Abstract
The objective of this study was to identify predictors of insulin independence and to establish the best clinical tools to follow patients after pancreatic islet transplantation (PIT). Sequential metabolic responses to intravenous (I.V.) glucose (I.V. glucose tolerance test [IVGTT]), arginine and glucose-potentiated argi-nine (glucose-potentiated arginine-induced insulin secretion [GPAIS]) were obtained from 30 patients. We determined the correlation between transplanted islet mass and islet engraftment and tested the ability of each assay to predict return to exogenous insulin therapy. We found transplanted islet mass within an average of 16 709 islet equivalents per kg body weight (IEQ/kg BW; range between 6602 and 29 614 IEQ/ kg BW) to be a poor predictor of insulin independence at 1 year, having a poor correlation between transplanted islet mass and islet engraftment. Acute insulin response to IVGTT (AIRGLU) and GPAIS (AIRmax) were the most accurate methods to determine sub-optimal islet mass engraftment. AIRGLU performed 3 months after transplant also proved to be a robust early metabolic marker to predict return to insulin therapy and its value was positively correlated with duration of insulin independence. In conclusion, AIRGLU is an early metabolic assay capable of anticipating loss of insulin independence at 1 year in T1D patients undergoing PIT and constitutes a valuable, simple and reliable method to follow patients after transplant.
Keywords: Clinical islet transplantation, insulin independence
Introduction
Pancreatic islet transplantation (PIT), a minimally invasive β-cell replacement approach for patients with type 1 diabetes (T1D) complicated by hypoglycemic unawareness, can restore near-normal glycemic control and alleviate severe hypoglycemic episodes. Although the currently accepted therapeutic approach of transplanting islets isolated from more than one donor pancreas has resulted in insulin independence for many recipients, the majority return to some insulin requirement even when persistent islet graft function is evident by C-peptide levels.
Assessment of β-cell secretory capacity from glucose potentiation of insulin or C-peptide release in response to a nonglucose secretagogue such as arginine is the most accurate method to determine functional islet β-cell mass in humans (1). Testing of β-cell function by measuring insulin or C-peptide responses to intravenous (I.V.) glucose (AIRGLU or ACRGLU) or arginine (AIRARG or ACRARG) alone has been used as a surrogate measure for β-cell secretory capacity in islet recipients (2,3). A clearer understanding of initial and long-term islet β-cell engraftment is necessary to improve long-term insulin independence.
To date, metabolic studies in PIT have revealed a markedly impaired first-phase insulin response to AIRGLU (4–6), a less impaired response to AIRARG (7) and a dramatically blunted β-cell response to glucose potentiation of arginine-induced secretion (GPAIS; AIRMAX; Refs. 8,9). AIRGLU is lost before AIRARG during β-cell mass reductions (10), likely because increases in fasting glucose desensitize β-cell response to glucose stimulation but potentiate β-cell response to arginine (11). As a surrogate measure for AIRMAX, AIRGLU has been considered a simple and sensitive indicator to assess early islet graft impairment, whereas AIRARG has been considered a more accurate test to predict surviving islet β-cell mass (3,7).
The purpose of this comparison was to establish predictors of insulin independence after PIT. To address this, sequential metabolic testing at 3, 6 and 12 months posttrans-plant was performed in 30 PIT recipients transplanted at three different institutions and the results compared to 10 matched control subjects to evaluate β-cell responsiveness to glucose, arginine and GPAIS.
This study also addresses four clinically important questions in the field of islet transplantation:
What is the metabolic impairment (β-cell secretory capacity) of insulin-independent PIT recipients versus well-matched nondiabetic controls?
Does transplanted islet mass correlate with insulin independence at 1 year?
Can insulin and C-peptide secretion be used as a clinical tool to predict subsequent exogenous insulin requirement?
Do patients who remain insulin independent 1 year after PIT have a greater engrafted islet mass than patients returning to insulin within 1-year posttransplant?
Materials and Methods
Subjects
Potential islet recipients (18–65 years; T1D > 5 years) were recruited using standard inclusion/exclusion criteria: (http://www.fda.gov/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/cellularandgenetherapy/ucm182440.htm). Inclusion criteria consisted of T1D with labile diabetes manifested by hypoglycemic unawareness complicated by frequent severe hypoglycemic episodes, recurrent ketoacidosis or already on immunosuppression for an existing kidney transplant. Thirty T1D subjects with longstanding C-peptide-negative disease were listed for PIT at the University of Miami, University of Pennsylvania and University of Wisconsin (UW; Table 1). Twenty-four patients underwent islet transplant alone (ITA) and six islet after kidney (IAK). All received immunosuppressive therapy based on modifications to the previously published Edmonton protocol (12). Briefly, IL-2 receptor blockade (1 mg/kg every 14 days for five consecutive doses) was given at transplant and steroid-free immunosuppression maintenance using tacrolimus and sirolimus (2–5 ng/mL and 10–14 ng/mL, respectively) for the first year. ITA and IAK recipients trough levels were managed similarly using sirolimus (8–12 ng/mL) and calcineurin inhibitors (tacrolimus 2–5 ng/mL). Four patients with well-functioning kidney allografts were corticosteroid-free for at least 9 months at enrollment. The additiona four patients received prednisone maintenance therapy of ≤5 mg/day. Patients receiving maintenance therapy with mycophenolate mofetil were maintained without dose modification. Eight subjects received a single dose of infliximab (Remicade®, Centocor, Malvera, PA, USA; 5 mg/kg), 2 h before first infusion.
Table 1.
Baseline demographic data for all islet transplant recipients (by center)
| Patients | Age (years) |
Gender (M/F) |
Weight (kg) |
BMI (kg/m2) | Diabetes (years) |
Insulin requirement pre-Tx (units/kg/day) |
Complications related to T1DM |
HbA1c pre-Tx (%) |
Hypoglycemia unawareness |
Creatinine clearance (mL/min) |
|---|---|---|---|---|---|---|---|---|---|---|
| Center 1 | ||||||||||
| 1 | 32 | M | 75.5 | 26.2 | 25 | 0.45 | R | 7.8 | Yes | 88.6 |
| 2 | 45 | M | 67.9 | 22.0 | 29 | 0.52 | R | 4.8 | Yes | 112 |
| 3 | 54 | M | 61.8 | 19.5 | 38 | 0.32 | None | 6.2 | Yes | 105.5 |
| 4 | 28 | M | 78.6 | 26.5 | 9 | 0.55 | None | 7.4 | Yes | 131.6 |
| 5 | 39 | F | 47.6 | 17.6 | 36 | 0.46 | R, K | 9.8 | Yes | 63.1 |
| 6 | 42 | F | 57.9 | 23.2 | 33 | 0.45 | R, K | 7.7 | Yes | 75.7 |
| Center 2 | ||||||||||
| 7 | 53 | M | 63 | 21.5 | 41 | 0.42 | R, N | 6.1 | Yes | 102 |
| 8 | 48 | F | 73 | 23.8 | 36 | 0.47 | None | 8.6 | Yes | 87 |
| 9 | 44 | M | 62 | 23.1 | 35 | 0.64 | R, N, K | 6.3 | Yes | 53 |
| 10 | 45 | F | 76 | 27.1 | 33 | 0.62 | R, N | 9.0 | Yes | 111 |
| 11 | 41 | F | 75 | 21.5 | 29 | 0.65 | N | 6.1 | Yes | 112 |
| 12 | 25 | M | 77 | 22 | 11 | 0.65 | None | 7.4 | Yes | 134 |
| 13 | 31 | M | 77 | 25.3 | 28 | 0.87 | None | 7.7 | Yes | 124 |
| 14 | 61 | F | 53 | 22 | 36 | 0.5 | R, N, K | 7.8 | Yes | 78 |
| 15 | 51 | F | 60 | 25.8 | 36 | 0.4 | R, N, K | 7.5 | Yes | 55 |
| 16 | 43 | F | 56 | 21.1 | 41 | 0.57 | R, N, K | 6.7 | Yes | 52 |
| Center 3 | ||||||||||
| 17 | 37 | M | 79 | 23.5 | 10 | 0.5 | None | 7.5 | Yes | 142 |
| 18 | 41 | F | 66 | 23.5 | 31 | 0.5 | R | 7.2 | Yes | 96 |
| 19 | 32 | F | 68 | 25.7 | 19 | 0.47 | R | 9.4 | Yes | 128 |
| 20 | 38 | M | 74 | 24.7 | 14 | 0.47 | None | 7.3 | Yes | 124 |
| 21 | 43 | F | 57 | 23.7 | 37 | 0.54 | R | 7.7 | Yes | 119 |
| 22 | 44 | F | 70 | 25.7 | 30 | 0.56 | R, K | 9.4 | Yes | 82 |
| 23 | 36 | M | 69 | 26.6 | 34 | 0.58 | R | 8.0 | Yes | 129 |
| 24 | 35 | M | 97 | 28 | 18 | 0.33 | R | 8.3 | Yes | 103 |
| 25 | 36 | M | 66 | 26 | 34 | 0.53 | R | 6.8 | Yes | 102 |
| 26 | 51 | M | 88 | 26 | 39 | 0.48 | None | 7.0 | Yes | 173 |
| 27 | 59 | M | 86 | 27 | 46 | 0.57 | R | 8.3 | Yes | 88 |
| 28 | 52 | F | 53 | 22 | 44 | 0.29 | R | 7.6 | Yes | 81 |
| 29 | 56 | F | 59 | 23 | 35 | 0.32 | None | Yes | 75 | |
| 30 | 40 | F | 60 | 23 | 12 | 0.55 | None | 7.1 | Yes | 125 |
Subjects were asked to record their daily insulin dose in self-monitoring diaries. Insulin dependence was defined as need for exogenous insulin to maintain HbA1c ≤ 6.5% and receiving exogenous insulin to maintain fasting capillary glucose level ≤140 mg/dL (7.8 mmol/L) at a minimum of 4 of 7 days per week, with 2-h postprandial capillary glucose levels not exceeding 180 mg/dL (10.0 mmol/L) more than three times per week. Subjects not receiving insulin who had HbA1c ≤ 6.5% with fasting and 2-h postprandial within target were deemed insulin independent. Ten controls matched for BMI, gender and age included seven historical controls (13) and three contemporaneous controls. Metabolic testing was approved by UW, Miami and Penn Health Sciences Institutional Review Boards, with written informed consent obtained from all subjects.
