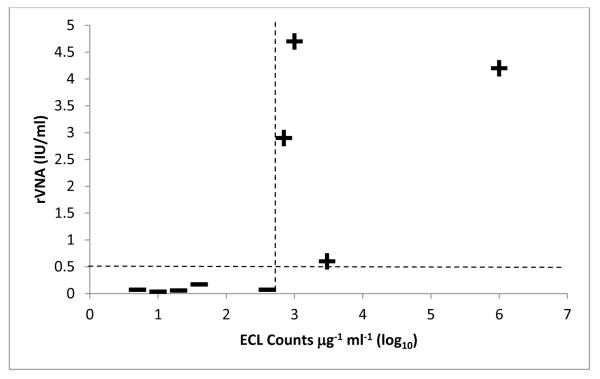
The ECL assay using MAb 2-21-14 was able to accurately distinguish vaccines that induced VNA titer >0.5 IU/ml from vaccines that induced VNA titer <0.5 IU/ml (Fig. 3). A weak correlation, R2 = 0.51, existed between the log transformed antigenicity values and VNA titers. The statistical cut-off, calculated from the mean antigenicity of non-immunogenic vaccines plus two standard deviations, was 440 counts μg−1 ml−1.
Figure 3. Comparison of rVNA and antigenicity measured by the ECL assay.
The geometric mean rVNA titer, measured 30 days after immunizing mice with selected rabies vaccines, was plotted against the log transformed ECL counts μg−1 ml−1. Vaccines were considered immunogenic (+) if the rVNA titer was >0.5 IU/ml, and non-immunogenic (—) if the rVNA titer was <0.5 IU/ml. The statistical cut-off for antigenicity was 440 counts μg−1 ml−1 (2.64 log10). The ECL assay was able to distinguish between vaccine lots that were close to the cut-off values for rVNA and antigenicity.
Discussion
This study describes the use of an ECL assay based on MSD technology to evaluate the immunogenic G content of RABV vaccines. The ECL assay has similar advantages and drawbacks as a traditional ELISA with the added benefit that absorption to the carbon electrode plate is more efficient than plastic and the detection method has higher sensitivity, lower background, and greater dynamic range [31-33]. The ECL assay is highly versatile and flexible in that multiple analyses can be completed on a single plate and even in a single well using multiplex technology considerably reducing the time required to evaluate vaccine lots. The ECL assay provides significant cost savings compared to in vivo tests such as the NIH test but is more costly than traditional ELISA.
Use of a single, conformational MAb such as 2-21-14 for antigen capture precludes capture of non-native G. The ECL assay and other in vitro methods measure binding antibodies; whereas in vivo tests measure neutralizing antibodies or survivorship which includes innate immunity. While some studies have reported good correlation between antigenicity and potency [18], the variability of in vivo tests, especially the NIH test, makes correlation difficult. Instead, a pass/fail approach can be adopted. For this study a vaccine was considered immunogenic if it induced a rVNA titer >0.5 IU/ml. Imovax® had the lowest antigenicity at 700 counts μg−1 ml−1 of the vaccines that were immunogenic, and CVS G (2011) had the highest antigenicity at 400 counts μg−1 ml−1 of the vaccines that were not immunogenic. The statistical cut-off of 440 counts μg−1 ml−1 falls within this empirical range. Using MAb 2-21-14 resulted in 100% concordance in this preliminary analysis, and borderline vaccine lots were accurately classified.
The use of RABV vaccines with adjuvant in animals remains a stumbling block for both traditional ELISA and the ECL assay. An antigen extraction procedure can be used to accurately measure the antigen content of adjuvanted vaccines [34]. This procedure is cumbersome, so a vaccine with adjuvant was tested without extraction to evaluate the ECL assay. When an experimental vaccine was mixed with aluminum hydroxide adjuvant, the ECL assay indicated a decrease in antigen content. Adjuvant did not significantly increase the rVNA response compared to the parent vaccine. However, the parent vaccine had low antigenicity, so addition of adjuvant was not beneficial. This highlights the broader issue of what role RABV vaccines containing adjuvant will play in the future of rabies control [35]. The ECL assay could be modified in the future to a direct detection method to evaluate vaccines with adjuvant [36].
