Abstract
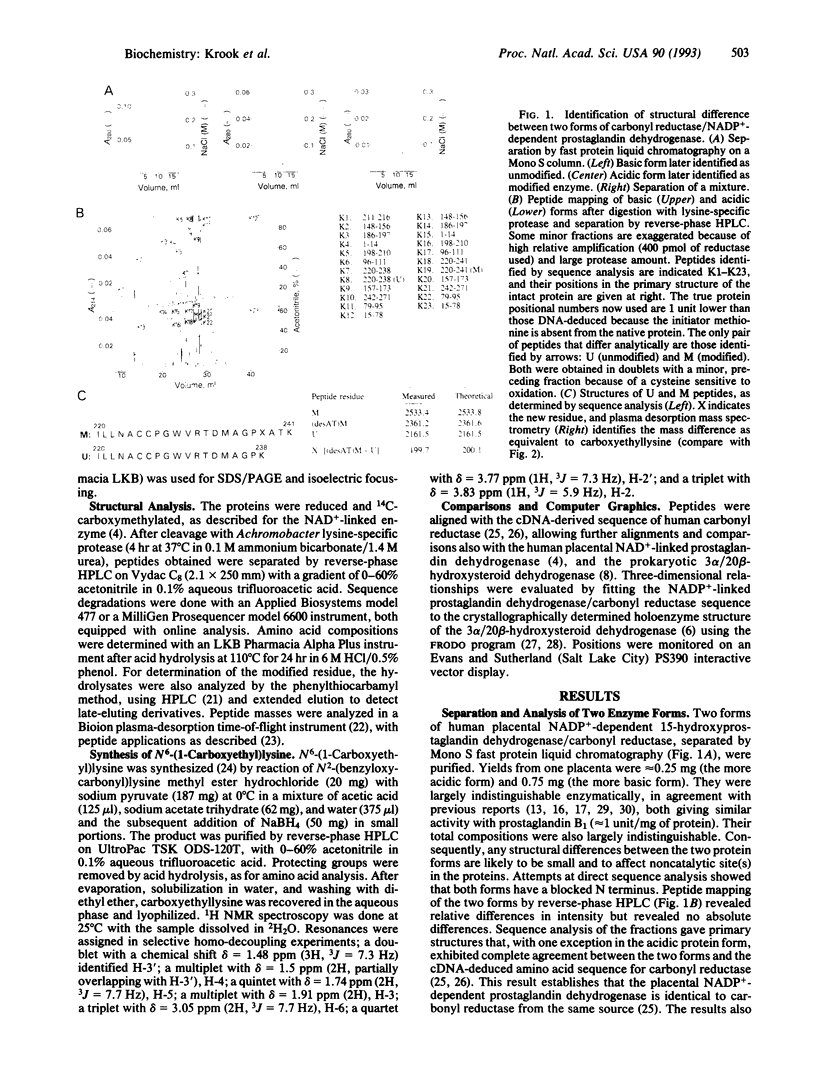
Two different forms of the monomeric NADP(+)-linked prostaglandin dehydrogenase/carbonyl reductase were purified from human placenta and shown to differ by the modification of a lysine residue. The modified and the unmodified proteins were reproducibly recovered in a ratio of approximately 1:3, and both were chemically stable. The modified form was more acidic (pI approximately 7.4 versus pI approximately 7.7) but indistinguishable from the unmodified form in specificity and activity. Amino acid analysis, sequence analysis, mass spectrometry, and chemical synthesis identified the modified residue as N6-(1-carboxyethyl)lysine with C-2 of propionic acid attached to the side-chain N of Lys-238. This compound can be formed from the lysine residue and pyruvate via a Schiff base and subsequent reduction. The enzyme and its NAD(+)-dependent counterpart are distantly related (23% residue identity) and have the same family assignment to short-chain dehydrogenases. Alignments and model-building into the tertiary structure of 3 alpha/20 beta-hydroxysteroid dehydrogenase show that carbonyl reductase has an extra loop (positions 149-189) that forms a separate extension and replaces a backbone C-terminal beta-strand. This change affects the substrate pocket, explaining the different substrate specificities but conserves residues of known functional importance. Carboxyethyllysine at position 238 corresponds to a proteolysis-sensitive position in several short-chain dehydrogenases, less well-defined in the model but close to a surface, and is compatible with the accessibility and enzyme properties observed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohren K. M., von Wartburg J. P., Wermuth B. Kinetics of carbonyl reductase from human brain. Biochem J. 1987 May 15;244(1):165–171. doi: 10.1042/bj2440165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang D. G., Sun M., Tai H. H. Prostaglandin 9-ketoreductase and type II 15-hydroxyprostaglandin dehydrogenase from swine kidney are alternate activities of a single enzyme protein. Biochem Biophys Res Commun. 1981 Mar 31;99(2):745–751. doi: 10.1016/0006-291x(81)91806-4. [DOI] [PubMed] [Google Scholar]
- Chang D. G., Tai H. H. Prostaglandin 9-ketoreductase/type II 15-hydroxyprostaglandin dehydrogenase is not a prostaglandin specific enzyme. Biochem Biophys Res Commun. 1981 Aug 14;101(3):898–904. doi: 10.1016/0006-291x(81)91834-9. [DOI] [PubMed] [Google Scholar]
- Curstedt T., Johansson J., Persson P., Eklund A., Robertson B., Löwenadler B., Jörnvall H. Hydrophobic surfactant-associated polypeptides: SP-C is a lipopeptide with two palmitoylated cysteine residues, whereas SP-B lacks covalently linked fatty acyl groups. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2985–2989. doi: 10.1073/pnas.87.8.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensor C. M., Tai H. H. Site-directed mutagenesis of the conserved tyrosine 151 of human placental NAD(+)-dependent 15-hydroxyprostaglandin dehydrogenase yields a catalytically inactive enzyme. Biochem Biophys Res Commun. 1991 Apr 30;176(2):840–845. doi: 10.1016/s0006-291x(05)80262-1. [DOI] [PubMed] [Google Scholar]
