Abstract
Objective
To provide family physicians with a background understanding of the epidemiology, pathogenesis, histology, and clinical approach to the diagnosis of alopecia areata (AA).
Sources of information
PubMed was searched for relevant articles regarding the pathogenesis, diagnosis, and prognosis of AA.
Main message
Alopecia areata is a form of autoimmune hair loss with a lifetime prevalence of approximately 2%. A personal or family history of concomitant autoimmune disorders, such as vitiligo or thyroid disease, might be noted in a small subset of patients. Diagnosis can often be made clinically, based on the characteristic nonscarring, circular areas of hair loss, with small “exclamation mark” hairs at the periphery in those with early stages of the condition. The diagnosis of more complex cases or unusual presentations can be facilitated by biopsy and histologic examination. The prognosis varies widely, and poor outcomes are associated with an early age of onset, extensive loss, the ophiasis variant, nail changes, a family history, or comorbid autoimmune disorders.
Conclusion
Alopecia areata is an autoimmune form of hair loss seen regularly in primary care. Family physicians are well placed to identify AA, characterize the severity of disease, and form an appropriate differential diagnosis. Further, they are able educate their patients about the clinical course of AA, as well as the overall prognosis, depending on the patient subtype.
Case
A 25-year-old man was getting his regular haircut when his barber pointed out several areas of hair loss. The man booked an appointment with his family physician who noted multiple circular areas of alopecia with small “exclamation mark” hairs at the periphery. During the assessment, the gentleman recalled some mild burning and itching at these sites in the preceding months. A diagnosis of alopecia areata (AA) was made.
Primary care physicians are often called upon to assess and treat hair loss. Alopecia areata is an autoimmune form of hair loss seen regularly in primary care. At any given time, AA is found in 0.1% to 0.2% of the population, as established by the NHANES-I (first National Health and Nutrition Examination Survey).1 All ethnic backgrounds appear to be similarly affected. Further, a retrospective population-based study looking at incidence rates revealed no difference between the sexes, but identified an individual’s lifetime chance of developing AA to be 1.7%.2 The onset of AA is typically before the age of 40 in 70% to 80% of those affected; however, a substantial proportion (48%) will show clinical signs during their first and second decades, making AA a common cause of hair loss in children.3,4
Sources of information
The PubMed database was searched up to November 14, 2014, for relevant articles regarding the pathogenesis, diagnosis, and prognosis of AA. Search terms used included alopecia areata, alopecia totalis, alopecia universalis, alopecia and review, alopecia areata and pathogenesis, and alopecia areata and prognosis.
Main message
Morphology
Although there are many clinical forms of AA, the condition typically presents as 1 or more well-defined round or oval patches of hair loss on the scalp (Figure 1).5,6 Toward the periphery of the area of hair loss, one can usually note characteristic, smaller 3- to 4-mm exclamation mark hairs (Figure 2).7,8 This exclamation mark appearance describes a damaged strand that is missing its distal end.8 It is thicker at its damaged section and notably thinner proximally as it enters the scalp.8,9 Exclamation mark hairs occur only in acute forms of AA and are not seen in patients with longstanding areas of hair loss.9
Figure 1.
