Abstract
Background
Acute bacterial cholangitis for the most part owing to common bile duct stones is common in gastroenterology practice and represents a potentially life-threatening condition often characterized by fever, abdominal pain, and jaundice (Charcot's triad) as well as confusion and septic shock (Reynolds' pentad).
Methods
This review is based on a systematic literature review in PubMed with the search items ‘cholangitis’, ‘choledocholithiasis’, ‘gallstone disease’, ‘biliary infection’, and ‘biliary sepsis’.
Results
Although most patients respond to empiric broad-spectrum antibiotic treatment, timely endoscopic biliary drainage depending on the severity of the disease is required to eliminate the underlying obstruction. Specific recommendations have been derived from the Tokyo guideline working group consensus 2006 and its update in 2013, albeit poorly evidence-based, providing a comprehensive overview of diagnosis, classification, risk stratification, and treatment algorithms in acute bacterial cholangitis.
Conclusion
Prompt clinical recognition and accurate diagnostic workup including adequate laboratory assessment and (aetiology-oriented) imaging are critical steps in the management of cholangitis. Treatment is directed at the two major interrelated pathophysiologic components, i.e. bacterial infection (immediate antimicrobial therapy) and bile duct obstruction (biliary drainage). As for the latter, transpapillary endoscopic drainage by stent or nasobiliary drain and/or same-session bile duct clearance, depending on individual disease severity, represent first-line treatment approaches.
Key Words: Cholangitis, Endoscopic retrograde cholangiography, Sepsis, Gallstone disease
Introduction
Gallstone disease ranks amongst the most prevalent gastrointestinal disorders in the Western world with estimates as high as 20% in European and Northern American populations, with rising incidence rates to be expected owing to the obesity epidemic [1]. Common bile duct (CBD) stones are to be expected in an estimated 10-20% of individuals with symptomatic gallstone disease, of whom 0.2% are at risk of acute bacterial cholangitis [2,3].
Acute bacterial cholangitis together with acute cholecystitis (both of whom may occur in concert in an individual) is a gastrointestinal emergency in the spectrum of acute biliary infection with high mortality rates and, thus, the need for straightforward diagnostic evaluation and immediate treatment initiation [4]. Acute cholangitis is characterized by acute inflammation and infection of the bile duct system with increased bacterial loads (biliary infection) and high intraductal pressure levels (biliary obstruction) favouring bacterial and endotoxin translocation into the vascular and lymphatic drainage (concept of cholangiovenous and cholangiolymphatic reflux, respectively). In conjunction with an increased permeability of the acutely inflamed biliary epithelium, the stage is set for potentially fatal complications such as biliary sepsis and hepatic abscess. The mere presence of bacteria in the biliary system, termed bacterobilia, is a sine qua non but by itself not sufficient to elicit cholangitis symptoms without coexistent obstruction due to effective antibacterial mechanical effects of bile flow and biliary immunoglobulin (Ig) A secretion protecting Kupffer cell function and integrity of biliary tight junctions [5]. The critical threshold of intrabiliary pressure above which biliary bacterial translocation into the systemic circulation occurs has been determined to be >20 cm H2O (normal: 7-14 cm H2O) [6,7]. As for biliary obstruction, the most frequent causes underlying mechanical cholestasis are (not mutually exclusive) choledocholithiasis as the leading cause, benign and malignant biliary stenosis, postoperative strictures, sclerosing cholangitis, and post-biliary instrumentation (table 1).
Table 1.
Potential causes of acute bacterial cholangitis (non-exhaustive selection)
| Choledocholithiasis |
| Congenital factors |
| Post-operative factors (bile duct injury, bilio-enteric anastomosis strictures, sump syndrome) |
| Inflammatory factors (parasitic infection, oriental cholangitis) |
| Malignant strictures (bile duct, gallbladder, ampullary, pancreatic malignancy) |
| Duodenal tumours |
| Pancreatitis |
| External compression, e.g. pericholecystic inflammatory changes, Mirizzi syndrome |
| Papillary stenosis |
| Duodenal diverticulum/Lemmel syndrome |
Despite the basically wide spectrum of potential causes of biliary obstruction, choledocholithiasis ranks first by far, which has been partly explained by cholesterol (secondary) bile duct stones being colonized by a bacterial biofilm, the clinical virulence of which is potentiated by obstruction-induced mucosal inflammatory cytokine production [8,9]. By contrast, primary bile duct stones result from biliary infection in the first place. In addition, there are several other risk factors associated with biliary infection such as recent infection elsewhere in the organism, advanced age >70 years, and presence of diabetes mellitus [10].
