Abstract
Objective
To contrast changes in clinical and kinematic measures of upper extremity movement in response to virtually simulated and traditionally presented rehabilitation interventions in persons with upper extremity hemiparesis due to chronic stroke.
Methods
This was a non-randomized controlled trial set in an ambulatory research facility. The participants were a volunteer sample of twenty one community-dwelling adults (mean age: 51±12 years) with residual hemiparesis due to stroke more than 6 months before enrollment (mean:74±48 months), recruited at support groups. Partial range, against gravity shoulder movement and at least 10° of active finger extension were required for inclusion. All subjects completed the study without adverse events. Interventions - A 2 weeks, 24-hour program of robotic/virtually simulated, arm and finger rehabilitation activities was compared to the same dose of traditionally presented arm and finger activities.
Results
Subjects in both groups demonstrated statistically significant improvements in the ability to interact with real-world objects as measured by the Wolf Motor Function Test (P=0.01). The robotic/virtually simulated activity (VR) group but not the traditional, repetitive task practice (RTP) group demonstrated significant improvements in peak reaching velocity (P=0.03) and finger extension excursion (P=0.03). Both groups also demonstrated similar improvements in kinematic measures of reaching and grasping performance such as increased shoulder and elbow excursion along with decreased trunk excursion.
Conclusions
Kinematic measurements identified differing adaptations to training that clinical measurements did not. These adaptations were targeted in the design of four of the six simulations performed by the simulated activity group. Finer grained measures may be necessary to accurately depict the relative benefits of dose matched motor interventions.
Keywords: Virtual reality, Robotics, Stroke, Kinematic measurements
Introduction
A significant proportion of the disability caused by stroke is the limited recovery of hand and arm function.1 The best evidence available suggests that rehabilitation interventions for the hemiparetic upper extremity (UE) must present training that is meaningful, consistently challenging, and highly repetitious.2 Repetitive task practice (RTP)-based approaches to rehabilitation fulfilling these requirements have demonstrated measurable improvements in motor function in persons with UE hemiparesis as measured by standardized clinical assessments,3 as well as kinematic assessments of reaching and prehension and measures of corticomotor excitability.4 Multiple investigators have developed and tested robotically facilitated and/or virtually simulated approaches to proximal UE rehabilitation.5–7 These systems allow for highly repetitious training that is intense and activity-based, while remaining manpower and space efficient.8 Several studies comparing proximal UE robotic or virtual reality (VR)-based interventions to traditionally presented RTP activities have produced comparable outcomes or identified modest advantages for technology-based rehabilitation in reaching kinematics, clinical tests of UE function, or both.9,10
Less information is available regarding technology-based training of the entire (proximal and distal) UE. Pilot studies establishing the feasibility of these systems describe clinical outcomes comparable or superior to those reported in studies of traditionally presented rehabilitation.11–14 A newer study by Klamroth-Marganska et al. compared an integrated upper extremity robotic intervention to a dose-matched program of RTP.15 They identified a small impairment level advantage for the virtually simulated/robotic training condition, but activity level changes were identical for the two groups. Even fewer studies have examined technology-based training of the hand; a majority are uncontrolled pilot studies describing feasibility and potentially promising clinical or kinematic motor outcomes.16 To date, no published studies have compared the effects of a balanced program of technology-based hand and arm training to a dose-matched program of traditionally presented RTP.
The interaction between the hand, arm, and objects is complex and integrated.17 This makes UE behaviors difficult to measure using clinical approaches that stress the functional outcomes of the movement. Measures that focus purely on task outcomes fail to differentiate between a patient’s regaining a more normal pattern of movement versus the development of an efficient, but abnormal compensatory strategy.18 Although some studies have shown good transfer of movement capabilities to real world function after VR training for lower extremity and proximal upper extremity,19,20 it is not well known whether training the hand in VR produces changes in movement that transfer to real-world hand–object interactions.
Several authors cite kinematic analysis as a means to identify the normalization of motor function in persons with stroke.18,21–23 None of the studies described above have compared the impact of technology-based and traditionally presented rehab interventions on kinematic measurements of the hand and arm of persons with hemiparesis as they interact with real world objects. We hypothesized that despite differences in testing and training conditions, that improvements in VR-based training would generalize to real-world object interaction at levels comparable to those elicited by traditionally presented RTP. We further hypothesized that different patterns of change in kinematic measures of UE–object interaction would emerge from the two types of training.