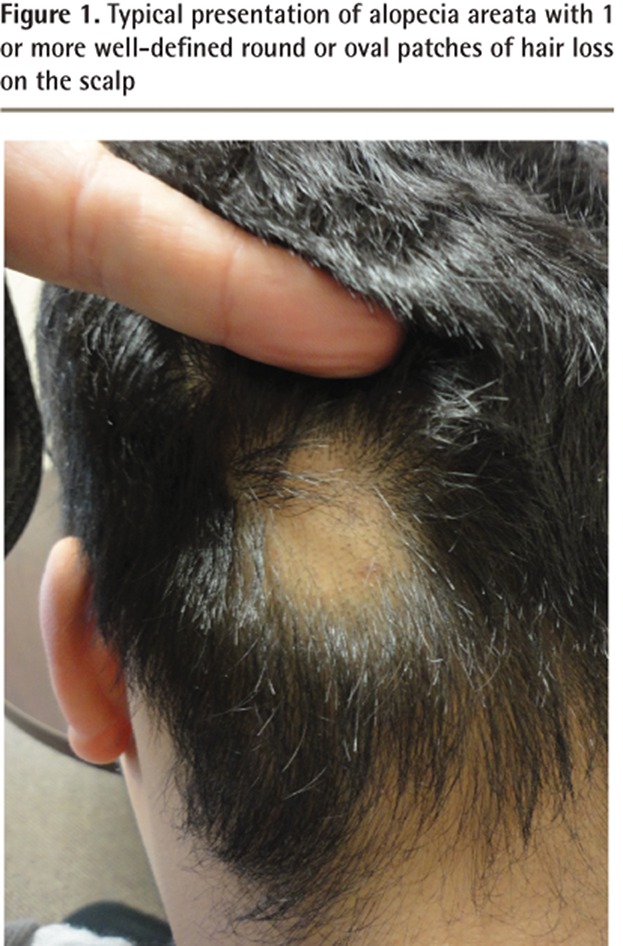
Typical presentation of alopecia areata with 1 or more well-defined round or oval patches of hair loss on the scalp
Figure 2.
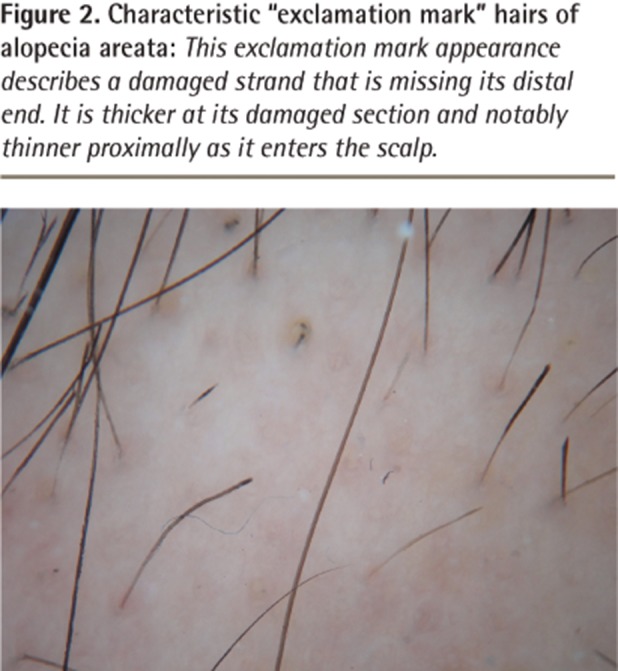
Characteristic “exclamation mark” hairs of alopecia areata: This exclamation mark appearance describes a damaged strand that is missing its distal end. It is thicker at its damaged section and notably thinner proximally as it enters the scalp.
Although spontaneous resolution and regrowth of the patch might occur in up to 50% to 80% of individuals in certain subgroups, it is also possible for additional patches to form, resulting in multiple areas of hair loss coalescing into a larger lesion or eventually involving the entire scalp, which is known as alopecia totalis (Figure 3).5,6,10,11 Other patterns of AA include the acute diffuse type, in which there is sudden hair loss all over the scalp, or the ophiasis pattern, in which hair is lost on the posterior and lateral aspects of the scalp (Figure 4).5,12,13
Figure 3.
