Abstract
Background
Based on controlled 36 h experiments a higher dietary protein intake causes a positive protein balance and a negative fat balance. A positive net protein balance may support fat free mass accrual. However, few data are available on the impact of more prolonged changes in habitual protein intake on whole-body protein metabolism and basal muscle protein synthesis rates.
Objective
To assess changes in whole-body protein turnover and basal muscle protein synthesis rates following 12 weeks of adaptation to a low versus high dietary protein intake.
Methods
A randomized parallel study was performed in 40 subjects who followed either a high protein (2.4 g protein/kg/d) or low protein (0.4 g protein/kg/d) energy-balanced diet (30/35/35% or 5/60/35% energy from protein/carbohydrate/fat) for a period of 12 weeks. A subgroup of 7 men and 8 women (body mass index: 22.8±2.3 kg/m2, age: 24.3±4.9 y) were selected to evaluate the impact of prolonged adaptation to either a high or low protein intake on whole body protein metabolism and basal muscle protein synthesis rates. After the diet, subjects received continuous infusions with L-[ring-2H5]phenylalanine and L-[ring-2H2]tyrosine in an overnight fasted state, with blood samples and muscle biopsies being collected to assess post-absorptive whole-body protein turnover and muscle protein synthesis rates in vivo in humans.
Results
After 12 weeks of intervention, whole-body protein balance in the fasted state was more negative in the high protein treatment when compared with the low protein treatment (-4.1±0.5 vs -2.7±0.6 μmol phenylalanine/kg/h;P<0.001). Whole-body protein breakdown (43.0±4.4 vs 37.8±3.8 μmol phenylalanine/kg/h;P<0.03), synthesis (38.9±4.2 vs 35.1±3.6 μmol phenylalanine/kg/h;P<0.01) and phenylalanine hydroxylation rates (4.1±0.6 vs 2.7±0.6 μmol phenylalanine/kg/h;P<0.001) were significantly higher in the high vs low protein group. Basal muscle protein synthesis rates were maintained on a low vs high protein diet (0.042±0.01 vs 0.045±0.01%/h;P = 0.620).
Conclusions
In the overnight fasted state, adaptation to a low-protein intake (0.4 g/kg/d) does not result in a more negative whole-body protein balance and does not lower basal muscle protein synthesis rates when compared to a high-protein intake.
Trial Registration
Clinicaltrials.gov NCT01551238.
Introduction
High-protein diets have attracted interest for many years because of their ability to preserve fat free mass (FFM) during negative energy balance [1, 2]. While being in a neutral or positive energy balance, a temporary increase in dietary protein consumption for 3 months can lead to an increase in FFM [3, 4], especially when combined with regular exercise [5]. Therefore, a temporary increase in dietary protein intake may act as a preventive measure to remain weight stable [6]. However, the impact of prolonged adaptation to a low or high protein intake on whole-body protein balance or muscle protein synthesis (MPS) has not been assessed. An increase in protein synthesis, accompanied by a simultaneous reduction in protein breakdown, due to increased protein consumption may be responsible for the preservation or increase of FFM, irrespective of energy balance.
Several studies [7–12] have shown that ingestion of dietary protein stimulates net muscle protein accretion. The post-prandial rise in circulating essential amino acids (EAA), and leucine in particular, has been identified as the key factor stimulating the post-prandial rise in MPS rate [8, 9]. In contrast to consumption of a high dietary protein diet, it is thought that a relatively low protein intake may lead to a decline in muscle protein synthesis, resulting in net protein loss. A diet providing 15 energy% protein, or an absolute amount of 0.8 g protein/kg/d, is recommended to maintain proper protein balance [13, 14]. Prolonged under-consumption of dietary protein has been suggested to induce muscle mass and strength loss. Nevertheless, despite a large amount of short-term studies investigating the impact of dietary protein consumption on whole-body protein turnover and MPS, few studies have examined the impact of prolonged adaptation to either a low or high protein intake on whole-body protein turnover and basal muscle protein synthesis rates. In the present substudy, we tested our hypothesis that consuming a diet low in dietary protein induces a negative whole-body protein balance and reduces basal muscle protein synthesis rates when compared with a high protein diet. We applied contemporary stable isotope methodology to assess the impact of a low versus high protein intake diet post-absorptive whole-body protein balance and fasting MPS rates in vivo in humans. Furthermore, we assessed 24 h whole-body protein balance by nitrogen balance following prolonged adaptation to a low and high protein intake diet.
Materials and Methods
Subjects
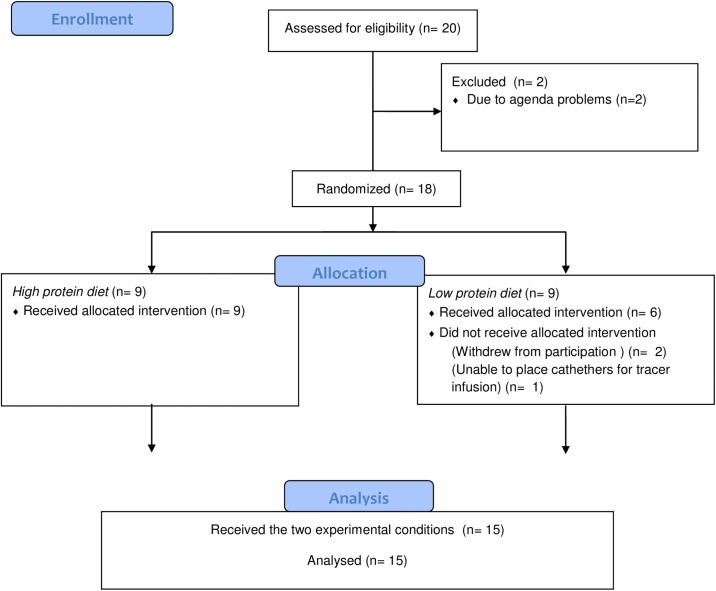
In the main study, Martens et al. (n = 37) [15] studied the impact of a high-protein (HP) diet compared with a low-protein (LP) diet on satiety and energy expenditure. They concluded that maintenance of energy expenditure, initial transient higher fullness, and a sustained positive protein balance on a HP vs LP diet in energy balance may prevent development of a positive energy balance and subsequent body weight gain. In a subgroup of subjects (n = 20) we determined post-absorptive whole-body protein metabolism and basal muscle protein synthesis rates to assess possible changes following 12 weeks of adaptation to a high vs low dietary protein intake. After drop-out of 5 subjects (2 subjects due to agenda problems, one subject we were not able to place the catheters and 2 subjects withdrew from the study without giving any reason), 15 subjects, (7 men and 8 women), aged 19 to 31 y; body mass index between 19 and 26 kg/m2) participated in the current substudy (Fig 1). The sample-size calculation was based on previous research from this lab on protein metabolism [16]. We calculated the sample size by using the following variables: the difference in fractional synthetic rate (FSR) of mixed muscle protein >20% and a SD of 15% with a type I error of 5% and a type II error of 10%. Power calculations showed that ≥ 9 subjects were needed, and therefore we decided to include 10 subjects per group. In the reference study, a crossover design with 10 healthy subjects was performed and resulted in significant effects of dietary protein consumption on muscle protein synthesis.