Islet monitoring: tests of β -cell function and secretory capacity
Most patients were monitored after islet transplantation using a similar follow-up protocol by the three different institutions. Patients were followed at transplant clinic at 2, 4, 8 and 12 weeks after each infusion and followed every 3 months. The timing of follow-up assessments was “reset” with additional transplants. Stimulation tests using glucose and/or arginine as secretagogues measuring insulin and C-peptide responses and changes over time were compared between islet recipients and controls. Sequential metabolic testing was performed at 3, 6 and 12 months post last islet infusion. Briefly, subjects fasted overnight before testing. Insulin-dependent subjects withheld long-acting insulin for 24 h and short-acting insulin for 12 h before testing. If necessary, I.V. insulin was administered overnight to maintain blood glucose concentration <7 mM and discontinued ≥45 min before testing. On the morning of the test, one additional catheter was placed in the contralateral hand vein for blood sampling and the hand placed in a thermoregulated box (50°C) to promote optimal arterialization of venous blood. Thirty patients were available at 3 months, 19 at 6 months and 27 at 12 months posttransplant. At each time point, blood samples were collected for glucose, insulin and C-peptide analysis. Plasma glucose was measured immediately using a YSI 2300 Stat Glucose Analyzer (Yellow Springs Instruments, Yellow Springs, OH, USA). Additional serum or plasma was collected for determination of immunoreactive insulin and C-peptide concentrations by commercial assays (Millipore, Bellerica, MA, USA). The samples were assessed by each institution and third-party validation of the data was performed independently by each laboratory (data not shown)
I.V. glucose tolerance test (IVGTT)
After overnight fasting and baseline blood sampling at −15, −10 and −5 min, 0.3 g/kg of 50% glucose was injected over a 1 -min period starting at t = 0. Additional blood samples were collected at t = 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 18 and 20, 30, 40 and 60 min after injection. Data from the first 10 min of the test were used to calculate incremental area under the curve (AUC) for insulin (AIRGLU) and C-peptide (ACRGLU) in response to I.V. glucose. AUC was calculated by the trapezoidal rule with the mean of the baseline values subtracted. IVGT was evaluated by glucose disappearance rate [Kg = ln(glucose)/min × 100], calculated as the slope of the natural log of glucose values between 10 and 20 min with least-squares linear regression (7,14).
Arginine stimulation test
The arginine stimulation test (AST) was performed at normoglycemia (~5 mM glucose) after overnight fasting. After baseline blood sampling at −15, −10 and −5 min, 5 g of l-arginine hydrochloride (10% solution; Rgene, Pharmacia Inc., Clayton, NC, USA) was given I.V. over a 30-s period. Blood samples were obtained at t = 2, 3, 4, 5, 7, 10, 15, 20, 25 and 30 min after injection to measure glucose, insulin and C-peptide. Data from t = 2–5 min were used to calculate incremental AUC with the mean of the baseline values subtracted for insulin (AIRARG) and C-peptide (ACRARG) by means of the trapezoidal rule (7,13,14).
Glucose-potentiated arginine test
After the AST, GPAIS was performed at hyperglycemia (>15 mM glucose) to calculate maximal acute insulin (AIRMAX) and C-peptide (ACRMAX) responses to arginine. Plasma glucose level was increased over 45 min to >15 mM using a modified hyperglycemic clamp technique with a priming rate of 20% dextrose solution infused over 15 min, subsequently modified based on plasma glucose determinations every 5 min to maintain the hyperglycemic level above 15 mM (15). After 45 min of hyperglycemic clamp, prestimulus blood samples were obtained at −10, −5 and 0min and a second 5 g arginine injection given. Samples and calculations were obtained at the same time intervals using the methodology described above for AST (7,13,14). Slope of glucose potentiation was calculated as previously published (1).
Secretory unit of islet transplant objects (SUITO) index
The SUITO index was calculated at 3, 6 and 12 months post last infusion as previously published (16–18).
Data analysis
AUC and statistical calculations were performed using GraphPad Prism 4 (GraphPad Software, La Jolla, CA, USA). Comparisons between groups were performed by one-way ANOVA and nonparametric ANOVA (Kruskal– Wallis) with post hoc testing by Bonferroni’s and Dunn’s Multiple Comparison Test, respectively. Student’s t–test was used to establish comparisons between the two groups. Results are shown as mean±SEM. Significance was established at a p-value <0.05. Receiver operator characteristics′ (ROC) AUC is equal to the probability that a classifier will rank a randomly chosen positive instance higher than a randomly chosen negative one. ROC analysis was implemented to most accurately calculate cutoff values for acute insulin and C-peptide responses derived from each metabolic test. Test cutoff values were selected based upon user-defined balance between the highest level of sensitivity and specificity, which produces the highest likelihood ratio (LR) of accurate discrimination between insulin-independent subjects and those who return to exogenous insulin therapy. An LR value >3 is considered clinically acceptable. For this purpose, all existing values at 3, 6 and 12 months from the three different institutions were used. For each assay, insulin requirement was noted at the time of assessment. The sensitivity, specificity, positive and negative predictive value of each test to predict need for insulin therapy was calculated.
Results
Subject characteristics
Demographics of PIT (n = 30) and their respective controls (n = 10) are summarized in Table 1. Twenty-five subjects achieved insulin independence. Mean follow-up after first islet infusion was almost 5 years (59.9 ± 22 months, range 28–89). Average islet equivalents (IEQ) infused was 16 483 ± 5951 IEQ/kg body weight (BW; Table 2). Donor demographics and islet quality are summarized in Table S1. Five subjects never achieved insulin independence despite initially reduced insulin requirement, improvement in HbA1c and elimination of hypoglycemic events. Eleven of 24 patients in the ITA group remain insulin independent for >1 year posttransplant. Five of six IAK patients were insulin independent for >1 year. HbA1c decreased from a pretransplant mean of 7.2 ± 1.2% to 5.82 ± 0.8% (p = 0.002) 3 months posttransplant and remained significantly lower than pretransplant through 12 months’ follow-up (Table 2).
Table 2.
Individual transplant characteristics and posttransplant hemoglobin A1C (HbA1c) level and insulin requirements for all patient (by center)
| Patient | Type of Tx |
Number of infusions |
Number of IEQ Tx/kg |
Pre-Tx HbA1c |
3 m HbA1c% |
6 m HbA1c% |
12 m HbA1c% |
12 m insulin |
|---|---|---|---|---|---|---|---|---|
| Center 1 | ||||||||
| 1 | ITA | 2 | 15 275 | 7.8 | 6.9 | 7.3 | 9.5 | 0.24 |
| 2 | ITA | 2 | 8541 | 4.8 | 6.0 | 6.7 | 6.4 | 0.26 |
| 3 | ITA | 1 | 6602 | 6.2 | 6.5 | 6.9 | 7.5 | 0 |
| 4 | ITA | 4 | 18 473 | 7.4 | 5.9 | 6.5 | 6.7 | 0.18 |
| 5 | IAK | 2 | 20 085 | 9.8 | 6.8 | 6.0 | 5.9 | 0 |
| 6 | IAK | 2 | 16 530 | 7.7 | 4.4 | 5.8 | 6.1 | 0.09 |
| Center 2 | ||||||||
| 7 | ITA | 3 | 20 466 | 6% | 5.3 | 6.7 | 6.7 | 0 |
| 8 | ITA | 2 | 11 440 | 8.9 | 7.9 | * | * | * |
| 9 | IAK | 3 | 17 886 | 7.5 | 4.5 | <4 | 4.8 | 0 |
| 10 | ITA | 2 | 31 810 | 7.4 | 5.8 | 6.8 | 6.9 | 0.34 |
| 11 | IAK | 2 | 25 430 | 7.7 | 6 | 6.1 | 5.3 | 0 |
| 12 | ITA | 2 | 25 817 | 7.3 | 5.2 | 5.6 | 5.6 | 0 |
| 13 | ITA | 3 | 29 614 | 7.1 | 7 | 7.4 | 8.4 | 0.19 |
| 14 | ITA | 3 | 27 419 | 8 | 5.8 | 5.6 | 6.3 | 0.26 |
| 15 | IAK | 2 | 19 187 | 6.7 | 5.8 | 6 | 5.9 | 0 |
| 16 | IAK | 2 | 18 397 | 7.5 | 5.8 | 6.1 | 5.6 | 0 |
| Center 3 | ||||||||
| 17 | ITA | 3 | 17 467 | 6.7 | 6.1 | 6.3 | 5.7 | 1.01 |
| 18 | ITA | 2 | 13 154 | 6.2 | 5.8 | 5.0 | 7.6 | 0.12 |
| 19 | ITA | 4 | 13 252 | 9.4 | 5.6 | 5.4 | 5.9 | 0 |
| 20 | ITA | 2 | 12 956 | 7.3 | 5.9 | 5.6 | 5.5 | 0 |
| 21 | ITA | 2 | 18 402 | 7.1 | 5.4 | 5.8 | 5.8 | 0.25 |
| 22 | ITA | 2 | 14 383 | 9.3 | 6.0 | 5.8 | 6.4 | 0.21 |
| 23 | ITA | 2 | 13 354 | 8.0 | 6.0 | 6.4 | 5.9 | 0 |
| 24 | ITA | 2 | 6250 | 7.4 | 5.5 | 5.6 | 5.6 | 0 |
| 25 | ITA | 3 | 15 482 | 6.8 | 5.7 | 5.6 | 6.2 | 0 |
| 26 | ITA | 3 | 9645 | 6.8 | 5.7 | 5.9 | 6.0 | 0 |
| 27 | ITA | 2 | 14 804 | 7.7 | 5.9 | 6.0 | 6.6 | 0.12 |
| 28 | ITA | 3 | 13 182 | 4.8 | 4.4 | 4.2 | 4.7 | 0 |
| 29 | ITA | 2 | 12 700 | 6.3 | 5.4 | 5.8 | 5.9 | 0 |
| 30 | ITA | 2 | 13 281 | 6.0 | 5.6 | 5.7 | 5.2 | 0 |
Subject 28, Center 3 had an artificially low HbA1c due to being on dapsone at the time of transplantation.