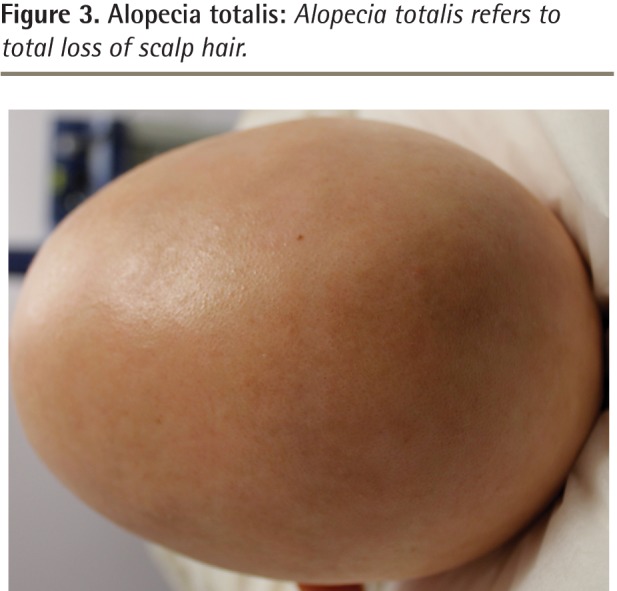
Alopecia totalis: Alopecia totalis refers to total loss of scalp hair.
Figure 4.
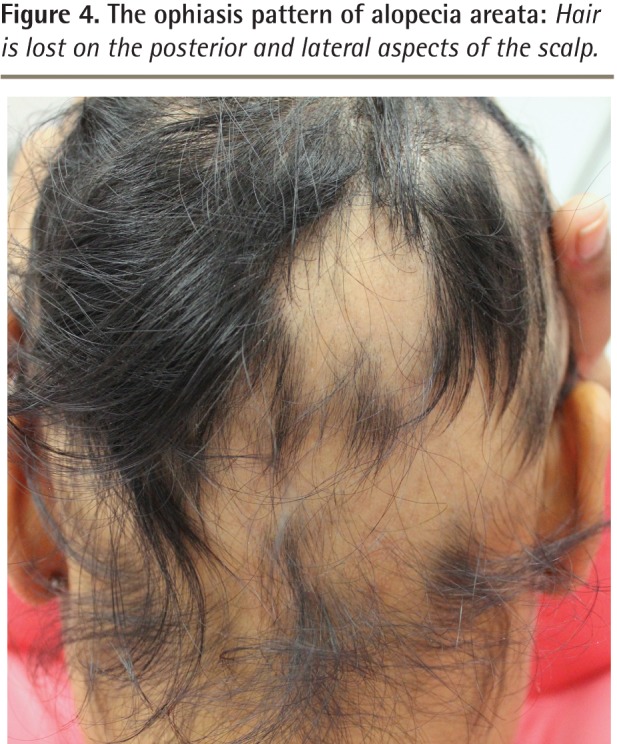
The ophiasis pattern of alopecia areata: Hair is lost on the posterior and lateral aspects of the scalp.
Hair loss can present on any part of the body and might involve areas such as the eyelashes, eyebrows, beard, and pubic area.5,13 The disease can progress to the point of global hair loss, after which it is known as alopecia universalis.5,13 Nail changes are present in up to 7% to 66% of those with AA, and although many types of changes are possible, nail pitting (Figure 5) is classically described.6,14–18
Figure 5.