Fig 1. Flow diagram (CONSORT) of a substudy in two diet groups (12 weeks High Protein or Low Protein diet), with n = 20 eligible men and women.
Subjects were recruited via advertisements in local newspapers and on notice boards at the university. Subject recruitment started in November 2012 and the study was conducted between January 2013 and September 2013. Subjects underwent a screening and all were in good health, non-smokers, not using medication (except for oral contraception) and moderate alcohol users (<10 drinks per week). None of them had a food allergy, gained or lost more than 3 kg in 6 months prior to the study, or were cognitive dietary restrained (F1>9) as assessed by a validated Dutch translation of the Three Factor Eating Questionnaire [17]. The validated Dutch translation of the Baecke Activity Questionnaire was used to measure habitual physical activity [18]. All procedures involving human subjects in this study, that were performed with a subgroup of subjects from the main study [15], were specifically approved by The Medical Ethical Committee of Maastricht University Medical Centre. This study was also conducted according to guidelines laid down in the Declaration of Helsinki. All subjects provided written informed consent. The main study [15] was registered at clinicaltrials.gov with identifier NCT01551238. The protocol for this trial and supporting CONSORT checklist are available as supporting information; see S1 Protocol and S1 CONSORT Checklist.
Study design
The study had a randomized, single-blinded, parallel design and consisted of a long-term (12 weeks) dietary intervention. Subjects were randomly divided in two groups that received either a HP (2.4 g protein/kg/d, 30/35/35% of energy from protein/carbohydrate/fat) or a LP energy-balanced diet (0.4 g protein/kg/d, 5/60/35% of energy from protein/carbohydrate/fat).
In order to maintain their diet at home, all subjects received a booklet containing individual guidelines with permitted and non-permitted foods and their corresponding portions, as well as three example menus. The example menus consisted of commercially available food products and were tailored to the energy requirements of each subject based on basal metabolic rate calculated with the Harris and Benedict equation [19] and multiplied with a physical activity level of 1.7 estimated by means of a computer simulation program [20]. At week 5 and 9 of the intervention period subjects visited the university to discuss the compliance to the dietary guidelines with a researcher. Subjects on the HP diet (30% of energy from protein) had to drink two protein shakes each day during 12 weeks. The supplemental protein was whey protein with α-lactalbumin (Hiprotal Whey Protein Alpha and Domo; FrieslandCampina). Subjects on the LP diet (60% of energy from carbohydrate) had to drink two carbohydrate shakes each day during 12 weeks. The supplemental carbohydrate was maltodextrin (Fantomalt, Nestle). During screening subjects had to rate the palatability of the shakes. Only subjects who rated the shakes as sufficiently palatable (VAS score ≥ 50 mm), and who were confident of being able to consume these daily during the study period were included in the study. The fat content between conditions was maintained at a constant proportion (35% of energy from fat). Subjects were instructed to keep their body weight stable. Subjects’ characteristics reported in Table 1were determined as described by Martens et al. [15].
Table 1. Subjects’ characteristics.
| Δ | HP | LP | Total | P-Value | |
|---|---|---|---|---|---|
| N (M/F) | 9 (4/5) | 6 (3/3) | 15 (7/8) | ||
| Age (Y) | 23.9±4.2 | 25.0±6.2 | 24.3±4.9 | 0.686 | |
| Height (m) | 1.70±0.08 | 1.70±0.09 | 1.70±0.09 | 0.899 | |
| Weight (kg) | 62.8±6.1 | 67.3±8.6 | 65.1±7.1 | 0.312 | |
| Δ Weight (kg) | +0.71±0.8 | +0.06±1.2 | +0.45±0.98 | 0.216 | |
| BMI (kg/m 2 ) | 22.1±2.4 | 23.3±2.2 | 22.6±2.3 | 0.373 | |
| Δ BMI (kg/m 2 ) | +0.26±0.30 | +0.04±0.39 | +0.17±0.34 | 0.903 | |
| FM% | 24.2±7.3 | 22.7±7.8 | 23.6±7.3 | 0.339 | |
| Δ FM (%) | +0.04±1.31 | +0.32±0.97 | +0.15±1.16 | 0.672 | |
| FFM% | 75.8±7.3 | 77.4±7.8 | 76.5±7.3 | 0.709 | |
| Δ FFM (%) | -0.04±1.31 | -0.32±0.97 | -0.15±1.16 | 0.672 | |
| PAL | 1.82±0.14 | 1.79±0.15 | 1.81±0.14 | 0.677 |
Δ changes over 12 weeks. BMI, Body Mass Index; FM, fat mass; FFM, fat free mass; PAL, physical activity level. These data concern the as analysed population and not the as randomised population. Values are expressed as mean ± SD. Data were analyzed with one-way ANOVA. Table adapted and modified from Martens et al. [15].