Subject 2, Center 2 withdrew from the study.
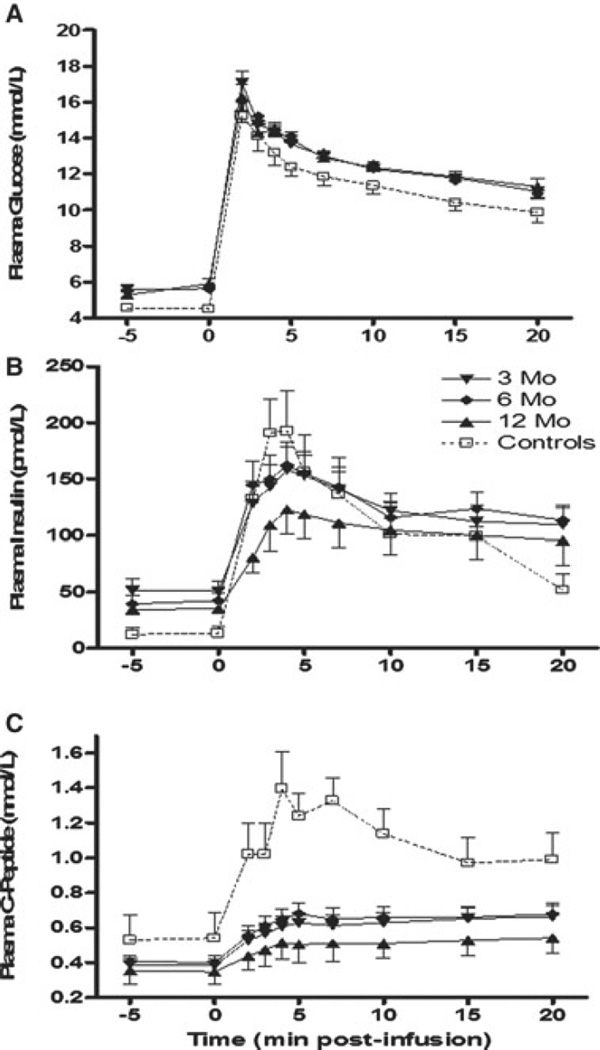
Recovery in glucose disposal rate (Kg) after PIT was observed after I.V. glucose bolus, with values similar to nondiabetic controls. First-phase insulin and C-peptide release after I.V. glucose stimulation are shown in Figure 1. AIRGLU and ACRGLU were significantly decreased (threefold lower) in islet recipients versus controls at 3, 6 and 12 months posttransplantation (p < 0.001; Table 3, Section A).
Figure 1. Intravenous glucose tolerance test in islet transplant recipients at 3, 6 and 12 months posttransplant.
(A) Glucose kinetics over 20 min after 300 mg/kg dextrose administration over 1 min starting at t = 0. (B) Levels of insulin and (C) C-peptide release were plotted against nondiabetic control subjects. Thirty patients were assessed at 3 months, 19 patients were assessed at 6 months and 27 patients were assessed at 12 months post last infusion. Data are expressed as mean ± SE.
Table 3.
Insulin and C-peptide response to glucose, arginine, glucose-potentiated arginine and SUITO Score at 3, 6, 12 and 24 months posttransplant. (A) Intravenous glucose tolerance test glucose (IVGTT) Glucose disposal and insulin and C-peptide secretion kinetics after stimulation with glucose. Incremental first-phase AUC and percentage over basal (means of the values from 2–10 minus basal value, divided by the basal value and expressed as percent). Kg was calculated based on the values for 10–20 min post glucose injection. (B) Arginine alone at 5mM glucose (ARG). Basal levels, incremental AUC from 2 to 5 min data and percentage of basal was calculated for plasma insulin and C-peptide after arginine stimulation. (C) Glucose potentiation of arginine-induced secretion (GPAIS) at 15 mM glucose. Basal levels, ncremental AUC from 2 to 5 min data and percentage of basal was calculated for plasma insulin and C-peptide after arginine stimulation at hyperglycemia
| N = 1 | 3 months n = 27 |
6 months n = 19 |
12 months n = 27 |
Nondiabetic control subjects n = 10* |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Section A: IVGTT | Basal | Incr. AUC | % Baseline | Basal | Incr. AUC | % Baseline | Basal | Incr. AUC | % Baseline | Basal | Incr. AUC | % Baseline |
| Insulin | 59.2 ±11.9 | 945.8 ±172 | 244 ±39* | 41.2±7 | 955 ±154.2 | 337±61* | 39.1 ±4.5 | 915.6±209 | 300.8 ±59* | 16±4 | 1448 ±243 | 1036 ±294 |
| C-peptide | 0.4 ±0.03 | 2.11 ±0.31* | 53 ±8.4* | 1.42 ±0.4** | 2.45 ±0.4* | 65±11* | 0.42 ±0.1 | 1.89 ±0.43* | 50 ± 11 * | 0.5 ±0.1 | 7.3± 1.2 | 160±71 |
| Kg | 1.33 + 0.1 | 1.38 + 0.1 | 1.4 + 0.2 | 1.4 + 0.2 | ||||||||
| n = 27 | N = 19 | N = 27 | n = 10 | |||||||||
| Section B: ARG | ||||||||||||
| Insulin | 63.3 ±11.9 | 538.7 ±60 | 257 ±29** | 70.8±19 | 693.8 ±124 | 288.5 ±43 | 52.6±6*** | 516.9±102 | 247 ±49 | 23±4 | 708 ±261 | 446 ±106 |
| C-peptide | 0.4 ±0.1 | 1 ±0.12* | 58±6*** | 0.54 ±0.1 | 1.36±0.2** | 60.7 ±9** | 0.5 ±0.1 | 1.12±0.2** | 56±5*** | 0.5 ±0.1 | 2.7 ±0.6 | 125 ±29 |
| n = 13 | n = 8 | n = 13 | n = 10 | |||||||||
| Section C: GPAIS | ||||||||||||
| Insulin | 150.8 ±48 | 2138±291** | 1385 ±333* | 116±36 | 2722 ±587** | 1794±343*** | 147.3 ±47 | 3308 ±942* | 746.8 ±239 | 168±27 | 6817±1527 | 4552 ±623 |
| C-peptide | 0.6 ±0.1 | 7.0±1.2*** | 334 ±34* | 0.7 ±0.2 | 10.1 ±2*** | 368 ±78*** | 0.7 ±0.2 | 6.0±2.6*** | 476 ±166** | 0.5 ±0.1 | 21.1 ±2.7 | 912 ±138 |
| N = 27 | N = 19 | N =27 | N = 10 | |||||||||
| Section D: SUITO | 3 months | 6 months | 12 months | Nondiabetic control subjects | ||||||||
| score | 49.2 + 6.0 | 60.6±7.3 | 67.5±15.0 | 132.5 + 93.5 | ||||||||
Nondiabetic subjects were used as a control group. Data are expressed as mean ± SEM.
p < 0.001 for this time point to nondiabetic controls.
p < 0.05 for this time point to nondiabetic controls.
p < 0.01 for this time point to nondiabetic controls.
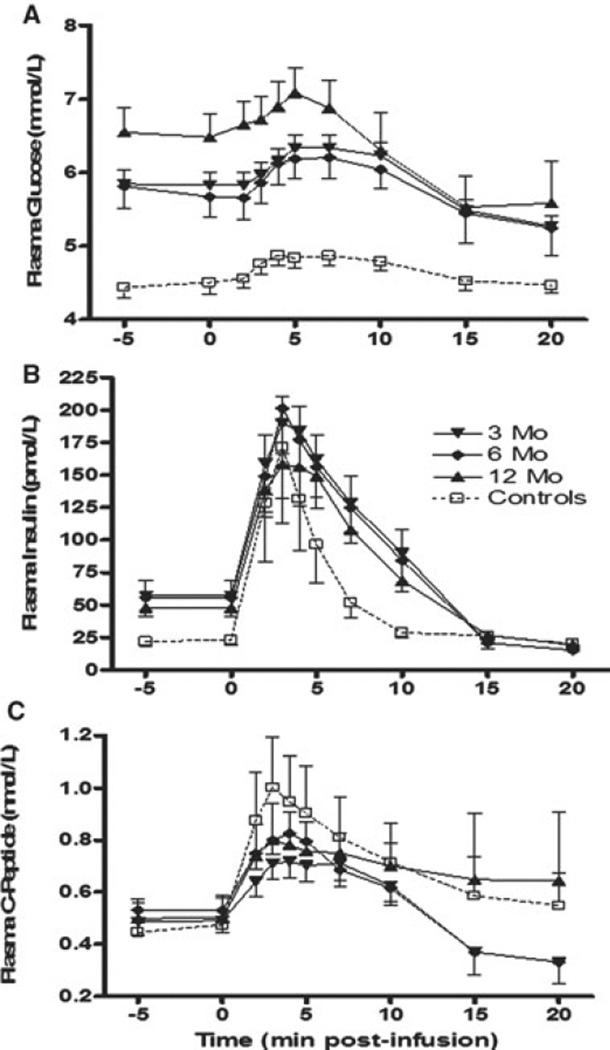
Figure 2 demonstrates insulin and C-peptide responses to arginine under normoglycemic conditions. AIRARG was approximately half in islet recipients versus controls at 3 months (p = 0.02), but not at 6 and 12 months (NS; Table 2, Section B). Similarly, ACRARG was significantly lower (twofold) in the transplant group versus controls at 3 months (p = 0.002) and at 6 and 12 months (p < 0.05; Table 3, Section B).