Methods
Subjects
Subjects were recruited through mailings, web sites, and support groups. Criteria for inclusion were: (1) between 18 and 80 years of age; (2) stroke in the chronic phase of recovery (greater than 6 months); (3) at least 20° of active wrist extension; and (4) at least 10° of active finger extension. Qualifying subjects were excluded if they were participating in therapy during the period of the study. Subjects with aphasia or significant hemi-sensory inattention or neglect were excluded as well. Subjects for the RTP phase of the study were recruited consecutively and subjects from the VR arm of the study were chosen from a single arm of a larger sample of subjects participating in a study of virtually simulated UE rehabilitation.24 All of the subjects that performed transfer task testing from each of the two samples are included in the analyses presented in this paper (see Table 1 for a description of clinical and demographic characteristics of the two groups). All subjects completed an informed consent process approved by the Institutional Review Boards of the New Jersey Institute of Technology and Rutgers, The State University of New Jersey.
Table 1.
Mean (SD) of initial subject characteristics
| VR | RTP | t/Fischer’s P | |
|---|---|---|---|
| Age (years) | 53 (11) | 50 (11) | 0.592 |
| UEFMA pre-test (max. =64) | 48 (6) | 52 (9) | 0.294 |
| WMFT pre-test (second) | 6.2 (1.8) | 4.6 (2.4) | 0.102 |
| Male/female | 6/4 | 4/7 | 0.391 |
| R CVA/L CVA | 4/6 | 7/4 | 0.307 |
| Premorbid handedness R/L | 10/0 | 11/0 | 1 |
| Dominant CVA/non-dominant CVA | 4/6 | 7/4 | 0.483 |
| UE Ashworth (16) | 4 (2) | 3 (3) | 0.832 |
| Ischemic CVA/hemorrhagic CVA | 7/3 | 9/2 | .644 |
| Time since CVA (month) | 60 (41) | 87 (56) | 0.236 |
Intervention schedule
Subjects in both intervention groups were trained for 3 hours per day, four consecutive days per week, for 2 weeks. The intensive 2-week training period was chosen based on the success of the EXCITE trial.3 Tolerance of 4-day training weeks and 3-hour training sessions in persons with chronic stroke was confirmed during design/pilot testing of the two interventions. Both intervention protocols were designed with specific plans to increase activity difficulty as subjects’ mastered tasks and to decrease activity difficulty if subject performance decreased within a session due to fatigue.
VR/robotically facilitated intervention
During this intervention, subjects interacted with virtual environments with haptic guidance in three-dimensional space provided by a robot. Subjects performed the same six simulations at each session. Half of each training session was spent performing hand simulations emphasizing hand opening and individual finger movement. For the other half of each session, subjects performed two arm simulations emphasizing three-dimensional reaching, and a third that emphasized forearm pronation with the arm elevated away from the trunk. Three activities simulated functional movements (hammering a peg, pressing piano keys, and placing cups on a shelf) and three were game-based activities (pong, piloting a space ship and blowing up targets). Initial difficulty level was determined on training day 1. Each of the simulations described below modified the difficulty of activities using an online algorithm based on patient performance or criteria based adjustments made between sets of an activity. Feedback on all simulations provides knowledge of results in the form of game scores or the number of successful repetitions performed during a given time period (see Ref. 24 for a detailed description).
Hardware
For arm training, we utilized the Haptic MASTER (Moog NCS, The Netherlands), an admittance-controlled robot with 6° of freedom. The Haptic MASTER acted as an interface between the subject and the virtual environments, provided assistance as needed to lower functioning subjects and added physical parameters such as the force of gravity and solid objects to the simulated activities (Fig. 1). For hand training, we utilized an integrated system of the CyberGlove (Immersion, USA) instrumented glove and a CyberGrasp, a cable-actuated exoskeleton robot which facilitated hand opening for lower functioning subjects (Immersion; see Fig. 2 and Ref. 24 for a detailed description).
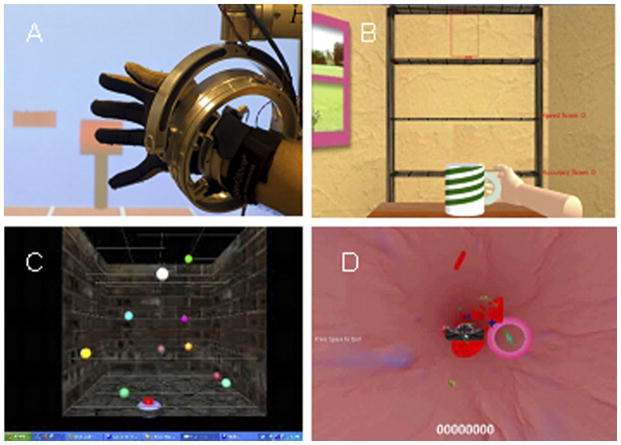
Figure 1.
