Abstract
Background
Vascular thrombosis is a well-known complication after simultaneous pancreas-kidney (SPK) transplantation procedures. The role of preoperative special coagulation studies to screen patients at high risk for vascular thrombosis is unclear and not well studied.
Methods
This study reports a retrospective medical record review of 83 SPK procedures performed between April 2007 and June 2013 in a single institution. All SPK transplantation recipients underwent preoperative screening for hypercoagulable state.
Results
Eighteen of 83 patients (21.69%) were diagnosed with vascular thrombosis of the pancreas. Of the 23 patients with at least 1 positive screening test, only 4 had a thrombotic event (17.39%). On the other hand, 14 of 60 patients with negative screening tests developed vascular thrombosis (23.33%). The hypercoagulable screening workup had a positive predictive value of 17.39% and a negative predictive value of 76.67%. The workup also demonstrated low sensitivity (22.22%) and specificity (70.77%).
Conclusion
No differences were seen in patient or graft survival between groups at 12 months. This retrospective study did not show any benefit of using special coagulation studies to rule out patients at risk for vascular thrombosis after SPK transplantation.
Keywords: Blood coagulation tests, kidney transplantation, mesenteric ischemia, organ transplantation, pancreas transplantation, thrombosis
INTRODUCTION
The first simultaneous pancreas-kidney (SPK) transplantation was performed in 1966, and since then more than 2,200 SPK transplantations have been performed in the United States.1 Vascular thrombosis is the major nonimmunologic cause of early pancreas allograft failure.2 The incidence of pancreas allograft failure secondary to vascular thrombosis is reported to be 4%-8% in SPK transplant recipients and 10%-12% following solitary pancreas transplantation.3 Indeed, the incidence of pancreas graft thrombosis in clinical pancreas transplantation has not changed since the complication of vascular thrombosis was first reported in the 11 pancreas transplantations done at the University of Minnesota in the mid-1960s.4
Vascular thrombosis can occur early or late after transplantation. Early thrombosis (within the first 2 weeks) is usually multifactorial and related to donor and recipient factors, preservation and procurement strategies, and technical aspects of pancreas transplantation.5 Donor factors such as increasing age, atherosclerosis, obesity, cerebrovascular event as a cause of death, and the presence of severe hypotension have all been cited as risk factors for pancreas graft thrombosis.6 The type of preservation solution and prolonged cold ischemia (>12-14 hours) may also play a role. In recipients, severe vascular disease, hypotension, orthostasis, and the presence of a thrombophilic state, whether definitively diagnosed or based on history, also increase the risk for thrombotic events.6 Diabetes is associated with abnormalities or defects in nearly all components of the coagulation system, including platelets, vascular endothelium, coagulation factors, anticoagulants, and fibrinolysis.7-9 Beyond 2 weeks, pancreas allograft thrombosis is usually mediated immunologically and associated with acute rejection of the allograft.10
Vascular thrombosis following SPK transplantation can be either arterial or venous; however, venous thrombosis is a more common event (2:1 ratio).11 Thrombosis may also be subclassified as either complete or partial. Whereas complete vascular occlusion generally results in graft loss, partially occlusive thrombosis is associated with a higher likelihood of pancreas graft survival.11,12 Because of the segmental nature of pancreas arterial anatomy, thrombosis of different arterial segments may lead to varying outcomes, and in some cases, the collateral circulation within the pancreas may prevent infarction. The occlusion of either the splenic or superior mesenteric artery may result in partial infarction of the graft. Likewise, different outcomes have been observed for splenic venous thrombosis compared with occlusion of the main portal vein in which graft loss is nearly universal.10,12
The risk for vascular thrombosis depends on the type of pancreas transplantation. Data from the Scientific Registry of Transplant Recipients and the International Pancreas Transplant Registry suggest that SPK transplant recipients have less pancreas graft thrombosis compared with pancreas after kidney or pancreas transplant alone recipients.13 The anticoagulant effects of uremia and the lower risk for acute rejection associated with SPK transplantation are the most commonly cited reasons for this difference.13 Surgical techniques to manage exocrine secretions may also be associated with variable risks for thrombosis. The incidence of thrombosis is slightly higher in the setting of enteric exocrine drainage compared with bladder drainage.13-15
We report our center's experience of using pretransplantation screening to diagnose a hypercoagulable condition in SPK transplant recipients to identify patients at high risk for vascular thrombosis.