Figure 2. Arginine stimulation test in islet transplant recipients at 3, 6 and 12 months posttransplant.
(A) Glucose kinetics over 20 min after 5 g arginine administration over 30 s starting at t = 0. (B) Levels of insulin and (C) C-peptide release were plotted against nondiabetic control subjects. Thirty patients were assessed at 3 months, 19 patients were assessed at 6 months and 27 patients were assessed at 12 months post last infusion. Data are expressed as mean ± SE.
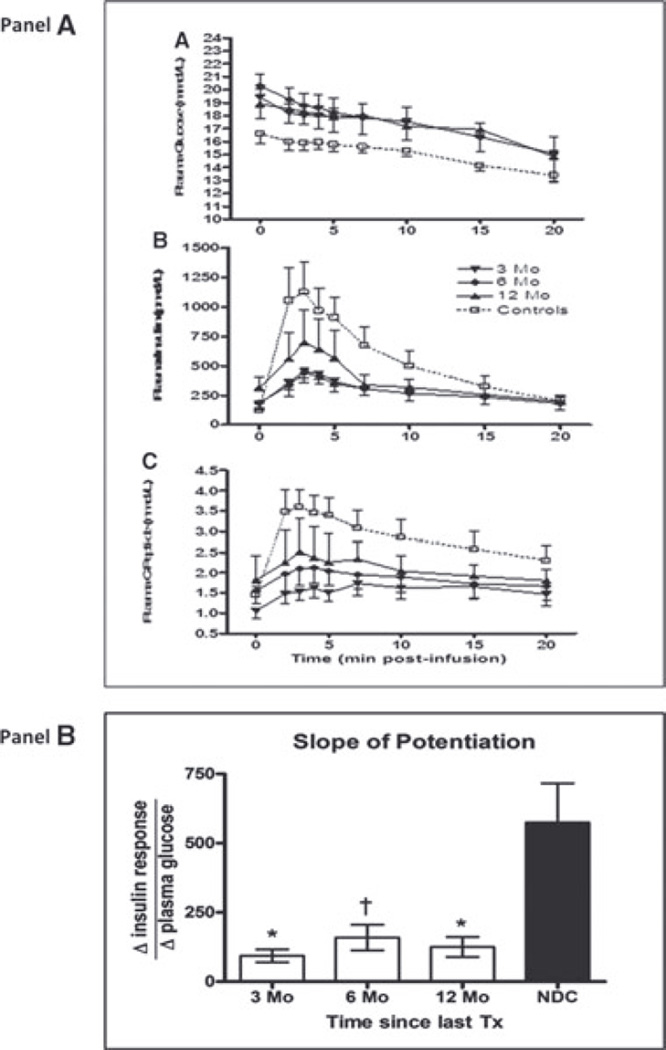
GPAIS is demonstrated in Figure 3. AIRMAX and ACRMAX were significantly decreased (threefold lower) in islet recipients versus controls at 3, 6 and 12 months posttrans-plantation (p < 0.0001; Table 3, Section C).
Figure 3. Panel (A): Glucose potentiation of arginine induced insulin secretion (GPAIS) in islet transplant recipients at 3, 6 and 12 months posttransplant.
Thirteen patients were assessed at 3 months, 8 patients were assessed at 6 months and 13 patients were assessed at 12 months post last infusion. (A) Glucose kinetics over 20 min after 5 g arginine administration over 30 s starting at t = 0. (B) Levels of insulin and (C) C-peptide release were plotted against nondiabetic control subjects. Data are expressed as mean ± SE. Panel (B): Slope of glucose-potentiation is calculated as the change in insulin release in response to arginine from the normoglycemic to the hyperglycemic condition, divided by the change in plasma glucose. The posttransplant calculated slope in PIT recipients was 1.2 ± 0.4 (p = 0.02) at 3 months, 1.2 ± 0.5 (p = 0.02) at 6 months, 1.6 ± 1.1 (p = 0.07) at 12 months and 1.8 ± 1.0 (p = 0.08) at 24 months, compared to a slope of 5.1 ± 1.4 for controls.
Slope of potentiation, an index of maximal β-cell secretory reserve (Figure 3, panel B), also demonstrated a reduced reserve in PIT compared to controls. At 3, 6 and 12 months, slope of potentiation is significantly lower in PIT (125.1 ± 36.3, 158 ± 46.3 and 93.2 ± 23.5) compared to Non-diabetic control group (NDC) (574.9 ± 142; p < 0.001, 0.01 and 0.001, respectively).
The SUITO index at 3, 6 and 12 months post last infusion (Table 3, Section D) is also reduced in islet recipients versus controls.
Islets transplanted as a predictor of insulin independence 1 year post last infusion
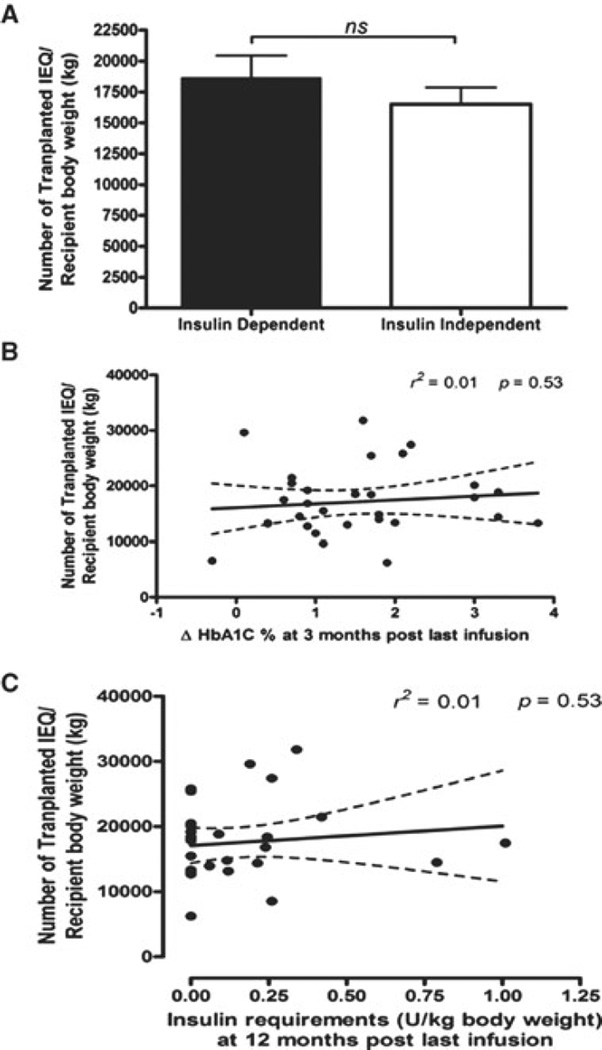
The number of IEQs per kilogram BW was compared between patients remaining insulin independent (16 515 ± 5229 IEQ/kg vs. 18 654 ± 6724 IEQ/kg) and those insulin dependent 1 year after last islet infusion (p= 0.345). No correlation was established between IEQ/kg BW and changes in HbA1c% at 3 months post last infusion (r2 = 0.01, p = 0.53). In addition, no correlation was established between IEQ/kg BW transplanted and units of exogenous insulin/kg BW required after the first year post last islet infusion (r2 = 0.01, p = 0.53; Figure 4).
Figure 4. Comparison of total transplanted islet mass and metabolic assessment in the posttransplant period.
Panel (A): Bar representation of transplanted islet mass and insulin requirement status at 12 months post last islet infusion. Insulin-dependent group (n = 14) and insulin-independent group (n = 15). Mean and SEM were calculated in each group. Statistical significance was considered at p < 0.05. Panel (B): Relation of total transplanted islet mass post last infusion with insulin secretion AUC at 12 months post last infusion (n = 29). Statistical significance was considered at p < 0.05. Panel (C): Relation of total transplanted islet mass post last infusion with HbA1C level at 3 months post last infusion normalized to pretransplant level (n = 29). Statistical significance was considered at p < 0.05.
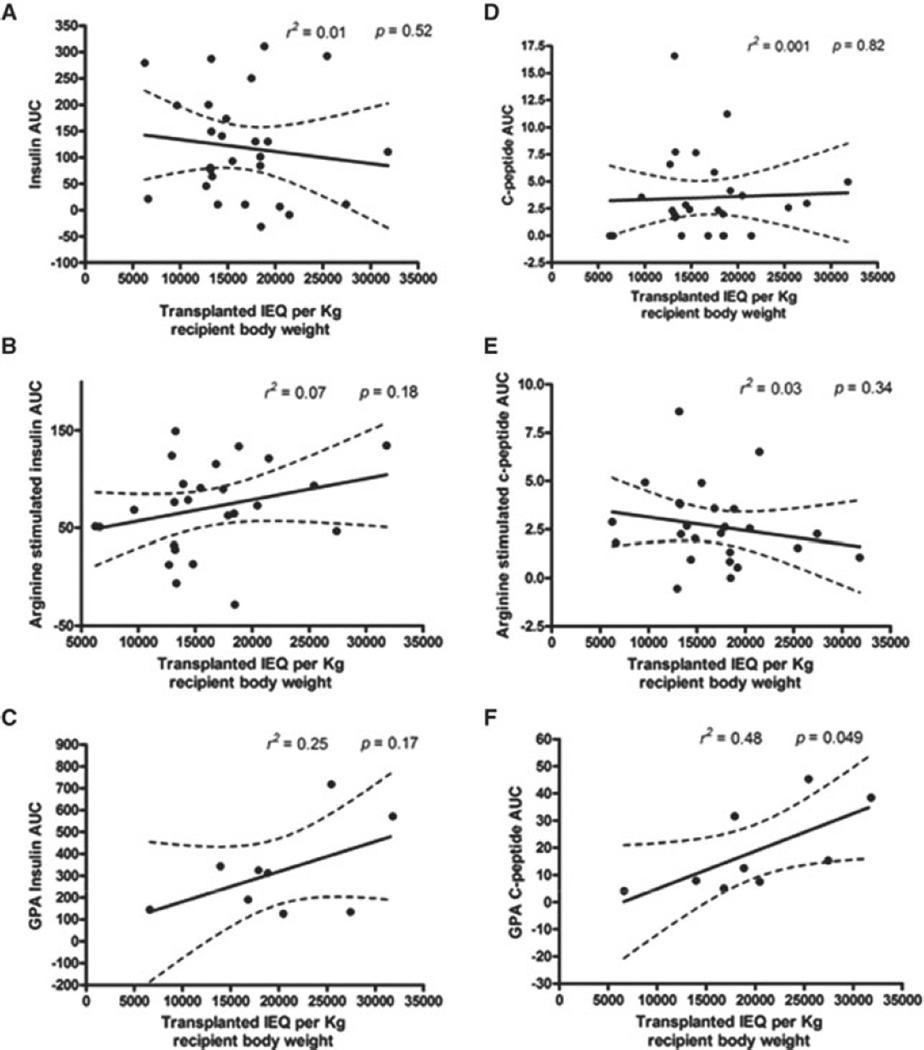
We then established whether a correlation might exist between IEQ/kg BW transplanted and functional islet mass engrafted. Insulin and C-peptide secretion at 3 months post last infusion were used as surrogate markers of β-cell mass engrafted. No correlation was identified between AIRGLU, ACRGLU, AIRARG, ACRARG, AIRMAX and IEQ/kg BW trans-planted(Figure 5). However, only ACRMAX and IEQ/kg BW of transplanted islets had a modest but significant correlation (r2 = 0.48, p = 0.049).