Screen shots of arm training simulations: (A) hammer task with NJIT RAVR system in foreground; (B) placing cups; (C) reach/touch; (D) blood cell.
Figure 2.
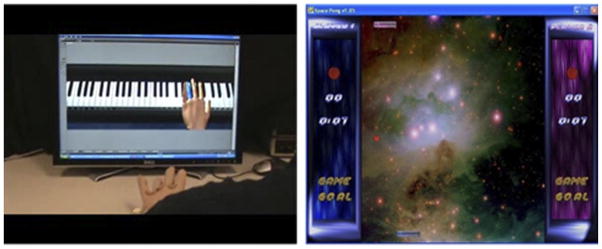
Screen shots of hand training simulations: (A) piano trainer with NJIT Trackglove system in the foreground; (B) space pong.
RTP intervention
Training activities included (1) reach and grasp of different sized objects; (2) coin sorting; (3) dressing; (4) writing/pre-writing; (5) carrying objects; (6) using a calculator or keyboard; (7) building with large and small objects; (8) cooking; (9) folding clothing; (10) Yahtzee; (11) velcro catching with impaired UE; and (12) feeding. Specific activities training hand opening (activities 1 and 9), individual finger movement (activities 2 and 6), three-dimensional reaching (activities 1, 3, and 11), and forearm pronation with the arm elevated away from the trunk (activities 8, 10, and 12) were chosen to ensure that the real-world RTP program trained movements were similar to those addressed by the robotic/VR intervention. In addition, training tasks involving specific objects (towels, cards, checkers, keys, etc.) used in the Wolf Motor Function Test (WMFT), or activities performed in the WMFT (checker stacking, simulated drinking, etc.), were avoided during RTP training in an attempt to reduce the similarity between the WMFT and the RTP program. Stations were supervised by a Licensed Physical Therapist or a Third-Year Doctor of Physical Therapy student. Each station presented a set of 8–12 specific activities that were graded by difficulty. Each subject practiced performing the most appropriate activity level at each station for approximately 15 minutes on each training day. Initial activity or difficulty level was determined on training day 1. Patients were presented with higher level difficulty tasks when they performed well and lower level tasks when they struggled. The therapist adapted tasks to accommodate the motor abilities of the subjects as necessary. Feedback provided to RTP subjects was limited to the number of successful repetitions; successful multistep tasks completed, or game score. This feedback was provided at the end of individual repetitions, individual multistep tasks, or game periods.
Outcome measurements
Upper Extremity Fugl–Meyer Assessment (UEFMA)
The UEFMA25 was performed using the expanded criteria described in Ref. 26. Higher scores indicate better performance. The published minimum detectable change for this measure is six points27 and the minimum clinically important difference is four points.28
WMFT
The 15 timed items from the WMFT were performed utilizing the procedures described in Ref. 29. The published minimum detectable change is 4.2 seconds and the minimum clinically important difference is 1.5 seconds for this measure.30
Reach to Grasp Test (RGT)
The RGT, a kinematic analysis of untrained UE reaching and object interaction,31 was used to attempt to identify the specific aspects of UE function that may have changed during each type of training.
data capture: Position of the subjects’ UE and trunk were recorded using electromagnetic sensors (trackSTAR™ system; Ascension Technologies, Inc.) attached with tape to the sternum, acromion, the lateral surface of the elbow, and mid-way between the radius and ulna on the dorsal surface of the wrist. Finger angles were recorded with a CyberGlove. The flexion/extension of the metacarpophalangeal, proximal interphalangeal, and distal interphalangeal joints of the index finger was utilized for analysis in this study.
task: Subjects were seated at a table with their arm against their trunk and their hand in front of their acromion. Subjects were presented objects of four different shapes and dimensions. Subjects were instructed to reach for, grasp and lift each of four objects from the table. Objects utilized were: a small disk (diameter=32 mm), a small box (l=95 mm, w=32 mm), a large disk (d=57 mm), and a large box (l=67 mm, w=57 mm).
-
variables:
Reach to lift time (RLT) encompasses the reach to grasp to lift portion of the task, which began when the hand moved away from the starting point and ended when the object left the support surface.
Peak velocity (PV) was attained during the reach to lift movement.
Reaching path length (RPL) was the distance the hand traveled from the starting point until it made contact with the object.