METHODS
This study is a retrospective medical record review of patients who underwent SPK transplantation between April 2007 and June 2013 in a single institution. The study was approved by the institutional review board.
Routine preoperative screening for hypercoagulable disorder included tests for prothombin time, activated partial thromboplastin time, international normalized ratio, antiphospholipid antibodies (IgG and IgM), lupus anticoagulant, protein C and protein S activity, factor V Leiden mutation, prothrombin G20210A mutation, and antithrombin III level. Diagnosis of thrombotic events was established by reviewing imaging studies including Doppler ultrasounds and computed tomography angiograms. We also reviewed inpatient hospital notes, discharge summaries, and outpatient clinic visits.
Organs were placed intraperitoneally with pancreas exocrine drainage to a jejunal loop without Roux-en-Y and venous drainage into the portal circulation. All patients had routine Doppler ultrasound of the pancreas and kidney allograft at days 1 and 7 post-SPK and whenever clinically indicated.
All patients received induction immunosuppression with antithymocyte globulin (rabbit antithymocyte globulin, 1.5 mg/kg × 3 doses). Maintenance immunosuppression consisted of tacrolimus, mycophenolate mofetil (MMF), and steroids. Tacrolimus troughs were maintained at 10-15 ng/mL during the first 3 months and 10-12 ng/mL until the end of the first year. MMF dose was maintained at 1,000 mg twice daily, and prednisone was tapered to 5 mg 3-6 months after the surgery.
At our center, we administer 325 mg of enteric-coated aspirin to all SPK transplant recipients. Patients with positive screening tests and/or history of thrombophilic disorders are usually referred to hematology for detailed evaluation. We have used anticoagulation on a case-by-case basis; however, no strict protocol exists regarding perioperative care of patients with a positive screening test. The duration of treatment depends on the clinical history of thrombophilia and the perceived risk of thrombosis as ascertained by the hematologist.
We reported partial and complete thrombosis involving either a pancreas or kidney allograft including the pancreatic conduit artery, superior mesenteric artery, splenic artery, portal vein, renal artery, renal vein, and/or iliac vessels. We also looked at transplantation kidney biopsy reports and the incidence of renal and pancreas allograft loss.
Demographic variables included age, sex, race, body mass index (BMI), and type of diabetes (type 1 vs type 2). For numeric variables (age and BMI), mean and standard deviation are reported. For categorical variables, occurrence and percentage are reported. Two-sample t test was employed for continuous variables such as age and BMI, and Fisher exact test was employed for categorical variables: sex, type of diabetes, and race. We calculated sensitivity, specificity, positive predictive value, and negative predictive value using standard methods. P value <0.05 was considered statistically significant.