Figure 5. Comparison of total transplanted islet mass and insulin and C-peptide levels after IVGTT, arginine stimulation test and glucose-potentiated arginine test.
Panel (A): Relation of total transplanted islet mass post last infusion with insulin secretion AUC after IVGTT at 3 months post last infusion (n = 27). Statistical significance was considered at p < 0.05. Panel (B): Relation of total transplanted islet mass post last infusion with insulin secretion AUC after arginine stimulation test at 3 months post last infusion (n = 25). Statistical significance was considered at p < 0.05. Panel (C): Relation of total transplanted islet mass post last infusion with insulin secretion AUC after glucose-potentiated arginine test at 3 months post last infusion (n = 9). Statistical significance was considered at p < 0.05. Panel (D): Relation of total transplanted islet mass post last infusion with c-peptide secretion AUC after IVGTT at 3 months post last infusion (n = 27). Statistical significance was considered at p < 0.05. Panel (E): Relation of total transplanted islet mass post last infusion with c-peptide secretion AUC after arginine stimulation test at 3 months post last infusion (n = 25). Statistical significance was considered at p < 0.05. Panel (F): Relation of total transplanted islet mass post last infusion with c-peptide secretion AUC after glucose-potentiated arginine test at 3 months post last infusion (n = 9). Statistical significance was considered at p < 0.05.
Establishment of cutoff values for acute insulin and C-peptide response for IVGTT, AST at normoglycemia and hyperglycemia levels corresponding to exogenous insulin use requirement
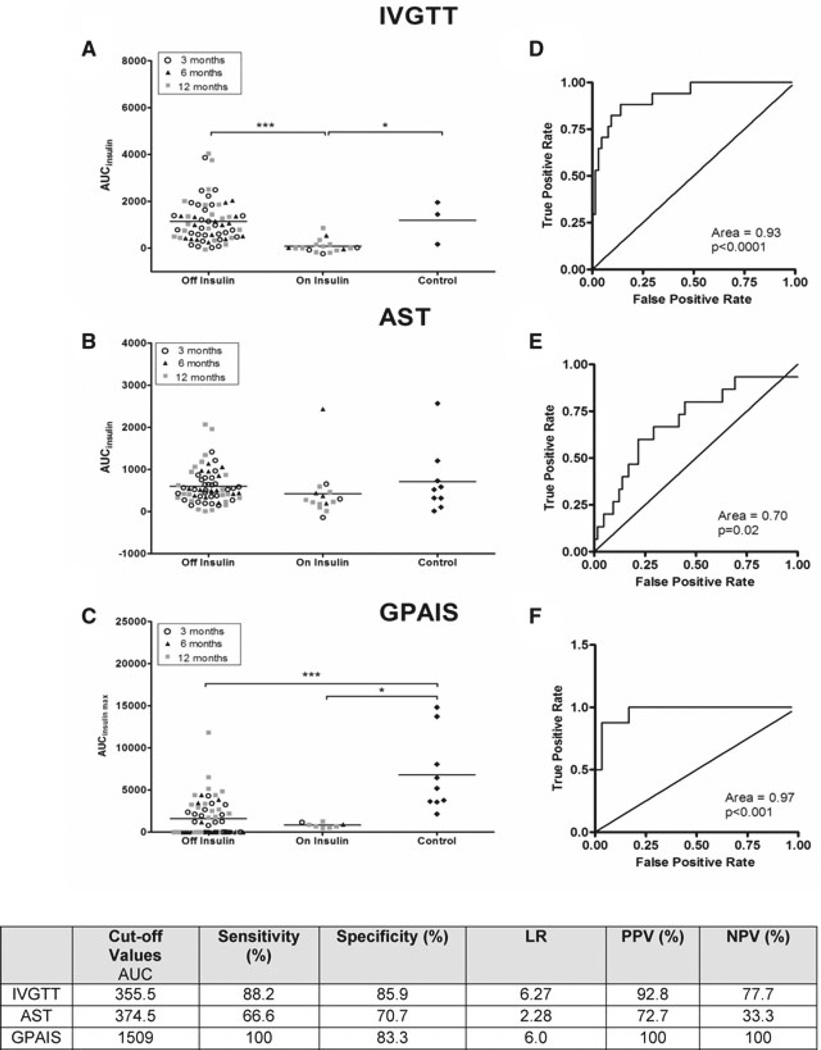
Figure 6 summarizes AIRGLU (panel A), AIRARG (panel B) and AIRMAX (panel C) for all subjects. Patients were stratified in two groups (off vs. on) according to insulin requirement at time of metabolic assay and compared to nondiabetic controls. AIRGLU was greater in patients remaining insulin independent versus those returning to insulin after PIT (1143 ± 113.2 vs. 81.6 ± 67.5 pmol/L min, p < 0.001). AIRARG values were no different in insulin-independent patients (599.8 ± 51.2 pmol/L min) compared to controls (708 ± 260 pmol/L min, p > 0.05). No differences were noted between patients on and off insulin after PIT (423.3 ± 53.4 pmol/L min, p > 0.05; panel B). AIRMAX was greater in the insulin-independent versus insulin-dependent groups (1623 ± 285 vs. 836 ± 100 pmol/L min), but significantly lower in both PIT groups compared to controls (6817 ± 1527 pmol/L min, p < 0.01 and p < 0.001, respectively).
Figure 6. Distribution plots for Insulin secretion using the ROC curve and analysis for IVGTT, arginine stimulation test and GPAIS.
Panels (A)–(C): All sequential AIRGLU, AIRARG and AIRMAX for UW patients during 24-month follow-up. Acute insulin response data were segregated according to exogenous insulin dependence. Mean and SEM were calculated in each group. Data are also stratified according to whether the test was performed 3, 6 or 12 months after the last islet transplantation. Statistically significant differences are expressed as *(p < 0.05), **(p < 0.01) and ***(p < 0.001). Panels (D)–(F) represent the receiver operator characteristic for AIRGLU, AIRARG and AIRMAX, respectively. It was determined at the time of each assay whether the patient required insulin to achieve normoglycemia and recorded as “On Insulin” or “Off Insulin.” The ROC graph recorded a point for each data pair (quantitative result, clinical outcome) as if it was the critical value for a predictive assay and considering the data set at that point as true positives and false positives. All data from sequential measurements at 3, 6 and 12 months post last islet infusion was included. Area under the ROC curve was then calculated. Tests which cannot discriminate between true and false positives show a sensitivity plot that is not significantly different from the line of identity and a p-value >0.05 when the AUC is calculated. Cutoff values that generate the highest sensitivity and specificity using the best likelihood ratios were chosen for each assay.
Figure 6 (panels D–F) represents ROC analysis used to calculate cutoff values for insulin in each metabolic test. The AUC for ROC analysis was similar between AIRGLU (0.93, p = 0.0001) and AIRMAX (0.95, p = 0.001); however, AIRGLU showed the best power of discrimination, with a LR of (6.27–) for a cutoff of 356 pmol/L min. This cutoff had a sensitivity of 88% a specificity of 85.9% with a positive and negative predictive value 93% and 78%, respectively. Very similar to AIRGLU, the AIRMAX also has an excellent clinically acceptable LR of 6.0, for a cutoff value of 1509 pmol/L min, with sensitivity and specificity of 100% and 83%, respectively, and positive and negative predictive value for both of 100% (n = 27).
In contrast, AIRARG has a clinically unacceptable LR (2.28), with a moderate sensitivity of 66.6% and specificity of 70.7%, a reasonable positive predictive value of 72.7%, but an unacceptable negative predictive value of 33%.