Reaching trajectory smoothness (RTS) was evaluated as a squared third derivative of the wrist position integrated along the wrist path and normalized to make it dimensionless.32
Trunk excursion (TE) during the reaching movement was measured as the length of the path covered by the magnetic tracker on the subjects’ sternum. This approach reflects a composite of all forward, lateral, and rotational movement of the trunk during reaching.23
Sagittal shoulder excursion (SS) was measured as the change in angle in the sagittal plane made by the position of the trackers on the elbow, the acromio-clavicular joint, and the sternum with the hand positioned on the starting point and the angle made by these trackers when the hand was on the object during grasping.
Transverse shoulder (TS) excursion was measured as the change in angle in the transverse plane made by the position of the trackers on the elbow, the acromio-clavicular joint, and the sternum with the hand positioned on the starting point and the angle made by these trackers when the hand was on the object during grasping.
Elbow excursion (EEx) was measured as the change in angle made by the position of the trackers on the wrist, elbow, and acromio-clavicular joint with the hand positioned on the starting point and the angle made by these trackers with the hand on the object during grasping.
Finger excursion (FEx) is the difference in the index finger angle measured with the hand at the starting point and the peak index finger extension angle measured during the reaching movement.
Data analysis
Shapiro–Wilks tests were utilized to confirm data normality.
Clinical tests
WMFT and UEFMA composite scores were evaluated with separate pre-planned ANOVAs for repeated measures, with a single between factor Training Group (VR, RTP) and a single within factor Test Time (Pre and Post).
RGT measures
Pre- and post-test averages for the two groups for each measure were evaluated with separate pre-planned ANOVAs for repeated measures, with a single between factor Training Group (VR, RTP) and one within factor Test Time (Pre and Post).
Results
The two groups of subjects did not differ significantly in demographics or measurements of stroke severity (Table 1). All 21 subjects completed 100% of the protocol without adverse events, overuse-related issues, or complaints of fatigue that negatively impacted their daily routines during the intervention period. All data were normally distributed. As a single, 21 subject sample subjects demonstrated statistically significant improvements in WMFT time and five kinematic measures with small to moderate effect sizes. Table 2 lists results of the repeated measures ANOVA for each clinical and kinematic variable and Table 3 lists the pre and post-test averages for each group.
Table 2.
ANOVA
| Main effect (Pre, Post)
|
Group by measurement time interaction
|
|||||
|---|---|---|---|---|---|---|
| Assessment | F(1,19) | P | ES | F(1,19) | P | ES |
| WMFT | 14.46 | 0.01 | 0.43 | 1.16 | 0.30 | |
| UEFMA | 6.1 | 0.23 | 0.04 | 0.84 | ||
| Reach to lift time | 20.10 | <0.01 | 0.44 | 3.20 | 0.09 | |
| Peak velocity | 5.33 | 0.03 | 0.22 | 5.83 | 0.03 | 0.23 |
| Reaching path length | 0.02 | 0.89 | 8.08 | 0.31 | ||
| Reaching trajectory smoothness | 0.53 | 0.37 | 0.11 | 0.72 | ||
| Trunk excursion | 4.90 | 0.04 | 0.21 | 0.57 | 0.46 | |
| Sagittal shoulder excursion | 5.51 | 0.03 | 0.23 | 0.15 | 0.90 | |
| Transverse shoulder excursion | 5.11 | 0.04 | 0.22 | 0.12 | 0.74 | |
| Elbow excursion | 1.53 | 0.23 | 0.05 | 0.82 | ||
| Finger excursion | 2.49 | 0.13 | 5.45 | 0.03 | 0.22 | |
Note: ES: effect size (partial eta-squared).
Table 3.
Mean±SD of clinical and kinematic parameters
| VR group (n=10)
|
RTP group (n=11)
|
|||||
|---|---|---|---|---|---|---|
| Assessment | Pre-test | Post-test | Difference | Pre-test | Post-test | Difference |
| WMFT (second) | 6.2±1.8 | 4.6±1.8 | 1.6±1.7 | 4.6±2.4 | 3.7±2.1 | 0.9±1.1 |
| UEFMA (/64) | 48±5.6 | 50±6.9 | 2.3±4.8 | 52±8.7 | 54±8.0 | 1.9±2.7 |
| Reach to lift time (second) | 1.9±.32 | 1.5±.35 | .35±.33 | 1.3±.5 | 1.1±.5 | .15±.11 |
| Peak velocity (m/s) | .14±.05 | .17±.03 | .03±.03 | .19±.05 | .19±.04 | 0±.02 |
| Reaching path length (cm) | 10.3±1.7 | 9.7±1.6 | .55±.99 | 10.0±2.8 | 9.4±2.8 | .62±.80 |
| Reaching trajectory smoothness | 74±24 | 64±40 | 9.8±41 | 41±37 | 36±33 | 4.8±14 |
| Trunk excursion (cm) | 2.6±1.6 | 2.3±1.0 | .37±1.5 | 2.9±1.4 | 2.1±1.1 | .21±.80 |
| Sagittal shoulder excursion (°) | 0.6±21 | 10.4±13.3 | 11±23 | 2.2±25 | 14±23 | 11±22 |
| Transverse shoulder excursion (°) | 6.6±16 | 12.0±9.0 | 5.4±13 | 4.1±10 | 8.1±8.9 | 4.0±4.5 |
| Elbow excursion (°) | 5.2±15 | 3.0±8.9 | 2.2±14 | 4.9±13 | 1.7±11 | 3.1±3.9 |
| Finger excursion (°) | 26±11 | 36±13.4 | 9.3±7.7 | 33±13 | 32±13 | 1.8±3.9 |
UEFMA scores
The VR groups initial mean scores were slightly lower at pre-test (VR=48.3±6.4, RTP=51.9±0). This difference was not statistically significant (Table 1). Both groups demonstrated small non-statistically significant improvements at the body function level as measured by the UEFMA. Group by Measurement Time interaction for this variable was not statistically significant (Table 2). Subjects in both groups demonstrated a mean improvement of approximately two points (VR=2.3±4.8, RTP=1.9±2.4).
