Review on the use of zebrafish to study the role of neutrophils in host defense against bacterial and fungal pathogens.
Keywords: infection, innate immunity
Abstract
Neutrophils are highly motile phagocytic cells that play a critical role in the immune response to infection. Zebrafish (Danio rerio) are increasingly used to study neutrophil function and host-pathogen interactions. The generation of transgenic zebrafish lines with fluorescently labeled leukocytes has made it possible to visualize the neutrophil response to infection in real time by use of optically transparent zebrafish larvae. In addition, the genetic tractability of zebrafish has allowed for the generation of models of inherited neutrophil disorders. In this review, we discuss several zebrafish models of infectious disease, both in the context of immunocompetent, as well as neutrophil-deficient hosts and how these models have shed light on neutrophil behavior during infection.
Introduction
Neutrophils are the most abundant type of circulating leukocyte in humans and zebrafish and are typically the first responders recruited to sites of tissue injury or infection [1, 2]. To traffic to a site of infection, neutrophils mobilize from hematopoietic tissue and travel through the vasculature. Once at the site of infection, these highly motile cells play a critical role in initial defense through phagocytosis of microbes, secretion of granule proteins and other antimicrobials, production of ROS, and release of NETs [3, 4]. Neutrophils also mediate the proinflammatory response to infection by releasing cytokines that recruit and activate other immune cells. The crucial importance of neutrophils in host defense against infection is demonstrated by enhanced susceptibility of neutropenic patients to a wide range of bacterial and fungal infections [2].
The progression of infectious disease is determined by dynamic and complex interactions between host defense systems and pathogen virulence factors. Whereas detailed in vitro analyses have provided critical insight into this delicate interaction, its complexity is more easily studied in vivo, especially when host and pathogen are amenable to genetic analysis. There has been increasing use of the larval zebrafish (D. rerio) to study infectious disease, as its optical accessibility and potential for genetic manipulations allow visualization of the immune response to infection inside a living, intact vertebrate host. Genetic tools in zebrafish allow for the generation of transgenic lines with fluorescently labeled cell populations, including neutrophils [5, 6] and macrophages [7]. The vertebrate innate immune system is highly conserved in zebrafish and includes: complement [8], TLRs [9, 10], macrophages [11], and neutrophils [1, 12]. The adaptive immune system is not functionally mature until 4–6 weeks postfertilization [13–15], and thus, the study of host-pathogen interactions in the embryonic and larval stages of development allows for an understanding of the specific contribution of innate immunity to host defense. Importantly, transient manipulation of gene expression is readily available in the larval zebrafish, including injection of synthetic mRNA for overexpression and injection of antisense morpholino oligonucleotides for gene suppression. Recently, the generation of null mutants has become easier and faster with the application of gene-editing tools, including CRISPR/ Cas [16] and TALENs [17].
Recent advances with the use of zebrafish to study macrophages during host-pathogen interactions were reviewed recently [18]. Here, we will focus on the role of neutrophils in host-pathogen interactions and how studies that use zebrafish have enhanced and complemented work done in mammalian models.
ZEBRAFISH NEUTROPHIL BIOLOGY AND IN VIVO VISUALIZATION OF NEUTROPHIL BEHAVIOR
Zebrafish neutrophil development
Myelopoiesis in zebrafish is genetically controlled by the transcription factor Pu.1/Spi1 [19]. Pu.1-expressing myeloid precursors are first detected ∼12 hpf [20]. Neutrophils originate in the developing larval zebrafish from the primitive and definitive waves of hematopoiesis. During the primitive wave, myeloid precursors arise in the rostral blood island (also known as the anterior lateral plate mesoderm) and migrate over the yolk sac, where they differentiate into primitive macrophages by 22 hpf [11]. A subset of these primitive macrophages then differentiates further into neutrophils by 33 hpf [12]. Definitive or multilineage hematopoiesis begins as early as 24 hpf when a transient population of pluripotent erythromyeloid progenitor cells differentiates within the posterior blood island, an area that later expands to become the CHT [21, 22]. These cells are soon replaced by another group of cells that migrates from the aorta-gonad-mesonephros through the blood to start colonizing the CHT by 48 hpf [23], where they also give rise to neutrophils [22]. Neutrophil distribution in the developing larva includes a population of neutrophils in the CHT, as well as a population of randomly migrating neutrophils in the head mesenchyme (Fig. 1A and Supplemental Video 1). By 4 dpf, the kidney marrow begins to mature and will become the site of definitive hematopoiesis in adult fish [24].
Figure 1. Live, in vivo imaging of neutrophil response to infection.
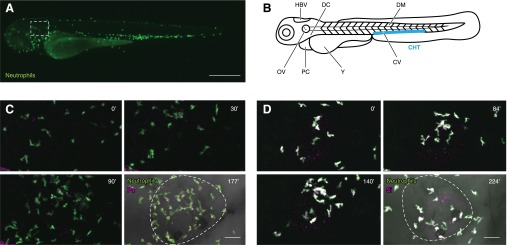
(A) Neutrophil (green) distribution at 3 dpf in a Tg(mpx:dendra2) larva. Neutrophils in the CHT, as well as randomly migrating neutrophils in the head, are shown in Supplemental Video 1. The box indicates the region around the OV (imaged in C and D). Original scale bar, 500 μm. (B) Commonly used sites of infection in embryonic and larval zebrafish. (C) Live imaging of a 3 dpf Tg(mpx:dendra2) larva infected with Pseudomonas aeruginosa (Pa) strain PAK (pMKB1::mCherry) in the OV (white dotted line). Four frames were extracted from a 3 h movie (see Supplemental Video 2) and show neutrophil (green) recruitment and phagocytosis of P. aeruginosa (magenta). Original scale bar, 40 μm. (D) Live imaging of a 3 dpf Tg(mpx:dendra2) larva infected with CellTracker Red-labeled Streptococcus iniae (Si) strain 9117 in the OV (white dotted line). Four frames were extracted from a 4 h movie (see Supplemental Video 3) and show neutrophil (green) recruitment and phagocytosis of S. iniae (magenta). Original scale bar, 40 μm.
Zebrafish macrophages and neutrophils are fully functional in the early developmental stages, capable of phagocytosis by 28–30 hpf [11, 12] and of generating a respiratory burst by 72 hpf [25]. They are also capable of NETosis [26], the release of decondensed chromatin, histones, and granule proteins into the extracellular space to trap and kill microbes physically by exposing them to high, local concentrations of antimicrobial molecules [27, 28]. Direct visualization of neutrophils in response to wounding has led to new insight into neutrophil behavior at sites of tissue damage. Indeed, a study of the neutrophil response to wounds in zebrafish provided the first direct demonstration that resolution of neutrophil infiltration at wounds can occur by neutrophil reverse migration and not apoptosis [5]. Reverse transmigration of neutrophils from a site of inflammation has also been reported with primary human neutrophils in vitro [29] and more recently in a mouse model of ischemia-reperfusion injury [30]. This highlights the advantage of the use of zebrafish as a model for visualizing neutrophil behavior in vivo and supports the idea that zebrafish represent an important model system that complements studies that use mouse and human neutrophils.
Tools for imaging neutrophils in zebrafish
Neutrophils can be imaged in whole, fixed zebrafish by use of Sudan Black, which stains the rod-shaped cytoplasmic granules irreversibly [12], or immunostaining. Differentiated neutrophils and macrophages express the pan-leukocyte marker L-plastin (lcp1) [31], and immunolabeling of L-plastin has been used to visualize leukocytes in fixed larvae [32]. For live in vivo imaging, the generation of transgenic lines that label zebrafish neutrophils has provided an important tool to image neutrophil behavior. Transgenic lines with fluorescently labeled leukocytes have been made possible, in part, by the Tol2 system, which allows for the efficient integration of exogenous DNA into the genome [33]. Photoconvertible proteins, such as Dendra2 [34, 35] or Kaede [36, 37], allow for the tracking of individual host cells over the course of infection. Fluorescent transgenic lines by use of the pu.1/spi1 promoter have been used to drive expression in all myeloid cell precursors [38, 39]. Reporter lines specific for macrophages or neutrophils have also been generated in zebrafish. Myeloperoxidase and lysozyme C are components of mammalian neutrophil granules [40], and the mpx [5, 6] and lyz [31, 41] promoters are generally neutrophil-specific in zebrafish. Although it has been shown that lyz marks neutrophils and macrophages at 32 hpf [42], lyz primarily labels neutrophils by 48 hpf [31, 41]. An additional transgenic line with expression in only a subset of neutrophils was generated by use of an enhancer trap screen [31]. Labeled neutrophils in this line are able to respond to wounds and bacterial infection and make up ∼50% of the neutrophil population. This suggests that different subsets of neutrophils serve distinct functions in zebrafish innate immunity. With the generation of a macrophage-specific reporter line [7, 43], double transgenic fish allow for the observation of neutrophil-macrophage interactions during inflammation and infection in intact hosts [7].
The use of fluorescently labeled microbes and phagocyte transgenic lines allows for the spatial and temporal imaging of host-pathogen interactions in a living animal (Fig. 1C and D and Supplemental Videos 2 and 3). As in mammalian models, localized and systemic infections can be modeled in the larval zebrafish, depending on the initial site of infection. A number of different anatomic sites in the larval zebrafish have been used to investigate the host response to systemic infection (Fig. 1B), but the most commonly used injection sites are the CV and DC. Localized infection in the HBV [44], OV [12], DM [45, 46], and PC [47] allows imaging of phagocyte recruitment. Visual analysis of phagocyte function in vivo is enhanced by the use of fluorescent reporter probes. During phagocytosis, pHrodo dye can be used to label microbes and will fluoresce when exposed to acidic environments, such as the leukocyte phagosome [48]. There are also probes for live imaging of the production of ROS [49, 50] and RNS [51, 52].
Depletion of neutrophils or macrophages can provide important information about phagocyte function during infection [53]. Neutrophils or macrophages can be depleted by use of zebrafish lines that express the bacterial nitroreductase under control of neutrophil- or macrophage-specific promoters. The Escherichia coli nitroreductase converts the drug metronidazole to a cytotoxic product, inducing cell death in expressing cells to achieve tissue-specific ablation [54, 55]. Importantly, it has been shown that specific ablation of neutrophils or macrophages by use of this technique has no effect on the other population [56]. Antisense morpholino oligonucleotides can also be used to deplete specific phagocyte populations. Morpholinos are nucleic acid analogs that act to block splicing or initiation of translation of the target mRNA. Limitations of morpholino technology include lack of tissue specificity and transient effects, typically wearing off after 3 dpf. Morpholinos targeting pu.1 deplete neutrophils and macrophages and are often used to examine the effect of myeloid cell loss on host defense. A morpholino targeting irf8 specifically depletes macrophages but not neutrophils. However, a caveat of this approach is that depletion of irf8 results in an expansion of the neutrophil population [57]. A morpholino target that allows specific depletion of neutrophils has not been reported. Several groups have used a morpholino targeting gcsfr/csf3r to reduce neutrophil numbers [51, 58], but gcsfr/csf3r is also expressed in a subset of monocytes and macrophages [59], and expression of mpeg1 is dependent on gcsfr/csf3r signaling [7]. Thus, although a gcsfr/csf3 morpholino largely reduces the neutrophil population, it also affects a subset of macrophages [60, 61].
MODELS OF PRIMARY IMMUNODEFICIENCY IN ZEBRAFISH
Defects in neutrophil function in humans lead to severe, recurrent bacterial and fungal infections. Human neutrophil disorders include neutrophil mobilization and adhesion defects, altered actin-based motility, and impaired microbial killing. The following is a summary of how zebrafish have been used to model human immune deficiencies that affect neutrophil function.
WHIM syndrome
WHIM syndrome is a primary immunodeficiency, a type of severe, chronic neutropenia with apoptosis of mature myeloid cells in the bone marrow [62, 63]. SDF1 is expressed in bone marrow stromal cells and binds the receptor CXCR4 [64], which is expressed on mature neutrophils in the bone marrow [65]. In WHIM syndrome, gain-of-function mutations associated with truncation of CXCR4 prevent CXCR4 internalization and result in the active retention of neutrophils in the bone marrow [65]. In a mouse model of WHIM syndrome, expression of the CXCR4 WHIM mutations in hematopoietic stem cells impairs neutrophil release from the bone marrow and increases neutrophil apoptosis [66], causing neutropenia and recapitulating the human disease. A zebrafish model of WHIM syndrome is also associated with reduced circulating neutrophils and recapitulates the neutropenia, although there was no reported effect on neutrophil apoptosis [67]. Endogenous expression of Sdf1a mRNA is concentrated in areas of neutrophil production in zebrafish embryos, both in the head and the CHT. Expression of the human WHIM mutations [68] in zebrafish neutrophils impairs Cxcr4b internalization and leads to retention of neutrophils in the CHT. Thus, WHIM neutrophils are not recruited to sites of wounding or infection, resulting in increased susceptibility to infection. Morpholino-mediated depletion of Sdf1a rescues neutrophil retention in the bone marrow [67], indicating that as in humans and mice, Sdf1a in zebrafish mediates the neutrophil retention signal. Thus, the zebrafish model of WHIM syndrome complements human and mouse WHIM models and provides the advantage of being amenable to direct visualization of the effects of altered chemokine signaling on neutrophil trafficking in vivo.
