Abstract
Background
Cesarean scar ectopic pregnancies are rare, with an incidence of approximately 1 in 2,000 pregnancies. The trauma of a cesarean section and a subsequent cesarean scar pregnancy can lead to the formation of an arteriovenous malformation (AVM). The resulting intractable bleeding is difficult to manage and can result in an emergent surgical intervention that could jeopardize a female's ability to become pregnant in the future.
Case Report
A 29-year-old female, gravida 2 para 1, with 1 prior low transverse cesarean section had a presumed cervical ectopic pregnancy treated with intramuscular methotrexate. Despite a negative serum beta human chorionic gonadotropin result, she had a persistent mass in the lower uterine segment of her cesarean section scar and was finally diagnosed with an AVM. We successfully preserved her fertility by performing a wedge resection of the AVM and using a novel technique of bilateral O'Leary sutures to occlude the ascending and descending branches of the uterine artery along with intramuscular vasopressin infiltration.
Conclusion
A multidisciplinary approach involving obstetrics/gynecology, interventional radiology, and anesthesiology allowed for a safe conservative surgical approach and the preservation of our patient's fertility.
Keywords: Arteriovenous malformations, cesarean section, pregnancy–ectopic
INTRODUCTION
Ectopic pregnancies complicate 1%-2% of all first-trimester pregnancies in the United States but still account for up to 6% of pregnancy-related deaths.1 While 95% of ectopic pregnancies implant in the fallopian tube, the remaining 5% are found elsewhere, including the ovary, peritoneal cavity, cervix, and cesarean section scar.
Arteriovenous malformations (AVMs) of the uterus are rare, and the incidence is unknown.2 They can be congenital, thought to be the result of abnormal embryologic differentiation, or acquired. The limited literature addressing AVMs of the uterus notes that acquired lesions usually occur after trauma or uterine instrumentation.3
Treatment of nontubal ectopic pregnancies can be perplexing because of the variety of anatomic implantation sites and associated implications. This report focuses on one such case and offers insight into management options for a patient with a rare, potentially fatal malformation who strongly desired fertility preservation.
CASE REPORT
A 29-year-old, gravida 2 para 1 patient with a history significant for 1 previous low transverse cesarean section presented to an outside hospital with a concern of persistent, light vaginal bleeding. Her serum beta human chorionic gonadotropin (hCG) level was 2,500 mIU/mL. A nonviable fetus measuring 7 weeks, 5 days of gestation by crown-rump length was noted very low in the uterus and thought likely to be in the cervix. Differential diagnoses included cervical pregnancy vs incomplete spontaneous abortion, and the patient was followed up with repeat ultrasound the next day. The follow-up ultrasound was unchanged, and the presumed diagnosis was a cervical ectopic pregnancy. The patient was treated with 1 dose of intramuscular methotrexate and responded appropriately, with a gradual serum beta hCG level reduction to <10 mIU/mL; however, repeat ultrasounds 1 and 2 months after the original diagnosis noted a persistent 4.8 × 4.9 cm cervical pregnancy despite the low beta hCG level. The patient was referred to Ochsner Clinic Foundation for further management.
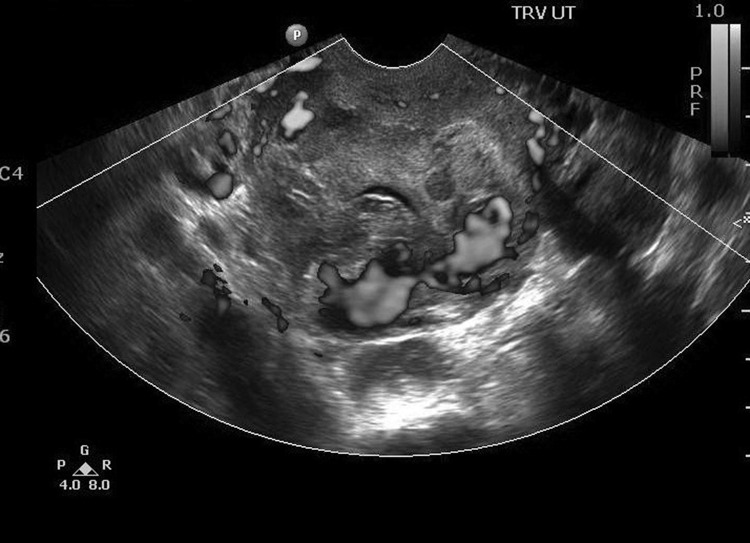
At the time of presentation, the patient reported 1 episode of heavy vaginal bleeding after intercourse the day prior and ongoing scant bleeding. During physical examination, a small amount of old blood was observed in the vagina, with no cervical lesions, cervical abnormalities, or active vaginal bleeding. Her hemoglobin and hematocrit values were 10.7 g/dL and 32.6%, respectively. Her beta hCG level was 3.5 mIU/mL, and an ultrasound performed by a maternal fetal medicine physician showed a large mass (6.0 × 4.6 cm) in the lower uterine segment/upper portion of the cervix that appeared to protrude from the lower uterine segment in the area of her cesarean section scar, most consistent with a cesarean scar pregnancy. In comparison with images obtained 2 weeks prior, the gestational sac previously visualized was now smaller and more irregularly shaped, consistent with the patient's report of a large amount of vaginal bleeding. Large areas of blood flow were noted, predominantly on the periphery of the mass (Figure 1). Differential diagnosis was expanded to include cesarean scar ectopic pregnancy in addition to the referring physician's diagnosis of cervical ectopic pregnancy. The patient strongly desired fertility preservation and opted for treatment via selective embolization of the pregnancy's blood supply.