RESULTS
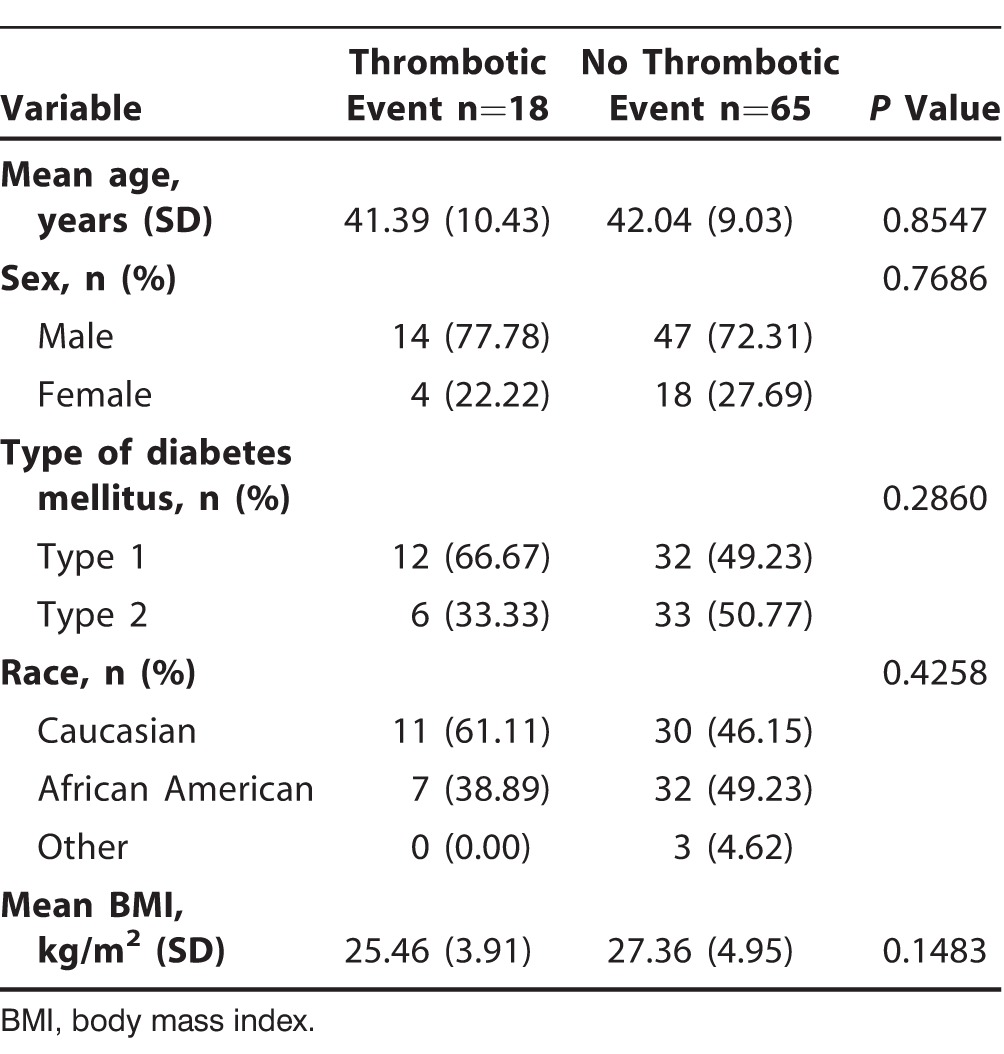
Ninety-six patients received SPK transplantation at our center between April 2007 and June 2013. All of the patients were dialysis dependent at the time of surgery. Of them, 13 patients were excluded because of incomplete data, and 83 patients were included for final analysis (Table 1). Eighteen of 83 (21.69%) patients were diagnosed with a thrombotic event, whereas 65 of 83 (78.31%) remained free of thrombotic complications. No statistical difference between the 2 groups was seen in age, sex, and BMI. An increased trend of vascular thrombosis in type 1 diabetics (66.67% vs 49.23%) and Caucasians (61.11% vs 46.15%) was noted; however, the P values were not significant.
Table 1.