LAD
LAD represents a group of human disorders characterized by an increase in circulating neutrophils (neutrophilia) and recurrent infections [69]. There are different types of LAD, depending on the underlying genetic defect (reviewed in ref. [70]). LAD IV [71] is a recently reported type of LAD identified in 2 patients with a dominant inhibitory mutation in the hematopoietic tissue-specific Rho GTPase, Rac2 [72–75], which is the GTP-binding protein in neutrophils [76] and plays important roles in neutrophil activation by regulating the actin cytoskeleton, cell migration, and NADPH oxidase activity [77]. Rac2-deficient mice display neutrophilia and impaired emigration out of the vasculature to sites of infection, resulting in increased susceptibility to infections, such as Aspergillus fumigatus [78]. The phenotype of Rac2-deficient mice is similar to the phenotype of human patients with a dominant inhibitory mutation in Rac2 (Rac2D57N). Neutrophils from these patients have impaired chemotaxis [73, 75], azurophilic granule secretion [72], and defects in superoxide generation [73, 74]. A zebrafish model of LAD IV (LAD fish) also recapitulates the neutrophilia and increased susceptibility to infection [79] seen in mammalian disease. Zebrafish neutrophils expressing the dominant inhibitory Rac2D57N mutation show defects in cell polarization and migration [79]. The zebrafish model provides new insight into the mechanisms that contribute to neutrophilia. LAD fish exhibit an increase in neutrophil mobilization from hematopoietic tissue and a defect in egress from the vasculature to sites of tissue damage, resulting in neutrophilia. Interestingly, neutrophil retention in the CHT in WHIM fish is attenuated by the expression of Rac2D57N in neutrophils [79], suggesting a critical role for Rac2-Cxcr4 signaling in retaining neutrophils in hematopoietic tissue. Thus, the zebrafish model of LAD recapitulates the human disease and provides new insight into the signaling mechanisms that mediate neutrophil retention in hematopoietic tissue.
WAS and X-linked neutropenia
Leukocyte trafficking to infection requires the ability of leukocytes to sense directional cues and modify their actin cytoskeleton to form an F-actin-rich leading edge. WASp is a key regulator of actin dynamics [80] that binds the actin-nucleating protein Arp2/3, resulting in actin polymerization at branch points along actin filaments [81]. Mutations in WASp result in a primary immunodeficiency, known as WAS, which is characterized by recurrent infections, autoimmunity, and bleeding disorders [82]. Zebrafish have 2 WASp orthologs in hematopoietic cells, zWASp1 and zWASp2, which are 52% and 41% similar to human WASp, respectively [83]. Morpholino-mediated depletion of zWASp1 results in impaired directed migration of neutrophils and macrophages to a tail wound as a result of defects in pseudopod selection and directed migration [83]. To validate the morpholino studies, a zWASp1 mutant was generated [83] by TILLING [84]. As in zWASp1 morphants, the zWASp1 null mutant has a normal number of neutrophils and macrophages, but these leukocytes exhibit impaired recruitment to a tailfin wound [85] with defects in pseudopod selection similar to in vitro studies [86, 87]. The WASp null mutant also has increased susceptibility to bacterial infection with Staphylococcus aureus, recapitulating the infection phenotype of WAS patients [85]. Constitutively activating mutations in the GTPase binding domain of WASp result in X-linked neutropenia [88] and have also been modeled in zebrafish larvae [85] to provide an in vivo model for human X-linked neutropenia.
CGD
CGD is an immunodeficiency characterized by inflammatory granulomatous lesions and enhanced susceptibility to bacterial and fungal infections. CGD is associated with mutations in the PHOX complex that impair the ability of leukocytes to produce an oxidative burst [89, 90]. Elements of the PHOX complex include the membrane-bound components p22phox and gp91phox, cytosolic components p47phox and p67phox, the regulatory units p40phox, and the small GTPase Rac1 or Rac2 [90]. Upon phagocyte activation, the cytosolic and membrane-bound components form the intact PHOX complex, leading to the generation of superoxide, which is converted to hydrogen peroxide by superoxide dismutase [90]. Hydrogen peroxide, in the presence of myeloperoxidase, can be converted further to other antimicrobial ROS, such as hypochlorous acid [40]. These ROS activate neutrophil granule proteins and contribute to microbial killing [91]. Although there is currently no transgenic model of CGD in zebrafish, the use of morpholinos targeting different components of the PHOX complex have been used successfully to model CGD [41, 92]. These studies demonstrate that phagocyte-mediated killing of Candida albicans [92] and Mycobacterium marinum [41] are dependent on their ability to generate an oxidative burst.
INSIGHTS INTO NEUTROPHIL-PATHOGEN INTERACTIONS BY USE OF ZEBRAFISH
Zebrafish have been used to model bacterial and fungal disease. For a comprehensive list of zebrafish infection models, see Table 1. Here, we focus on studies that address how neutrophils contribute to host defense to specific infections.
TABLE 1.
The role of neutrophils in zebrafish models of infection
| Pathogen | Age of zebrafish | Route of infection | Role of neutrophils |
|---|---|---|---|
| Bacterium | |||
| Aeromonas hydrophila | Adult | Immersion [93]; i.p. [94, 95] | N/A |
| Larva | Immersion [96, 97] | N/A | |
| Aeromonas salmonicida | Adult | Immersion after woundinga [98] | N/A |
| Aeromonas veronii | Larva | Immersion [99] | N/A |
| Bacillus sp. | Adult | Mixed with feed [100] | N/A |
| Bacillus sphaericus | Adult | Immersion [101] | N/A |
| Bacillus subtilis | Embryo | CV [11]; DC [102] | Recruitment and phagocytosis [46] |
| Larva | DM [46] | ||
| Bacillus thuringiensis | Adult | Immersion [101] | N/A |
| Bartonella henselae | Embryo | CV, DC, Y [103] | Recruitment and phagocytosis [103] |
| Burkholderia cenocepacia | Adult | i.p. [104] | N/A |
| Embryo | CV [105, 106] | Some recruitment and phagocytosis, intracellular niche for bacteria [105] | |
| Citrobacter freundii | Adult | Immersion [107] | N/A |
| Edwardsiella ictaluri | Adult | i.m. [108]; immersion [108] | Leukocyte recruitment [108] |
| Edwardsiella tarda | Adult | i.m. [109, 110]; immersion [111, 112]; i.p. [113, 114] | Infection causes up-regulation in genes involved in neutrophil recruitment [112] |
| Embryo | CV [115, 116]; DC [117]; immersion [111, 115] | N/A | |
| Enterococcus faecalis | Embryo | DC [118] | Phagocytosis, pu.1 morphants have increased mortality [118] |
| E. coli | Embryo | CV [11, 12, 119]; DC/PC [47, 120–122]; mesenchyme between eye and OV [123]; notochord [124] | Recruitment [12, 47, 124] mediated by Cxcl8 [123]; phagocytosis [12]; degranulation, production of IL-1β [124]; pu.1 morphants have increased mortality [47] |
| Larva | Immersion [97, 125]; OV [12] | ||
| Flavobacterium columnare | Adult | i.m., immersion after wounding,a i.p. [126] | N/A |
| Embryo | Immersion [127] | N/A | |
| Flavobacterium johnsoniae | Adult | i.m., immersion after wounding,a i.p. [126] | N/A |
| Francisella spp. | Adult | i.p. [128] | N/A |
| Embryo | DC, DM, OV [129] | Recruitment, phagocytosis is more efficient when microbe is attached to surface [129] | |
| Haemophilus influenzae | Embryo | DC [117] | N/A |
| Lactococcus garvieae | Adult | i.p. [130] | Infiltration into optic lobes, lamina propria, and gills [130] |
| Leptospira interrogans | Embryo | CV, HBV [131] | Recruitment and phagocytosis by phagocytes [131] |
| Listeria monocytogenes | Adult | i.p. [132] | N/A |
| Embryo | CV [133] | Limited phagocytosis, engulfed bacteria are always in vacuoles, usually degraded, neutrophils contact infected macrophages [133] | |
| Listonella anguillarum | Adult | i.p. [134] | Up-regulation of neutrophil genes following infection (e.g., mpx and lyz) [134] |
| Larva | Immersion [135] | N/A | |
| Mycobacterium abscessus | Embryo | CV [136] | Recruitment to macrophage granulomas, phagocytosis [136] |
| M. marinum | Adult | Immersion [137]; oral intubation [137]; i.p. [137–150] | Infection induces up-regulation of neutrophil genes following infection [139–141] |
| Embryo | CV [41, 44, 116, 147, 151–162]; HBV [41, 44, 141, 147, 150, 152–154, 156–158]; Y [162, 163] | Rare recruitment and phagocytosis at infection site [41]; recruitment and scavenger role at granuloma [41]; pu.1 morphants have increased mortality [154]; RNS production [25]; oxidative burst [41] | |
| Larva | Tail fin [164] | Rare recruitment [164] | |
| Mycobacterium peregrinum | Adult | Immersion, i.p., oral intubation [137] | N/A |
| P. aeruginosa | Embryo | CV [165–167]; DC [117, 168, 169]; HBV [117] | Recruitment [117]; phagocytosis [117, 165, 168]; pu.1 morphants have increased mortality [165, 168] |
| Larva | Immersion [96, 97, 125]; OV [79, 170, 171]; Y [170] | Recruitment [79, 170, 171]; phagocytosis [170]; LAD model supports critical role of neutrophils for survival [79] | |
| Pseudomonas fluorescens | Larva | Immersion [99] | N/A |
| Salmonella arizonae | Embryo | CV [44] | N/A |
| Salmonella enterica serovar Typhimurium | Embryo | CV [116, 119, 172–175]; HBV [49, 51]; OV [176]; DM [176]; Y [177] | Recruitment [49, 51, 176]; infection induces neutrophil production [51] |
| Larva | Immersion [178] | N/A | |
| Shigella flexneri | Larva | CV [179] | Some phagocytosis, efficient killing of engulfed bacteria, scavenger role [179] |
| S. aureus | Embryo | CV [180]; eye [180]; HBV [180]; DC [56, 180, 181]; PC [180–182]; Y [181, 182] | Recruitment [180]; phagocytosis [56, 180]; possible intracellular niche for bacteria [56]; neutrophil ablation increases susceptibility to infection [5]; pu.1 and irf8 morphants have increased mortality [56] |
| Staphylococcus epidermidis | Embryo | Y [163] | N/A |
| Staphylococcus chromogenes | Adult | Immersion after woundinga [107] | N/A |
| Stenotrophomonas maltophilia | Adult | i.p. [183, 184] | N/A |
| Streptococcus agalactiae | Adult | i.m. [185]; i.p. [186–188] | N/A |
| Streptococcus equi | Adult | i.m., i.p. [189] | Leukocyte recruitment [189] |
| S. iniae | Adult | i.m., immersion after wounding,a i.p. [190–193] | Leukocyte recruitment [190, 191] |
| Larva | OV [43] | Recruitment, phagocytosis, LAD model supports critical role of neutrophils for survival [43] | |
| Streptococcus pnuemoniae | Adult | i.m., i.p. [194] | N/A |
| Embryo | DC [195] | Phagocytosis by Spi1+ cells, pu.1 and WASp morphants have increased mortality [195] | |
| Streptococcus pyogenes | Adult | i.p. [191, 196–198]; i.m. [45, 191, 196–200] | Lack of infiltrating leukocytes [191, 196–198] |
| Embryo | Y [45] | N/A | |
| Larva | DM [45] | Lack of neutrophil recruitment [45]; recruitment to SLS mutant [45] | |
| Streptococcus suis | Adult | i.p. [201–206] | N/A |
| Vibrio alginlyticus | Adult | i.m. [207] | N/A |
| Vibrio anguillarum | Larva | Immersion [208] | N/A |
| Vibrio vulnificus | Adult | i.p. [209] | N/A |
| Vibrio cholerae | Adult | Oral gavage, immersion [210] | N/A |
| Larva | Immersion [210] | N/A | |
| Yersinia ruckeri | Embryo | CV [211] | N/A |
| Intestinal microbiota | Larva | Immersion [96, 99, 125, 212–214] | Presence of microbiota results in: establishment of neutrophil homeostasis in the gut [99, 212, 213]; enhanced neutrophil recruitment to wound [212, 214]; neutrophil priming to produce H2O2 upon PMA stimulation [214] |
| Human intestinal microbiota | Larva | Injection into gut, immersion [215] | N/A |
| Mouse intestinal microbiota | Larva | Immersion [96] | N/A |
| Fungus | |||
| A. fumigatus | Embryo | HBV [216] | Recruitment to hyphae but not conidia, LAD model supports critical role of neutrophils for survival [216] |
| C. albicans | Adult | i.p. [217, 218] | N/A |
| Embryo | HBV [92, 217, 219]; Y [217] | Recruitment [219, 220] mediated by NADPH oxidase [219]; phagocytosis and killing of yeast cells [92] | |
| Larva | Swimbladder (injection) [219]; swimbladder (immersion) [220] | Recruitment [220] | |
| Penicillium marneffei (spores) | Larva | Somatic muscle [7] | Phagocytosis [7] |
As described in ref. [190].