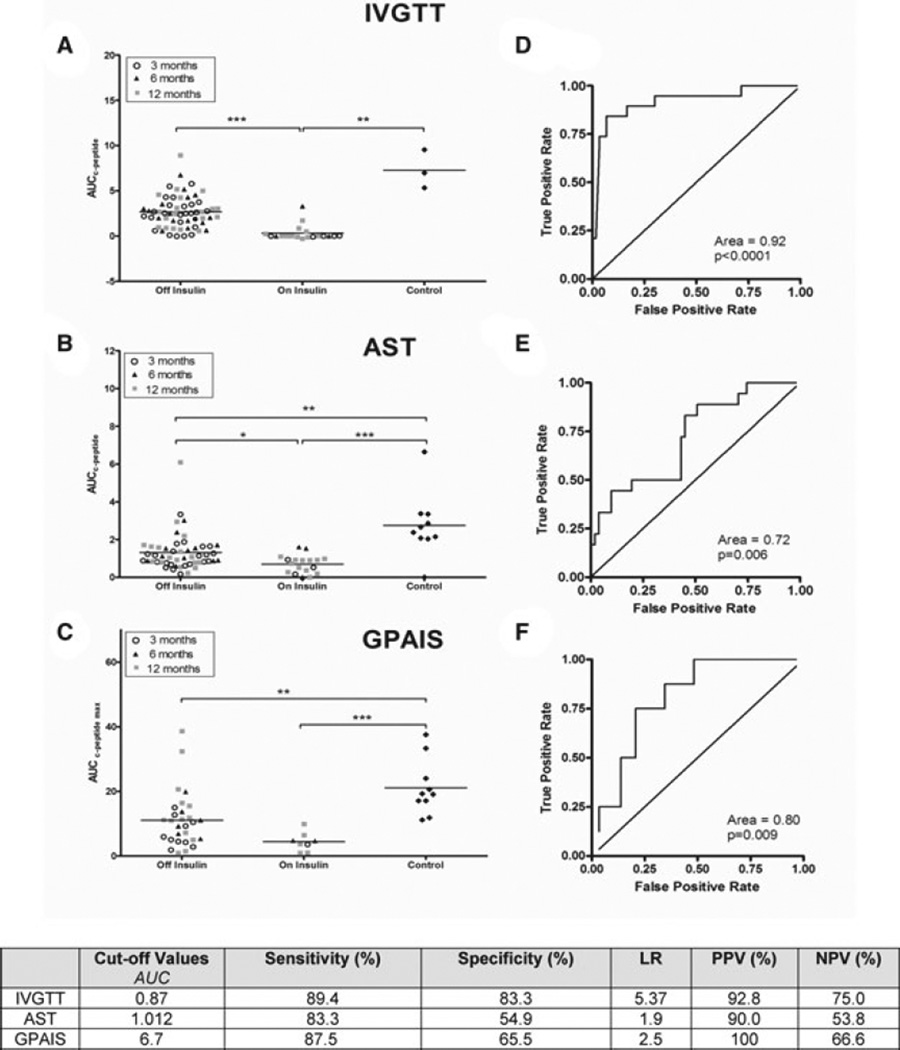
Figure 7 summarizes acute C-peptide response to I.V. glucose (panel A), arginine (panel B) and GPAIS (panel C). ACRGLU was the only test capable of discriminating differences between PIT recipients off and on exogenous insulin (2.67 ± 0.22 vs. 0.32 ± 0.2 nmol/L min, p < 0.001). A statistically greater AUC was seen in the nondiabetic control group (7.28 ± 1.22 nmol/L mi) compared to PIT recipients on or off exogenous insulin (p < 0.001 for both). In contrast, ACRARG and ACRMAX are only capable of differentiating between islet recipients and their controls (p < 0.001 for both), but not between insulin-independent patients versus patients returning to exogenous insulin after PIT (panels B and C).
Figure 7. Distribution plots for C-peptide secretion using the ROC curve and analysis for IVGTT, arginine stimulation test and GPAIS.
Panels (A)–(C): All sequential ACRGLU, ACRARG and ACRMAX for UW patients during 24-month follow-up. Acute C-peptide response data was segregated according to their exogenous insulin dependency. Mean and SEM were calculated in each group. Data is also stratified according to whether the test was performed 3, 6 or 12 months after the last islet transplantation. Statistically significant differences were expressed as *(p < 0.05), **(p < 0.01) and ***(p < 0.001). Panels (D)–(F) represent the receiver operator characteristics for ACRGLU, ACRARG and ACRMAX, respectively. Tests, which cannot discriminate between true and false positives, show a sensitivity plot that is not significantly different from the line of identity and a p-value >0.05 when the AUC is calculated. All data from sequential measurements at 3, 6 and 12 months post last islet infusion was used. Area under the ROC curve was then calculated. Cutoff values that generated the highest sensitivity and specificity using the best likelihood ratios were chosen for each assay.
Figure 7 (panels D–F) represents ROC analysis to calculate the cutoff value for each test. The ACRGLU ROC has an AUC of 0.92 (p < 0.0001). For an ACRGLU cutoff value of 0.87, this test has the highest sensitivity and specificity (89% and 83%, respectively) and the best LR (5.37), with a positive and negative predictive value of 93% and 75%, respectively. Neither ACRARG nor ACRMAX provides a useful cutoff value differentiating patients requiring exogenous insulin therapy compared to those who do not. The positive and negative predictive value for each test was calculated based on data available at 12 months and summarized at the bottom of Figure 7.
Is lower islet β -cell mass engraftment at 3 months post-PIT associated with return to insulin dependence by 12 months?
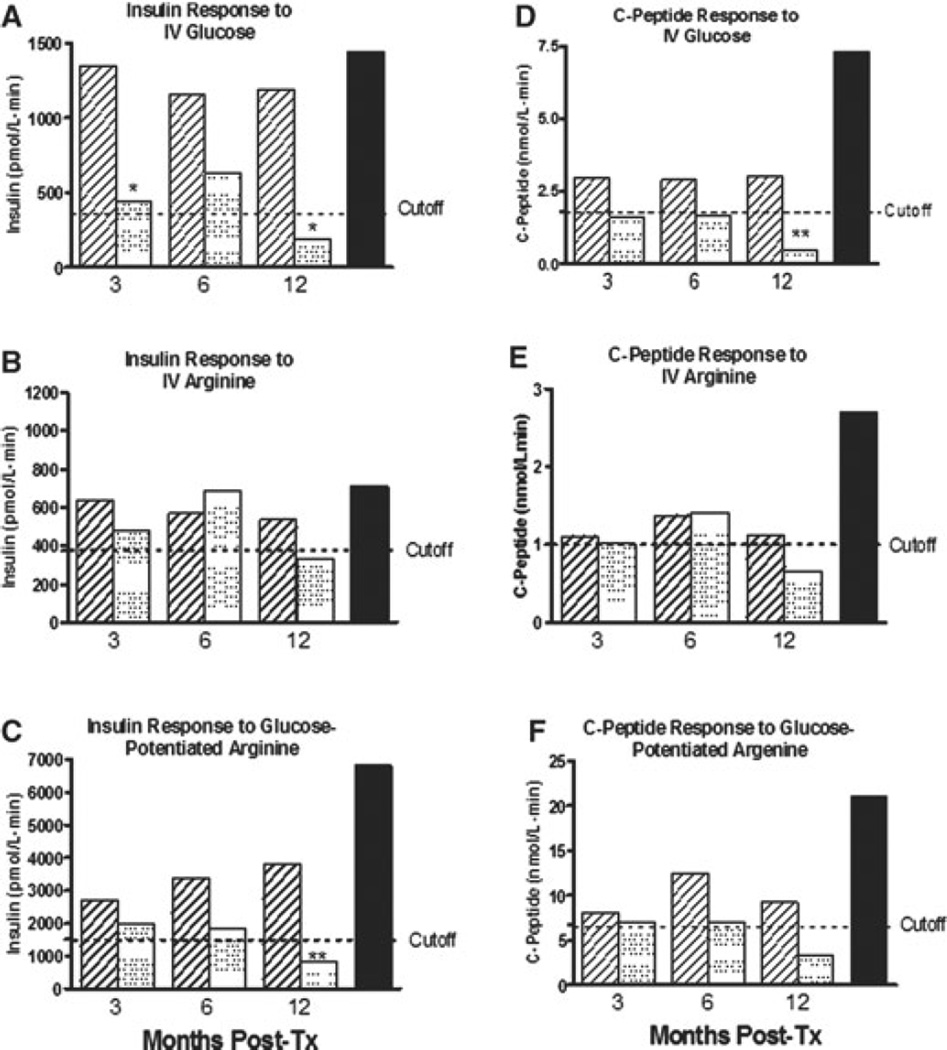
When PIT recipients were separated into two groups (insulin independent 1 year posttransplant vs. partial β-cell function as determined by detectable fasting C-peptide but requiring exogenous insulin at 1 year), a difference in AIRGLU at 3 months was observed between the two subgroups (Figure 8, upper panel).
Figure 8. Comparison between acute insulin and peptide response in 32 islet transplant recipients from three institutions.
Patients were divided according to their exogenous insulin requirement 12 months posttransplant. Acute insulin and C-peptide response is expressed as mean ± SEM. Cutoff values from Figures 6 and 7 are overlapped with the bars, representative of the data. Statistically significant differences were expressed as *(p < 0.05) and **(p < 0.01). Nondiabetic controls are represented for comparison. Panels (A) and (B): Bar representation of the AIRGLU and ACRGLU response at 3, 6 and 12 months after last transplant. Panels (C) and (D): Bar representation of the AIRARG and ACRARG response at 3, 6 and 12 months after last transplant. Panels (E) and (F): Bar representation of the AIRMAX and ACRMAX response at 3, 6 and 12 months after last transplant.
In contrast, no significant differences at 3 months were seen when AIRARG, ACRARG, AIRMAX and ACRMAX results were compared between on and off insulin at 1 year (Figure 8, middle panel).
Discussion
Clinical trials have shown that insulin independence can be consistently achieved when a sufficient number of islets is implanted (>10 000 IEQ/kg recipient BW; Ref. 19). However, the correlation between islet mass transplanted and engraftment and the potential to predict the stage of insulin independence beyond 1 year after transplantation based on number of transplanted islets remains unknown. Transplanting the largest possible number of islets is considered among the most important factors for success, but other factors, including quality of transplanted islets (20), instant blood-mediated inflammatory response (21) and inefficient neovascularization of the graft (22), account for the lack of correlation between transplanted islet mass and engraftment. Interestingly, our data shows that transplanted mass (IEQ/kg BW) is an unreliable predictor of insulin independence and correlates poorly with function at 1 year post-transplant.