Baseline Demographics of Patients with Simultaneous Pancreas-Kidney Transplantation (n=83)
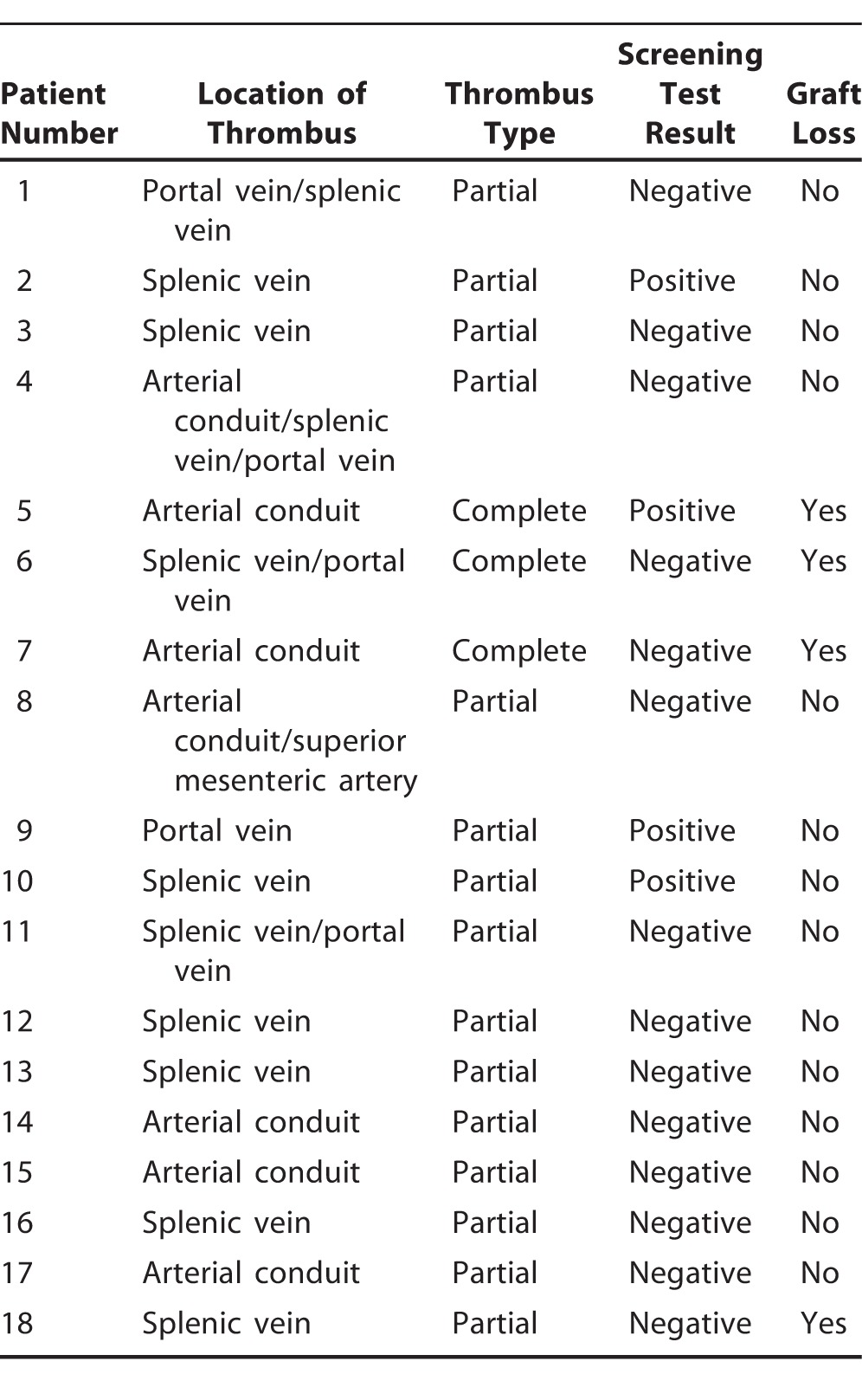
The figure is a schematic illustration of the study design. Of the 83 patients whose data were available for final analysis, 23 (27.71%) patients had at least 1 positive screening test. One patient had 3 positive screening tests, and 2 patients had 2 positive screening tests each. Among the 23 patients with at least 1 positive screening test, only 4 (17.39%) had a thrombotic event, while 14 of 60 (23.33%) patients with negative screening tests were diagnosed with vascular thrombosis. Twelve of the 18 patients with a thrombotic event presented with venous thrombosis involving the splenic vein, portal vein, or both. Most of the vascular thromboses were partial and nonocclusive (Table 2).