P. aeruginosa
P. aeruginosa, a gram-negative bacterium, is an important, opportunistic pathogen of humans that causes localized and systemic disease. Most clinical cases of P. aeruginosa infection are associated with some compromise in immune function as a result of illness, injury, or underlying immune disorders, such as neutropenia [221]. Chronic infection caused by P. aeruginosa is the leading cause of pulmonary infection in cystic fibrosis patients [221]. In mammalian P. aeruginosa infection, neutrophils are known to be critical for host protection. Mice depleted of neutrophils succumb to localized infection within 2 days [222]. Human neutropenic hosts are also hypersensitive to Pseudomonas infection [223]. Although a number of plant and animal models have been used to study P. aeruginosa virulence, including Arabidopsis thaliana [224], Caenorhabditis elegans [225], Drosophila melanogaster [226], and mice [222], zebrafish have been developed more recently as a model to study P. aeruginosa infection by use of localized [79, 170] and systemic infection [165]. Zebrafish, like humans, are also relatively resistant to systemic infection with P. aeruginosa, and large inocula are needed to induce infection [165]. Localized injection of Pseudomonas into the OV of zebrafish larvae induces neutrophil recruitment (Fig. 1C; and Supplemental Video 2), and ∼65% of the neutrophils contain phagocytosed bacteria [170]. Neutrophils and macrophages phagocytose and kill P. aeruginosa, and depletion of phagocytes results in increased susceptibility to infection [165]. Neutrophils are critical in the host response to localized Pseudomonas infection, as shown by 2 zebrafish models of neutrophil deficiency: LAD [79] and WHIM syndrome [41]. Although LAD and WHIM fish have normal macrophage responses to infection [41, 43, 79], localized infection with P. aeruginosa results in increased mortality, likely as a result of impaired neutrophil function [41, 79]. This supports a more critical role for neutrophils than macrophages in the host defense against Pseudomonas infection in zebrafish, similar to humans. In a zebrafish model of CGD, larvae deficient in 2 key subunits of the NADPH oxidase complex (gp91phox and p22phox) have increased susceptibility to P. aeruginosa infection, suggesting that neutrophil ROS signaling is likely important for host defense to P. aeruginosa [41]. Oxidative killing is similarly important in mice, as p47phox -deficient animals have an increased bacterial burden [227]. The Pseudomonas-zebrafish infection model has also provided new insight into the signaling mechanisms that mediate neutrophil recruitment to localized infection. Unlike wound recruitment, neutrophil recruitment to OV P. aeruginosa infection does not require tissue-generated hydrogen peroxide [170, 228]. Similar to mice [222], neutrophil recruitment to P. aeruginosa infection requires Cxcr2-Cxcl8 signaling [171].
S. iniae
S. iniae is a natural fish pathogen and causes systemic disease, characterized by meningitis and sepsis in fish and humans. S. iniae causes 30–50% mortality in affected fish ponds, resulting in $100 million in annual losses for the aquaculture industry [229–231]. Localized S. iniae infection induces recruitment of leukocytes to infection sites in mice and zebrafish [43, 190, 191]. In mice and adult zebrafish, infection spreads systemically and results in rapid host mortality [190, 191]. Localized OV infection with S. iniae is also extremely lethal in larvae, with only 10 CFUs capable of causing significant host mortality [43]. In contrast, the capsule-deficient cpsA mutant is nonpathogenic in zebrafish [43, 192, 193], supporting the importance of the polysaccharide capsule in protecting this bacterium from the host immune response. Following OV injection with wild-type S. iniae or the cpsA mutant, neutrophils exhibit a type of behavior referred to as “neutrophil swarming” that has also been described in mice [232], in which there is initial chemotaxis of nearby neutrophils, followed by a second phase of amplified recruitment and clustering of neutrophils. Besides recruitment, macrophages and neutrophils (Fig. 1D and Supplemental Video 3) efficiently phagocytose wild-type S. iniae, as well as the cpsA mutant. This finding is in contrast to results with E. coli injected into fluid-filled cavities, such as the OV, where neutrophils, unlike macrophages, are inefficient at phagocytosing bacteria [46]. However, it has been shown that neutrophils are, in fact, capable of phagocytosing certain microbes, such as S. iniae [43] and P. aeruginosa [41, 170], even in these closed cavities. Thus, it appears that the efficiency of neutrophil phagocytosis may be dependent on the type of pathogen. Interestingly, infection of LAD fish shows that neutrophils are necessary for host survival in response to wild-type S. iniae but not the cpsA mutant. These findings suggest a protective role for neutrophils in wild-type S. iniae but not cpsA mutant infection. However, when neutrophils and macrophages are depleted, there is an increased susceptibility to the cpsA mutant, suggesting an important role for macrophages in this context. Interestingly, the closely related S. pyogenes has developed a mechanism for inhibiting neutrophil recruitment. Studies in mice were complemented by live imaging studies in zebrafish larvae to show that S. pyogenes expresses the cytolytic toxin SLS that limits neutrophil recruitment and optimizes pathogen dissemination [45]. SLS-deficient bacteria recruit more neutrophils and have reduced lethality of infection in zebrafish [45]. This is a nice example of how pathogens develop strategies to limit neutrophil recruitment to promote pathogen growth.
M. marinum
M. marinum is a natural fish pathogen and a close genetic relative of Mtb [233], the etiology of human TB. In adult [148, 234] and larval zebrafish [44] models of infection, M. marinum causes chronic disease with necrotic, granulomatous lesions, similar in structure to those induced by Mtb in humans. In the larval zebrafish model, macrophages not only form granuloma-like aggregates with similar histology to TB granulomas but also induce mycobacterial expression of granuloma-specific genes [44]. The optical accessibility of the larval zebrafish has provided important, new insights into host [41, 149–151, 235] and pathogen [152, 153, 236]. With the allowance of repeated live imaging of infected larvae, the zebrafish model has changed the widespread view of the granuloma as a strictly host-protective structure. In a series of studies, it was found that a mycobacterial virulence determinant, the ESX-1/RD1 secretion system, drives the formation of early granulomas [152] through the secretion of ESAT-6, which induces production of MMP9 by nearby epithelial cells [153]. This localized MMP9 enhances recruitment of macrophages to foci of infection, enhancing granuloma formation [153]. The appearance of granulomas coincides with and contributes to a marked increase in bacterial proliferation [152], in contrast to the previous idea that granulomas are primarily host-protective structures. The larval zebrafish model of M. marinum infection has also helped to uncover a host-protective role for neutrophils in the early granuloma. Although neutrophils are present at sites of TB infection, such as in the cerebrospinal fluid of TB meningitis patients [237] or in the granulomas of pulmonary TB patients [238], previously, there has been a limited understanding of their role in mycobacterial pathogenesis. Some in vitro studies show that isolated human neutrophils kill Mtb [239, 240], whereas others seem to demonstrate their inability to do so [241]. In mouse models, the role of neutrophils in mycobacterial infection is also unclear. A study by Pedrosa et al. [242] indicates that neutrophils play a protective role in systemic Mtb infection that is independent of their phagocytic ability. Although neutrophils are recruited to infection foci, bacilli are only found inside macrophages but not neutrophils [242]. However, depletion of neutrophils results in increased severity of infection [242]. Thus, instead of a direct role for neutrophils in bacterial killing, these findings suggest an immunomodulatory role for neutrophils in the immune response to Mtb [242]. Sugawara et al. [243] also demonstrated a protective role for neutrophils in a rat model where neutrophil depletion results in worsened disease, whereas LPS-induced neutrophilia actually prevents early mycobacterial infection. However, other in vivo studies have suggested that neutrophils have a detrimental effect on the host response to Mtb [154]. Recent work that uses the zebrafish model has helped to clarify the role of neutrophils in mycobacterial infection. Systemic infection with M. marinum in zebrafish embryos results in the recruitment of many macrophages and few neutrophils [41, 244]. Of the neutrophils recruited, very few become infected with M. marinum [244]. Live imaging reveals that neutrophils traffic into granulomas and interact closely with infected macrophages and can become infected by engulfing macrophages that contain M. marinum. Treatment with a pan-caspase inhibitor that blocks apoptosis in zebrafish [245] impairs neutrophil recruitment to granulomas, suggesting that neutrophils respond to signals released from apoptotic macrophages. This scavenging role for neutrophils is surprising, as macrophages have long been thought to be the sole phagocyte responsible for removing apoptotic cells and cell debris during resolution of inflammation (described in zebrafish in [7] and reviewed in ref. [246]). Neutrophils also act as scavengers in a zebrafish model of systemic S. flexneri infection [179]. This illustrates the power of the use of zebrafish to dissect the role of immune cells in a living, intact animal. The importance of neutrophils to host defense against mycobacterial infection has been demonstrated directly by the use of the neutropenic WHIM zebrafish [67], which show increased susceptibility to M. marinum infection [41]. Infection of fish depleted of 2 components of the NADPH oxidase complex (gp91phox and p22phox) also results in increased susceptibility to infection compared with controls [41]. This NADPH oxidase-dependent killing of mycobacteria may provide a possible explanation for the long-standing observation of the susceptibility of CGD patients to TB.
Fungal disease
C. albicans and A. fumigatus undergo morphologic changes that induce specific immune responses. In zebrafish larvae, C. albicans yeast can germinate to form hyphae, but this switch in morphology is inhibited after phagocytosis of the yeast by neutrophils and macrophages [92]. The NADPH oxidase complex is necessary to control this change in morphology [92, 219]. However, the specific role of neutrophils in controlling these morphologic changes remains unclear. A morphology-specific innate immune response occurs to the opportunistic human pathogen A. fumigatus in zebrafish [216]; macrophages are recruited to spores and hyphae, whereas neutrophils are recruited only to hyphae. The infectious particles of Aspergillus are airborne asexual spores called conidia, and because of the prevalence of Aspergillus in the environment, the average individual is thought to inhale as many as several hundred conidia per day [247]. Immunocompromised populations, particularly neutropenic patients, are at risk for developing the often-fatal disease, Invasive Aspergillosis, characterized by conidial germination into tissue-penetrating hyphae [248, 249]. Like humans and mice, immunocompetent fish are not susceptible to A. fumigatus infection [216], but depletion of neutrophils and macrophages in zebrafish increases susceptibility to infection and results in conidial germination into tissue-penetrating hyphae, similar to aspergillosis in humans. LAD zebrafish with impaired neutrophil function develop invasive disease and have impaired survival with A. fumigatus [216]. Interestingly, the inability of neutrophils to phagocytose A. fumigatus conidia is pathogen-specific, as neutrophils respond to and phagocytose C. albicans yeast forms [92]. Schaffner et al. [250] also demonstrated distinct roles for neutrophils and monocytes in targeting hyphae and conidia, respectively, in a mouse model of Aspergillus infection, which is consistent with observations made in zebrafish. Accordingly, neutropenic mice are unable to control hyphal growth [250]. Isolated human neutrophils also show a higher induction of NETosis in response to hyphae compared with conidia [251], and hyphal growth is inhibited by NET production [252]. Taken together, the zebrafish model of fungal disease highlights the conservation of the innate immune response to different morphologic forms of Aspergillus species, and the optical accessibility provided by zebrafish has helped to uncover the distinct responses of neutrophils to different morphologic forms of fungi in vivo.
CONCLUDING REMARKS
Zebrafish represent a powerful model system to study the role of neutrophils in host-pathogen interactions that complements studies in mice and humans. Challenges remain in dissecting how neutrophils modulate disease, as neutrophils can have beneficial and detrimental effects. For example, in a mouse model of S. aureus infection, neutrophils crawling in capillaries around the site of infection have a potentially beneficial role early on in the infection in limiting bacterial dissemination, but continued presence at the infection site results in increased tissue injury [253]. It has been shown in mice and zebrafish that neutrophils can traffic away from sites of tissue damage in a process known as neutrophil reverse migration [5, 30]. Although the contribution of neutrophil reverse migration in the context of infection remains unclear, the genetic tractability and optical transparency of zebrafish larvae can help elucidate novel mechanisms of neutrophil behavior in response to tissue damage and infection. It is possible that reverse migration of neutrophils may serve as a mechanism of systemic activation of the immune response [34]. Alternatively, infected neutrophils may provide an intracellular niche for persisting microbes, serving as "Trojan Horses" to disseminate bacteria from the initial infection site, as has been suggested with S. aureus infection in zebrafish [56]. The use of labeled bacteria and transgenic fish lines with labeled phagocytes will provide further insight into the temporal and spatial interactions between neutrophils and macrophages during infection. Finally, zebrafish models of neutropenia and other human neutrophil disorders have made it possible to elucidate the specific role of neutrophils in host defense to different types of bacterial and fungal infections. Future studies will be needed to determine if these models can be used to advance treatments that target innate immunity and infection. Recent studies that use small molecule screening in zebrafish mycobacterial models have raised the possibility that zebrafish infection models can be used to identify new therapeutic strategies to treat infectious disease [254]. It will be particularly intriguing to understand how the inflammatory status affects response to a particular therapy, with the aim toward host-directed therapeutic strategies.
AUTHORSHIP
E.A.H. performed the experiments and wrote the paper; A.H. cowrote the paper.
Acknowledgments
This work was supported by National Institute of General Medical Sciences (NIGMS) U.S. National Institutes of Health Grant R01 GM074827 (to A.H.). The authors thank Benjamin P. Knox, Emily E. Rosowski, William J. B. Vincent, and J. Muse Davis for their critical reading of the manuscript and lab members for zebrafish care and maintenance.
Glossary
- Cas
CRISPR-associated
- CGD
chronic granulomatous disease
- CHT
caudal hematopoietic tissue
- CRISPR
clustered regularly interspaced short palindromic repeats
- CV
caudal vein
- DC
duct of Cuvier
- DM
dorsal muscle
- dpf
days postfertilization
- ESAT-6
early secretory antigenic target of Mycobacterium tuberculosis
- ESX-1
early secretory antigenic target 6 system 1
- HBV
hind brain ventricle
- hpf
hours postfertilization
- irf8
IFN regulatory factor 8
- LAD
leukocyte adhesion deficiency
- lyz
lysozyme C
- MMP9
matrix metalloproteinase 9
- mpeg1
macrophage expressed gene 1
- mpx
myeloid peroxidase
- Mtb
Mycobacterium tuberculosis
- NET
neutrophil extracellular trap
- OV
otic vesicle
- PC
pericardial cavity
- PHOX
phagocyte NADPH oxidase complex
- Rac1/2
Ras-related C3 botulinum toxin substrate 1/2
- RD1
region of difference 1
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SDF1
stromal cell-derived factor 1
- SLS
streptolysin S
- TB
tuberculosis
- TALEN
transcription activator-like endonucleases
- WAS
Wiskott Aldrich syndrome
- WASp
Wiskott-Aldrich Syndrome protein
- WHIM
warts, hypogammaglobulinemia, infections, and myelokathexis
- Y
yolk sac
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The authors declare no competing financial interests.