WMFT scores
The VR group’s initial mean scores were slightly slower at pre-test (VR=6.2±1.8, RTP=4.6±2.4). This difference was not statistically significant (Table 1). The overall improvement in the WMFT scores was significant, with a large effect size (Table 2). Both groups demonstrated statistically significant improvements at the activity level as measured by the WMFT. Subjects in the VR group demonstrated a larger mean improvement from pre to post-test (VR=1.6±1.8, RTP=0.9±1.1). Group by Measurement Time interaction for this variable was not statistically significant (Table 2). Two individual VR group subjects and two individual RTP group subjects were able to complete WMFT items at post-test that they were unable to complete at pre-test. This type of improvement has been cited as clinically important change as well.3
RTG testing
As a single group, subjects demonstrated a statistically significant improvement in the speed of reaching and grasping, with significant main effects of training on peak hand velocity and time to object lift (Table 2). In addition, both groups showed essentially identical trunk and proximal UE adaptations to training during the reach to grasp movement testing, with statistically significant decreases in trunk excursion and statistically significant increases in sagittal and transverse shoulder excursion for the entire 21 subject sample (Table 2). No significant Group by Measurement Time interactions were observed for any of these variables. In contrast, the Group by Measurement Time interaction for the amount of finger extension during grasping was statistically significant. VR group subjects increased the maximum finger extension they achieved during the reach to grasp movement by an average of ten degrees. This change was statistically significant. The RTP subject’s hand opening performance did not change from pre- to post-test substantially.
Changes in measures of hand kinematics varied more. Pre-test scores for RLT were slower for the VR group. Subjects in the VR group demonstrated a statistically significant 800 ms improvement in RLT, while subjects in the RTP group demonstrated non-significant improvements, but this difference in responses to training was not statistically significant (Group by Measurement Time interaction, P=0.09). Pre-test scores for PV were slower for the VR group when compared to RTP group (P=0.018). VR group subjects demonstrated statistically significant improvements in PV. RTP group’s subjects did not demonstrate improvements in PV. Group by Measurement Time interaction for PV was significant (P=0.03). Subjects in both groups demonstrated shorter and smoother hand trajectories after training, but these changes did not reach the level of significance.
Discussion
Pre- and post-testing data suggest that our hypothesis may have been accurate. Both groups demonstrated statistically significant improvements in the ability to interact with real-world objects as measured by WMFT. Additionally, several individual subjects in each group also demonstrated clinically important differences in this measure, but neither training group demonstrated superior improvements. Finally, the VR group also demonstrated improvement in several kinematic measurements collected during un-trained transfer tasks (reaching and grasping of real-world objects). This improvement was equal or better than shown by the RTP group. This similarity in clinical outcome shown by the VR group occurred in spite of the fact that, unlike the VR training, the RTP training conditions share commonalities with the WMFT and RTG test in terms of normal tactile input and visual perspective as well as the absence of sensorimotor transformations. However, these similarities in testing and training conditions for RTP subjects did not result in larger gains compared to a dose matched program of simulated activities. We feel that this suggests that improvements in motor function elicited by practicing simulated activities might transfer to real-world task performance as well as training in the real world.
The VR training approach produced improvements in hand opening and reaching velocity that were superior to RTP training to a statistically significant degree. This difference could be due to the ability of simulated activities to train the specific constructs using adaptive online and offline algorithms that cannot be used in real-world training. An alternative explanation of this effect could be that real-world and simulated training programs were not perfectly matched as they related to these two constructs. A better controlled study of training for these two specific movement constructs will be needed to evaluate this conclusion definitively.