Figure.
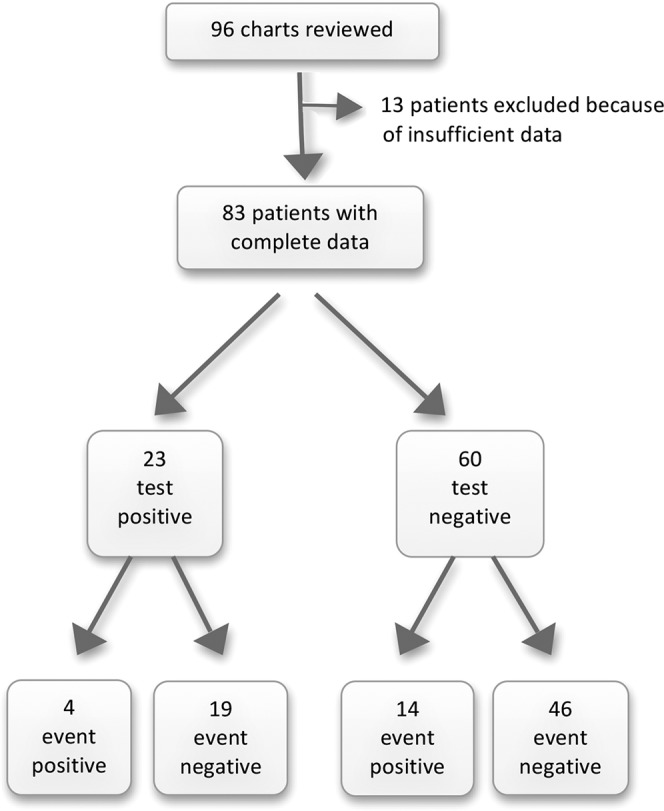
Schematic illustration of the study.
Table 2.
Thrombosis and Graft Outcomes
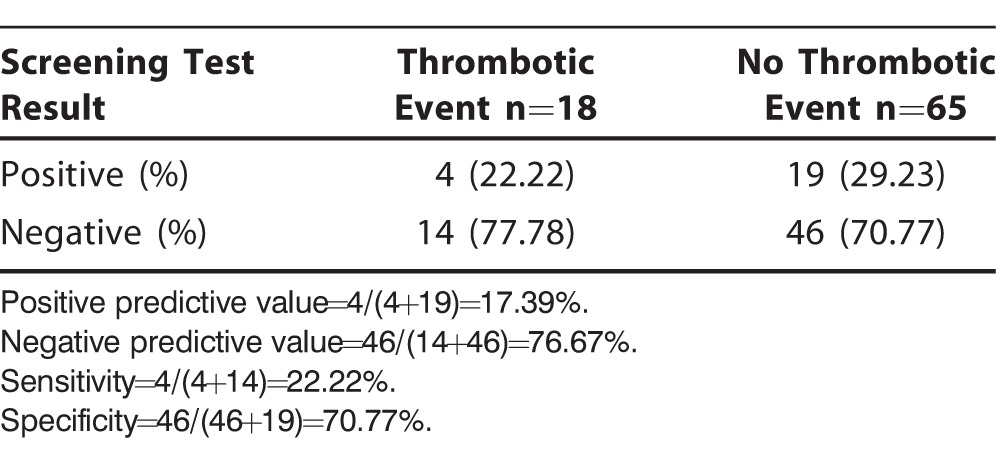
The screening workup had a positive predictive value of 17.39% and a negative predictive value of 76.67%. Sensitivity of the screening panel to rule out a thrombotic event after SPK transplantation was 22.22%, and specificity was 70.77% (Table 3).