Figure 1.
Ultrasound shows large areas of blood flow, predominantly on the periphery of the large mass (6.0 × 4.6 cm) in the lower uterine segment in the area of the patient's cesarean section scar.
The interventional radiology department was consulted for uterine artery embolization. During the attempt at selective embolization, the femoral artery approach arteriogram revealed rapid venous outflow from the hypervascular uterine lesion into either the right ovarian vein, internal iliac vein to the inferior vena cava, or dilated lumbar veins. Because of concern for an AVM or arteriovenous fistula, the procedure was aborted, and magnetic resonance imaging (MRI) was recommended for further evaluation. The MRI was significant for a large area of heterogeneity involving the anterior lower uterine segment and a thinned anterior uterine wall with abnormal, large serpiginous vessels confined by the uterine wall, possibly in the cesarean scar, with findings related to an AVM or arteriovenous fistula (Figure 2). After confirmation, embolization was deemed inappropriate, and the treatment options offered to the patient were wedge resection of the lower uterine segment or hysterectomy. The patient strongly desired uterine preservation and chose to proceed with a uterine wedge resection.
Figure 2.
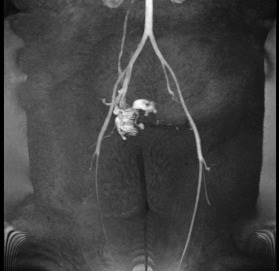
Magnetic resonance angiogram demonstrates multiple vessels tangled in the lower pelvis arising from the right internal iliac artery, corresponding to the arteriovenous malformation or arteriovenous fistula seen on magnetic resonance imaging.
The patient presented to the emergency department the evening prior to the planned surgery with reported heavy vaginal bleeding, weakness, and dizziness. Clotted blood in the vagina without any brisk bleeding was observed during physical examination. Hemoglobin and hematocrit values were significantly decreased to 6.9 g/dL and 21.7%, respectively. The patient was admitted and transfused with 2 units of packed red blood cells with plans to proceed with surgery in the morning as originally scheduled.
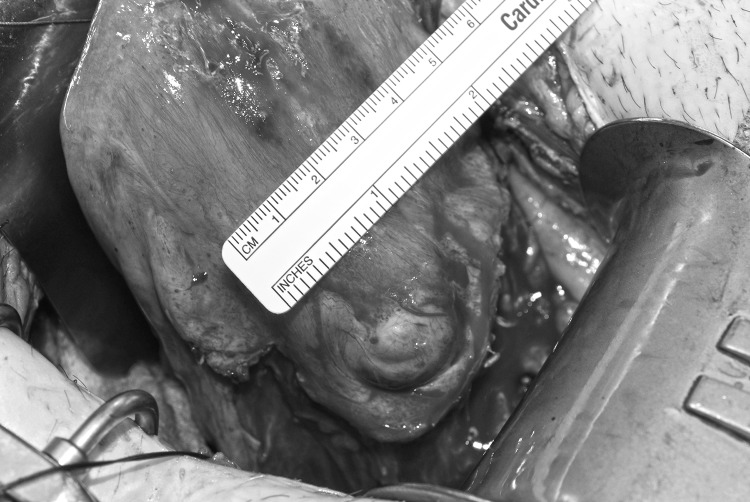
The patient underwent an exploratory laparotomy with a wedge resection of the lower uterine segment. A central venous catheter was placed prior to the start of the operation in case of the need for rapid fluid resuscitation. Pfannenstiel incision was used to enter the abdominal cavity, and findings included a 6-week sized, mobile uterus with a 3 × 4 cm AVM in the lower uterine segment (Figure 3). Dense bladder adhesions to the lower uterine segment were dissected to clearly isolate the AVM. Next, the uterine arteries were ligated bilaterally with O'Leary sutures. Vasopressin was injected around the mass, and a No. 11 scalpel blade was used to score the uterus in an elliptic fashion around the AVM, with this incision carried posteriorly into the endometrial cavity. A significant amount of bright red, freshly clotted blood was present within the uterus, indicative of bleeding during the surgery, and the patient received an additional 3 units of packed red blood cells and 2 units of fresh frozen plasma intraoperatively. A 2-layer closure was used for the uterine incision with deep 0-chromic simple interrupted sutures and 0-chromic running locked sutures for the superficial layer. Hemostasis was noted prior to closing the abdominal incision, and the total estimated blood loss during the procedure was 400 mL. Cystoscopy at the end of the case did not suggest any ureteral or bladder injury. Surgical pathology confirmed a partially thrombosed AVM with dense fibrosis and scattered chronic inflammation without any products of conception.