Biomarker of protein intake and 24-h protein turnover
Nitrogen excretion was used as biomarker for protein intake (to measure compliance) and to estimate 24-h protein turnover. Subjects collected their 24-h urine at five different time points during the 12 week period. Collection started after the first voiding in the morning at 0800h and lasted until the next day at 0800h including the first voiding. The total volume of the 24-h urine was recorded. Urine was collected in 2-L urine bottles with 10 mL of diluted hydrochloric acid (4 mmol/L) added to prevent nitrogen loss through evaporation. Urine was gently mixed, and samples were taken and stored at -20°C until analysis. Nitrogen concentrations were measured with a nitrogen analyzer (CHO-O-Rapid; Hereaus). 24-h protein turnover was calculated using the prescribed protein intake and measured urinary nitrogen excretion data.
Test day
The experiment started at 0800 am, when overnight-fasted subjects arrived at the laboratory by car or public transportation. A polytetrafluoroethylene catheter was inserted into an antecubital vein for stable isotope infusion. A second polytetrafluoroethylene catheter was inserted in a heated dorsal hand vein of the contralateral arm and placed in a hot box (60°C) for arterialized blood sampling [21]. After basal blood collection (t = 0 min), plasma phenylalanine (Phe) and tyrosine (Tyr) pools were primed with a single intravenous dose of L-[ring-2H5] Phe (2 μmol/kg) and L-[ring-2H2]Tyr (0.615 μmol/kg), after which a continuous L-[ring-2H5]Phe and L-[ring-2H2]Tyr infusion was started (0.050±0.0005 and 0.015±0.0001 μmol/kg/min, respectively). After resting in a supine position for 120 min, a second arterialized blood sample was drawn, and a muscle biopsy was collected from the vastus lateralis muscle (t = 120 min). Additional arterialized blood samples (8 mL) were collected at t = 180, 240, and 300 min with a second muscle biopsy, taken from the contralateral leg, at t = 300 min, which marked the end of the basal fasting period as well as the experiment. Blood samples were collected in tubes containing EDTA and centrifuged at 1000g for 10 min at 4°C. Aliquots of plasma were frozen in liquid nitrogen and stored at –80°C. Muscle biopsies were obtained from the middle region of the vastus lateralis, 15 cm above the patella and ~3 cm below entry through the fascia, by using the percutaneous needle biopsy technique [22]. Muscle samples were dissected carefully and freed from any visible non-muscle material. Muscle samples were immediately frozen in liquid nitrogen and stored at –80°C until additional analysis.
Plasma analysis
Plasma glucose (Uni Kit III, 07367204; Roche) concentrations were analyzed with a COBAS-FARA semiautomatic analyzer (Roche). Insulin was analyzed by using a radioimmunoassay (Insulin RIA kit; LINCO Research Inc). Plasma (100 mL) for amino acid analyses was deproteinized on ice with 10 mg dry 5-sulphosalicylic acid, mixed, and the clear supernatant fluid was collected after centrifugation. Plasma amino acid concentrations were determined by using HPLC after precolumn derivatization with o-phthaldialdehyde [23]. For plasma enrichment measurements, plasma Phe and Tyr were derivatized to their t-butyldimethylsilyl derivatives and analyzed by using gas chromatography–mass spectrometry (GC-MS) (Agilent 6890N GC/5973N MSD; Agilent) by using selected ion monitoring of masses 336 and 341 for unlabeled and labeled (ring-2H5) Phe, respectively; and masses 466, 468, and 470 for unlabeled and labeled (ring-2H2 and ring-2H4) Tyr, respectively [24]. Thereafter, ratios of labeled: unlabeled derivatives were analyzed by using gas chromatography–combustion isotope ratio mass spectrometry (FinniganMAT 252; ThermoFisher Scientific). Standard regression curves were applied in all isotopic enrichment analyses to assess the linearity of the mass spectrometer and to control for the loss of tracer.
Muscle analysis
For the measurement of L-[ring-2H5]Phe enrichment in the muscle tissue–free amino acid pool and mixed muscle protein, 55 mg wet muscle was freeze-dried. Collagen, blood, and other non-muscle fiber material were removed from muscle fibers under a light microscope. The isolated muscle fiber mass (10–15 mg) was weighed, and 8 volumes (8x dry weight of isolated muscle fibers x wet:dry ratio) of ice-cold 2% perchloric acid were added. The tissue was homogenized and centrifuged. The supernatant fluid was collected and processed in the same manner as plasma samples, such that tissue-free L-[ring-2H5]Phe enrichments could be measured by using their t-butyldimethylsilyl derivatives on a GC-MS.
The protein pellet was washed with 3 additional 1.5-mL washes of 2% perchloric acid, dried, and hydrolyzed in 6 mol/L HCl at 120°C for 15–18h. The hydrolyzed protein fraction was dried under a nitrogen stream while heated to 120°C, and a 50% acetic acid solution was added, and the hydrolyzed protein was passed over a Dowex exchange resin (AG 50W-X8, 100–200 mesh hydrogen form; Biorad) by using 2 mol/L NH4OH. The eluate was collected, and L-[ring-2H5]Phe was derivatized to N-methyl-N-tert-butyldimethylsilyltrifluoroacetamidephenylethyl- amine [25]. Thereafter, ratios of labeled:unlabeled derivatives were determined by using GC-MS. Standard regression curves were applied to assess the linearity of the mass spectrometer and to control for the loss of tracer.