REFERENCES
- 1.Lieschke G. J., Oates A. C., Crowhurst M. O., Ward A. C., Layton J. E. (2001) Morphologic and functional characterization of granulocytes and macrophages in embryonic and adult zebrafish. Blood 98, 3087–3096. [DOI] [PubMed] [Google Scholar]
- 2.Amulic B., Cazalet C., Hayes G. L., Metzler K. D., Zychlinsky A. (2012) Neutrophil function: from mechanisms to disease. Annu. Rev. Immunol. 30, 459–489. [DOI] [PubMed] [Google Scholar]
- 3.Borregaard N. (2010) Neutrophils, from marrow to microbes. Immunity 33, 657–670. [DOI] [PubMed] [Google Scholar]
- 4.Nathan C. (2006) Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6, 173–182. [DOI] [PubMed] [Google Scholar]
- 5.Mathias J. R., Perrin B. J., Liu T. X., Kanki J., Look A. T., Huttenlocher A. (2006) Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J. Leukoc. Biol. 80, 1281–1288. [DOI] [PubMed] [Google Scholar]
- 6.Renshaw S. A., Loynes C. A., Trushell D. M. I., Elworthy S., Ingham P. W., Whyte M. K. B. (2006) A transgenic zebrafish model of neutrophilic inflammation. Blood 108, 3976–3978. [DOI] [PubMed] [Google Scholar]
- 7.Ellett F., Pase L., Hayman J. W., Andrianopoulos A., Lieschke G. J. (2011) mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 117, e49–e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seeger A., Mayer W. E., Klein J. (1996) A complement factor B-like cDNA clone from the zebrafish (Brachydanio rerio). Mol. Immunol. 33, 511–520. [DOI] [PubMed] [Google Scholar]
- 9.Jault C., Pichon L., Chluba J. (2004) Toll-like receptor gene family and TIR-domain adapters in Danio rerio. Mol. Immunol. 40, 759–771. [DOI] [PubMed] [Google Scholar]
- 10.Meijer A. H., Gabby Krens S. F., Medina Rodriguez I. A., He S., Bitter W., Ewa Snaar-Jagalska B., Spaink H. P. (2004) Expression analysis of the Toll-like receptor and TIR domain adaptor families of zebrafish. Mol. Immunol. 40, 773–783. [DOI] [PubMed] [Google Scholar]
- 11.Herbomel P., Thisse B., Thisse C. (1999) Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development 126, 3735–3745. [DOI] [PubMed] [Google Scholar]
- 12.Le Guyader D., Redd M. J., Colucci-Guyon E., Murayama E., Kissa K., Briolat V., Mordelet E., Zapata A., Shinomiya H., Herbomel P. (2008) Origins and unconventional behavior of neutrophils in developing zebrafish. Blood 111, 132–141. [DOI] [PubMed] [Google Scholar]
- 13.Lam S. H., Chua H. L., Gong Z., Lam T. J., Sin Y. M. (2004) Development and maturation of the immune system in zebrafish, Danio rerio: a gene expression profiling, in situ hybridization and immunological study. Dev. Comp. Immunol. 28, 9–28. [DOI] [PubMed] [Google Scholar]
- 14.Danilova N., Steiner L. A. (2002) B cells develop in the zebrafish pancreas. Proc. Natl. Acad. Sci. USA 99, 13711–13716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willett C. E., Cortes A., Zuasti A., Zapata A. G. (1999) Early hematopoiesis and developing lymphoid organs in the zebrafish. Dev. Dyn. 214, 323–336. [DOI] [PubMed] [Google Scholar]
- 16.Hwang W. Y., Fu Y., Reyon D., Maeder M. L., Tsai S. Q., Sander J. D., Peterson R. T., Yeh J.-R. J., Joung J. K. (2013) Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 31, 227–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang W. Y., Peterson R. T., Yeh J.-R. J. (2014) Methods for targeted mutagenesis in zebrafish using TALENs. Methods 69, 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torraca V., Masud S., Spaink H. P., Meijer A. H. (2014) Macrophage-pathogen interactions in infectious diseases: new therapeutic insights from the zebrafish host model. Dis. Model. Mech. 7, 785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhodes J., Hagen A., Hsu K., Deng M., Liu T. X., Look A. T., Kanki J. P. (2005) Interplay of pu.1 and gata1 determines myelo-erythroid progenitor cell fate in zebrafish. Dev. Cell 8, 97–108. [DOI] [PubMed] [Google Scholar]
- 20.Lieschke G. J., Oates A. C., Paw B. H., Thompson M. A., Hall N. E., Ward A. C., Ho R. K., Zon L. I., Layton J. E. (2002) Zebrafish SPI-1 (PU.1) marks a site of myeloid development independent of primitive erythropoiesis: implications for axial patterning. Dev. Biol. 246, 274–295. [DOI] [PubMed] [Google Scholar]
- 21.Bertrand J. Y., Kim A. D., Violette E. P., Stachura D. L., Cisson J. L., Traver D. (2007) Definitive hematopoiesis initiates through a committed erythromyeloid progenitor in the zebrafish embryo. Development 134, 4147–4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murayama E., Kissa K., Zapata A., Mordelet E., Briolat V., Lin H.-F., Handin R. I., Herbomel P. (2006) Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity 25, 963–975. [DOI] [PubMed] [Google Scholar]
- 23.Jin H., Sood R., Xu J., Zhen F., English M. A., Liu P. P., Wen Z. (2009) Definitive hematopoietic stem/progenitor cells manifest distinct differentiation output in the zebrafish VDA and PBI. Development 136, 647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennett C. M., Kanki J. P., Rhodes J., Liu T. X., Paw B. H., Kieran M. W., Langenau D. M., Delahaye-Brown A., Zon L. I., Fleming M. D., Look A. T. (2001) Myelopoiesis in the zebrafish, Danio rerio. Blood 98, 643–651. [DOI] [PubMed] [Google Scholar]
- 25.Hermann A. C., Millard P. J., Blake S. L., Kim C. H. (2004) Development of a respiratory burst assay using zebrafish kidneys and embryos. J. Immunol. Methods 292, 119–129. [DOI] [PubMed] [Google Scholar]
- 26.Palić D., Andreasen C. B., Ostojić J., Tell R. M., Roth J. A. (2007) Zebrafish (Danio rerio) whole kidney assays to measure neutrophil extracellular trap release and degranulation of primary granules. J. Immunol. Methods 319, 87–97. [DOI] [PubMed] [Google Scholar]
- 27.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D. S., Weinrauch Y., Zychlinsky A. (2004) Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535. [DOI] [PubMed] [Google Scholar]
- 28.Fuchs T. A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., Weinrauch Y., Brinkmann V., Zychlinsky A. (2007) Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176, 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buckley C. D., Ross E. A., McGettrick H. M., Osborne C. E., Haworth O., Schmutz C., Stone P. C. W., Salmon M., Matharu N. M., Vohra R. K., Nash G. B., Rainger G. E. (2006) Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. J. Leukoc. Biol. 79, 303–311. [DOI] [PubMed] [Google Scholar]
- 30.Woodfin A., Voisin M.-B., Beyrau M., Colom B., Caille D., Diapouli F.-M., Nash G. B., Chavakis T., Albelda S. M., Rainger G. E., Meda P., Imhof B. A., Nourshargh S. (2011) The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat. Immunol. 12, 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meijer A. H., van der Sar A. M., Cunha C., Lamers G. E. M., Laplante M. A., Kikuta H., Bitter W., Becker T. S., Spaink H. P. (2008) Identification and real-time imaging of a myc-expressing neutrophil population involved in inflammation and mycobacterial granuloma formation in zebrafish. Dev. Comp. Immunol. 32, 36–49. [DOI] [PubMed] [Google Scholar]
- 32.Mathias J. R., Dodd M. E., Walters K. B., Yoo S. K., Ranheim E. A., Huttenlocher A. (2009) Characterization of zebrafish larval inflammatory macrophages. Dev. Comp. Immunol. 33, 1212–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urasaki A., Morvan G., Kawakami K. (2006) Functional dissection of the Tol2 transposable element identified the minimal cis-sequence and a highly repetitive sequence in the subterminal region essential for transposition. Genetics 174, 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoo S. K., Huttenlocher A. (2011) Spatiotemporal photolabeling of neutrophil trafficking during inflammation in live zebrafish. J. Leukoc. Biol. 89, 661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gurskaya N. G., Verkhusha V. V., Shcheglov A. S., Staroverov D. B., Chepurnykh T. V., Fradkov A. F., Lukyanov S., Lukyanov K. A. (2006) Engineering of a monomeric green-to-red photoactivatable fluorescent protein induced by blue light. Nat. Biotechnol. 24, 461–465. [DOI] [PubMed] [Google Scholar]
- 36.Hatta K., Tsujii H., Omura T. (2006) Cell tracking using a photoconvertible fluorescent protein. Nat. Protoc. 1, 960–967. [DOI] [PubMed] [Google Scholar]
- 37.Ando R., Hama H., Yamamoto-Hino M., Mizuno H., Miyawaki A. (2002) An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc. Natl. Acad. Sci. USA 99, 12651–12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward A. C., McPhee D. O., Condron M. M., Varma S., Cody S. H., Onnebo S. M. N., Paw B. H., Zon L. I., Lieschke G. J. (2003) The zebrafish spi1 promoter drives myeloid-specific expression in stable transgenic fish. Blood 102, 3238–3240. [DOI] [PubMed] [Google Scholar]
- 39.Hsu K., Traver D., Kutok J. L., Hagen A., Liu T. X., Paw B. H., Rhodes J., Berman J. N., Zon L. I., Kanki J. P., Look A. T. (2004) The pu.1 promoter drives myeloid gene expression in zebrafish. Blood 104, 1291–1297. [DOI] [PubMed] [Google Scholar]
- 40.Klebanoff S. J. (2005) Myeloperoxidase: friend and foe. J. Leukoc. Biol. 77, 598–625. [DOI] [PubMed] [Google Scholar]
- 41.Yang C.-T., Cambier C. J., Davis J. M., Hall C. J., Crosier P. S., Ramakrishnan L. (2012) Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocytosed from infected macrophages. Cell Host Microbe 12, 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall C., Flores M. V., Storm T., Crosier K., Crosier P. (2007) The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC Dev. Biol. 7, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harvie E. A., Green J. M., Neely M. N., Huttenlocher A. (2013) Innate immune response to Streptococcus iniae infection in zebrafish larvae. Infect. Immun. 81, 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis J. M., Clay H., Lewis J. L., Ghori N., Herbomel P., Ramakrishnan L. (2002) Real-time visualization of mycobacterium-macrophage interactions leading to initiation of granuloma formation in zebrafish embryos. Immunity 17, 693–702. [DOI] [PubMed] [Google Scholar]
- 45.Lin A., Loughman J. A., Zinselmeyer B. H., Miller M. J., Caparon M. G. (2009) Streptolysin S inhibits neutrophil recruitment during the early stages of Streptococcus pyogenes infection. Infect. Immun. 77, 5190–5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colucci-Guyon E., Tinevez J. Y., Renshaw S. A., Herbomel P. (2011) Strategies of professional phagocytes in vivo: unlike macrophages, neutrophils engulf only surface-associated microbes. J. Cell Sci. 124, 3053–3059. [DOI] [PubMed] [Google Scholar]
- 47.Wiles T. J., Bower J. M., Redd M. J., Mulvey M. A. (2009) Use of zebrafish to probe the divergent virulence potentials and toxin requirements of extraintestinal pathogenic Escherichia coli. PLoS Pathog. 5, e1000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hall C., Flores M. V., Chien A., Davidson A., Crosier K., Crosier P. (2009) Transgenic zebrafish reporter lines reveal conserved Toll-like receptor signaling potential in embryonic myeloid leukocytes and adult immune cell lineages. J. Leukoc. Biol. 85, 751–765. [DOI] [PubMed] [Google Scholar]
- 49.Hall C. J., Boyle R. H., Astin J. W., Flores M. V., Oehlers S. H., Sanderson L. E., Ellett F., Lieschke G. J., Crosier K. E., Crosier P. S. (2013) Immunoresponsive gene 1 augments bactericidal activity of macrophage-lineage cells by regulating β-oxidation-dependent mitochondrial ROS production. Cell Metab. 18, 265–278. [DOI] [PubMed] [Google Scholar]
- 50.Hall C. J., Boyle R. H., Sun X., Wicker S. M., Misa J. P., Krissansen G. W., Print C. G., Crosier K. E., Crosier P. S. (2014) Epidermal cells help coordinate leukocyte migration during inflammation through fatty acid-fuelled matrix metalloproteinase production. Nat. Commun. 5, 3880. [DOI] [PubMed] [Google Scholar]
- 51.Hall C. J., Flores M. V., Oehlers S. H., Sanderson L. E., Lam E. Y., Crosier K. E., Crosier P. S. (2012) Infection-responsive expansion of the hematopoietic stem and progenitor cell compartment in zebrafish is dependent upon inducible nitric oxide. Cell Stem Cell 10, 198–209. [DOI] [PubMed] [Google Scholar]
- 52.Elks P. M., Brizee S., van der Vaart M., Walmsley S. R., van Eeden F. J., Renshaw S. A., Meijer A. H. (2013) Hypoxia inducible factor signaling modulates susceptibility to mycobacterial infection via a nitric oxide dependent mechanism. PLoS Pathog. 9, e1003789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gray C., Loynes C. A., Whyte M. K. B., Crossman D. C., Renshaw S. A., Chico T. J. A. (2011) Simultaneous intravital imaging of macrophage and neutrophil behaviour during inflammation using a novel transgenic zebrafish. Thromb. Haemost. 105, 811–819. [DOI] [PubMed] [Google Scholar]