Figure 3.
A 3 × 4 cm arteriovenous malformation is visible in the lower uterine segment.
The patient's postoperative course was uncomplicated. Vaginal bleeding decreased significantly, and postoperative hemoglobin/hematocrit levels were stable. She was discharged on postoperative day 2 in stable condition. In addition to bleeding and pelvic rest precautions, the patient was advised to avoid pregnancy for at least 6 months to allow the lower uterine segment time to heal, and she was also made aware of the need for a cesarean section for future pregnancies. For contraception, she decided to use an intrauterine device that was successfully placed several weeks after her surgery. To date, we are unaware of subsequent pregnancies or pregnancy outcomes for this patient.
DISCUSSION
Of the few reported cases of uterine AVMs, acquired occurrences are more common than congenital incidences.4 AVMs usually follow uterine trauma, such as cesarean section, pelvic surgery, or uterine curettage, and form during tissue healing when an artery and veins connect abnormally. Infection, retained products of conception, gestational trophoblastic disease, gynecologic malignancies, and exposure to diethylstilbestrol are additional identified risk factors for acquired uterine AVMs.4
The presenting scenario of uterine AVM is often intractable bleeding during pregnancy or after uterine trauma. Ultrasound, computed tomography, and MRI are frequently used to evaluate bleeding and suspected AVMs, but angiography is the gold standard for diagnosis.5 The treatment of choice for postmenopausal patients is hysterectomy; however, stable patients who wish to preserve fertility have options.4 Expectant management and/or medical management with oral contraceptive pills or methylergonovine maleate have been described as successful options, as have invasive options such as arterial embolization, hysteroscopy AVM coagulation, laparoscopic uterine vessel cauterization, surgical uterine artery ligation, and AVM excision.2
While cesarean scar pregnancy is observed more often than uterine AVM, it is still a rare occurrence. The reported incidence of cesarean scar pregnancies is on the rise, accompanying increased cesarean section rates, with a 2007 report concluding that approximately 1 in 2,000 pregnancies is complicated by cesarean scar implantations.6 Presentation is most common between 5 and 16 weeks of gestation, with the most common chief concern being painless light vaginal bleeding. Ultrasound is the first-line imaging modality for diagnosis in the hemodynamically stable patient, with color flow Doppler ultrasound, MRI, diagnostic hysteroscopy, and diagnostic laparoscopy possibly useful adjuncts for diagnosis and treatment planning. Treatment should include measures to prevent rupture of the sac, to remove the gestational sac, and to preserve fertility. Treatment options are numerous and include expectant management (although not advised); conservative medical treatment (methotrexate only); combined medical treatment (local injection of potassium chloride, methotrexate, and mifepristone); and medical treatment combined with cesarean surgical sac aspiration, uterine curettage, hysteroscopic evacuation, laparoscopic removal, open surgical treatment, or hysterectomy.6
The incidence of cesarean scar pregnancy complicated by uterine AVM is unknown, and we found only 5 case reports to date.7-10 All treatments described in prior cases included uterine artery or selective arterial embolization. Methotrexate, uterine curettage, and wedge resection are also described as successful adjuvant treatment methods.