Calculations
The intravenous infusion of L-[ring-2H5]Phe and L-[ring-2H2]Tyr, and arterialized blood sampling were used to assess whole-body protein metabolism in steady state conditions. The total Phe rate of appearance (Ra) was calculated by using modified Steele’s equations [26, 27]. These variables were calculated as follows:
where F is the intravenous tracer infusion rate (μmol/kg/min), pV (0.125) is the distribution volume for Phe [27]. C(t) is the mean plasma Phe concentration between two consecutive time points. dE iv/dt represents the time-dependent variations of plasma Phe enrichment derived from the intravenous tracer, and E iv(t) is the mean plasma Phe enrichment from the intravenous tracer between 2 consecutive time points. Total R a represents the plasma entry of Phe derived from whole-body protein breakdown. The total rate of disappearance of Phe (total R d) equals the Phe-to-Tyr conversion rate (first step in Phe oxidation) and utilization for protein synthesis. These variables were calculated as follows:
where Phe R d and Tyr R a are the flux rates for Phe and Tyr, respectively; E t(t) and E p(t) are the mean plasma enrichments of L-[ring-2H2]Tyr and L-[ring-2H5]Phe, respectively; and F p is the infusion rate of the Phe tracer. The FSR (in %/h) was calculated by using the precursor-product method [24]:
where ΔE p is the Δ increment of muscle protein-bound L-[ring-2H5]Phe during the incorporation period. E precursor is the average plasma L-[ring-2H5]Phe enrichment during the time period for determination of amino acid incorporation, and t indicates the time interval (h) between biopsies.
Statistical analysis
A one-way ANOVA was used to assess differences in subject characteristics, hormone, amino acid concentrations and basal FSRs between treatments. Further, a 2-factor ANOVA with time as factor 1 and treatment as factor 2 was used to assess differences between treatments over time (time x treatment interaction) for plasma Phe, Tyr, Leu concentrations and plasma enrichments, as well as whole-body protein metabolism. In case of a significant time x treatment interaction, pairwise comparisons for individual time points were applied to locate differences between treatments. A 2-factor repeated measures ANOVA was used to assess differences over time and between treatments for 24-h protein turnover (protein intake, excretion and balance). Finally, Gender comparisons for whole-body protein metabolism and basal FSRs were made using a 2-factor ANOVA with gender as factor 1 and treatment as factor 2 (gender x treatment interaction). Statistical significance was set at P<0.05. All calculations were performed with the SPSS 20.0 software package (SPSS Inc). All data are expressed as means ± SEMs. For whole-body protein metabolism, FSR and 24 h protein turnover data we also report the 95% confidence interval as well as the mean difference, SEMs and the 95% confidence interval of the mean difference.
Results
All individual data points behind means, medians and variance measures presented in the results, tables and figures are available in S1 File.
Plasma analyses
Basal plasma insulin (10.9 ± 1.8 vs 11.0 ± 1.7 mU/l; P = 0.879) and glucose (4.95 ± 0.18 vs 4.62 ± 0.11 mmol/l; P = 0.153) concentrations did not differ between the HP vs LP group after 12 weeks.
Plasma Phe is illustrated over time in Fig 2A. After 12 weeks on a HP vs LP diet plasma Phe levels were not significantly different between groups (P = 0.282). Plasma Tyr (P<0.05) and Leu (P<0.03) concentrations were significantly increased after the HP diet compared with the LP diet. At week 12, basal EAA (P = 0.551), basal branched-chain amino acids (P = 0.402) and basal total amino acids (P = 0.535) concentrations in plasma were not different between the HP vs LP group (Table 2).
Fig 2. Mean (+SEM) plasma Phe (A) concentration (μmol L-1) and plasma L-[ring-2H5]Phe (B) enrichment (MPE) after 12 weeks on a HP (grey) vs LP (black) diet.
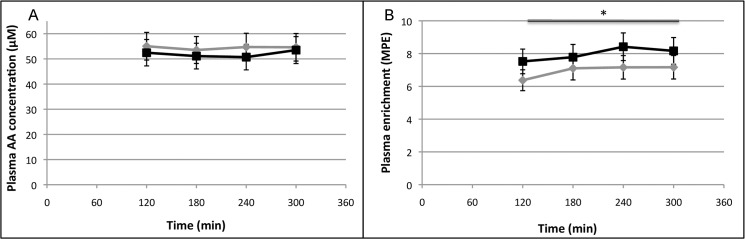
n = 15. Data were analyzed with a two-factor ANOVA (time x treatment). *Treatment effect P<0.001.
Table 2. Hormone and amino acids concentrations in plasma at baseline.
| HP | LP | P-value | |
|---|---|---|---|
| Glucose (mmol L -1 ) | 4.9 ± 0.2 | 4.6 ± 0.1 | 0.948 |
| Insulin (mU L -1 ) | 10.9 ± 1.8 | 11.0 ± 1.7 | 0.202 |
| Phenylalanine (μmol L -1 ) | 55 ± 2 | 52 ± 4 | 0.121 |
| Tyrosine (μmol L -1 ) | 54 ± 3 | 46 ± 3 | <0.01 |
| Leucine (μmol L -1 ) | 122 ± 5 | 105 ± 8 | <0.01 |
| Total BCAA (μmol L -1 ) | 457 ± 42 | 374 ± 41 | 0.199 |
| Total EAA (μmol L -1 ) | 969 ± 75 | 880 ± 54 | 0.402 |
| Total AA (μmol L -1 ) | 2566 ± 148 | 2642 ± 117 | 0.717 |
BCAA, branched chain amino acids; EAA, essential amino acids; AA, amino acids. Values are expressed as mean ± SEM. Data were analyzed with one-way ANOVA.
Fig 2B shows the time course of the plasma L-[ring 2H5]phenylalanine enrichment. Plasma L-[ring 2H5]phenylalanine enrichments were significantly higher in the LP group compared with the HP group (P<0.001).