Targeted, repetitive training of finger extension may have been the cause of the superiority in improved hand opening shown by the VR training group. Two simulations specifically targeted finger extension. The piano simulation utilized a targeted, adaptive algorithm that aggressively shaped finger fractionation in order to successfully press the keys on the virtual piano. Although the piano key press requires finger flexion, the algorithm necessitates active extension of the other three fingers to maximize the distance between the active finger and the rest of the fingers in order to elicit the sound, thus making this simulation a strong stimulus for improving finger extension. The simulation forces subjects to increase their performance in this construct as soon as they achieve a level of consistency. While the RTP program presented subjects with activities that required finger extension to complete them, none required a progressive increase in finger extension for continued success. In addition, the space pong simulation required modulation of flexion and extension of the fingers. Importantly, this simulation allows for the magnification of gain of trace finger extension into a meaningful activity. This provides patients with lower initial levels of finger extension an opportunity to successfully use the simulation and train finger extension before they could do this is in a real-world functional context. In contrast, RTP group subjects with minimal finger extension would need to use compensatory strategies to accomplish tasks that they could not perform due to limited finger extension.
VR group subjects demonstrated slightly larger improvements in the total time to complete the reach to grasp movement (RLT). In addition, the peak velocity attained during the reaching movement (PV) was improved substantially by VR subjects and essentially, no change was demonstrated in this construct by RTP group members. Two possible explanations for these differences are readily apparent. Initial scores were slower for the VR group. VR group subjects may have presented to us with a greater potential for improvement because they started the study moving more slowly. Alternatively, two of the simulations utilized by the VR group emphasized fast proximal movements. All VR group subjects were able to perform both of these simulations. While some of the RTP group subjects that were able, performed a single station (due to clinic space limitations) that incorporated catching a thrown object, a majority of the RTP group subjects needed to perform an alternate activity. The scaling afforded by simulating the activity may have allowed a larger proportion of VR subjects to train faster proximal movements, resulting in larger improvements in reaching velocity.
Study limitations
Four factors limit the generalizability of this research. The first is the small sample size. The second is the non-randomized recruitment of subjects. The strength of this argument is weakened by the similarity in the clinical measures of the two groups at baseline, but a randomized controlled comparison of these two approaches to training will be necessary before definitive conclusions can be drawn. Third, the subjects in this group are moderately to mildly impaired; thus, ceiling effects may have limited the amount of overall change in UEFMA scores. Lastly, a more severely impaired group may have demonstrated a more divergent set of adaptations to training.
Conclusions
To date, many authors have described similar magnitude of change in clinical test outcomes when comparing traditionally presented and virtually simulated activities designed to rehabilitate hemi-paretic upper extremities. The clinically tested outcomes of this study reveal a similar pattern. However, finer grained tests examining changes in movement patterns elicited, like those presented in this paper, may reveal additive effects of simulated training for specific movement patterns like the differences in hand opening or reaching speed improvement identified in this study. Success algorithms and gain scaling are two approaches that allow virtually simulated activities to shape specific movement abilities. The ability to target specific movement parameters using these approaches may result in simulated activities proving to be more effective than traditionally presented activities for improving specific aspects of movement. Utilizing kinematic measures to compare training outcomes related to specific movement abilities may be necessary to compare the relative effectiveness of this approach. In addition, investigators interested in simulated rehabilitation activities may make more effective contributions to rehabilitation science by focusing on simulating activities that are difficult to train in the early stages of recovery or activities that are time and space inefficient, when utilizing traditional equipment.
Acknowledgments
Funding
This work was supported in part by NIH grant HD 58301 and National Institute on Disability and Rehabilitation Research Grant H133E050011.
Footnotes
Contributors
All authors read and approved the final manuscript. GGF participated in the robotic/VR system design, RTP treatment design, study design, subject recruitment, data collection, data analysis, initial manuscript preparation and manuscript revision. ASM participated in the robotic/VR system design, study design, data collection, data analysis, initial manuscript preparation and manuscript revision. QQ participated in the robotic/VR system design, data collection, data analysis and initial manuscript preparation. MR participated in data analysis and initial manuscript preparation. AV participated in RTP treatment design, study design, subject recruitment, data collection, initial manuscript preparation and manuscript revision. SVA participated in the robotic/VR system design, study design, data collection, data analysis, initial manuscript preparation and manuscript revision processes.
Conflicts of Interest
All authors report that they have no conflicts of interest to declare.