Table 3.
Performance of Hypercoagulable Workup
Six pancreas allografts failed during the study period (data not shown). Four of the 6 graft losses occurred in patients with vascular thrombosis; however, no difference in graft and patient survival was seen at 12 months between the 2 groups.
DISCUSSION
Pancreas graft thrombosis remains one of the most common reasons for pancreas allograft loss. Thrombectomy may salvage the pancreas graft if performed early; however, pancreas graft loss is the most commonly observed scenario in cases with total venous or arterial occlusion.16,17 In addition to graft loss, vascular thrombosis leads to prolonged hospital stay and increased readmission rates and contributes to increased patient morbidity.17,18
Patients undergoing SPK transplantation may have all the risk factors associated with formation of a thrombus. Their blood flow may be low because of low venous pressure system, microvascular disease, and perioperative hypotension. If the vascular endothelium is injured because of ischemia-reperfusion injury, the patient may be more susceptible to thrombosis. Last, thrombophilia and hypercoagulable states are prevalent in patients with diabetes.19,20
Most transplantation centers in the United States administer some form of anticoagulation following SPK transplantation. Low-dose aspirin is generally recommended for all pancreas recipients, although no literature supports its use. Unfractionated heparin or low molecular weight heparin (LMWH; enoxaparin) may also be used to reduce the risk of vascular thrombosis after pancreas transplantation, although the risk of perioperative bleeding remains significant.21,22 The dose of LMWH needs to be adjusted in patients with renal insufficiency, and the change in renal function following SPK transplantation makes the dosing of LMWH problematic.23-25 Warfarin is typically not recommended in the immediate posttransplantation period; however, it is frequently used as a long-term anticoagulant for prophylaxis of vascular thrombosis in patients with thrombophilic disorders.
Surprisingly, risk stratification for thrombotic complications after SPK transplantation has not been given much attention. To the best of our knowledge, the role of screening tests to rule out a hypercoagulable disorder has not been studied in this population of patients, and our study is the first to address this question. It is pertinent to evaluate transplant candidates with thrombophilia and identify patients at highest risk for thrombotic complications. However, such an extensive preoperative workup represents a significant financial burden for the institution and increases the cost of the pretransplantation evaluation. In the absence of data supporting the use of pretransplantation screening for a hypercoagulable disorder, we considered it important to look at the incidence of vascular thrombosis and graft failure in this population.
Our study failed to show any demonstrable effect of using screening tests to predict a thrombotic event after SPK transplantation. Sensitivity of the testing panel was poor because we only correctly identified 22.22% of event-positive patients with a high false-negative rate (77.78%, type II error). The testing panel performed slightly better in excluding a thrombotic event (specificity 70.77%); however, the false-positive rate was 29.23% (type I error).
In our study, the hypercoagulable screening testing had a low positive predictive value of 17.39%, meaning that only 17.39% of the patients with ≥1 positive screening tests developed vascular thrombus. This result may be because of the relatively low prevalence of vascular thrombosis in this population. Negative predictive value on the screening panel performed better in that 76.67% of the patients with negative screening test results remained event-free; however, negative predictive value is not sufficiently reliable to be used as a screening tool.
Based on our results, we do not recommend a pretransplantation hypercoagulable screening workup because of the low yield and unfavorable cost-benefit ratio. The use of anticoagulation in the perioperative period should be individualized and based on each center's experience. High-risk populations, such as patients with a history of thrombophilic disorders, multiple abortions, systemic lupus erythematosus, or multiple clotted accesses, may be screened prior to SPK transplantation. In the early posttransplantation period, a high index of suspicion and the routine use of Doppler ultrasound to evaluate pancreas perfusion are also highly recommended.