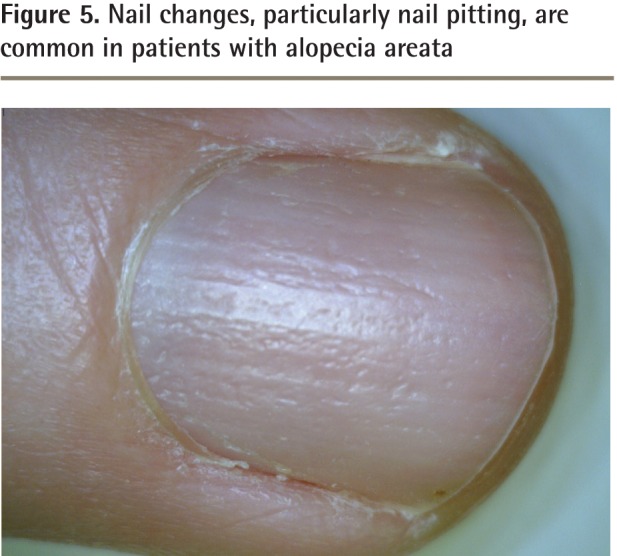
Nail changes, particularly nail pitting, are common in patients with alopecia areata
Histology
Hair follicles from patients with AA exhibit both abnormal hair cycling and inflammation. Normally, a hair follicle goes through 3 phases during its growth cycle: anagen, catagen, and telogen. The hair shaft elongates during the anagen phase. It can remain in this phase for a period of months to years.9 Next the follicle enters a 2- to 4-week period known as the catagen phase, during which it prepares to enter the “resting phase” known as the telogen phase.9 This final part of the follicular growth cycle takes approximately 3 months to complete, after which the hair shaft is lost as a “club hair” and the hair follicle begins the cycle again.9
In AA, inflammation causes a large proportion of hair follicles to shift from the anagen phase to the telogen phase. In the acute stages of AA, most hair follicles are still in the anagen phase. If one were to perform a scalp biopsy at this stage, the histologic examination would reveal an excessive amount of lymphocytes in and around the hair follicle. While the normal ratio of anagen to telogen hairs in the scalp is usually 80:20, patients with AA exhibit a 60:40 or even 50:50 anagen to telogen ratio, and in some cases the number of telogen hairs might dominate.8,9,19–22
Prognosis
The natural course of AA is unpredictable. Some patients might experience only a single episode of hair loss during their lifetimes, while others experience multiple recurrences. Further variation occurs during recovery. Some patients have full hair regrowth, yet others remain the same or experience further hair loss.10,11,23
Several epidemiologic studies have identified prognostic factors associated with poor regrowth in individuals affected by AA (Box 1).23–28 In general, hair regrowth is possible and known to be inversely correlated with the extent of hair loss. A study of 191 patients showed regrowth in roughly 40% to 70% of patients in those classified as limited-patch stage.23 However, the greater the area of involvement (alopecia totalis or universalis), the less likely the chance of complete regrowth, and the more likely AA will progress over time.23,24 One exception would be individuals who rapidly lose their hair (acute diffuse and total alopecia variant). Despite substantial hair loss, chances of regrowth are high in this subtype.12 Other poor prognostic indicators include those who experience the ophiasis variant or note coexisting nail changes.25 Compared with adults, early onset of AA in childhood often results in both a greater degree of and progression of AA.23,26 A history of atopy and the coexistence of other autoimmune diseases also confer a poor outcome.25
Box 1. Prognostic factors associated with poor outcomes in individuals with alopecia areata.