Ethics Approval
Protocol and consent process was approved by the Institutional Review Boards of Rutgers, The State University of New Jersey and New Jersey Institute of Technology.
VR group subjects were participants in Trial # NCT01072461, registered at clinicaltrials.gov.
References
- 1.Kwakkel G, Kollen B. Predicting improvement in the upper paretic limb after stroke: a longitudinal prospective study. Restor Neurol Neurosci. 2007;25(5):453–460. [PubMed] [Google Scholar]
- 2.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51(1):S225–S239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- 3.Wolf S, Winstein C, Miller J, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 4.Boake C, Noser EA, Ro T, et al. Constraint-induced movement therapy during early stroke rehabilitation. Neurorehabil Neural Repair. 2007;21(1):14–24. doi: 10.1177/1545968306291858. [DOI] [PubMed] [Google Scholar]
- 5.Maciejasz P, Eschweiler J, Gerlach-Hahn K, Jansen-Troy A, Leonhardt S. A survey on robotic devices for upper limb rehabilitation. J Neuroeng Rehabil. 2014;11(1):3. doi: 10.1186/1743-0003-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laver KE, George S, Thomas S, Deutsch JE, Crotty M. Virtual reality for stroke rehabilitation. Cochrane Database Syst Rev. 2011;(9):CD008349. doi: 10.1002/14651858.CD008349.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Kwakkel G, Kollen B, Krebs H. Effects of robot-assisted therapy on upper limb recovery after stroke: a systematic review. Neurorehabil Neural Repair. 2008;22(2):111–121. doi: 10.1177/1545968307305457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fluet GG, Merians AS, Qinyin Q, Adamovich SV. Sensorimotor training in virtual environments produces similar outcomes to real world training with greater efficiency. Paper presented at: International Conference on Virtual Rehabilitation (ICVR); 2013; August 26–29; Philadelphia, PA, USA. 2013. [Google Scholar]
- 9.Saposnik G, Teasell R, Mamdani M, et al. Effectiveness of virtual reality using Wii gaming technology in stroke rehabilitation: a pilot randomized clinical trial and proof of principle. Stroke. 2013;41(7):1477–1484. doi: 10.1161/STROKEAHA.110.584979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henderson A, Korner-Bitensky N, Levin M. Virtual reality in stroke rehabilitation: a systematic review of its effectiveness for upper limb motor recovery. Top Stroke Rehabil. 2007;14(2):52–61. doi: 10.1310/tsr1402-52. [DOI] [PubMed] [Google Scholar]
- 11.Merians A, Fluet GG, Qiu Q, Saleh S, Lafond I, Adamovich SV. Robotically facilitated virtual rehabilitation of arm transport integrated with finger movement in persons with hemiparesis. J Neuroeng Rehabil. 2011;8:27. doi: 10.1186/1743-0003-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Housman S, Scott K, Reinkensmeyer D. A randomized controlled trial of gravity-supported, computer-enhanced arm exercise for individuals with severe hemiparesis. Neurorehabil Neural Repair. 2009;23(5):505–514. doi: 10.1177/1545968308331148. [DOI] [PubMed] [Google Scholar]
- 13.Krebs HI, Mernoff S, Fasoli SE, Hughes R, Stein J, Hogan N. A comparison of functional and impairment-based robotic training in severe to moderate chronic stroke: a pilot study. NeuroRehabilitation. 2008;23(1):81–87. [PMC free article] [PubMed] [Google Scholar]
- 14.Brokaw EB, Nichols D, Holley RJ, Lum PS. Robotic therapy provides a stimulus for upper limb motor recovery after stroke that is complementary to and distinct from conventional therapy. Neurorehabil Neural Repair. 2014;28(4):367–376. doi: 10.1177/1545968313510974. [DOI] [PubMed] [Google Scholar]
- 15.Klamroth-Marganska V, Blanco J, Campen K, et al. Three-dimensional, task-specific robot therapy of the arm after stroke: a multicentre, parallel-group randomised trial. Lancet Neurol. 2014;13(2):159–166. doi: 10.1016/S1474-4422(13)70305-3. [DOI] [PubMed] [Google Scholar]
- 16.Balasubramanian S, Klein J, Burdet E. Robot-assisted rehabilitation of hand function. Curr Opin Neurol. 2013;23(6):661–670. doi: 10.1097/WCO.0b013e32833e99a4. [DOI] [PubMed] [Google Scholar]