We recognize the limitations of our retrospective study without a control group. Also, factors such as ischemia-reperfusion injury, cold ischemia time, and postoperative hypotension, which could potentially cause vascular thrombosis, could not be taken into account because of the retrospective nature of the study. The positive predictive value and negative predictive value of the testing may also be skewed because of the relatively low prevalence of vascular thrombosis in patients with SPK transplantations.
CONCLUSION
In conclusion, our study showed no benefit of pretransplantation screening for vascular thrombosis with special coagulation studies in patients undergoing SPK transplantation. The decision to screen and administer anticoagulants to potential SPK transplantation recipients should be made on clinical grounds.
ACKNOWLEDGMENTS
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1.Kim YH, Park JB, Lee SS, Byun JH, Kim SC, Han DJ. How to avoid graft thrombosis requiring graftectomy: immediate posttransplant CT angiography in pancreas transplantation. Transplantation. 2012 Nov 15;94(9):925–930. doi: 10.1097/TP.0b013e3182692b4d. [DOI] [PubMed] [Google Scholar]
- 2.Ramessur Chandran S, Kanellis J, Polkinghorne KR, Saunder AC, Mulley WR. Early pancreas allograft thrombosis. Clin Transplant. 2013 May-Jun;27(3):410–416. doi: 10.1111/ctr.12105. [DOI] [PubMed] [Google Scholar]
- 3.Stratt RJ. Prevention of thrombosis after pancreas transplantation. 2005. Medscape Transplantation. April 29, http://www.medscape.com/viewarticle/503838. Accessed November 26, 2014. [Google Scholar]
- 4.Lillehei RC, Simmons RL, Najarian JS, et al. Pancreatico-duodenal allotransplantation: experimental and clinical experience. Ann Surg. 1970 Sep;172(3):405–436. doi: 10.1097/00000658-197009000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farney AC, Rogers J, Stratta RJ. Pancreas graft thrombosis: causes, prevention, diagnosis, and intervention. Curr Opin Organ Transplant. 2012 Feb;17(1):87–92. doi: 10.1097/MOT.0b013e32834ee717. [DOI] [PubMed] [Google Scholar]
- 6.Burke GW, 3rd, Ciancio G, Figueiro J, et al. Hypercoagulable state associated with kidney-pancreas transplantation. Thromboelastogram-directed anti-coagulation and implications for future therapy. Clin Transplant. 2004 Aug;18(4):423–428. doi: 10.1111/j.1399-0012.2004.00183.x. [DOI] [PubMed] [Google Scholar]
- 7.Carr ME. Diabetes mellitus: a hypercoagulable state. J Diabetes Complications. 2001 Jan-Feb;15(1):44–54. doi: 10.1016/s1056-8727(00)00132-x. Review. [DOI] [PubMed] [Google Scholar]
- 8.Glassman AB. Platelet abnormalities in diabetes mellitus. Ann Clin Lab Sci. 1993 Jan-Feb;23(1):47–50. [PubMed] [Google Scholar]
- 9.Piemontino U, Ceriello A, Di Minno G. Hemostatic and metabolic abnormalities in diabetes mellitus. The search for a link. Haematologica. 1994 Jul-Aug;79(4):387–392. [PubMed] [Google Scholar]
- 10.Drachenberg CB, Papadimitriou JC, Farney A, et al. Pancreas transplantation: the histologic morphology of graft loss and clinical correlations. Transplantation. 2001 Jun 27;71(12):1784–1791. doi: 10.1097/00007890-200106270-00014. [DOI] [PubMed] [Google Scholar]
- 11.Ciancio G, Cespedes M, Olson L, Miller J, Burke GW. Partial venous thrombosis of the pancreatic allografts after simultaneous pancreas-kidney transplantation. Clin Transplant. 2000 Oct;14(5):464–471. doi: 10.1034/j.1399-0012.2000.140504.x. [DOI] [PubMed] [Google Scholar]