The ECL assay was able to accurately predict the immunogenicity of the depleted commercial vaccines in that one retained antigenicity and immunogenicity while the other lost antigenicity in the ECL assay and immunogenicity in vivo. In addition, the experimental vaccine provided by Fraunhofer® had low antigenicity in the ECL assay and low immunogenicity in vivo. We predict that this vaccine would also be sub-potent in the NIH test. Given that only one immunization was given for the in vivo efficacy test, the performance of this vaccine may improve with a different regimen and additional immunizations. While the experimental vaccine provided by Medicago® had high antigenicity, this vaccine was not tested in vivo. Based on the antigenicity, we predict this vaccine would be immunogenic in vivo and potent if tested in the NIH test. The number of vaccine lots tested in vitro and in vivo should be increased by approximately 20 lots to reach acceptance using a pass/fail approach. However, preliminary evidence suggests the ECL assay has utility to predict in vivo immunogenicity from in vitro antigenicity, and could prove useful in the ongoing search for an international replacement to the outdated NIH test in both human and veterinary medicine [37].
Acknowledgements
We thank Franhofer® and Medicago® for providing the experimental vaccines. We thank J Blanton, DB Green, L Greenberg, F Jackson, M Niezgoda, MO Osinubi, ST Taylor, A Wadhwa, and X Wu for assistance. This work was supported in part by an appointment to the Research Participation Program at CDC administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and CDC.
Abbreviations
- RABV
rabies virus
- ECL
electrochemiluminescent
- MAb
monoclonal antibodies
- rVNA
rabies virus neutralizing antibody
- IU
international units
- ED50
effective dose
- G
glycoprotein
- MSD
Meso Scale Discovery
- IM
intramuscular
- RFFIT
rapid fluorescent focus inhibition test
- p.i.
post challenge or post infection
Footnotes
Disclaimer
Use of trade names and commercial sources are for identification only and do not imply endorsement by the US Department of Health and Human Services or US Department of Energy. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of their institutions.
References
- [1].Tsiang H, Koulakoff A, Bizzini B, Berwald-Netter Y. Neurotropism of rabies virus: an in vitro study. J Neuropathol Exp Neurol. 1983;42(4):439–52. doi: 10.1097/00005072-198307000-00006. [DOI] [PubMed] [Google Scholar]
- [2].Baloul L, Camelo S, Lafon M. Up-regulation of Fas ligand (FasL) in the central nervous system: a mechanism of immune evasion by rabies virus. J NeuroVirol. 2004;10(6):372–82. doi: 10.1080/13550280490521122. [DOI] [PubMed] [Google Scholar]
- [3].Horton D, McElhinney L, Marston D, Wood J, Russell C, Lewis N, et al. Quantifying antigenic relationships among the lyssaviruses. J Virol. 2010;84(22):11841–8. doi: 10.1128/JVI.01153-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].WHO . Expert Committee on Biological Standardization Thirty-seventh report. World Health Organization; Geneva: 1987. WHO Technical Report Series. [PubMed] [Google Scholar]
- [5].WHO . WHO Expert Committee on Rabies: Eighth Report. World Health Organization; 1992. [PubMed] [Google Scholar]
- [6].Wilbur L, Aubert M. The NIH test for potency. In: Meslin FX, Kaplan MM, Koprowski H, editors. Laboratory techniques in rabies. 4th ed World Health Organization; Geneva: 1996. pp. 360–8. [Google Scholar]
- [7].Barth R, Diderrich G, Weinmann E. NIH test, a problematic method for testing potency of inactivated rabies vaccine. Vaccine. 1988 Aug;6(4):369–77. doi: 10.1016/0264-410x(88)90185-5. [DOI] [PubMed] [Google Scholar]
- [8].Jivapaisarnpong T, Schofield T, Krause PR. A vaccine measured with a highly variable assay: rabies. Biologicals. 2009 Nov;37(6):412–5. doi: 10.1016/j.biologicals.2009.08.002. discussion 21-3. [DOI] [PubMed] [Google Scholar]