Whole-body protein metabolism
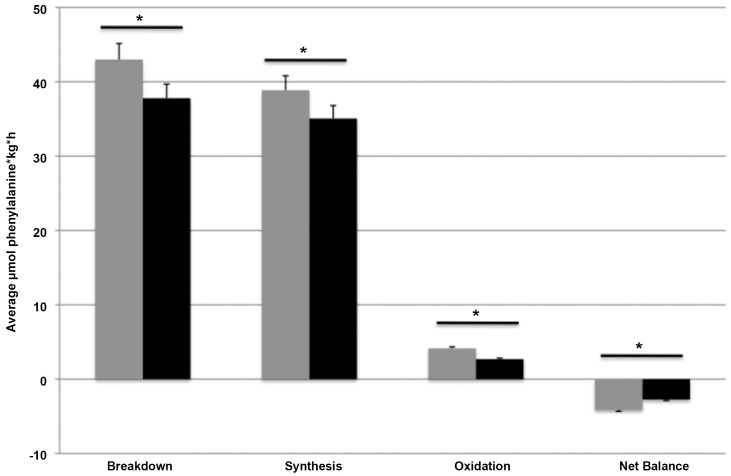
Whole-body protein synthesis, breakdown, oxidation and net balance in the fasted state are expressed as the AUC in Fig 3. Whole-body protein synthesis, reflected by Phe-utilization and expressed as the average of total Phe Rd minus the conversion rate of Phe into Tyr, was significantly higher in the HP group (38.9±4.2 μmol Phe/kg/h, 95%CI: 36.1–42.0; P<0.01) vs LP group (35.1±3.6 μmol Phe/kg/h, 95%CI: 31.5–38.6; P<0.01), with a mean difference of 4.01±2.10 μmol Phe/kg/h, 95%CI: -0.60–8.63; P = 0.008. Phenylalanine Rd was also increased in the HP-group (43.0±4.4 μmol Phe/kg/h, 95%CI: 40.3–46.1; P<0.03) vs the LP group (37.8±3.8 μmol Phe/kg/h, 95%CI: 34.3–41.3; P<0.03), with a mean difference of 5.44±2.08 μmol Phe/kg/h, 95%CI: 0.86–10.02; P = 0.024.
Fig 3. Mean (+SEM) basal whole-body protein metabolism expressed as the AUC (μmol Phe/kg/h) after 12 weeks on a HP (grey) vs LP (black) diet.
n = 15. Data were analyzed with a two-factor ANOVA (time x treatment). *Treatment effect P<0.001.
Whole-body protein oxidation, which was expressed as the average of the Phe-to-Tyr conversion rate, was greater in the HP group (4.1±0.6 μmol Phe/kg/h, 95%CI: 3.85–4.53; P<0.001) vs the LP group (2.7±0.6 μmol Phe/kg/h, 95%CI: 2.29–3.11; P<0.001), with a mean difference of 1.49±0.24 μmol Phe/kg/h, 95%CI: 0.96–2.03; P = 0.000. The deduction of synthesis minus breakdown, resulting in whole-body protein balance, was more negative in the HP group (-4.1±0.5 μmol Phe/kg/h, 95%CI: -4.50 –-3.84; P<0.001) compared with the LP group (-2.7±0.6 μmol Phe/kg/h, 95%CI: -3.14 –-2.34; P<0.001), with a mean difference of -1.43±0.24 μmol Phe/kg/h, 95%CI: -1.95 –-0.91; P = 0.000.
Whole-body protein metabolism differed significantly between men and women with higher protein synthesis (P<0.05), protein breakdown (P<0.01) and protein oxidation (P<0.001) rates as well as a more negative protein net balance (P<0.001) in men compared with women. When expressed per kg FFM, only protein synthesis and protein breakdown rates differed between genders, with higher protein synthesis (P<0.03) and protein breakdown (P = 0.05) rates for women. There were no significant interactions between gender and treatment for whole-body protein metabolism
Muscle tracer analysis and mixed-muscle protein synthesis rates
The increment in muscle protein bound L-[ring-2H5]Phe enrichment between the first and the second biopsy did not differ between the HP group and the LP group (0.0094±0.0023 vs 0.0101±0.032 MPE; P = 0.395).
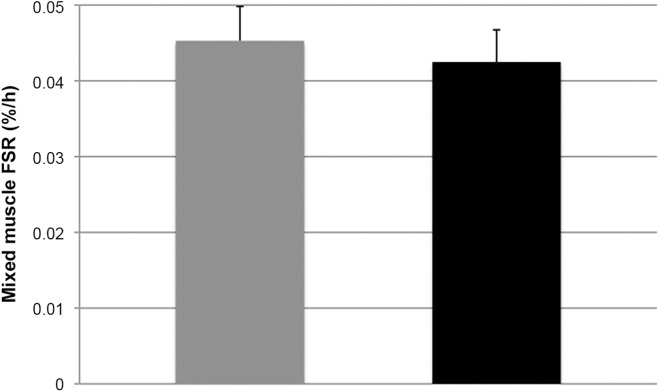
Post-absorptive mixed MPS rates (Fig 4) did not differ following 12 weeks on either the HP diet (0.042±0.01%/h, 95%CI: 0.039–0.051; P = 0.620) or LP diet (0.045±0.01%/h, 95%CI: 0.035–0.050; P = 0.620), with a mean difference of 0.002±0.004%/h, 95%CI: -0.007–0.012; P = 0.621. FSRs were significantly higher in women vs men (P<0.01), without any significant interaction for gender vs treatment.
Fig 4. Mean (+SEM) mixed muscle protein fractional synthesis rates (%/h) in the basal state after 12 weeks on a HP (grey) vs LP (black) diet.
n = 15. Data were analyzed with a one-way ANOVA.