- 12.Fisher RA, Munda R, Madden R. Pancreas transplant functional salvage after segmental vascular thrombosis. Transplant Proc. 1993 Apr;25(2):2138–2140. [PubMed] [Google Scholar]
- 13.Gruessner AC, Sutherland DE. Pancreas transplant outcomes for United States (US) and non-US cases as reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR) as of June 2004. Clin Transplant. 2005 Aug;19(4):433–455. doi: 10.1111/j.1399-0012.2005.00378.x. [DOI] [PubMed] [Google Scholar]
- 14.Gruessner AC, Sutherland DE. Pancreas transplant outcomes for United States (US) and non-US cases as reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR) as of October 2002. Clin Transpl. 2002:41–77. [PubMed] [Google Scholar]
- 15.Gruessner AC, Sutherland DE. Analysis of United States (US) and non-US pancreas transplants as reported to the International Pancreas Transplant Registry (IPTR) and to the United Network for Organ Sharing (UNOS) Clin Transpl. 1998:53–73. [PubMed] [Google Scholar]
- 16.Martins L, Henriques AC, Dias L, et al. Pancreas-kidney transplantation: complications and readmissions in 9-years of follow-up. Transplant Proc. 2010 Mar;42(2):552–554. doi: 10.1016/j.transproceed.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto I, Shinzeki M, Asari S, et al. Functioning pancreas graft with thromboses of splenic and superior mesenteric arteries after simultaneous pancreas-kidney transplantation: a case report. Transplant Proc. 2014 Apr;46(3):989–991. doi: 10.1016/j.transproceed.2013.09.054. [DOI] [PubMed] [Google Scholar]
- 18.Troppmann C, Gruessner AC, Benedetti E, et al. Vascular graft thrombosis after pancreatic transplantation: univariate and multivariate operative and nonoperative risk factor analysis. J Am Coll Surg. 1996 Apr;182(4):285–316. [PubMed] [Google Scholar]
- 19.Benz S, Busing M, Kruger B, et al. Pancreas graft thrombosis: is there a role for trypsin. Pancreas. 2004 Jan;28(1):75–79. doi: 10.1097/00006676-200401000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Okabe Y, Kitada H, Miura Y, et al. Pancreas transplantation: a single-institution experience in Japan. Surg Today. 2013 Dec;43(12):1406–1411. doi: 10.1007/s00595-013-0516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shullo MA, Rose ML, Vivas C, et al. Hemorrhagic complications of enoxaparin and aspirin in patients with kidney transplants. Pharmacotherapy. 2002 Feb;22(2):184–187. doi: 10.1592/phco.22.3.184.33541. [DOI] [PubMed] [Google Scholar]
- 22.Cadroy Y, Pourrat J, Baladre MF, et al. Delayed elimination of enoxaparin in patients with chronic renal insufficiency. Thromb Res. 1991 Aug 1;63(3):385–390. doi: 10.1016/0049-3848(91)90141-i. [DOI] [PubMed] [Google Scholar]
- 23.Lachish T, Rudensky B, Slotki I, Zevin S. Enoxaparin dosage adjustment in patients with severe renal failure: antifactor xa concentrations and safety. Pharmacotherapy. 2007 Oct;27(10):1347–1352. doi: 10.1592/phco.27.10.1347. [DOI] [PubMed] [Google Scholar]
- 24.Crowther M, Lim W. Low molecular weight heparin and bleeding in patients with chronic renal failure. Curr Opin Pulm Med. 2007 Sep;13(5):409–413. doi: 10.1097/MCP.0b013e328216430d. [DOI] [PubMed] [Google Scholar]
- 25.Hulot JS, Montalescot G, Lechat P, Collet JP, Ankri A, Urien S. Dosing strategy in patients with renal failure receiving enoxaparin for the treatment of non-ST-segment elevation acute coronary syndrome. Clin Pharmacol Ther. 2005 Jun;77(6):542–552. doi: 10.1016/j.clpt.2005.02.012. [DOI] [PubMed] [Google Scholar]