- [9].Smith TG, Wu X, Franka R, Rupprecht CE. Design of future rabies biologics and antiviral drugs. Adv Virus Res. 2011;79:345–63. doi: 10.1016/B978-0-12-387040-7.00016-0. [DOI] [PubMed] [Google Scholar]
- [10].Fitzgerald EA, Needy CF. Use of the single radial immunodiffusion test as a replacement for the NIH mouse potency test for rabies vaccine. Dev Biol Stand. 1986;64:73–9. [PubMed] [Google Scholar]
- [11].Ferguson M, Schild GC. A single-radial-immunodiffusion technique for the assay of rabies glycoprotein antigen: application for potency tests of vaccines against rabies. The Journal of general virology. 1982 Mar;59(Pt 1):197–201. doi: 10.1099/0022-1317-59-1-197. [DOI] [PubMed] [Google Scholar]
- [12].Atanasiu P, Perrin P, Delagneau JF. Use of an enzyme immunoassay with protein A for rabies antigen and antibody determination. Developments in biological standardization. 1980;46:207–15. [PubMed] [Google Scholar]
- [13].van der Marel P, van Wezel AL. Quantitative determination of rabies antigen by ELISA. Developments in biological standardization. 1981;50:267–75. [PubMed] [Google Scholar]
- [14].Lafon M, Perrin P, Versmisse P, Sureau P. Use of a monoclonal antibody for quantitation of rabies vaccine glycoprotein by enzyme immunoassay. J Biol Stand. 1985 Oct;13(4):295–301. doi: 10.1016/s0092-1157(85)80042-1. [DOI] [PubMed] [Google Scholar]
- [15].Thraenhart O, Ramakrishnan K. Standardization of an enzyme immunoassay for the in vitro potency assay of inactivated tissue culture rabies vaccines: determination of the rabies virus glycoprotein with polyclonal antisera. J Biol Stand. 1989 Oct;17(4):291–309. doi: 10.1016/s0092-1157(89)80001-0. [DOI] [PubMed] [Google Scholar]
- [16].Perrin P, Morgeaux S, Sureau P. In vitro rabies vaccine potency appraisal by ELISA: advantages of the immunocapture method with a neutralizing anti-glycoprotein monoclonal antibody. Biologicals. 1990 Oct;18(4):321–30. doi: 10.1016/1045-1056(90)90037-z. [DOI] [PubMed] [Google Scholar]
- [17].Fournier-Caruana J, Poirier B, Haond G, Jallet C, Fuchs F, Tordo N, et al. Inactivated rabies vaccine control and release: use of an ELISA method. Biologicals. 2003 Mar;31(1):9–16. doi: 10.1016/s1045-1056(02)00070-2. [DOI] [PubMed] [Google Scholar]
- [18].Nagarajan T, Reddy GS, Mohana Subramanian B, Rajalakshmi S, Thiagarajan D, Tordo N, et al. A simple immuno-capture ELISA to estimate rabies viral glycoprotein antigen in vaccine manufacture. Biologicals. 2006 Mar;34(1):21–7. doi: 10.1016/j.biologicals.2005.07.004. [DOI] [PubMed] [Google Scholar]
- [19].Jallet C, Jacob Y, Bahloul C, Drings A, Desmezieres E, Tordo N, et al. Chimeric lyssavirus glycoproteins with increased immunological potential. J Virol. 1999 Jan;73(1):225–33. doi: 10.1128/jvi.73.1.225-233.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Desmezieres E, Maillard AP, Gaudin Y, Tordo N, Perrin P. Differential stability and fusion activity of Lyssavirus glycoprotein trimers. Virus Res. 2003 Feb;91(2):181–7. doi: 10.1016/s0168-1702(02)00267-8. [DOI] [PubMed] [Google Scholar]
- [21].Sissoeff L, Mousli M, England P, Tuffereau C. Stable trimerization of recombinant rabies virus glycoprotein ectodomain is required for interaction with the p75NTR receptor. J Gen Virol. 2005 Sep;86(Pt 9):2543–52. doi: 10.1099/vir.0.81063-0. [DOI] [PubMed] [Google Scholar]
- [22].Mousli M, Turki I, Kharmachi H, Saadi M, Dellagi K. Recombinant single-chain Fv antibody fragment-alkaline phosphatase conjugate: a novel in vitro tool to estimate rabies viral glycoprotein antigen in vaccine manufacture. J Virol Methods. 2007 Dec;146(1-2):246–56. doi: 10.1016/j.jviromet.2007.07.015. [DOI] [PubMed] [Google Scholar]