24h-nitrogen retention
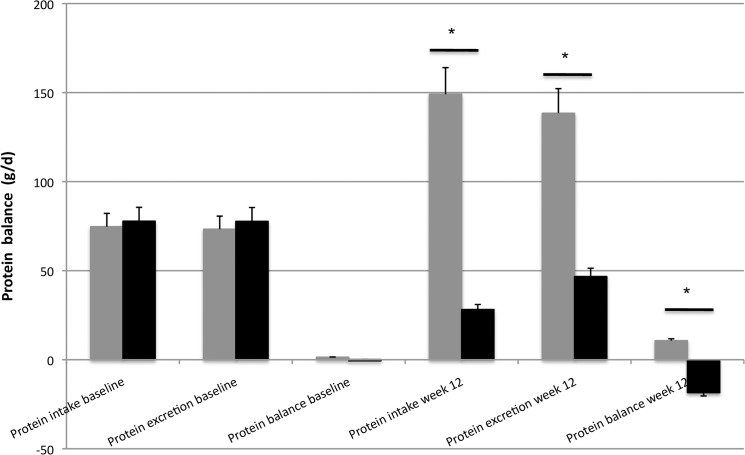
In Fig 5we present results for this subgroup, taken from Martens et al. [15] providing estimates of 24h- whole body protein turnover, based on nitrogen retention data. 24h-protein turnover, based on protein intake and nitrogen excretion, did not differ between the HP (0.1 ± 7.7 g/d, 95%CI: -11.7–12.7; P = 0.892) vs LP (1.4 ± 5.1 g/d, 95%CI: -14.7–15.0; P = 0.892) diet groups at baseline, with a mean difference of 0.41 ± 8.7 g/d, 95%CI: -18.8–19.6; P = 0.964. During the following 12 weeks, protein intake and nitrogen excretion were significantly increased in the HP (P<0.001) and significantly decreased in the LP (P<0.03) diet group compared with baseline. Therefore, 24h-protein turnover at week 12 was significantly different between the HP (10.7 ± 5.2 g/d, 95%CI: -0.50–21.2; P<0.01) vs LP diet groups (-18.5 ± 4.4 g/d, 95%CI: -31.7 –-5.3; P<0.01), with a mean difference of 28.9 ± 7.7 g/d, 95%CI: 11.8–46.0; P = 0.003. Moreover, the observed change between baseline and week 12 was significantly different between the HP (9.3 ± 5.9 g/d, 95%CI: -4.2–23.8; P<0.03) and the LP treatment (-18.6 ± 8.5 g/d, 95%CI: -35.7 –-1.6; P<0.03), with a mean difference of 28.5 ± 10.0 g/d, 95%CI: 6.4–50.5; P = 0.016.
Fig 5. Mean (+SEM) protein intake, excretion and balance (g/d) at baseline and after 12 weeks on a HP (grey) vs LP (black) diet.
n = 15. Data were analyzed with a two-factor repeated measures ANOVA (time x treatment). *Treatment effect P<0.001. #Time effect P<0.03. Adapted and modified from Martens et al. [15].
Protein intake (P<0.001) and nitrogen excretion (P<0.01) were significantly different between men and women. However, 24h-protein turnover (P = 0.485) as well as the changes over time (P = 0.626) did not differ between both genders
Discussion
In the current study, continuous intravenous infusions of L-[ring-2H5]Phenylalanine and L-[ring-2H2]Tyrosine were used to asses whole-body protein metabolism and MPS rates in the basal fasted state, in subjects that followed either a HP diet or LP diet for 12 weeks. Prolonged adaptation to a low dietary protein intake may lead to muscle loss due to a negative whole-body protein balance and a decline in basal muscle protein synthesis rates [13]. The results from the present study show that basal whole-body protein synthesis, breakdown, and oxidation rates were modified following three months on a LP when compared with a HP diet. Protein turnover was significantly lower with significant lower protein synthesis, protein breakdown, and protein oxidation rates on the low protein intake diet. Despite the lower protein turnover rates, post-absorptive whole-body net protein balance had not declined following prolonged adaptation to the low when compared with the high protein intake diet (Fig 3).
Post-absorptive plasma phenylalanine kinetics can be used to provide some insight in whole-body protein turnover rates. When combined with the intravenous infusion of a tyrosine tracer, phenylalanine oxidation rates can be assessed via the rate of phenylalanine hydrolysis to tyrosine [28, 29]. Despite obvious limitations [28, 29], phenylalanine kinetics provide us with a good estimate of potential differences in basal protein breakdown, synthesis, and oxidation rates on a whole body level. However, these measurements of basal protein synthesis and protein breakdown rates do not necessarily reflect skeletal muscle tissue protein metabolism. Therefore, we also collected multiple muscle tissue biopsies to measure fasting mixed muscle protein fractional synthesis rates. In line with the absence of a decline in post-absorptive whole-body protein balance following adaptation to a low protein intake, we did not detect a decline in basal mixed muscle protein synthesis rates when comparing muscle protein synthesis rates after the low protein versu high protein intake diet. To our surprise, prolonged habituation to a diet providing only 0.4 g protein per kg body mass per day did not lower basal muscle protein synthesis rates, with muscle protein synthesis rates being maintained at the same level observed in the high protein intake diet (Fig 4). The observation that post-absorptive whole-body protein balance as well as mixed muscle protein synthesis rates were not reduced on the low protein intake regimen seems to be in line with the observations in the full study, in which no changes in body composition were observed following 12 weeks of adaptation to a low or high protein intake with respect to body weight, FFM and fat mass. Apparently, prolonged adaptation to a low protein intake reduces whole-body protein turnover but does not seem to compromise whole-body protein balance, basal muscle protein synthesis rates or skeletal muscle mass maintenance. This study is the first to show that on a low protein diet (0.4 g/kg/d) body mass and fat free mass can be preserved by lowering whole-body turnover and maintaining basal muscle protein turnover rates. Future work is required to investigate the factors responsible for orchestrating such a protein conservation strategy.
Though we show that prolonged adaptation to a (very) low protein intake diet (0.4 g/kg/d) allows maintenance of post-absorptive whole body protein balance as well as basal muscle protein synthesis rates, we can only speculate on the impact of prolonged adaptation to a low compared with a high protein intake on 24 h whole-body protein balance as well as post-prandial muscle protein synthesis rates. It could be speculated that a low versus high protein intake diet modulates dietary protein digestion and amino acid absorption, peripheral amino acid uptake, muscle protein synthesis and breakdown in an effort to maintain a proper post-prandial muscle protein synthetic response to feeding and, as such, allow skeletal muscle maintenance. In the full study [15] we performed 24 h nitrogen balance analyses as a means to verify adherence and compliance to the low and high protein intake diet. We could use these data to get some insight in the overall 24 h whole-body protein balance following ingestion of a low or high protein diet. The 24 h nitrogen excretion data revealed a more positive whole-body protein balance in the HP versus LP diet [15], suggesting greater tissue protein accretion following the high protein intake diet. However, this seems to be at odds with the absence of any significant changes in body mass or fat free mass over the 12 week time span in both dietary conditions [15]. However, the more positive whole-body protein balance on the high protein versus low protein intake diet when assessed using 24 h nitrogen balance is likely attributed to the applied methodology. Previous work has already shown implausible high nitrogen retention rates when ingesting large amounts of protein [30–32].