The following factors are associated with poorer outcomes:
|
Pathogenesis
The exact cause of AA is still unknown. The current body of evidence supports an autoimmune origin and strong genetic contribution, further modified by unknown environmental influences.29
Genetic contribution: Multiple genetic factors contribute to the development of AA. A positive family history is evident in approximately 10% to 25% of cases.6,30,31 Further support for a genetic contribution comes from a concordant twin study by Rodriguez et al, in which the percentage of monozygotes (42%) who both had AA was much greater than that of the dizygotes (10%).29
Molecular genetics: Extensive work has been done to determine the molecular basis of AA. Alopecia areata is considered to be a T cell–mediated autoimmune disease.19,32 It is important to understand that the hair follicle is fundamentally considered a site of immune privilege, whereby a number of mechanisms tightly control immunologic access and prevent it from autoimmune attack under normal conditions. The development of AA is thought to result from a breakdown of this immune-privileged site.33
A groundbreaking study from 2010 provided a deeper understanding of the genetic basis of AA. By comparing 1054 patient cases and 3278 controls, Petukhova et al discovered 139 single-nucleotide polymorphisms related to the development of AA.34 Further investigation located 8 genes highly correlated with the risk of disease. Interestingly, several of these “susceptibility loci” are thought to play a role in other autoimmune processes such as type 1 diabetes and rheumatoid arthritis, helping to once again strengthen the autoimmune hypothesis for AA.34
Evaluation and differential diagnosis
Multiple hair loss disorders can mimic AA. During examination one should consider possibilities such as trichotillomania, tinea capitis, scarring alopecia, and telogen effluvium.7 However, the diagnosis of AA can usually be made clinically owing to the characteristic appearance of the hair loss regions.7
Individuals with trichotillomania have broken hairs of different lengths and might or might not report a history of hair pulling when asked. Many also have concomitant psychiatric disorders.35 Tinea capitis is a fungal infection of the scalp. Examination typically reveals hair loss associated with scale. A definitive diagnosis can be made by performing a scalp scraping of the affected region and sending it for culture.36 Scarring alopecias, particularly a condition known as pseudopelade, can closely mimic AA. However, the areas of hair loss are permanent, and in this particular condition the hair follicle openings are not visible upon clinical examination. If there is any concern, a scalp biopsy can be performed looking for the previously mentioned characteristics in order to differentiate the 2 conditions.37
Associated disorders
A review of the National Alopecia Areata Registry revealed that approximately 16% of AA patients had other coexisting autoimmune conditions.28 Specifically, rates of thyroid disease in these patients are thought to range from approximately 8% to 28% and vitiligo from 2.5% to 4.1%.24,27,38,39 Concomitant type 1 diabetes mellitus (0.6%) and lupus erythematosus (0.6%) were noted in a review of 513 patients performed by Goh et al.27 Atopy is also strongly correlated with AA, showing comorbidity rates of up to 40%, compared with the background rate in the general population of 20%.28,40
Laboratory evaluation
A biopsy is not necessary in most cases of AA. Unusual forms of AA, including diffuse AA, might closely mimic other conditions, making a biopsy helpful in these specific situations. While we routinely check complete blood count and ferritin, thyroid-stimulating hormone, and 25-hydroxyvitamin D levels in AA, at present there are no evidence-based guidelines to direct the workup or studies highlighting the cost-effectiveness of screening for concomitant disorders. Preliminary small studies show a possible increased risk of 25-hydroxyvitamin D deficiency in AA; however, more extensive research is still required.41,42 Therefore, we recommend a full and comprehensive review of systems be used to guide any further workup that might be required.
Revisiting the case
This 25-year-old gentleman had a classic presentation of AA in the multiple-patch stage. Further evaluation revealed a history of atopy, and examination revealed nail pitting. Findings from routine bloodwork did not reveal any other concomitant disorders. The man is upset by his hair loss and wants to understand his treatment options.
Conclusion
Hair loss is often encountered in primary care and it can be very distressing to patients. Family physicians are well positioned to identify AA, counsel patients, and initiate treatment. Treatment options are discussed in part 2 of this review (page 757).43
EDITOR’S KEY POINTS
Alopecia areata is an autoimmune form of hair loss with a lifetime prevalence of about 2%. Most cases can be recognized by well-defined patches of hair loss with “exclamation mark” hairs at the periphery.
Scalp biopsy is typically not required to make the diagnosis; however, if one is performed, results will often show a lymphocytic infiltrate around the bulb region of the hair follicle.
Alopecia areata has a genetic predisposition and can be associated with other autoimmune disorders.
Footnotes
This article is eligible for Mainpro-M1 credits. To earn credits, go to www.cfp.ca and click on the Mainpro link.
This article has been peer reviewed.
La traduction en français de cet article se trouve à www.cfp.ca dans la table des matières du numéro de septembre 2015 à la page e401.
Contributors
Both authors contributed to the literature review, analysis, and interpretation, and to preparing the manuscript for submission.