Diminished first-phase insulin response to I.V. glucose is recognized as an early marker of β-cell dysfunction, appearing before significant impairment in glucose tolerance. Our PIT recipients showed a decreased first-phase insulin release, paralleling findings in other populations at increased risk for overt diabetes development (23,24). Sufficient functional islet β-cell mass is necessary for restoration of first-phase insulin release which is not accomplished after PIT using an “Edmonton-like” immunosuppressive protocol. In addition, we have also demonstrated that AIRARG is neither sensitive nor specific enough to unmask differences in functional β-cell mass between islet recipients remaining insulin independent and those returning to insulin, as previously demonstrated in streptozotocin-induced β-cell loss in nonhuman primates (25,26). Similar findings of preserved AIRARG when the response to AIRGLU is minimal or absent have also been reported by Rickels et al. in PIT (3), early T1D (10), type 2 diabetes (11,27) and in partially functioning solid organ pancreas transplants (2). Our results are also analogous to previous findings in which β-cell response to GPAIS correlated best with directly measured β-cell mass (26,28), as AIRMAX provided the greatest discrimination in functional β-cell mass between islet recipients and nondiabetic controls and the highest sensitivity and specificity to discriminate between patients that are insulin independent and those who return to insulin therapy.
We sought to provide cutoff values for each individual metabolic test paralleling its accuracy in predicting insulin dependence. Based on ROC analysis, our results clearly indicate AIRGLU and AIRMAX provide similar AUC. Similarly, the LR to discriminate between the insulin-independent and insulin-dependent subgroups are alike for AIRGLU LR of 6.27, for a cutoff value of 356 pmol/L min, compared to an LR of 6 for a cutoff value of 1509 pmol/L min for the AIRMAX. Both sensitivity and specificity are within acceptable range for clinical use, with excellent positive and negative predictive values. In contrast, AIRARG is not an acceptable method to follow clinical islet transplantation.
For C-peptide measurement, ACRGLU provides the only clinically acceptable method to differentiate between insulin-independent recipients versus those who return to insulin (AUC = 0.92, p = 0.0001) with a cutoff value between 0.87 nmol/L min, representing LR of 5.37. The cutoff values established for ACRARG and ACRMAX did not sufficiently discriminate between patients on and off insulin.
The rationale to determine cutoff values was intended to provide a standardized tool to guide clinicians on the significance/use of their results, specifically for PIT recipients. If this cutoff value was to be used prospectively, it could provide guidance in the following areas: (1) to define the best time for retransplantation; (2) to initiate potential therapeutic interventions aimed at preserving or increasing islet β-cell mass and/or function and (3) to define a surrogate end point for future clinical trials in which the benefit of a therapeutic intervention may be measured with short-term follow-up. Clearly, usage of these values must be rigorously tested with a larger database and with different immunosuppressive protocols.
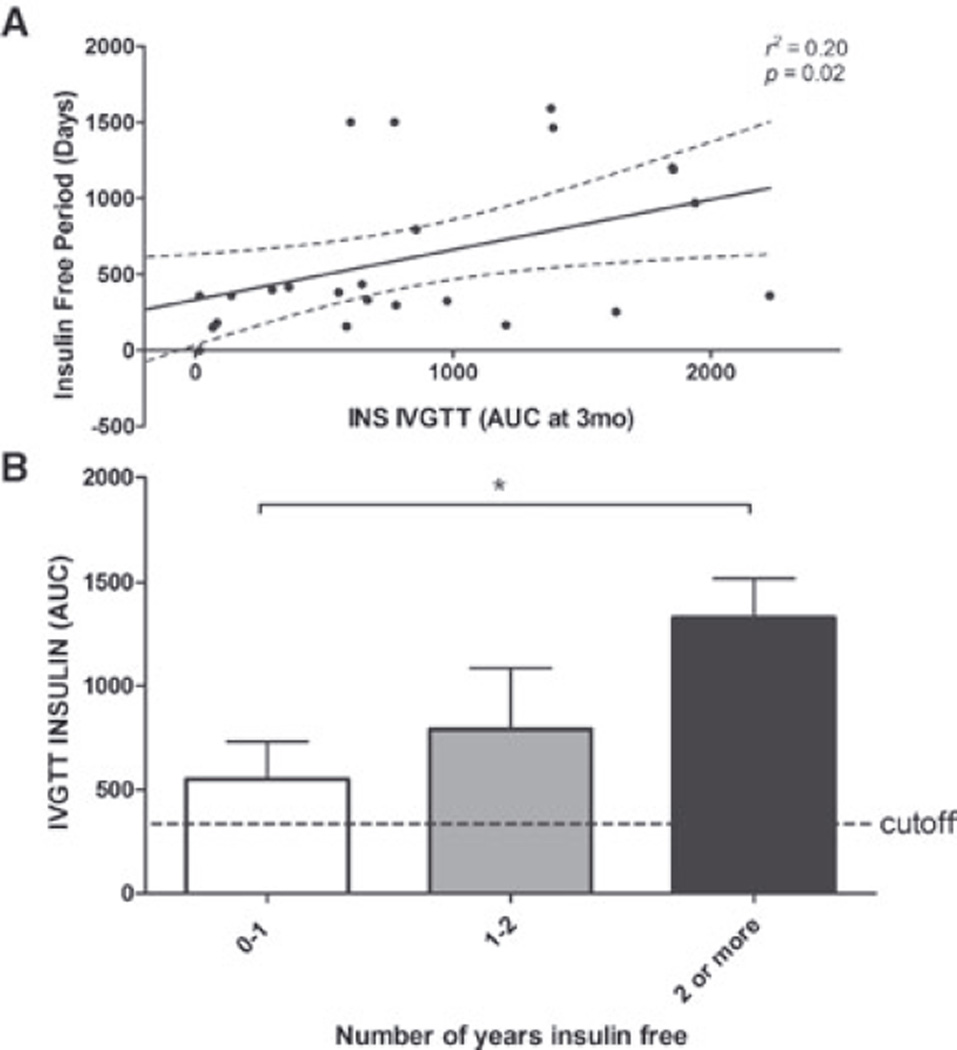
Our results also demonstrate that AIRGLU is of great value in discriminating changes in functional β-cell mass over time. Based on AIRGLU, we determined that exogenous insulin dependence 12 months postPIT was associated with lower islet mass engraftment 3 months after islet infusion (Figure 8). In addition, a positive correlation was observed between islet mass engraftment-detected AIRGLU and days of insulin independency (Figure 9).
Figure 9. Insulin IVGTT performed at 3 months as a predictor of posttransplant insulin independence and long-term graft function.
Panel (A): Correlation between IVGTT acute insulin response at 3 months and posttransplant insulin independence. Panel (B): Comparison between IVGTT insulin AUC performed at 3 months and long-term insulin independence. Data are mean ± SEM. Statistical significant differences expressed as *(p < 0.05), **(p < 0.01) and ***(p < 0.001).
We have also extended our analysis to compare IVGTT to previously simplified indexes that have proven to correlate with insulin independence (16). Specifically, we have observed that the SUITO index calculated at 3 months post islet transplantation correlated well with AUC for blood glucose, AIRGLU and ACRGLU after IVGTT changes posttransplant and for HbA1c changes at 12 months after last infusion. However, the SUITO index calculated at 3 months failed to predict patients who required exogenous insulin therapy at 12 months. Another important finding in our analysis is that the average SUITO index at 3, 6 and 12 months in patients who were insulin dependent were significantly greater than a SUITO index of 26, the established cutoff proposed as a discriminator between insulin-independent and insulin-dependent patients (Figures S1 and S2).
Study limitations are based on the restricted number of patients available to establish the predictive model described here, the lack of an alternative population of islet transplant recipients to validate the results, the short-term metabolic follow-up (12 months) and the inability to compare our data with other simplified beta scores published previously (29). Despite these limitations, this manuscript represents the largest series of sequential metabolic testing evaluating β-cell secretory capacity as a surrogate marker of functional β-cell mass.
In conclusion, these data support that AIRGLU and AIRMAX were estimated to be the two best methods to determine insufficient islet mass engraftment associated with return to insulin dependence within 1 year after PIT. In addition, our findings demonstrate that AIRGLU is the best method to serve as an early metabolic marker anticipating loss of insulin independence in T1D islet allograft recipients. In light of the observed results, wide availability and simple methodology, we strongly support the usage of AIRGLU as the optimal method to follow patients after islet transplantation.
While acknowledging that the loss of islet mass over time is likely multifactorial, return to insulin dependence in some islet recipients may be due to impaired β-cell secretory capacity related to insufficient initially engrafted β-cell mass, leading to progressive β-cell functional deterioration.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health #U42RR0234001 (PI LAF) and internal funding from the UW Department of Surgery, Division of Transplantation. In addition, this work was made possible by grants to the University of Pennsylvania: U42RR016600 (to A.N.), UL1RR024134 (Penn Clinical & Translational Research Center), P30DK19525 (Penn Diabetes Endocrinology Research Center) and Juvenile Diabetes Research Foundation International 4–2005-351. This work was also supported by NIH grants to the University of Miami: MO1RR16587, 1RO1-DK55347, IU42 RR016603 (to C.R.), Juvenile Diabetes Research Foundation International 4–200-946 and the Diabetes Research Institute Foundation.
We are indebted to the transplant recipients and the healthy nondiabetic control subjects for their participation in this study. Special thanks to UW, University of Miami and University of Pennsylvania General Clinical Research Centers for the execution of the metabolic studies, and the UW Organ Procurement Organization and all OPOs which provided the pancreata for islet isolation. We also express our deep gratitude to the UW Islet Core Facility members for manufacturing the islets for transplantation. Special thanks to Kristi Schneider, Melissa Moss, Elisa Park and Mallory Sears for their technical support. We also would like to acknowledge the generous support of Wyeth Pharmaceuticals (now Pfizer) for providing the rapamycin used by our study patients.