Evidently, nitrogen retention data should be interpreted with much caution when using them as a proxy for changes in whole-body or muscle protein metabolism. Clearly, more work is required to assess whether the maintenance of post-absorptive whole body protein balance and basal muscle protein synthesis rates are accompanied by changes in the (muscle) protein synthetic response to feeding [33].
Though we did not intend to assess potential gender differences in basal protein balance and fasting MPS rates, we did observe differences with respect to whole-body protein balance and mixed muscle protein synthesis rates between men and women. Despite the small number of men and women, our data show that post-absorptive whole-body and muscle protein synthesis rates where greater in women compared with men after correction for differences in fat free mass. This seems to be in line with some [34] but certainly not all researchers, who generally fail to detect any major gender differences with respect to post-absorptive muscle protein synthesis rates [35–38].
In conclusion, prolonged adaptation to a low dietary protein intake lowers fasting, whole-body protein turnover rates, but does not compromise post-absorptive whole-body net protein balance. Post-absorptive skeletal muscle protein synthesis rates are maintained even when consuming a (very) low protein intake diet (0.4 g/kg/d).
Supporting Information
(DOCX)
(PDF)
Data has been presented in HP and LP diet groups and we also discriminated between males and females. Subject codes have been removed to ensure privacy. Tab 1 contains the data of the plasma analyses. Tab 2 and 3 contain the whole-body kinetics. Tab 4 contains the eventual outcomes of the whole-body protein metabolism. Tab 5 contains the muscle biopsy data the muscle tracer analysis and mixed-muscle protein synthesis rates. Tab 6 contains the 24 h protein turnover data.
(XLSX)
Acknowledgments
We gratefully acknowledge the help of Givan Paulus, Kirsten van der Beek and Tim Snijders with the collection of muscle tissue. Moreover, we would like to thank Stephan van Vliet and Stefan Camps for their assistance during the experiments.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 266408. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Soenen S, Martens EA, Hochstenbach-Waelen A, Lemmens SG, Westerterp-Plantenga MS. Normal protein intake is required for body weight loss and weight maintenance, and elevated protein intake for additional preservation of resting energy expenditure and fat free mass. J Nutr. 2013; 143(5):591–6. 10.3945/jn.112.167593 [DOI] [PubMed] [Google Scholar]
- 2. Westerterp-Plantenga MS, Lemmens SG, Westerterp KR. Dietary protein—its role in satiety, energetics, weight loss and health. Br J Nutr. 2012; 108 Suppl 2:S105–12. 10.1017/S0007114512002589 [DOI] [PubMed] [Google Scholar]
- 3. Bray GA, Smith SR, de Jonge L, Xie H, Rood J, Martin CK et al. Effect of dietary protein content on weight gain, energy expenditure, and body composition during overeating: a randomized controlled trial. JAMA. 2012; 307(1):47–55. 10.1001/jama.2011.1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Soenen S, Westerterp-Plantenga MS. Changes in body fat percentage during body weight stable conditions of increased daily protein intake vs. control. Physiol Behav. 2010; 101(5):635–8. 10.1016/j.physbeh.2010.09.014 [DOI] [PubMed] [Google Scholar]
- 5. Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr. 2012; 96(6):1454–64. 10.3945/ajcn.112.037556 [DOI] [PubMed] [Google Scholar]
- 6. Summerbell CD, Douthwaite W, Whittaker V, Ells LJ, Hillier F, Smith S et al. The association between diet and physical activity and subsequent excess weight gain and obesity assessed at 5 years of age or older: a systematic review of the epidemiological evidence. Int J Obes (Lond). 2009; 33 Suppl 3:S1–92. [DOI] [PubMed] [Google Scholar]
- 7. Wolfe RR. Regulation of muscle protein by amino acids. J Nutr. 2002; 132(10):3219S–24S. [DOI] [PubMed] [Google Scholar]
- 8. Dideriksen K, Reitelseder S, Holm L. Influence of amino acids, dietary protein, and physical activity on muscle mass development in humans. Nutrients. 2013;s 5(3):852–76. 10.3390/nu5030852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Symons TB, Sheffield-Moore M, Wolfe RR, Paddon-Jones D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Diet Assoc. 2009; 109(9):1582–6. 10.1016/j.jada.2009.06.369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koopman R, Verdijk L, Manders RJ, Gijsen AP, Gorselink M, Pijpers E et al. Co-ingestion of protein and leucine stimulates muscle protein synthesis rates to the same extent in young and elderly lean men. Am J Clin Nutr. 2006; 84(3):623–32. [DOI] [PubMed] [Google Scholar]
- 11. Pennings B, Boirie Y, Senden JM, Gijsen AP, Kuipers H, van Loon LJ. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr. 2011; 93(5):997–1005. 10.3945/ajcn.110.008102 [DOI] [PubMed] [Google Scholar]
- 12. van Loon LJ, Gibala MJ. Dietary protein to support muscle hypertrophy. Nestle Nutr Inst Workshop Ser. 2011; 69:79–89. 10.1159/000329287 [DOI] [PubMed] [Google Scholar]
- 13. Westerterp-Plantenga MS, Nieuwenhuizen A, Tome D, Soenen S, Westerterp KR. Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr. 2009; 29:21–41. 10.1146/annurev-nutr-080508-141056 [DOI] [PubMed] [Google Scholar]
- 14.Division FS. Available: http://www.dietaryfiberfood.com/protein-requirement.php.