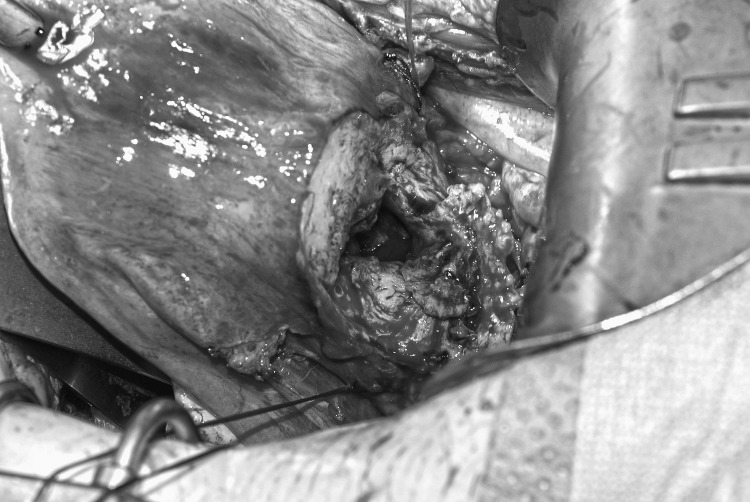
The AVM in this patient could not be treated with selective embolization, prompting the decision for surgical management. To control bleeding during the wedge resection of the uterus, O'Leary sutures, traditionally used at the time of cesarean section for management of uterine artery lacerations from hysterotomy extensions, were placed on the ascending and descending uterine arteries above and below the lateral edges of the AVM (Figure 4). This suture placement helped decrease pulse pressure and allowed for safe excision and repair. Additionally, there were no long-term consequences to the uterus from the O'Leary sutures because of good collateral blood supply and the nonpermanent nature of the suture. The uterus was closed in a multilayer fashion to develop a strong lower uterine segment that would not rupture with subsequent pregnancies. The patient was counseled on the importance of spacing the pregnancy interval after uterine repair to allow adequate healing.
Figure 4.
Bilateral O'Leary sutures, traditionally used at the time of cesarean section for management of uterine artery lacerations from hysterotomy extensions, were placed on the ascending and descending uterine arteries above and below the lateral edges of the arteriovenous malformation.
CONCLUSION
This case is unique because of the rare incidence of cesarean scar pregnancy and AVM complication and because the treatment was performed without prior uterine artery or selective embolization, unlike the cases presented in the literature. Our case shows that safe, effective surgical management can be provided and the uterus can be preserved for a patient with a potentially morbid uterine AVM at the cesarean scar site. With the advent of improved suturing techniques to ensure a double-layer closure of the lower uterine segment available in the laparoscopic and robotic platforms, a minimally invasive approach using our technique could be considered in the future. Also, a multidisciplinary approach involving obstetrics/gynecology, interventional radiology, and anesthesiology allowed for a safe conservative surgical approach.
ACKNOWLEDGMENTS
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1.Hoffman BL, Schaffer JI, Halvorson LM, Bradshaw KD, Cunningham F, Calver LE. Williams Gynecology. New York, NY: McGraw-Hill; 2012. [Google Scholar]
- 2.Vijayakumar A, Srinivas A, Chandrashekar BM, Vijayakumar A. Uterine vascular lesions. Rev Obstet Gynecol. 2013;6(2):69–79. [PMC free article] [PubMed] [Google Scholar]
- 3.Moulder JK, Garrett LA, Salazar GM, Goodman A. The role of radical surgery in the management of acquired uterine arteriovenous malformation. Case Rep Oncol. 2013 Jun 1;6((s)):303–310. doi: 10.1159/000351609. doi: 10.1159/000351609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashim H, Nawawi O. Uterine arteriovenous malformation. Malays J Med Sci. 2013 Mar;20(2):76–80. [PMC free article] [PubMed] [Google Scholar]
- 5.Grivell RM, Reid KM, Mellor A. Uterine arteriovenous malformations: a review of the current literature. Obstet Gynecol Surv. 2005 Nov;60(11):761–767. doi: 10.1097/01.ogx.0000183684.67656.ba. [DOI] [PubMed] [Google Scholar]
- 6.Ash A, Smith A, Maxwell D. Caesarean scar pregnancy. BJOG. 2007 Mar;114(3):253–263. doi: 10.1111/j.1471-0528.2006.01237.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim D, Moon NR, Lee SR, et al. Acquired uterine arteriovenous malformation in a cesarean scar pregnancy. Taiwan J Obstet Gynecol. 2013 Dec;52(4):590–592. doi: 10.1016/j.tjog.2013.10.026. doi: 10.1016/j.tjog.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Akbayir O, Gedikbasi A, Akyol A, Ucar A, Saygi-Ozyurt S, Gulkilik A. Cesarean scar pregnancy: a rare cause of uterine arteriovenous malformation. J Clin Ultrasound. 2011 Nov-Dec;39(9):534–538. doi: 10.1002/jcu.20848. doi: 10.1002/jcu.20848. [DOI] [PubMed] [Google Scholar]
- 9.Kochhar PK, Sarangal M, Gupta U. Conservative management of cesarean scar pregnancy with uterine arteriovenous malformation: a case report. J Reprod Med. 2013 Jan-Feb;58((1-2)):81–84. [PubMed] [Google Scholar]
- 10.Rygh AB, Greve OJ, Fjetland L, Berland JM, Eggebø TM. Arteriovenous malformation as a consequence of a scar pregnancy. Acta Obstet Gynecol Scand. 2009;88(7):853–855. doi: 10.1080/00016340902971466. doi: 10.1080/00016340902971466. [DOI] [PubMed] [Google Scholar]