- 54.Curado S., Anderson R. M., Jungblut B., Mumm J., Schroeter E., Stainier D. Y. R. (2007) Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev. Dyn. 236, 1025–1035. [DOI] [PubMed] [Google Scholar]
- 55.Curado S., Stainier D. Y. R., Anderson R. M. (2008) Nitroreductase-mediated cell/tissue ablation in zebrafish: a spatially and temporally controlled ablation method with applications in developmental and regeneration studies. Nat. Protoc. 3, 948–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prajsnar T. K., Hamilton R., Garcia-Lara J., McVicker G., Williams A., Boots M., Foster S. J., Renshaw S. A. (2012) A privileged intraphagocyte niche is responsible for disseminated infection of Staphylococcus aureus in a zebrafish model. Cell. Microbiol. 14, 1600–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li L., Jin H., Xu J., Shi Y., Wen Z. (2011) Irf8 regulates macrophage versus neutrophil fate during zebrafish primitive myelopoiesis. Blood 117, 1359–1369. [DOI] [PubMed] [Google Scholar]
- 58.Palha N., Guivel-Benhassine F., Briolat V., Lutfalla G., Sourisseau M., Ellett F., Wang C.-H., Lieschke G. J., Herbomel P., Schwartz O., Levraud J.-P. (2013) Real-time whole-body visualization of Chikungunya virus infection and host interferon response in zebrafish. PLoS Pathog. 9, e1003619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stachura D. L., Svoboda O., Campbell C. A., Espín-Palazón R., Lau R. P., Zon L. I., Bartunek P., Traver D. (2013) The zebrafish granulocyte colony-stimulating factors (Gcsfs): 2 paralogous cytokines and their roles in hematopoietic development and maintenance. Blood 122, 3918–3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liongue C., Hall C. J., O’Connell B. A., Crosier P., Ward A. C. (2009) Zebrafish granulocyte colony-stimulating factor receptor signaling promotes myelopoiesis and myeloid cell migration. Blood 113, 2535–2546. [DOI] [PubMed] [Google Scholar]
- 61.Feng Y., Renshaw S., Martin P. (2012) Live imaging of tumor initiation in zebrafish larvae reveals a trophic role for leukocyte-derived PGE2. Curr. Biol. 22, 1253–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wetzler M., Talpaz M., Kleinerman E. S., King A., Huh Y. O., Gutterman J. U., Kurzrock R. (1990) A new familial immunodeficiency disorder characterized by severe neutropenia, a defective marrow release mechanism, and hypogammaglobulinemia. Am. J. Med. 89, 663–672. [DOI] [PubMed] [Google Scholar]
- 63.Gorlin R. J., Gelb B., Diaz G. A., Lofsness K. G., Pittelkow M. R., Fenyk J. R. Jr. (2000) WHIM syndrome, an autosomal dominant disorder: clinical, hematological, and molecular studies. Am. J. Med. Genet. 91, 368–376. [PubMed] [Google Scholar]
- 64.Oberlin E., Amara A., Bachelerie F., Bessia C., Virelizier J. L., Arenzana-Seisdedos F., Schwartz O., Heard J. M., Clark-Lewis I., Legler D. F., Loetscher M., Baggiolini M., Moser B. (1996) The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature 382, 833–835. [DOI] [PubMed] [Google Scholar]
- 65.Furze R. C., Rankin S. M. (2008) Neutrophil mobilization and clearance in the bone marrow. Immunology 125, 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kawai T., Choi U., Cardwell L., DeRavin S. S., Naumann N., Whiting-Theobald N. L., Linton G. F., Moon J., Murphy P. M., Malech H. L. (2007) WHIM syndrome myelokathexis reproduced in the NOD/SCID mouse xenotransplant model engrafted with healthy human stem cells transduced with C-terminus-truncated CXCR4. Blood 109, 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walters K. B., Green J. M., Surfus J. C., Yoo S. K., Huttenlocher A. (2010) Live imaging of neutrophil motility in a zebrafish model of WHIM syndrome. Blood 116, 2803–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hernandez P. A., Gorlin R. J., Lukens J. N., Taniuchi S., Bohinjec J., Francois F., Klotman M. E., Diaz G. A. (2003) Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat. Genet. 34, 70–74. [DOI] [PubMed] [Google Scholar]
- 69.Etzioni A., Alon R. (2004) Leukocyte adhesion deficiency III: a group of integrin activation defects in hematopoietic lineage cells. Curr. Opin. Allergy Clin. Immunol. 4, 485–490. [DOI] [PubMed] [Google Scholar]
- 70.Etzioni A. (2010) Defects in the leukocyte adhesion cascade. Clin. Rev. Allergy Immunol. 38, 54–60. [DOI] [PubMed] [Google Scholar]
- 71.Pai S.-Y., Kim C., Williams D. A. (2010) Rac GTPases in human diseases. Dis. Markers 29, 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ambruso D. R., Knall C., Abell A. N., Panepinto J., Kurkchubasche A., Thurman G., Gonzalez-Aller C., Hiester A., deBoer M., Harbeck R. J., Oyer R., Johnson G. L., Roos D. (2000) Human neutrophil immunodeficiency syndrome is associated with an inhibitory Rac2 mutation. Proc. Natl. Acad. Sci. USA 97, 4654–4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Williams D. A., Tao W., Yang F., Kim C., Gu Y., Mansfield P., Levine J. E., Petryniak B., Derrow C. W., Harris C., Jia B., Zheng Y., Ambruso D. R., Lowe J. B., Atkinson S. J., Dinauer M. C., Boxer L. (2000) Dominant negative mutation of the hematopoietic-specific Rho GTPase, Rac2, is associated with a human phagocyte immunodeficiency. Blood 96, 1646–1654. [PubMed] [Google Scholar]
- 74.Accetta D., Syverson G., Bonacci B., Reddy S., Bengtson C., Surfus J., Harbeck R., Huttenlocher A., Grossman W., Routes J., Verbsky J. (2011) Human phagocyte defect caused by a Rac2 mutation detected by means of neonatal screening for T-cell lymphopenia. J. Allergy Clin. Immunol. 127, 535–538, e1–e2. [DOI] [PubMed] [Google Scholar]
- 75.Berthier E., Surfus J., Verbsky J., Huttenlocher A., Beebe D. (2010) An arrayed high-content chemotaxis assay for patient diagnosis. Integr Biol (Camb) 2, 630–638. [DOI] [PubMed] [Google Scholar]
- 76.Heyworth P. G., Bohl B. P., Bokoch G. M., Curnutte J. T. (1994) Rac translocates independently of the neutrophil NADPH oxidase components p47phox and p67phox. Evidence for its interaction with flavocytochrome b558. J. Biol. Chem. 269, 30749–30752. [PubMed] [Google Scholar]
- 77.Kaibuchi K., Kuroda S., Amano M. (1999) Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu. Rev. Biochem. 68, 459–486. [DOI] [PubMed] [Google Scholar]
- 78.Roberts A. W., Kim C., Zhen L., Lowe J. B., Kapur R., Petryniak B., Spaetti A., Pollock J. D., Borneo J. B., Bradford G. B., Atkinson S. J., Dinauer M. C., Williams D. A. (1999) Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity 10, 183–196. [DOI] [PubMed] [Google Scholar]
- 79.Deng Q., Yoo S. K., Cavnar P. J., Green J. M., Huttenlocher A. (2011) Dual roles for Rac2 in neutrophil motility and active retention in zebrafish hematopoietic tissue. Dev. Cell 21, 735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Derry J. M. J., Ochs H. D., Francke U. (1994) Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell 78, 635–644. [DOI] [PubMed] [Google Scholar]
- 81.Takenawa T., Suetsugu S. (2007) The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat. Rev. Mol. Cell Biol. 8, 37–48. [DOI] [PubMed] [Google Scholar]
- 82.Ochs H. D., Thrasher A. J. (2006) The Wiskott-Aldrich syndrome. J. Allergy Clin. Immunol. 117, 725–738, quiz 739. [DOI] [PubMed] [Google Scholar]
- 83.Cvejic A., Hall C., Bak-Maier M., Flores M. V., Crosier P., Redd M. J., Martin P. (2008) Analysis of WASp function during the wound inflammatory response—live-imaging studies in zebrafish larvae. J. Cell Sci. 121, 3196–3206. [DOI] [PubMed] [Google Scholar]
- 84.Wienholds E., van Eeden F., Kosters M., Mudde J., Plasterk R. H. A., Cuppen E. (2003) Efficient target-selected mutagenesis in zebrafish. Genome Res. 13, 2700–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jones R. A., Feng Y., Worth A. J., Thrasher A. J., Burns S. O., Martin P. (2013) Modelling of human Wiskott-Aldrich syndrome protein mutants in zebrafish larvae using in vivo live imaging. J. Cell Sci. 126, 4077–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ishihara D., Dovas A., Park H., Isaac B. M., Cox D. (2012) The chemotactic defect in Wiskott-Aldrich syndrome macrophages is due to the reduced persistence of directional protrusions. PLoS ONE 7, e30033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zicha D., Allen W. E., Brickell P. M., Kinnon C., Dunn G. A., Jones G. E., Thrasher A. J. (1998) Chemotaxis of macrophages is abolished in the Wiskott-Aldrich syndrome. Br. J. Haematol. 101, 659–665. [DOI] [PubMed] [Google Scholar]
- 88.Devriendt K., Kim A. S., Mathijs G., Frints S. G. M., Schwartz M., Van Den Oord J. J., Verhoef G. E. G., Boogaerts M. A., Fryns J.-P., You D., Rosen M. K., Vandenberghe P. (2001) Constitutively activating mutation in WASP causes X-linked severe congenital neutropenia. Nat. Genet. 27, 313–317. [DOI] [PubMed] [Google Scholar]
- 89.Segal B. H., Leto T. L., Gallin J. I., Malech H. L., Holland S. M. (2000) Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore) 79, 170–200. [DOI] [PubMed] [Google Scholar]
- 90.Holland S. M. (2010) Chronic granulomatous disease. Clin. Rev. Allergy Immunol. 38, 3–10. [DOI] [PubMed] [Google Scholar]
- 91.Reeves E. P., Lu H., Jacobs H. L., Messina C. G. M., Bolsover S., Gabella G., Potma E. O., Warley A., Roes J., Segal A. W. (2002) Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416, 291–297. [DOI] [PubMed] [Google Scholar]
- 92.Brothers K. M., Newman Z. R., Wheeler R. T. (2011) Live imaging of disseminated candidiasis in zebrafish reveals role of phagocyte oxidase in limiting filamentous growth. Eukaryot. Cell 10, 932–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lü A. J., Hu X. C., Wang Y., Zhu A. H., Shen L. L., Tian J., Feng Z. Z., Feng Z. J. (2015) Skin immune response in the zebrafish, Danio rerio (Hamilton), to Aeromonas hydrophila infection: a transcriptional profiling approach. J. Fish Dis. 38, 137–150. [DOI] [PubMed] [Google Scholar]
- 94.Li J., Ni X. D., Liu Y. J., Lu C. P. (2011) Detection of three virulence genes alt, ahp and aerA in Aeromonas hydrophila and their relationship with actual virulence to zebrafish. J. Appl. Microbiol. 110, 823–830. [DOI] [PubMed] [Google Scholar]
- 95.Cantas L., Midtlyng P. J., Sørum H. (2012) Impact of antibiotic treatments on the expression of the R plasmid tra genes and on the host innate immune activity during pRAS1 bearing Aeromonas hydrophila infection in zebrafish (Danio rerio). BMC Microbiol. 12, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rawls J. F., Samuel B. S., Gordon J. I. (2004) Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc. Natl. Acad. Sci. USA 101, 4596–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rawls J. F., Mahowald M. A., Ley R. E., Gordon J. I. (2006) Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell 127, 423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin B., Chen S., Cao Z., Lin Y., Mo D., Zhang H., Gu J., Dong M., Liu Z., Xu A. (2007) Acute phase response in zebrafish upon Aeromonas salmonicida and Staphylococcus aureus infection: striking similarities and obvious differences with mammals. Mol. Immunol. 44, 295–301. [DOI] [PubMed] [Google Scholar]
- 99.Bates J. M., Mittge E., Kuhlman J., Baden K. N., Cheesman S. E., Guillemin K. (2006) Distinct signals from the microbiota promote different aspects of zebrafish gut differentiation. Dev. Biol. 297, 374–386. [DOI] [PubMed] [Google Scholar]
- 100.Chu W., Zhou S., Zhu W., Zhuang X. (2014) Quorum quenching bacteria Bacillus sp. QSI-1 protect zebrafish (Danio rerio) from Aeromonas hydrophila infection. Sci. Rep. 4, 5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grisolia C. K., Oliveira-Filho E. C., Ramos F. R., Lopes M. C., Muniz D. H. F., Monnerat R. G. (2009) Acute toxicity and cytotoxicity of Bacillus thuringiensis and Bacillus sphaericus strains on fish and mouse bone marrow. Ecotoxicology 18, 22–26. [DOI] [PubMed] [Google Scholar]
- 102.Li X., Wang S., Qi J., Echtenkamp S. F., Chatterjee R., Wang M., Boons G.-J., Dziarski R., Gupta D. (2007) Zebrafish peptidoglycan recognition proteins are bactericidal amidases essential for defense against bacterial infections. Immunity 27, 518–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lima A., Cha B. J., Amin J., Smith L. K., Anderson B. (2014) Zebrafish embryo model of Bartonella henselae infection. Zebrafish 11, 434–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Deng Y., Boon C., Eberl L., Zhang L. H. (2009) Differential modulation of Burkholderia cenocepacia virulence and energy metabolism by the quorum-sensing signal BDSF and its synthase. J. Bacteriol. 191, 7270–7278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vergunst A. C., Meijer A. H., Renshaw S. A., O’Callaghan D. (2010) Burkholderia cenocepacia creates an intramacrophage replication niche in zebrafish embryos, followed by bacterial dissemination and establishment of systemic infection. Infect. Immun. 78, 1495–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Agnoli K., Schwager S., Uehlinger S., Vergunst A., Viteri D. F., Nguyen D. T., Sokol P. A., Carlier A., Eberl L. (2012) Exposing the third chromosome of Burkholderia cepacia complex strains as a virulence plasmid. Mol. Microbiol. 83, 362–378. [DOI] [PubMed] [Google Scholar]