Competing interests
None declared
References
- 1.Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128(5):702. doi: 10.1001/archderm.1992.01680150136027. [DOI] [PubMed] [Google Scholar]
- 2.Safavi KH, Muller SA, Suman VJ, Moshell AN, Melton LJ., 3rd Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70(7):628–33. doi: 10.4065/70.7.628. [DOI] [PubMed] [Google Scholar]
- 3.Shellow WV, Edwards JE, Koo JY. Profile of alopecia areata: a questionnaire analysis of patient and family. Int J Dermatol. 1992;31(3):186–9. doi: 10.1111/j.1365-4362.1992.tb03932.x. [DOI] [PubMed] [Google Scholar]
- 4.Kyriakis KP, Paltatzidou K, Kosma E, Sofouri E, Tadros A, Rachioti E. Alopecia areata prevalence by gender and age. J Eur Acad Dermatol Venereol. 2009;23(5):572–3. doi: 10.1111/j.1468-3083.2008.02956.x. Epub 2008 Sep 8. [DOI] [PubMed] [Google Scholar]
- 5.Anderson I. Alopecia areata: a clinical study. Br Med J. 1950;2(4691):1250–2. doi: 10.1136/bmj.2.4691.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller SA, Winkelmann RK. Alopecia areata. An evaluation of 736 patients. Arch Dermatol. 1963;88:290–7. doi: 10.1001/archderm.1963.01590210048007. [DOI] [PubMed] [Google Scholar]
- 7.Messenger AG, McKillop J, Farrant P, McDonagh AJ, Sladden M. British Association of Dermatologists’ guidelines for the management of alopecia areata 2012. Br J Dermatol. 2012;166(5):916–26. doi: 10.1111/j.1365-2133.2012.10955.x. [DOI] [PubMed] [Google Scholar]
- 8.Eckert J, Church RE, Ebling FJ. The pathogenesis of alopecia areata. Br J Dermatol. 1968;80(4):203–10. doi: 10.1111/j.1365-2133.1968.tb11960.x. [DOI] [PubMed] [Google Scholar]
- 9.Sperling LC, Lupton GP. Histopathology of non-scarring alopecia. J Cutan Pathol. 1995;22(2):97–114. doi: 10.1111/j.1600-0560.1995.tb01391.x. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda T. A new classification of alopecia areata. Dermatologica. 1965;131(6):421–45. doi: 10.1159/000254503. [DOI] [PubMed] [Google Scholar]
- 11.Walker SA, Rothman S. A statistical study and consideration of endocrine influences. J Invest Dermatol. 1950;14(6):403–13. doi: 10.1038/jid.1950.52. [DOI] [PubMed] [Google Scholar]
- 12.Lew BL, Shin MK, Sim WY. Acute diffuse and total alopecia: a new subtype of alopecia areata with a favorable prognosis. J Am Acad Dermatol. 2009;60(1):85–93. doi: 10.1016/j.jaad.2008.08.045. Epub 2008 Nov 6. [DOI] [PubMed] [Google Scholar]
- 13.Finner AM. Alopecia areata: clinical presentation, diagnosis, and unusual cases. Dermatol Ther. 2011;24(3):348–54. doi: 10.1111/j.1529-8019.2011.01413.x. [DOI] [PubMed] [Google Scholar]
- 14.Kasumagic-Halilovic E, Prohic A. Nail changes in alopecia areata: frequency and clinical presentation. J Eur Acad Dermatol Venereol. 2009;23(2):240–1. doi: 10.1111/j.1468-3083.2008.02830.x. Epub 2008 Jun 1. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi V, Baruah MC, Bhattacharaya SN. Nail changes in alopecia areata: incidence and pattern. Indian J Dermatol Venereol Leprol. 2003;69(2):114–5. [PubMed] [Google Scholar]