- [23].Nimmagadda SV, Aavula SM, Biradhar N, Rao VS, Shanmugham R, Chandran D, et al. Recombinant diabody-based immunocapture enzyme-linked immunosorbent assay for quantification of rabies virus glycoprotein. Clin Vaccine Immunol. 2010 Aug;17(8):1261–8. doi: 10.1128/CVI.00204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Marissen WE, Kramer RA, Rice A, Weldon WC, Niezgoda M, Faber M, et al. Novel rabies virus-neutralizing epitope recognized by human monoclonal antibody: fine mapping and escape mutant analysis. J Virol. 2005 Apr;79(8):4672–8. doi: 10.1128/JVI.79.8.4672-4678.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ellison JA, Johnson SR, Kuzmina N, Gilbert A, Carson WC, VerCauteren KC, et al. Multidisciplinary approach to epizootiology and pathogenesis of bat rabies viruses in the United States. Zoonoses Public Health. 2013 Feb;60(1):46–57. doi: 10.1111/zph.12019. [DOI] [PubMed] [Google Scholar]
- [26].Dietzschold B. Techniques for the purification of rabies virus, its subunits and recombinant products. In: Meslin FX, Kaplan MM, Koprowski H, editors. Laboratory techniques in rabies. 4th ed World Health Organization; Geneva: 1996. pp. 175–80. [Google Scholar]
- [27].Smith JS, Yager PA, Baer GM. A rapid reproducible test for determining rabies neutralizing antibody. Bull World Health Organ. 1973 May;48(5):535–41. [PMC free article] [PubMed] [Google Scholar]
- [28].Kuzmin IV, Niezgoda M, Franka R, Agwanda B, Markotter W, Beagley JC, et al. Lagos bat virus in Kenya. J Clin Microbiol. 2008 Apr;46(4):1451–61. doi: 10.1128/JCM.00016-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dean DJ, Abelseth MK, Atanasiu P. The fluorescent antibody test. In: Meslin FX, Kaplan MM, Koprowski H, editors. Laboratory techniques in rabies. 4th ed World Health Organization; Geneva: 1996. pp. 88–95. [Google Scholar]
- [30].Wunderli PS, Dreesen DW, Miller TJ, Baer GM. The rabies peripheral challenge test: more accurate determination of vaccine potency. Vaccine. 2006 Nov 30;24(49-50):7115–23. doi: 10.1016/j.vaccine.2006.06.078. [DOI] [PubMed] [Google Scholar]
- [31].Thompson DK, Huffman KM, Kraus WE, Kraus VB. Critical appraisal of four IL-6 immunoassays. PLoS One. 2012;7(2):e30659. doi: 10.1371/journal.pone.0030659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Blackburn GF, Shah HP, Kenten JH, Leland J, Kamin RA, Link J, et al. Electrochemiluminescence detection for development of immunoassays and DNA probe assays for clinical diagnostics. Clin Chem. 1991 Sep;37(9):1534–9. [PubMed] [Google Scholar]
- [33].Guglielmo-Viret V, Thullier P. Comparison of an electrochemiluminescence assay in plate format over a colorimetric ELISA, for the detection of ricin B chain (RCA-B) J Immunol Methods. 2007 Dec 1;328(1-2):70–8. doi: 10.1016/j.jim.2007.08.003. [DOI] [PubMed] [Google Scholar]
- [34].Katz J. Desorption of porcine parvovirus from aluminum hydroxide adjuvant with subsequent viral immunoassay or hemagglutination assay. Vet Res Commun. 1987;11(1):83–92. doi: 10.1007/BF00361329. [DOI] [PubMed] [Google Scholar]
- [35].Wu X, Smith TG, Rupprecht CE. From brain passage to cell adaptation: the road of human rabies vaccine development. Expert Rev Vaccines. 2011 Nov;10(11):1597–608. doi: 10.1586/erv.11.140. [DOI] [PubMed] [Google Scholar]
- [36].Zhu D, Huang S, Gebregeorgis E, McClellan H, Dai W, Miller L, et al. Development of a Direct Alhydrogel Formulation Immunoassay (DAFIA) J Immunol Methods. 2009 May 15;344(1):73–8. doi: 10.1016/j.jim.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Stokes W, McFarland R, Kulpa-Eddy J, Gatewood D, Levis R, Halder M, et al. Report on the international workshop on alternative methods for human and veterinary rabies vaccine testing: state of the science and planning the way forward. Biologicals. 2012 Sep;40(5):369–81. doi: 10.1016/j.biologicals.2012.07.005. [DOI] [PubMed] [Google Scholar]