- 107.Lü A., Hu X., Wang Y., Shen X., Zhu A., Shen L., Ming Q., Feng Z. (2013) Comparative analysis of the acute response of zebrafish Danio rerio skin to two different bacterial infections. J. Aquat. Anim. Health 25, 243–251. [DOI] [PubMed] [Google Scholar]
- 108.Petrie-Hanson L., Romano C. L., Mackey R. B., Khosravi P., Hohn C. M., Boyle C. R. (2007) Evaluation of zebrafish Danio rerio as a model for enteric septicemia of catfish (ESC). J. Aquat. Anim. Health 19, 151–158. [DOI] [PubMed] [Google Scholar]
- 109.Xiao J., Chen T., Wang Q., Zhang Y. (2012) Comparative analysis of the roles of catalases KatB and KatG in the physiological fitness and pathogenesis of fish pathogen Edwardsiella tarda. Lett. Appl. Microbiol. 54, 425–432. [DOI] [PubMed] [Google Scholar]
- 110.Wang X., Wang Q., Xiao J., Liu Q., Wu H., Xu L., Zhang Y. (2009) Edwardsiella tarda T6SS component evpP is regulated by esrB and iron, and plays essential roles in the invasion of fish. Fish Shellfish Immunol. 27, 469–477. [DOI] [PubMed] [Google Scholar]
- 111.Pressley M. E., Phelan P. E. III, Witten P. E., Mellon M. T., Kim C. H. (2005) Pathogenesis and inflammatory response to Edwardsiella tarda infection in the zebrafish. Dev. Comp. Immunol. 29, 501–513. [DOI] [PubMed] [Google Scholar]
- 112.Liu X., Chang X., Wu H., Xiao J., Gao Y., Zhang Y. (2014) Role of intestinal inflammation in predisposition of Edwardsiella tarda infection in zebrafish (Danio rerio). Fish Shellfish Immunol. 41, 271–278. [DOI] [PubMed] [Google Scholar]
- 113.Dong X., Fan X., Wang B., Shi X., Zhang X. H. (2013) Invasin of Edwardsiella tarda is essential for its haemolytic activity, biofilm formation and virulence towards fish. J. Appl. Microbiol. 115, 12–19. [DOI] [PubMed] [Google Scholar]
- 114.Yang D., Liu Q., Ni C., Li S., Wu H., Wang Q., Xiao J., Zhang Y. (2013) Gene expression profiling in live attenuated Edwardsiella tarda vaccine immunized and challenged zebrafish: insights into the basic mechanisms of protection seen in immunized fish. Dev. Comp. Immunol. 40, 132–141. [DOI] [PubMed] [Google Scholar]
- 115.Van Soest J. J., Stockhammer O. W., Ordas A., Bloemberg G. V., Spaink H. P., Meijer A. H. (2011) Comparison of static immersion and intravenous injection systems for exposure of zebrafish embryos to the natural pathogen Edwardsiella tarda. BMC Immunol. 12, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Van der Vaart M., van Soest J. J., Spaink H. P., Meijer A. H. (2013) Functional analysis of a zebrafish myd88 mutant identifies key transcriptional components of the innate immune system. Dis. Model. Mech. 6, 841–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Phennicie R. T., Sullivan M. J., Singer J. T., Yoder J. A., Kim C. H. (2010) Specific resistance to Pseudomonas aeruginosa infection in zebrafish is mediated by the cystic fibrosis transmembrane conductance regulator. Infect. Immun. 78, 4542–4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Prajsnar T. K., Renshaw S. A., Ogryzko N. V., Foster S. J., Serror P., Mesnage S. (2013) Zebrafish as a novel vertebrate model to dissect enterococcal pathogenesis. Infect. Immun. 81, 4271–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Van der Sar A. M., Musters R. J. P., van Eeden F. J. M., Appelmelk B. J., Vandenbroucke-Grauls C. M. J. E., Bitter W. (2003) Zebrafish embryos as a model host for the real time analysis of Salmonella typhimurium infections. Cell. Microbiol. 5, 601–611. [DOI] [PubMed] [Google Scholar]
- 120.Lavigne J.-P., Vergunst A. C., Goret L., Sotto A., Combescure C., Blanco J., O’Callaghan D., Nicolas-Chanoine M.-H. (2012) Virulence potential and genomic mapping of the worldwide clone Escherichia coli ST131. PLoS ONE 7, e34294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vigil P. D., Wiles T. J., Engstrom M. D., Prasov L., Mulvey M. A., Mobley H. L. T. (2012) The repeat-in-toxin family member TosA mediates adherence of uropathogenic Escherichia coli and survival during bacteremia. Infect. Immun. 80, 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wiles T. J., Norton J. P., Smith S. N., Lewis A. J., Mobley H. L. T., Casjens S. R., Mulvey M. A. (2013) A phyletically rare gene promotes the niche-specific fitness of an E. coli pathogen during bacteremia. PLoS Pathog. 9, e1003175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sarris M., Masson J.-B., Maurin D., Van der Aa L. M., Boudinot P., Lortat-Jacob H., Herbomel P. (2012) Inflammatory chemokines direct and restrict leukocyte migration within live tissues as glycan-bound gradients. Curr. Biol. 22, 2375–2382. [DOI] [PubMed] [Google Scholar]
- 124.Nguyen-Chi M., Phan Q. T., Gonzalez C., Dubremetz J.-F., Levraud J.-P., Lutfalla G. (2014) Transient infection of the zebrafish notochord with E. coli induces chronic inflammation. Dis. Model. Mech. 7, 871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rawls J. F., Mahowald M. A., Goodman A. L., Trent C. M., Gordon J. I. (2007) In vivo imaging and genetic analysis link bacterial motility and symbiosis in the zebrafish gut. Proc. Natl. Acad. Sci. USA 104, 7622–7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moyer T. R., Hunnicutt D. W. (2007) Susceptibility of zebra fish Danio rerio to infection by Flavobacterium columnare and F. johnsoniae. Dis. Aquat. Organ. 76, 39–44. [DOI] [PubMed] [Google Scholar]
- 127.Chang M. X., Nie P. (2008) RNAi suppression of zebrafish peptidoglycan recognition protein 6 (zfPGRP6) mediated differentially expressed genes involved in Toll-like receptor signaling pathway and caused increased susceptibility to Flavobacterium columnare. Vet. Immunol. Immunopathol. 124, 295–301. [DOI] [PubMed] [Google Scholar]
- 128.Vojtech L. N., Sanders G. E., Conway C., Ostland V., Hansen J. D. (2009) Host immune response and acute disease in a zebrafish model of Francisella pathogenesis. Infect. Immun. 77, 914–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brudal E., Ulanova L. S. O., O Lampe E., Rishovd A. L., Griffiths G., Winther-Larsen H. C. (2014) Establishment of three Francisella infections in zebrafish embryos at different temperatures. Infect. Immun. 82, 2180–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Aguado-Urda M., Rodríguez-Bertos A., de las Heras A. I., Blanco M. M., Acosta F., Cid R., Fernández-Garayzábal J. F., Gibello A. (2014) Experimental Lactococcus garvieae infection in zebrafish and first evidence of its ability to invade non-phagocytic cells. Vet. Microbiol. 171, 248–254. [DOI] [PubMed] [Google Scholar]
- 131.Davis J. M., Haake D. A., Ramakrishnan L. (2009) Leptospira interrogans stably infects zebrafish embryos, altering phagocyte behavior and homing to specific tissues. PLoS Negl. Trop. Dis. 3, e463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Menudier A., Rougier F. P., Bosgiraud C. (1996) Comparative virulence between different strains of Listeria in zebrafish (Brachydanio rerio) and mice. Pathol. Biol. (Paris) 44, 783–789. [PubMed] [Google Scholar]
- 133.Levraud J.-P., Disson O., Kissa K., Bonne I., Cossart P., Herbomel P., Lecuit M. (2009) Real-time observation of listeria monocytogenes-phagocyte interactions in living zebrafish larvae. Infect. Immun. 77, 3651–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rojo I., de Ilárduya O. M., Estonba A., Pardo M. A. (2007) Innate immune gene expression in individual zebrafish after Listonella anguillarum inoculation. Fish Shellfish Immunol. 23, 1285–1293. [DOI] [PubMed] [Google Scholar]
- 135.Oehlers S. H. B., Flores M. V., Hall C. J., O’Toole R., Swift S., Crosier K. E., Crosier P. S. (2010) Expression of zebrafish cxcl8 (interleukin-8) and its receptors during development and in response to immune stimulation. Dev. Comp. Immunol. 34, 352–359. [DOI] [PubMed] [Google Scholar]
- 136.Bernut A., Herrmann J. L., Kissa K., Dubremetz J. F., Gaillard J. L., Lutfalla G., Kremer L. (2014) Mycobacterium abscessus cording prevents phagocytosis and promotes abscess formation. Proc. Natl. Acad. Sci. USA 111, E943–E952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Harriff M. J., Bermudez L. E., Kent M. L. (2007) Experimental exposure of zebrafish, Danio rerio (Hamilton), to Mycobacterium marinum and Mycobacterium peregrinum reveals the gastrointestinal tract as the primary route of infection: a potential model for environmental mycobacterial infection. J. Fish Dis. 30, 587–600. [DOI] [PubMed] [Google Scholar]
- 138.Gao L.-Y., Pak M., Kish R., Kajihara K., Brown E. J. (2006) A mycobacterial operon essential for virulence in vivo and invasion and intracellular persistence in macrophages. Infect. Immun. 74, 1757–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Meijer A. H., Verbeek F. J., Salas-Vidal E., Corredor-Adámez M., Bussman J., van der Sar A. M., Otto G. W., Geisler R., Spaink H. P. (2005) Transcriptome profiling of adult zebrafish at the late stage of chronic tuberculosis due to Mycobacterium marinum infection. Mol. Immunol. 42, 1185–1203. [DOI] [PubMed] [Google Scholar]
- 140.Hammarén M. M., Oksanen K. E., Nisula H. M., Luukinen B. V., Pesu M., Rämet M., Parikka M. (2014) Adequate Th2-type response associates with restricted bacterial growth in latent mycobacterial infection of zebrafish. PLoS Pathog. 10, e1004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stoop E. J. M., Mishra A. K., Driessen N. N., van Stempvoort G., Bouchier P., Verboom T., van Leeuwen L. M., Sparrius M., Raadsen S. A., van Zon M., van der Wel N. N., Besra G. S., Geurtsen J., Bitter W., Appelmelk B. J., van der Sar A. M. (2013) Mannan core branching of lipo(arabino)mannan is required for mycobacterial virulence in the context of innate immunity. Cell. Microbiol. 15, 2093–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Watkins B. Y., Joshi S. A., Ball D. A., Leggett H., Park S., Kim J., Austin C. D., Paler-Martinez A., Xu M., Downing K. H., Brown E. J. (2012) Mycobacterium marinum SecA2 promotes stable granulomas and induces tumor necrosis factor alpha in vivo. Infect. Immun. 80, 3512–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Parikka M., Hammarén M. M., Harjula S.-K. E., Halfpenny N. J. A., Oksanen K. E., Lahtinen M. J., Pajula E. T., Iivanainen A., Pesu M., Rämet M. (2012) Mycobacterium marinum causes a latent infection that can be reactivated by gamma irradiation in adult zebrafish. PLoS Pathog. 8, e1002944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sun G., Chen C., Li J., Gui X., Li X., Chen L., Peng F., Mei J., Gao Q. (2011) Discriminatory potential of a novel set of Variable Number of Tandem Repeats for genotyping Mycobacterium marinum. Vet. Microbiol. 152, 200–204. [DOI] [PubMed] [Google Scholar]
- 145.Salas-Vidal E., Meijer A. H., Cheng X., Spaink H. P. (2005) Genomic annotation and expression analysis of the zebrafish Rho small GTPase family during development and bacterial infection. Genomics 86, 25–37. [DOI] [PubMed] [Google Scholar]
- 146.Van der Sar A. M., Abdallah A. M., Sparrius M., Reinders E., Vandenbroucke-Grauls C. M. J. E., Bitter W. (2004) Mycobacterium marinum strains can be divided into two distinct types based on genetic diversity and virulence. Infect. Immun. 72, 6306–6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Van Leeuwen L. M., van der Kuip M., Youssef S. A., de Bruin A., Bitter W., van Furth A. M., van der Sar A. M. (2014) Modeling tuberculous meningitis in zebrafish using Mycobacterium marinum. Dis. Model. Mech. 7, 1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Swaim L. E., Connolly L. E., Volkman H. E., Humbert O., Born D. E., Ramakrishnan L. (2006) Mycobacterium marinum infection of adult zebrafish causes caseating granulomatous tuberculosis and is moderated by adaptive immunity. Infect. Immun. 74, 6108–6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Van der Sar A. M., Spaink H. P., Zakrzewska A., Bitter W., Meijer A. H. (2009) Specificity of the zebrafish host transcriptome response to acute and chronic mycobacterial infection and the role of innate and adaptive immune components. Mol. Immunol. 46, 2317–2332. [DOI] [PubMed] [Google Scholar]