- 16.Nanda A, Al-Fouzan AS, Al-Hasawi F. Alopecia areata in children: a clinical profile. Pediatr Dermatol. 2002;19(6):482–5. doi: 10.1046/j.1525-1470.2002.00215.x. [DOI] [PubMed] [Google Scholar]
- 17.Sharma VK, Dawn G, Muralidhar S, Kumar B. Nail changes in 1000 Indian patients with alopecia areata. J Eur Acad Dermatol Venereol. 1998;10(2):189–91. doi: 10.1111/j.1468-3083.1998.tb00727.x. [DOI] [PubMed] [Google Scholar]
- 18.Baran R, Dawber RP. Diseases of nails and their management. Oxford, UK: Blackwell Scientific; 1984. Alopecia areata; pp. 165–7. [Google Scholar]
- 19.Whiting DA. Histopathologic features of alopecia areata: a new look. Arch Dermatol. 2003;139(12):1555–9. doi: 10.1001/archderm.139.12.1555. [DOI] [PubMed] [Google Scholar]
- 20.Whiting DA. The histopathology of alopecia areata in vertical and horizontal sections. Dermatol Ther. 2001;14(4):297–305. [Google Scholar]
- 21.Van Scott EJ. Morphologic changes in pilosebaceous units and anagen hairs in alopecia areata. J Invest Dermatol. 1958;31(1):35–43. [PubMed] [Google Scholar]
- 22.Messenger AG, Slater DN, Bleehen SS. Alopecia areata: alterations in the hair growth cycle and correlation with the follicular pathology. Br J Dermatol. 1986;114(3):337–47. doi: 10.1111/j.1365-2133.1986.tb02825.x. [DOI] [PubMed] [Google Scholar]
- 23.Tosti A, Bellavista S, Iorizzo M. Alopecia areata: a long term follow-up study of 191 patients. J Am Acad Dermatol. 2006;55(3):438–41. doi: 10.1016/j.jaad.2006.05.008. Epub 2006 Jun 27. [DOI] [PubMed] [Google Scholar]
- 24.Tan E, Tay YK, Goh CL, Chin Giam Y. The pattern and profile of alopecia areata in Singapore—a study of 219 Asians. Int J Dermatol. 2002;41(11):748–53. doi: 10.1046/j.1365-4362.2002.01357.x. [DOI] [PubMed] [Google Scholar]
- 25.De Waard-van der Spek FB, Oranje AP, De Raeymaecker DM, Peereboom-Wynia JD. Juvenile versus maturity-onset alopecia areata—a comparative retrospective clinical study. Clin Exp Dermatol. 1989;14(6):429–33. doi: 10.1111/j.1365-2230.1989.tb02604.x. [DOI] [PubMed] [Google Scholar]
- 26.Yang S, Yang J, Liu JB, Wang HY, Yang Q, Gao M, et al. The genetic epidemiology of alopecia areata in China. Br J Dermatol. 2004;151(1):16–23. doi: 10.1111/j.1365-2133.2004.05915.x. [DOI] [PubMed] [Google Scholar]
- 27.Goh C, Finkel M, Christos PJ, Sinha AA. Profile of 513 patients with alopecia areata: associations of disease subtypes with atopy, autoimmune disease and positive family history. J Eur Acad Dermatol Venereol. 2006;20(9):1055–60. doi: 10.1111/j.1468-3083.2006.01676.x. [DOI] [PubMed] [Google Scholar]
- 28.Barahmani N, Schabath MB, Duvic M, National Alopecia Areata Registry History of atopy or autoimmunity increases risk of alopecia areata. J Am Acad Dermatol. 2009;61(4):581–91. doi: 10.1016/j.jaad.2009.04.031. Epub 2009 Jul 11. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez TA, Fernandes KE, Dresser KL, Duvic M, National Alopecia Areata Registry Concordance rate of alopecia areata in identical twins supports both genetic and environmental factors. J Am Acad Dermatol. 2010;62(3):525–7. doi: 10.1016/j.jaad.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Friedmann PS. Alopecia areata and auto-immunity. Br J Dermatol. 1981;105(2):153–7. doi: 10.1111/j.1365-2133.1981.tb01200.x. [DOI] [PubMed] [Google Scholar]