- 15. Martens EA, Gonnissen HK, Gatta-Cherifi B, Janssens PL, Westerterp-Plantenga MS. Maintenance of energy expenditure on high-protein vs high-carbohydrate diets at a constant body weight may prevent a positive energy balance. Clin Nutr. 2014; November 8 pii: S0261-5614(14)00259-3. 10.1016/j.clnu.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 16. Koopman R, Crombach N, Gijsen AP, Walrand S, Fauquant J, Kies AK et al. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am J Clin Nutr. 2009; 90:106–15. 10.3945/ajcn.2009.27474 [DOI] [PubMed] [Google Scholar]
- 17. Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985; 29(1):71–83. [DOI] [PubMed] [Google Scholar]
- 18. Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982; 36(5):936–42. [DOI] [PubMed] [Google Scholar]
- 19. Harris JA, Benedict FG. A Biometric Study of Human Basal Metabolism. Proc Natl Acad Sci U S A. 1918; 4(12):370–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Westerterp KR, Donkers JH, Fredrix EW, Boekhoudt P. Energy intake, physical activity and body weight: a simulation model. Br J Nutr. 1995; 73(3):337–47. [DOI] [PubMed] [Google Scholar]
- 21. Abumrad NN, Rabin D, Diamond MP, Lacy WW. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism. 1981; 30(9):936–40. [DOI] [PubMed] [Google Scholar]
- 22. Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975; 35(7):609–16. [PubMed] [Google Scholar]
- 23. van Eijk HM, Rooyakkers DR, Deutz NE. Rapid routine determination of amino acids in plasma by high-performance liquid chromatography with a 2–3 microns Spherisorb ODS II column. J Chromatogr. 1993; 620(1):143–8. [DOI] [PubMed] [Google Scholar]
- 24. Wolfe RR, Chinkes DL. Isotope tracers in metabolic research New York: Wiley-Liss; 2005. [Google Scholar]
- 25. Husek P. Amino acid derivatization and analysis in five minutes. FEBS lett. 1991; 280(2):354–6. [DOI] [PubMed] [Google Scholar]
- 26. Dangin M, Guillet C, Garcia-Rodenas C, Gachon P, Bouteloup-Demange C, Reiffers-Magnani K et al. The rate of protein digestion affects protein gain differently during aging in humans. J Physiol. 2003; 549(Pt 2):635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boirie Y, Gachon P, Corny S, Fauquant J, Maubois JL, Beaufrere B. Acute postprandial changes in leucine metabolism as assessed with an intrinsically labeled milk protein. Am J Physiol. 1996; 271(6 Pt 1):E1083–91. [DOI] [PubMed] [Google Scholar]
- 28. Millward DJ, Price GM, Pacy PJ, Halliday D. Whole body protein and amino acid turnover in man: what can we measure with confidence? Proc Nutr Soc. 1991; 50: 197–216. [DOI] [PubMed] [Google Scholar]
- 29. Matthews DE. An overview of phenylalanine and tyrosine kinetics in humans. J Nutr, 2007. June; 137 (6 Suppl 1): 1549S–1575S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hegsted DM. Balance studies. J Nutr. 1976; 106: 307–311. [Google Scholar]
- 31. Young VR. Nutritional balance studies: Indicators of human requirements or of adaptive mechanisms? J Nutr 1986; 116: 700–703. [DOI] [PubMed] [Google Scholar]
- 32. Young VR, Gucalp C, Rand WM, Matthews DE, Bier DM. Leucine kinetics during three weeks at submaintenance-to-maintenance intakes of leucine in men: Adaptation and accommodation. Hum Nutr Clin Nutr. 1987: 41: 1–18. [PubMed] [Google Scholar]
- 33. Burd NA, Gorissen SH, van Loon LJ. Anabolic resistance of muscle protein synthesis with aging. Exerc Sport Sci Rev. 2013; 41(3):169–73. 10.1097/JES.0b013e318292f3d5 [DOI] [PubMed] [Google Scholar]
- 34. Henderson GC, Dhatariya K, Ford GC, Klaus KA, Basu R, Rizza RA et al. Higher muscle protein synthesis in women than men across the lifespan, and failure of androgen administration to amend age-related decrements. FASEB J. 2009; 23(2):631–41. 10.1096/fj.08-117200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fujita S, Rasmussen BB, Bell JA, Cadenas JG, Volpi E. Basal muscle intracellular amino acid kinetics in women and men. Am J Physiol Endocrinol Metab. 2007; 292(1):E77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jahn LA, Barrett EJ, Genco ML, Wei L, Spraggins TA, Fryburg DA. Tissue composition affects measures of postabsorptive human skeletal muscle metabolism: comparison across genders. J Clin Endocrinol Metab. 1999; 84(3):1007–10. [DOI] [PubMed] [Google Scholar]
- 37. Smith GI, Atherton P, Reeds DN, Mohammed BS, Jaffery H, Rankin D et al. No major sex differences in muscle protein synthesis rates in the postabsorptive state and during hyperinsulinemia-hyperaminoacidemia in middle-aged adults. J Appl Physiol. 2009; 107(4):1308–15. 10.1152/japplphysiol.00348.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith GI, Reeds DN, Hall AM, Chambers KT, Finck BN, Mittendorfer B. Sexually dimorphic effect of aging on skeletal muscle protein synthesis. Biol Sex Differ. 2012; 3(1):11 10.1186/2042-6410-3-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(PDF)
Data has been presented in HP and LP diet groups and we also discriminated between males and females. Subject codes have been removed to ensure privacy. Tab 1 contains the data of the plasma analyses. Tab 2 and 3 contain the whole-body kinetics. Tab 4 contains the eventual outcomes of the whole-body protein metabolism. Tab 5 contains the muscle biopsy data the muscle tracer analysis and mixed-muscle protein synthesis rates. Tab 6 contains the 24 h protein turnover data.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.