- 150.Clay H., Volkman H. E., Ramakrishnan L. (2008) Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death. Immunity 29, 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Roca F. J., Ramakrishnan L. (2013) TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell 153, 521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Volkman H. E., Clay H., Beery D., Chang J. C. W., Sherman D. R., Ramakrishnan L. (2004) Tuberculous granuloma formation is enhanced by a mycobacterium virulence determinant. PLoS Biol. 2, e367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Volkman H. E., Pozos T. C., Zheng J., Davis J. M., Rawls J. F., Ramakrishnan L. (2010) Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science 327, 466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nandi B., Behar S. M. (2011) Regulation of neutrophils by interferon-γ limits lung inflammation during tuberculosis infection. J. Exp. Med. 208, 2251–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Alibaud L., Rombouts Y., Trivelli X., Burguière A., Cirillo S. L. G., Cirillo J. D., Dubremetz J.-F., Guérardel Y., Lutfalla G., Kremer L. (2011) A Mycobacterium marinum TesA mutant defective for major cell wall-associated lipids is highly attenuated in Dictyostelium discoideum and zebrafish embryos. Mol. Microbiol. 80, 919–934. [DOI] [PubMed] [Google Scholar]
- 156.Davis J. M., Ramakrishnan L. (2009) The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell 136, 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tobin D. M., Vary J. C. Jr., Ray J. P., Walsh G. S., Dunstan S. J., Bang N. D., Hagge D. A., Khadge S., King M.-C., Hawn T. R., Moens C. B., Ramakrishnan L. (2010) The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell 140, 717–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cosma C. L., Klein K., Kim R., Beery D., Ramakrishnan L. (2006) Mycobacterium marinum Erp is a virulence determinant required for cell wall integrity and intracellular survival. Infect. Immun. 74, 3125–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Van der Woude A. D., Sarkar D., Bhatt A., Sparrius M., Raadsen S. A., Boon L., Geurtsen J., van der Sar A. M., Luirink J., Houben E. N. G., Besra G. S., Bitter W. (2012) Unexpected link between lipooligosaccharide biosynthesis and surface protein release in Mycobacterium marinum. J. Biol. Chem. 287, 20417–20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ordas A., Kanwal Z., Lindenberg V., Rougeot J., Mink M., Spaink H. P., Meijer A. H. (2013) MicroRNA-146 function in the innate immune transcriptome response of zebrafish embryos to Salmonella typhimurium infection. BMC Genomics 14, 696–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Takaki K., Cosma C. L., Troll M. A., Ramakrishnan L. (2012) An in vivo platform for rapid high-throughput antitubercular drug discovery. Cell Reports 2, 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Carvalho R., de Sonneville J., Stockhammer O. W., Savage N. D. L., Veneman W. J., Ottenhoff T. H. M., Dirks R. P., Meijer A. H., Spaink H. P. (2011) A high-throughput screen for tuberculosis progression. PLoS ONE 6, e16779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Veneman W. J., Marín-Juez R., de Sonneville J., Ordas A., Jong-Raadsen S., Meijer A. H., Spaink H. P. (2014) Establishment and optimization of a high throughput setup to study Staphylococcus epidermidis and Mycobacterium marinum infection as a model for drug discovery. J. Vis. Exp. 88, e51649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hosseini R., Lamers G. E., Hodzic Z., Meijer A. H., Schaaf M. J., Spaink H. P. (2014) Correlative light and electron microscopy imaging of autophagy in a zebrafish infection model. Autophagy 10, 1844–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Brannon M. K., Davis J. M., Mathias J. R., Hall C. J., Emerson J. C., Crosier P. S., Huttenlocher A., Ramakrishnan L., Moskowitz S. M. (2009) Pseudomonas aeruginosa type III secretion system interacts with phagocytes to modulate systemic infection of zebrafish embryos. Cell. Microbiol. 11, 755–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Reen F. J., Haynes J. M., Mooij M. J., O’Gara F. (2013) A non-classical LysR-type transcriptional regulator PA2206 is required for an effective oxidative stress response in Pseudomonas aeruginosa. PLoS ONE 8, e54479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Llamas M. A., van der Sar A., Chu B. C. H., Sparrius M., Vogel H. J., Bitter W. (2009) A novel extracytoplasmic function (ECF) sigma factor regulates virulence in Pseudomonas aeruginosa. PLoS Pathog. 5, e1000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Clatworthy A. E., Lee J. S. W., Leibman M., Kostun Z., Davidson A. J., Hung D. T. (2009) Pseudomonas aeruginosa infection of zebrafish involves both host and pathogen determinants. Infect. Immun. 77, 1293–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chand N. S., Lee J. S. W., Clatworthy A. E., Golas A. J., Smith R. S., Hung D. T. (2011) The sensor kinase KinB regulates virulence in acute Pseudomonas aeruginosa infection. J. Bacteriol. 193, 2989–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Deng Q., Harvie E. A., Huttenlocher A. (2012) Distinct signalling mechanisms mediate neutrophil attraction to bacterial infection and tissue injury. Cell. Microbiol. 14, 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Deng Q., Sarris M., Bennin D. A., Green J. M., Herbomel P., Huttenlocher A. (2013) Localized bacterial infection induces systemic activation of neutrophils through Cxcr2 signaling in zebrafish. J. Leukoc. Biol. 93, 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kanwal Z., Zakrzewska A., den Hertog J., Spaink H. P., Schaaf M. J., Meijer A. H. (2013) Deficiency in hematopoietic phosphatase ptpn6/Shp1 hyperactivates the innate immune system and impairs control of bacterial infections in zebrafish embryos. J. Immunol. 190, 1631–1645. [DOI] [PubMed] [Google Scholar]
- 173.Van der Sar A. M., Stockhammer O. W., van der Laan C., Spaink H. P., Bitter W., Meijer A. H. (2006) MyD88 innate immune function in a zebrafish embryo infection model. Infect. Immun. 74, 2436–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Stockhammer O. W., Zakrzewska A., Hegedûs Z., Spaink H. P., Meijer A. H. (2009) Transcriptome profiling and functional analyses of the zebrafish embryonic innate immune response to Salmonella infection. J. Immunol. 182, 5641–5653. [DOI] [PubMed] [Google Scholar]
- 175.Ordas A., Hegedus Z., Henkel C. V., Stockhammer O. W., Butler D., Jansen H. J., Racz P., Mink M., Spaink H. P., Meijer A. H. (2011) Deep sequencing of the innate immune transcriptomic response of zebrafish embryos to Salmonella infection. Fish Shellfish Immunol. 31, 716–724. [DOI] [PubMed] [Google Scholar]
- 176.Benard E. L., van der Sar A. M., Ellett F., Lieschke G. J., Spaink H. P., Meijer A. H. (2012) Infection of zebrafish embryos with intracellular bacterial pathogens. J. Vis. Exp. 61, 3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Oehlers S. H., Flores M. V., Hall C. J., Swift S., Crosier K. E., Crosier P. S. (2011) The inflammatory bowel disease (IBD) susceptibility genes NOD1 and NOD2 have conserved anti-bacterial roles in zebrafish. Dis. Model. Mech. 4, 832–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Flores M. V., Crawford K. C., Pullin L. M., Hall C. J., Crosier K. E., Crosier P. S. (2010) Dual oxidase in the intestinal epithelium of zebrafish larvae has anti-bacterial properties. Biochem. Biophys. Res. Commun. 400, 164–168. [DOI] [PubMed] [Google Scholar]
- 179.Mostowy S., Boucontet L., Mazon Moya M. J., Sirianni A., Boudinot P., Hollinshead M., Cossart P., Herbomel P., Levraud J.-P., Colucci-Guyon E. (2013) The zebrafish as a new model for the in vivo study of Shigella flexneri interaction with phagocytes and bacterial autophagy. PLoS Pathog. 9, e1003588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Li Y.-J., Hu B. (2012) Establishment of multi-site infection model in zebrafish larvae for studying Staphylococcus aureus infectious disease. J. Genet. Genomics 39, 521–534. [DOI] [PubMed] [Google Scholar]
- 181.Prajsnar T. K., Cunliffe V. T., Foster S. J., Renshaw S. A. (2008) A novel vertebrate model of Staphylococcus aureus infection reveals phagocyte-dependent resistance of zebrafish to non-host specialized pathogens. Cell. Microbiol. 10, 2312–2325. [DOI] [PubMed] [Google Scholar]
- 182.Bao Y., Li Y., Jiang Q., Zhao L., Xue T., Hu B., Sun B. (2013) Methylthioadenosine/S-adenosylhomocysteine nucleosidase (Pfs) of Staphylococcus aureus is essential for the virulence independent of LuxS/AI-2 system. Int. J. Med. Microbiol. 303, 190–200. [DOI] [PubMed] [Google Scholar]
- 183.Huedo P., Yero D., Martínez-Servat S., Estibariz I., Planell R., Martínez P., Ruyra A., Roher N., Roca I., Vila J., Daura X., Gibert I. (2014) Two different rpf clusters distributed among a population of Stenotrophomonas maltophilia clinical strains display differential diffusible signal factor production and virulence regulation. J. Bacteriol. 196, 2431–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ferrer-Navarro M., Planell R., Yero D., Mongiardini E., Torrent G., Huedo P., Martínez P., Roher N., Mackenzie S., Gibert I., Daura X. (2013) Abundance of the quorum-sensing factor Ax21 in four strains of Stenotrophomonas maltophilia correlates with mortality rate in a new zebrafish model of infection. PLoS ONE 8, e67207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hanson B. R., Runft D. L., Streeter C., Kumar A., Carion T. W., Neely M. N. (2012) Functional analysis of the CpsA protein of Streptococcus agalactiae. J. Bacteriol. 194, 1668–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang Z., Guo C., Xu Y., Liu G., Lu C., Liu Y. (2014) Two novel functions of hyaluronidase from Streptococcus agalactiae are enhanced intracellular survival and inhibition of proinflammatory cytokine expression. Infect. Immun. 82, 2615–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liu G., Zhang W., Liu Y., Yao H., Lu C., Xu P. (2014) Identification of a virulence-related surface protein XF in piscine Streptococcus agalactiae by pre-absorbed immunoproteomics. BMC Vet. Res. 10, 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Patterson H., Saralahti A., Parikka M., Dramsi S., Trieu-Cuot P., Poyart C., Rounioja S., Rämet M. (2012) Adult zebrafish model of bacterial meningitis in Streptococcus agalactiae infection. Dev. Comp. Immunol. 38, 447–455. [DOI] [PubMed] [Google Scholar]
- 189.Borst L. B., Patterson S. K., Lanka S., Suyemoto M. M., Maddox C. W. (2013) Zebrafish (Danio rerio) as a screen for attenuation of Lancefield group C streptococci and a model for streptococcal pathogenesis. Vet. Pathol. 50, 457–467. [DOI] [PubMed] [Google Scholar]
- 190.Neely M. N., Pfeifer J. D., Caparon M. (2002) Streptococcus-zebrafish model of bacterial pathogenesis. Infect. Immun. 70, 3904–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Miller J. D., Neely M. N. (2004) Zebrafish as a model host for streptococcal pathogenesis. Acta Trop. 91, 53–68. [DOI] [PubMed] [Google Scholar]
- 192.Lowe B. A., Miller J. D., Neely M. N. (2007) Analysis of the polysaccharide capsule of the systemic pathogen Streptococcus iniae and its implications in virulence. Infect. Immun. 75, 1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Miller J. D., Neely M. N. (2005) Large-scale screen highlights the importance of capsule for virulence in the zoonotic pathogen Streptococcus iniae. Infect. Immun. 73, 921–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Saralahti A., Piippo H., Parikka M., Henriques-Normark B., Rämet M., Rounioja S. (2014) Adult zebrafish model for pneumococcal pathogenesis. Dev. Comp. Immunol. 42, 345–353. [DOI] [PubMed] [Google Scholar]
- 195.Rounioja S., Saralahti A., Rantala L., Parikka M., Henriques-Normark B., Silvennoinen O., Rämet M. (2012) Defense of zebrafish embryos against Streptococcus pneumoniae infection is dependent on the phagocytic activity of leukocytes. Dev. Comp. Immunol. 36, 342–348. [DOI] [PubMed] [Google Scholar]
- 196.Montañez G. E., Neely M. N., Eichenbaum Z. (2005) The streptococcal iron uptake (Siu) transporter is required for iron uptake and virulence in a zebrafish infection model. Microbiology 151, 3749–3757. [DOI] [PubMed] [Google Scholar]
- 197.Phelps H. A., Runft D. L., Neely M. N.. 2005. Adult Zebrafish Model of Streptococcal Infection. Current Protocols in Microbiology, John Wiley & Sons, ; Hoboken, NJ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bates C. S., Toukoki C., Neely M. N., Eichenbaum Z. (2005) Characterization of MtsR, a new metal regulator in group A streptococcus, involved in iron acquisition and virulence. Infect. Immun. 73, 5743–5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kizy A. E., Neely M. N. (2009) First Streptococcus pyogenes signature-tagged mutagenesis screen identifies novel virulence determinants. Infect. Immun. 77, 1854–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Fisher M., Huang Y. S., Li X., McIver K. S., Toukoki C., Eichenbaum Z. (2008) Shr is a broad-spectrum surface receptor that contributes to adherence and virulence in group A streptococcus. Infect. Immun. 76, 5006–5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Li Y., Zheng J.-X., Zhang H., Fan H.-J., Lu C.-P. (2014) Effect of Tran on virulence through regulating metabolism and stress tolerance of Streptococcus suis serotype 2. Microbiol. Res. 169, 666–674. [DOI] [PubMed] [Google Scholar]