- Flinta C., Persson B., Jörnvall H., von Heijne G. Sequence determinants of cytosolic N-terminal protein processing. Eur J Biochem. 1986 Jan 2;154(1):193–196. doi: 10.1111/j.1432-1033.1986.tb09378.x. [DOI] [PubMed] [Google Scholar]
- Forrest G. L., Akman S., Krutzik S., Paxton R. J., Sparkes R. S., Doroshow J., Felsted R. L., Glover C. J., Mohandas T., Bachur N. R. Induction of a human carbonyl reductase gene located on chromosome 21. Biochim Biophys Acta. 1990 Apr 6;1048(2-3):149–155. doi: 10.1016/0167-4781(90)90050-c. [DOI] [PubMed] [Google Scholar]
- Ghosh D., Weeks C. M., Grochulski P., Duax W. L., Erman M., Rimsay R. L., Orr J. C. Three-dimensional structure of holo 3 alpha,20 beta-hydroxysteroid dehydrogenase: a member of a short-chain dehydrogenase family. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10064–10068. doi: 10.1073/pnas.88.22.10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Israelsson U. Metabolism of prostaglandin E2 in guinea pig liver. I. Identification of seven metabolites. J Biol Chem. 1970 Oct 10;245(19):5107–5114. [PubMed] [Google Scholar]
- Hara A., Deyashiki Y., Nakagawa M., Nakayama T., Sawada H. Isolation of proteins with carbonyl reductase activity and prostaglandin-9-ketoreductase activity from chicken kidney. J Biochem. 1982 Dec;92(6):1753–1762. doi: 10.1093/oxfordjournals.jbchem.a134105. [DOI] [PubMed] [Google Scholar]
- Hassid A., Levine L. Multiple molecular forms of prostaglandin 15-hydroxydehydrogenase and 9-ketoreductase in chicken kidney. Prostaglandins. 1977 Mar;13(3):503–516. doi: 10.1016/0090-6980(77)90028-4. [DOI] [PubMed] [Google Scholar]
- Jarabak J., Luncsford A., Berkowitz D. Substrate specificity of three prostaglandin dehydrogenases. Prostaglandins. 1983 Dec;26(6):849–868. doi: 10.1016/0090-6980(83)90149-1. [DOI] [PubMed] [Google Scholar]
- Jones T. A. Diffraction methods for biological macromolecules. Interactive computer graphics: FRODO. Methods Enzymol. 1985;115:157–171. doi: 10.1016/0076-6879(85)15014-7. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Thirup S. Using known substructures in protein model building and crystallography. EMBO J. 1986 Apr;5(4):819–822. doi: 10.1002/j.1460-2075.1986.tb04287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook M., Marekov L., Jörnvall H. Purification and structural characterization of placental NAD(+)-linked 15-hydroxyprostaglandin dehydrogenase. The primary structure reveals the enzyme to belong to the short-chain alcohol dehydrogenase family. Biochemistry. 1990 Jan 23;29(3):738–743. doi: 10.1021/bi00455a021. [DOI] [PubMed] [Google Scholar]
- Krook M., Prozorovski V., Atrian S., Gonzàlez-Duarte R., Jörnvall H. Short-chain dehydrogenases. Proteolysis and chemical modification of prokaryotic 3 alpha/20 beta-hydroxysteroid, insect alcohol and human 15-hydroxyprostaglandin dehydrogenases. Eur J Biochem. 1992 Oct 1;209(1):233–239. doi: 10.1111/j.1432-1033.1992.tb17281.x. [DOI] [PubMed] [Google Scholar]
- Krüger S., Schlegel W. Prostaglandin-E2 9-ketoreductase from human uterine decidua vera. Eur J Biochem. 1986 Jun 16;157(3):481–485. doi: 10.1111/j.1432-1033.1986.tb09692.x. [DOI] [PubMed] [Google Scholar]