- 17.Michaelsen SM, Magdalon EC, Levin MF. Grip aperture scaling to object size in chronic stroke. Motor Control. 2009;13(2):197–217. doi: 10.1123/mcj.13.2.197. [DOI] [PubMed] [Google Scholar]
- 18.Krakauer JW, Carmichael ST, Corbett D, Wittenberg GF. Getting neurorehabilitation right: what can be learned from animal models? Neurorehabil Neural Repair. 2012;26(8):923–931. doi: 10.1177/1545968312440745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirelman A, Bonato P, Deutsch JE. Effects of training with a robot-virtual reality system compared with a robot alone on the gait of individuals after stroke. Stroke. 2009;40(1):169–174. doi: 10.1161/STROKEAHA.108.516328. [DOI] [PubMed] [Google Scholar]
- 20.Subramanian SK, Lourenco CB, Chilingaryan G, Sveistrup H, Levin MF. Arm motor recovery using a virtual reality intervention in chronic stroke: randomized control trial. Neurorehabil Neural Repair. 2013;27(1):13–23. doi: 10.1177/1545968312449695. [DOI] [PubMed] [Google Scholar]
- 21.van Kordelaar J, van Wegen EEH, Nijland RHM, Daffertshofer A, Kwakkel G. Understanding adaptive motor control of the paretic upper limb early poststroke: the EXPLICIT-stroke Program. Neurorehabil Neural Repair. 2013;27(9):854–863. doi: 10.1177/1545968313496327. [DOI] [PubMed] [Google Scholar]
- 22.Lum P, Mulroy S, Amdur R, Requejo P, Prilutsky B, Dromerick A. Gains in upper extremity function after stroke via recovery or compensation: potential differential effects on amount of real-world limb use. Top Stroke Rehabil. 2009;16(4):237–253. doi: 10.1310/tsr1604-237. [DOI] [PubMed] [Google Scholar]
- 23.Wu CY, Liing RJ, Chen HC, Chen CL, Lin KC. Arm and trunk movement kinematics during seated reaching within and beyond arm’s length in people with stroke: a validity study. Phys Ther. 2014;94(6):845–856. doi: 10.2522/ptj.20130101. [DOI] [PubMed] [Google Scholar]
- 24.Fluet GG, Merians AS, Qiu Q, Davidow A, Adamovich SV. Comparing integrated training of the hand and arm with isolated training of the same effectors in persons with stroke using haptically rendered virtual environments, a randomized clinical trial. J Neuroeng Rehabil. 2014;11(1):126. doi: 10.1186/1743-0003-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fugl-Meyer AR. Post-stroke hemiplegia assessment of physical properties. Scand J Rehabil Med Suppl. 1980;7:85–93. [PubMed] [Google Scholar]
- 26.Dobkin BH. Recommendations for publishing case studies of cell transplantation for spinal cord injury. Neurorehabil Neural Repair. 2010;24(8):687–691. doi: 10.1177/1545968310377508. [DOI] [PubMed] [Google Scholar]
- 27.Lin JH, Hsu MJ, Sheu CF, et al. Psychometric comparisons of 4 measures for assessing upper-extremity function in people with stroke. Phys Ther. 2009;89(8):840–850. doi: 10.2522/ptj.20080285. [DOI] [PubMed] [Google Scholar]
- 28.Page SJ, Fulk GD, Boyne P. Clinically important differences for the upper-extremity Fugl–Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther. 2012;92(6):791–798. doi: 10.2522/ptj.20110009. [DOI] [PubMed] [Google Scholar]
- 29.Wolf SL, Thompson PA, Morris DM, et al. The EXCITE trial: attributes of the Wolf Motor Function Test in patients with subacute stroke. Neurorehabil Neural Repair. 2005;19(3):194–205. doi: 10.1177/1545968305276663. [DOI] [PubMed] [Google Scholar]
- 30.Hsieh YW, Wu CY, Lin KC, Chang YF, Chen CL, Liu JS. Responsiveness and validity of three outcome measures of motor function after stroke rehabilitation. Stroke. 2009;40(4):1386–1391. doi: 10.1161/STROKEAHA.108.530584. [DOI] [PubMed] [Google Scholar]
- 31.Schettino LF, Adamovich SV, Hening W, Tunik E, Sage J, Poizner H. Hand preshaping in Parkinson’s disease: effects of visual feedback and medication state. Exp Brain Res. 2006;168(1–2):186–202. doi: 10.1007/s00221-005-0080-4. [DOI] [PubMed] [Google Scholar]
- 32.Adamovich SV, Berkinblit MB, Hening W, Sage J, Poizner H. The interaction of visual and proprioceptive inputs in pointing to actual and remembered targets in Parkinson’s disease. Neuroscience. 2001;104(4):1027–1041. doi: 10.1016/s0306-4522(01)00099-9. [DOI] [PubMed] [Google Scholar]