- 202.Zhang H., Ma Z., Li Y., Zheng J., Yi L., Fan H., Lu C. (2013) Identification of a novel collagen type І-binding protein from Streptococcus suis serotype 2. Vet. J. 197, 406–414. [DOI] [PubMed] [Google Scholar]
- 203.Ju C. X., Gu H. W., Lu C. P. (2012) Characterization and functional analysis of atl, a novel gene encoding autolysin in Streptococcus suis. J. Bacteriol. 194, 1464–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wang Y., Zhang W., Wu Z., Zhu X., Lu C. (2011) Functional analysis of luxS in Streptococcus suis reveals a key role in biofilm formation and virulence. Vet. Microbiol. 152, 151–160. [DOI] [PubMed] [Google Scholar]
- 205.Zhang H., Fan H., Lu C. (2010) Identification of a novel virulence-related gene in Streptococcus suis type 2 strains. Curr. Microbiol. 61, 494–499. [DOI] [PubMed] [Google Scholar]
- 206.Wu Z., Zhang W., Lu Y., Lu C. (2010) Transcriptome profiling of zebrafish infected with Streptococcus suis. Microb. Pathog. 48, 178–187. [DOI] [PubMed] [Google Scholar]
- 207.He H., Wang Q., Sheng L., Liu Q., Zhang Y. (2011) Functional characterization of Vibrio alginolyticus twin-arginine translocation system: its roles in biofilm formation, extracellular protease activity, and virulence towards fish. Curr. Microbiol. 62, 1193–1199. [DOI] [PubMed] [Google Scholar]
- 208.O’Toole R., Von Hofsten J., Rosqvist R., Olsson P. E., Wolf-Watz H. (2004) Visualisation of zebrafish infection by GFP-labelled Vibrio anguillarum. Microb. Pathog. 37, 41–46. [DOI] [PubMed] [Google Scholar]
- 209.Pan C.-Y., Wu J.-L., Hui C.-F., Lin C.-H., Chen J.-Y. (2011) Insights into the antibacterial and immunomodulatory functions of the antimicrobial peptide, epinecidin-1, against Vibrio vulnificus infection in zebrafish. Fish Shellfish Immunol. 31, 1019–1025. [DOI] [PubMed] [Google Scholar]
- 210.Runft D. L., Mitchell K. C., Abuaita B. H., Allen J. P., Bajer S., Ginsburg K., Neely M. N., Withey J. H. (2014) Zebrafish as a natural host model for Vibrio cholerae colonization and transmission. Appl. Environ. Microbiol. 80, 1710–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sieger D., Stein C., Neifer D., van der Sar A. M., Leptin M. (2009) The role of gamma interferon in innate immunity in the zebrafish embryo. Dis. Model. Mech. 2, 571–581. [DOI] [PubMed] [Google Scholar]
- 212.Kanther M., Tomkovich S., Xiaolun S., Grosser M. R., Koo J., Flynn E. J. III, Jobin C., Rawls J. F. (2014) Commensal microbiota stimulate systemic neutrophil migration through induction of serum amyloid A. Cell. Microbiol. 16, 1053–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bates J. M., Akerlund J., Mittge E., Guillemin K. (2007) Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe 2, 371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Galindo-Villegas J., García-Moreno D., de Oliveira S., Meseguer J., Mulero V. (2012) Regulation of immunity and disease resistance by commensal microbes and chromatin modifications during zebrafish development. Proc. Natl. Acad. Sci. USA 109, E2605–E2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Toh M. C., Goodyear M., Daigneault M., Allen-Vercoe E., Van Raay T. J. (2013) Colonizing the embryonic zebrafish gut with anaerobic bacteria derived from the human gastrointestinal tract. Zebrafish 10, 194–198. [DOI] [PubMed] [Google Scholar]
- 216.Knox B. P., Deng Q., Rood M., Eickhoff J. C., Keller N. P., Huttenlocher A. (2014) Distinct innate immune phagocyte responses to Aspergillus fumigatus conidia and hyphae in zebrafish larvae. Eukaryot. Cell 13, 1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chao C. C., Hsu P. C., Jen C. F., Chen I. H., Wang C. H., Chan H. C., Tsai P. W., Tung K. C., Wang C. H., Lan C. Y., Chuang Y. J. (2010) Zebrafish as a model host for Candida albicans infection. Infect. Immun. 78, 2512–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kuo Z.-Y., Chuang Y.-J., Chao C.-C., Liu F.-C., Lan C.-Y., Chen B.-S. (2013) Identification of infection- and defense-related genes via a dynamic host-pathogen interaction network using a Candida albicans-zebrafish infection model. J. Innate Immun. 5, 137–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Brothers K. M., Gratacap R. L., Barker S. E., Newman Z. R., Norum A., Wheeler R. T. (2013) NADPH oxidase-driven phagocyte recruitment controls Candida albicans filamentous growth and prevents mortality. PLoS Pathog. 9, e1003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gratacap R. L., Rawls J. F., Wheeler R. T. (2013) Mucosal candidiasis elicits NF-κB activation, proinflammatory gene expression and localized neutrophilia in zebrafish. Dis. Model. Mech. 6, 1260–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lyczak J. B., Cannon C. L., Pier G. B. (2000) Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2, 1051–1060. [DOI] [PubMed] [Google Scholar]
- 222.Tsai W. C., Strieter R. M., Mehrad B., Newstead M. W., Zeng X., Standiford T. J. (2000) CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect. Immun. 68, 4289–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sadikot R. T., Blackwell T. S., Christman J. W., Prince A. S. (2005) Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am. J. Respir. Crit. Care Med. 171, 1209–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Rahme L. G., Stevens E. J., Wolfort S. F., Shao J., Tompkins R. G., Ausubel F. M. (1995) Common virulence factors for bacterial pathogenicity in plants and animals. Science 268, 1899–1902. [DOI] [PubMed] [Google Scholar]
- 225.Tan M.-W., Mahajan-Miklos S., Ausubel F. M. (1999) Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc. Natl. Acad. Sci. USA 96, 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.D’Argenio D. A., Gallagher L. A., Berg C. A., Manoil C. (2001) Drosophila as a model host for Pseudomonas aeruginosa infection. J. Bacteriol. 183, 1466–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sadikot R. T., Zeng H., Yull F. E., Li B., Cheng D.-S., Kernodle D. S., Jansen E. D., Contag C. H., Segal B. H., Holland S. M., Blackwell T. S., Christman J. W. (2004) p47phox deficiency impairs NF-kappa B activation and host defense in Pseudomonas pneumonia. J. Immunol. 172, 1801–1808. [DOI] [PubMed] [Google Scholar]
- 228.Niethammer P., Grabher C., Look A. T., Mitchison T. J. (2009) A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 459, 996–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Agnew W., Barnes A. C. (2007) Streptococcus iniae: an aquatic pathogen of global veterinary significance and a challenging candidate for reliable vaccination. Vet. Microbiol. 122, 1–15. [DOI] [PubMed] [Google Scholar]
- 230.Eldar A., Bejerano Y., Livoff A., Horovitcz A., Bercovier H. (1995) Experimental streptococcal meningo-encephalitis in cultured fish. Vet. Microbiol. 43, 33–40. [DOI] [PubMed] [Google Scholar]
- 231.Shoemaker C. A., Klesius P. H., Evans J. J. (2001) Prevalence of Streptococcus iniae in tilapia, hybrid striped bass, and channel catfish on commercial fish farms in the United States. Am. J. Vet. Res. 62, 174–177. [DOI] [PubMed] [Google Scholar]
- 232.Lämmermann T., Afonso P. V., Angermann B. R., Wang J. M., Kastenmüller W., Parent C. A., Germain R. N. (2013) Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498, 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Tønjum T., Welty D. B., Jantzen E., Small P. L. (1998) Differentiation of Mycobacterium ulcerans, M. marinum, and M. haemophilum: mapping of their relationships to M. tuberculosis by fatty acid profile analysis, DNA-DNA hybridization, and 16S rRNA gene sequence analysis. J. Clin. Microbiol. 36, 918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Prouty M. G., Correa N. E., Barker L. P., Jagadeeswaran P., Klose K. E. (2003) Zebrafish-Mycobacterium marinum model for mycobacterial pathogenesis. FEMS Microbiol. Lett. 225, 177–182. [DOI] [PubMed] [Google Scholar]
- 235.Hegedus Z., Zakrzewska A., Agoston V. C., Ordas A., Rácz P., Mink M., Spaink H. P., Meijer A. H. (2009) Deep sequencing of the zebrafish transcriptome response to mycobacterium infection. Mol. Immunol. 46, 2918–2930. [DOI] [PubMed] [Google Scholar]
- 236.Adams K. N., Takaki K., Connolly L. E., Wiedenhoft H., Winglee K., Humbert O., Edelstein P. H., Cosma C. L., Ramakrishnan L. (2011) Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism. Cell 145, 39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Thwaites G. E., Chau T. T. H., Stepniewska K., Phu N. H., Chuong L. V., Sinh D. X., White N. J., Parry C. M., Farrar J. J. (2002) Diagnosis of adult tuberculous meningitis by use of clinical and laboratory features. Lancet 360, 1287–1292. [DOI] [PubMed] [Google Scholar]
- 238.Lowe D. M., Redford P. S., Wilkinson R. J., O’Garra A., Martineau A. R. (2012) Neutrophils in tuberculosis: friend or foe? Trends Immunol. 33, 14–25. [DOI] [PubMed] [Google Scholar]
- 239.Jones G. S., Amirault H. J., Andersen B. R. (1990) Killing of Mycobacterium tuberculosis by neutrophils: a nonoxidative process. J. Infect. Dis. 162, 700–704. [DOI] [PubMed] [Google Scholar]
- 240.Brown A. E., Holzer T. J., Andersen B. R. (1987) Capacity of human neutrophils to kill Mycobacterium tuberculosis. J. Infect. Dis. 156, 985–989. [DOI] [PubMed] [Google Scholar]
- 241.Corleis B., Korbel D., Wilson R., Bylund J., Chee R., Schaible U. E. (2012) Escape of Mycobacterium tuberculosis from oxidative killing by neutrophils. Cell. Microbiol. 14, 1109–1121. [DOI] [PubMed] [Google Scholar]
- 242.Pedrosa J., Saunders B. M., Appelberg R., Orme I. M., Silva M. T., Cooper A. M. (2000) Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect. Immun. 68, 577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sugawara I., Udagawa T., Yamada H. (2004) Rat neutrophils prevent the development of tuberculosis. Infect. Immun. 72, 1804–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Clay H., Davis J. M., Beery D., Huttenlocher A., Lyons S. E., Ramakrishnan L. (2007) Dichotomous role of the macrophage in early Mycobacterium marinum infection of the zebrafish. Cell Host Microbe 2, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Walters K. B., Dodd M. E., Mathias J. R., Gallagher A. J., Bennin D. A., Rhodes J., Kanki J. P., Look A. T., Grinblat Y., Huttenlocher A. (2009) Muscle degeneration and leukocyte infiltration caused by mutation of zebrafish Fad24. Dev. Dyn. 238, 86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Silva M. T. (2011) Macrophage phagocytosis of neutrophils at inflammatory/infectious foci: a cooperative mechanism in the control of infection and infectious inflammation. J. Leukoc. Biol. 89, 675–683. [DOI] [PubMed] [Google Scholar]
- 247.Latgé J.-P. (1999) Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12, 310–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Marr K. A., Carter R. A., Boeckh M., Martin P., Corey L. (2002) Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood 100, 4358–4366. [DOI] [PubMed] [Google Scholar]
- 249.Denning D. W. (1998) Invasive aspergillosis. Clin. Infect. Dis. 26, 781–803, quiz 804–805. [DOI] [PubMed] [Google Scholar]
- 250.Schaffner A., Douglas H., Braude A. (1982) Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J. Clin. Invest. 69, 617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Bruns S., Kniemeyer O., Hasenberg M., Aimanianda V., Nietzsche S., Thywissen A., Jeron A., Latgé J. P., Brakhage A. A., Gunzer M. (2010) Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathog. 6, e1000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.McCormick A., Heesemann L., Wagener J., Marcos V., Hartl D., Loeffler J., Heesemann J., Ebel F. (2010) NETs formed by human neutrophils inhibit growth of the pathogenic mold Aspergillus fumigatus. Microbes Infect. 12, 928–936. [DOI] [PubMed] [Google Scholar]
- 253.Harding M. G., Zhang K., Conly J., Kubes P. (2014) Neutrophil crawling in capillaries; a novel immune response to Staphylococcus aureus. PLoS Pathog. 10, e1004379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Tobin D. M., Roca F. J., Oh S. F., McFarland R., Vickery T. W., Ray J. P., Ko D. C., Zou Y., Bang N. D., Chau T. T. H., Vary J. C., Hawn T. R., Dunstan S. J., Farrar J. J., Thwaites G. E., King M.-C., Serhan C. N., Ramakrishnan L. (2012) Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell 148, 434–446. [DOI] [PMC free article] [PubMed] [Google Scholar]