- Lee S. C., Levine L. Prostaglandin metabolism. II. Identification of two 15-hydroxyprostaglandin dehydrogenase types. J Biol Chem. 1975 Jan 25;250(2):548–552. [PubMed] [Google Scholar]
- Lin Y. M., Jarabak J. Isolation of two proteins with 9-ketoprostaglandin reductase and NADP-linked 15-hydroxyprostaglandin dehydrogenase activities and studies on their inhibition. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1227–1234. doi: 10.1016/0006-291x(78)91267-6. [DOI] [PubMed] [Google Scholar]
- Marekov L., Krook M., Jörnvall H. Prokaryotic 20 beta-hydroxysteroid dehydrogenase is an enzyme of the 'short-chain, non-metalloenzyme' alcohol dehydrogenase type. FEBS Lett. 1990 Jun 18;266(1-2):51–54. doi: 10.1016/0014-5793(90)81504-h. [DOI] [PubMed] [Google Scholar]
- Persson B., Krook M., Jörnvall H. Characteristics of short-chain alcohol dehydrogenases and related enzymes. Eur J Biochem. 1991 Sep 1;200(2):537–543. doi: 10.1111/j.1432-1033.1991.tb16215.x. [DOI] [PubMed] [Google Scholar]
- Schieber A., Frank R. W., Ghisla S. Purification and properties of prostaglandin 9-ketoreductase from pig and human kidney. Identity with human carbonyl reductase. Eur J Biochem. 1992 Jun 1;206(2):491–502. doi: 10.1111/j.1432-1033.1992.tb16952.x. [DOI] [PubMed] [Google Scholar]
- Thompson J., Donkersloot J. A. N-(carboxyalkyl)amino acids: occurrence, synthesis, and functions. Annu Rev Biochem. 1992;61:517–557. doi: 10.1146/annurev.bi.61.070192.002505. [DOI] [PubMed] [Google Scholar]
- Toft B. S., Hansen H. S. Glutathione-prostaglandin A1 conjugate as substrate in the purification of prostaglandin 9-ketoreductase from rabbit kidney. Prostaglandins. 1980 Oct;20(4):735–746. doi: 10.1016/0090-6980(80)90112-4. [DOI] [PubMed] [Google Scholar]
- Varughese K. I., Skinner M. M., Whiteley J. M., Matthews D. A., Xuong N. H. Crystal structure of rat liver dihydropteridine reductase. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6080–6084. doi: 10.1073/pnas.89.13.6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wermuth B., Bohren K. M., Heinemann G., von Wartburg J. P., Gabbay K. H. Human carbonyl reductase. Nucleotide sequence analysis of a cDNA and amino acid sequence of the encoded protein. J Biol Chem. 1988 Nov 5;263(31):16185–16188. [PubMed] [Google Scholar]
- Wermuth B. NADP-dependent 15-hydroxyprostaglandin dehydrogenase is homologous to NAD-dependent 15-hydroxyprostaglandin dehydrogenase and other short-chain alcohol dehydrogenases. Prostaglandins. 1992 Jul;44(1):5–9. doi: 10.1016/0090-6980(92)90102-y. [DOI] [PubMed] [Google Scholar]
- Wermuth B. Purification and properties of an NADPH-dependent carbonyl reductase from human brain. Relationship to prostaglandin 9-ketoreductase and xenobiotic ketone reductase. J Biol Chem. 1981 Feb 10;256(3):1206–1213. [PubMed] [Google Scholar]
- Westbrook C., Lin Y. M., Jarabak J. NADP-linked 15-hydroxyprostaglandin dehydrogenase from human placenta: partial purification and characterization of the enzyme and identification of an inhibitor in placental tissue. Biochem Biophys Res Commun. 1977 Jun 6;76(3):943–949. doi: 10.1016/0006-291x(77)91593-5. [DOI] [PubMed] [Google Scholar]