Abbreviations
- ACRARG
acute C-peptide response to arginine
- ACRMAX
acute C-peptide response to glucose-potentiated arginine-induced insulin secretion
- ACRGLU
acute C-peptide response to glucose
- Kg
glucose disappearance rate
- AIRARG
acute insulin response to arginine
- AIRGLU
acute insulin response to glucose
- AIRMAX
acute insulin response to glucose-potentiated arginine-induced insulin secretion
- AST
arginine stimulation test
- AUC
area under the curve
- DBD
donation after brain death
- DCD
donation after cardiocirculatory death
- GPAIS
glucose-potentiated arginine-induced insulin secretion
- IAK
islet after kidney
- ITA
islet transplant alone
- IVGTT
I.V. glucose tolerance test
- K
nephropathy
- LR
likelihood ratio
- N
neuropathy
- PIT
pancreatic islet transplantation
- R
retinopathy
- ROC
receiver operator characteristics
- SUITO
secretory unit of islet transplant objects
- T1D
type 1 diabetes
- UW
University of Wisconsin
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Figure S1: Correlation analysis between SUITO index (fasting C-peptide [ng/mL]/[fasting blood glucose (mg/dL) – 63] × 1500) and (A) relative insulin requirement (ratio between pretransplant and posttransplant daily insulin requirements at 12 months), (B) relative HbA1c level (ratio between pretransplant and 12-month posttransplant HbA1c level), (C) blood glucose (AUC first phase) during IVGTT at 12 months posttransplant, (D) insulin release (AUC first phase) during IVGTT at 12 months posttransplant and (E) C-peptide release (AUC first phase) during IVGTT at 12 months posttransplant. Data shown represent slope and 95% confidence intervals for each data set. Statistical significance was considered at p < 0.05.
Figure S2: (A) SUITO index calculated at 3, 6 and 12 months posttransplant as a predictor of insulin independence at 12 months. Data are mean ± SEM. Nonparametric t-test used to establish differences between groups. A p-value < 0.05 was considered significant. (B) Receiver operator characteristic analysis of SUITO Index as a predictor of insulin independence at 12 months posttransplant. The ROC graph recorded a point for each data pair (clinical outcome) as if it was the critical value for a predictive assay and considering the data set at that point as true positives and false positives. Area under the ROC curve was then calculated. Tests that cannot discriminate between true and false positives show a sensitivity plot that is not significantly different from the line of identity and a p-value > 0.05 when the AUC is calculated. Cutoff values that generate maximized likelihood ratios for each assay are shown along with the sensitivity and specificity of the assay using that cutoff and the AUC for the plotted ROC graph for the assay.
Table S1: Descriptive statistics of pancreas donor demographics. Data are mean ± SEM and range where applicable
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Kahn SE, Carr DB, Faulenbach MV, Utzschneider KM. An examination of beta-cell function measures and their potential use for estimating beta-cell mass. Diabetes Obes Metab. 2008;10(Suppl 4):63–76. doi: 10.1111/j.1463-1326.2008.00945.x. [DOI] [PubMed] [Google Scholar]
- 2.Robertson RP. Consequences on beta-cell function and reserve after long-term pancreas transplantation. Diabetes. 2004;53:633–644. doi: 10.2337/diabetes.53.3.633. [DOI] [PubMed] [Google Scholar]
- 3.Rickels MR, Naji A, Teff KL. Acute insulin responses to glucose and arginine as predictors of beta-cell secretory capacity in human islet transplantation. Transplantation. 2007;84:1357–1360. doi: 10.1097/01.tp.0000287595.16442.a7. [DOI] [PubMed] [Google Scholar]
- 4.Luzi L, Hering BJ, Socci C, et al. Metabolic effects of successful intraportal islet transplantation in insulin-dependent diabetes mellitus. J Clin Invest. 1996;97:2611–2618. doi: 10.1172/JCI118710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luzi L. Metabolic strategies to predict and improve intrahepatic islet graft function. J Mol Med. 1999;77:49–56. doi: 10.1007/s001090050300. [DOI] [PubMed] [Google Scholar]
- 6.Baidal DA, Faradji RN, Messinger S, et al. Early metabolic markers of islet allograft dysfunction. Transplantation. 2009;87:689–697. doi: 10.1097/TP.0b013e318195c249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan EA, Lakey JR, Paty BW, et al. Successful islet transplantation: Continued insulin reserve provides long-term glycemic control. Diabetes. 2002;51:2148–2157. doi: 10.2337/diabetes.51.7.2148. [DOI] [PubMed] [Google Scholar]
- 8.Rickels MR, Schutta MH, Markmann JF, Barker CF, Naji A, Teff KL. {beta}-Cell function following human islet transplantation for type 1 diabetes. Diabetes. 2005;54:100–106. doi: 10.2337/diabetes.54.1.100. [DOI] [PubMed] [Google Scholar]
- 9.Keymeulen B, Gillard P, Mathieu C, et al. Correlation between beta cell mass and glycemic control in type 1 diabetic recipients of islet cell graft. Proc Natl Acad Sci U S A. 2006;103:17444–17449. doi: 10.1073/pnas.0608141103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganda OP, Srikanta S, Brink SJ, et al. Differential sensitivity to beta-cell secretagogues in “early,” type I diabetes mellitus. Diabetes. 1984;33:516–521. doi: 10.2337/diab.33.6.516. [DOI] [PubMed] [Google Scholar]
- 11.Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D., Jr Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest. 1984;74:1318–1328. doi: 10.1172/JCI111542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using aglucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez LA, Lehmann R, Luzi L, et al. The effects of maintenance doses of FK506 versus cyclosporin A on glucose and lipid metabolism after orthotopic liver transplantation. Transplantation. 1999;68:1532–1541. doi: 10.1097/00007890-199911270-00017. [DOI] [PubMed] [Google Scholar]
- 14.Rickels MR, Schutta MH, Mueller R, et al. Islet cell hormonal responses to hypoglycemia after human islet transplantation for type 1 diabetes. Diabetes. 2005;54:3205–3211. doi: 10.2337/diabetes.54.11.3205. [DOI] [PubMed] [Google Scholar]
- 15.Robertson RP. Estimation of beta-cell mass by metabolic tests: Necessary, but how sufficient? Diabetes. 2007;56:2420–2424. doi: 10.2337/db07-0742. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto S, Noguchi H, Takita M, Shimoda M, Tamura Y, Olsen G, et al. Excellence of suito index for assessing clinical outcome of islet transplantation. Transplant Proc. 2010;42:2062–2064. doi: 10.1016/j.transproceed.2010.05.079. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto S, Noguchi H, Takita M, et al. Super-high-dose islet transplantation is associated with high SUITO index and prolonged insulin independence: A case report. Transplant Proc. 42:2156–2158. doi: 10.1016/j.transproceed.2010.05.095. [DOI] [PubMed] [Google Scholar]
- 18.Takita M, Matsumoto S, Noguchi H, et al. Secretory unit of islet transplant objects (SUITO) index can predict outcome of intravenous glucose tolerance test. Transplant Proc. 2010;42:2065–2067. doi: 10.1016/j.transproceed.2010.05.102. [DOI] [PubMed] [Google Scholar]
- 19.Shapiro AM, Ricordi C, Hering B. Edmonton’s islet success has indeed been replicated elsewhere. Lancet. 2003;362:1242. doi: 10.1016/S0140-6736(03)14526-6. [DOI] [PubMed] [Google Scholar]
- 20.Hanson MS, Park EE, Sears ML, et al. A simplified approach to human islet quality assessment. Transplantation. 2010;89:1178–1188. doi: 10.1097/TP.0b013e3181d54bce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson H, Lukinius A, Moberg L, et al. Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes. 2005;54:1755–1762. doi: 10.2337/diabetes.54.6.1755. [DOI] [PubMed] [Google Scholar]
- 22.Brissova M, Powers AC. Revascularization of transplanted islets: Can it be improved? Diabetes. 2008;57:2269–2271. doi: 10.2337/db08-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berkowitz K, Peters R, Kjos SL, et al. Effect of troglitazone on insulin sensitivity and pancreatic beta-cell function in women at high risk for NIDDM. Diabetes. 1996;45:1572–1579. doi: 10.2337/diab.45.11.1572. [DOI] [PubMed] [Google Scholar]
- 24.Cavaghan MK, Ehrmann DA, Byrne MM, Polonsky KS. Treatment with the oral antidiabetic agent troglitazone improves beta cell responses to glucose in subjects with impaired glucose tolerance. J Clin Invest. 1997;100:530–537. doi: 10.1172/JCI119562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCulloch DK, Koerker DJ, Kahn SE, Bonner-Weir S, Palmer JP. Correlations of in vivo beta-cell function tests with beta-cell mass and pancreatic insulin content in streptozocin-administered baboons. Diabetes. 1991;40:673–679. doi: 10.2337/diab.40.6.673. [DOI] [PubMed] [Google Scholar]
- 26.Larsen MO, Rolin B, Sturis J, et al. Measurements of insulin responses as predictive markers of pancreatic beta-cell mass in normal and beta-cell-reduced lean and obese Gottingen minipigs in vivo. Am J Physiol Endocrinol Metab. 2006;290:E670–E677. doi: 10.1152/ajpendo.00251.2005. [DOI] [PubMed] [Google Scholar]
- 27.Robertson RP, Porte D., Jr The glucose receptor. A defective mechanism in diabetes mellitus distinct from the beta adrenergic receptor. J Clin Invest. 1973;52:870–876. doi: 10.1172/JCI107251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tobin BW, Lewis JT, Chen DZ, Finegood DT. Insulin secretory function in relation to transplanted islet mass in STZ-induced diabetic rats. Diabetes. 1993;42:98–105. doi: 10.2337/diab.42.1.98. [DOI] [PubMed] [Google Scholar]
- 29.Ryan EA, Paty BW, Senior PA, Lakey JR, Bigam D, Shapiro AM. Beta-score: An assessment of beta-cell function after islet transplantation. Diabetes Care. 2005;28:343–347. doi: 10.2337/diacare.28.2.343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.