- 31.Blaumeiser B, van der Goot I, Fimmers R, Hanneken S, Ritzmann S, Seymons K, et al. Familial aggregation of alopecia areata. J Am Acad Dermatol. 2006;54(4):627–32. doi: 10.1016/j.jaad.2005.12.007. Epub 2006 Jan 23. [DOI] [PubMed] [Google Scholar]
- 32.Todes-Taylor N, Turner R, Wood GS, Stratte PT, Morhenn VB. T cell subpopulations in alopecia areata. J Am Acad Dermatol. 1984;11(2 Pt 1):216–23. doi: 10.1016/s0190-9622(84)70152-6. [DOI] [PubMed] [Google Scholar]
- 33.Christoph T, Müller-Röver S, Audring H, Tobin DJ, Hermes B, Cotsarelis G, et al. The human hair follicle immune system: cellular composition and immune privilege. Br J Dermatol. 2000;142(5):862–73. doi: 10.1046/j.1365-2133.2000.03464.x. [DOI] [PubMed] [Google Scholar]
- 34.Petukhova L, Duvic M, Hordinsky M, Norris D, Price V, Shimomura Y, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466(7302):113–7. doi: 10.1038/nature09114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walsh KH, McDougle CJ. Trichotillomania. Presentation, etiology, diagnosis and therapy. Am J Clin Dermatol. 2001;2(5):327–33. doi: 10.2165/00128071-200102050-00007. [DOI] [PubMed] [Google Scholar]
- 36.Pomeranz AJ, Sabnis SS. Tinea capitis: epidemiology, diagnosis and management strategies. Paediatr Drugs. 2002;4(12):779–83. doi: 10.2165/00128072-200204120-00002. [DOI] [PubMed] [Google Scholar]
- 37.Alzolibani AA, Kang H, Otberg N, Shapiro J. Pseudopelade of Brocq. Dermatol Ther. 2008;21(4):257–63. doi: 10.1111/j.1529-8019.2008.00207.x. [DOI] [PubMed] [Google Scholar]
- 38.Seyrafi H, Akhiani M, Abbasi H, Mirpour S, Gholamrezanezhad A. Evaluation of the profile of alopecia areata and the prevalence of thyroid function test abnormalities and serum autoantibodies in Iranian patients. BMC Dermatol. 2005;5:11. doi: 10.1186/1471-5945-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cunliffe WJ, Hall R, Stevenson CJ, Weightman D. Alopecia areata, thyroid disease and autoimmunity. Br J Dermatol. 1969;81(12):877–81. doi: 10.1111/j.1365-2133.1969.tb15967.x. [DOI] [PubMed] [Google Scholar]
- 40.Hordinsky M, Ericson M. Autoimmunity: alopecia areata. J Investig Dermatol Symp Proc. 2004;9(1):73–8. doi: 10.1111/j.1087-0024.2004.00835.x. [DOI] [PubMed] [Google Scholar]
- 41.Mahamid M, Abu-Elhija O, Samamra M, Mahamid A, Nseir W. Association between vitamin D levels and alopecia areata. Isr Med Assoc J. 2014;16(6):367–70. [PubMed] [Google Scholar]
- 42.D’Ovidio R, Vessio M, d’Ovidio FD. Reduced level of 25-hydroxyvitamin D in chronic/relapsing alopecia areata. Dermatoendocrinol. 2013;5(2):271–3. doi: 10.4161/derm.24411. Epub 2013 Jan 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spano F, Donovan JC. Alopecia areata. Part 2: treatment. Can Fam Physician. 2015;61:757–61. (Eng), e406–11 (Fr). [PMC free article] [PubMed] [Google Scholar]