Clinical Presentation and Diagnosis
The clinical presentation is prototypically characterized by the so-called Charcot's triad as of 1887 which comprises intermittent fever, right upper quadrant (RUQ) pain, and jaundice (‘hepatic fever’ as by Dr. Jean-Martin Charcot). In 1959, Reynolds expanded the description of acute cholangitis by adding lethargy/mental confusion and shock indicative of ongoing biliary sepsis, thus dubbed the Reynolds' pentad [11]. However, an unequivocal diagnosis of acute cholangitis calls for confirmation of the underlying biliary source of the systemic infection, e.g. by aspiration of purulent bile during endoscopic retrograde cholangiography (ERC) representing the formal (albeit insensitive) gold standard. Since no non-invasive methods exist to obtain bile samples for analysis, in clinical practice the diagnosis relies on clinical findings, e.g. presence of the Charcot's triad, and additional laboratory assessment focusing on signs of systemic infection and cholestasis. Likewise, alternative diagnoses, e.g. other abdominal infectious foci with septic cholestasis, have to be adequately excluded. Of interest, in a prospective study assessing the diagnostic power of the Charcot triad only 22% of patients exhibiting suppurative bile fluids on surgical choledochotomy complied with all three Charcot criteria, thus indicating that its diagnostic utility remains suboptimal [12]. In general, Charcot's triad exhibits a high specificity while sensitivity is low. An estimated 90% of patients present with fever, and 60-70% are jaundiced. In an occasional patient, pain may be the only complaint, which, however, can also be lacking altogether especially in the elderly population [13,14]. Early diagnosis and timely treatment initiation is critical for an individual patient's prognosis, which is particularly important in the elderly population with often atypical presentations and, thus, considerable delays in diagnosis.
The Tokyo Guidelines
Against this background, in the Tokyo guidelines derived from an international meeting in 2006 and updated in 2013, much progress has been made in the definition and classification of acute cholangitis.
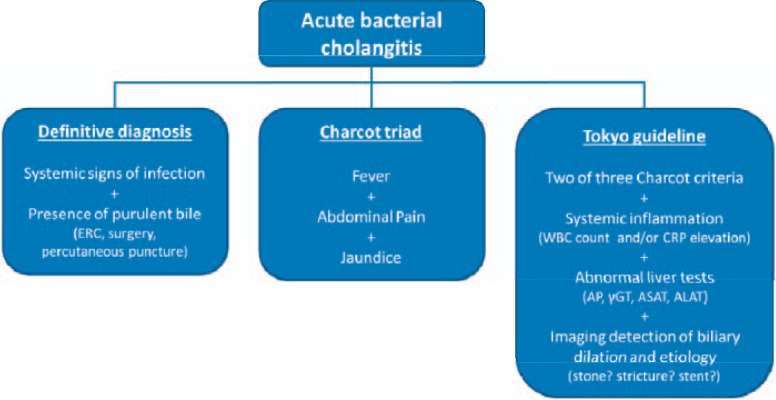
Figure 1 depicts the clinical diagnosis of acute cholangitis beyond the (impractical) evidence of purulent bile and the (unreliable) presence of Charcot's triad to incorporate laboratory evidence of infection and cholestasis as well as (aetiology-oriented) imaging studies, evaluating for dilatation of the biliary system as a minimum requirement and, more specific, existence of biliary stones, strictures, and stents, for example. As for clinical presentation, and in extension of the Charcot criteria, the Tokyo guideline participants have proposed a valid clinical cholangitis suspicion as per two or more of the following findings: history of biliary disease, fever/chills, jaundice, and right upper or upper abdominal pain [15].
Fig. 1.
Diagnostic algorithm in acute bacterial cholangitis. ERC = Endoscopic retrograde cholangiography; WBC = white blood cell; CRP = C-reactive protein; AP = alkaline phosphatase; γGT = γ-glutamyltransferase; ASAT = aspartate aminotransferase; ALAT = alanine aminotransferase.
Imaging Studies
There are various modalities available for imaging of the biliary tract, all of which harbour different benefits and caveats, with the most powerful being endoscopic ultrasound (EUS) and magnetic resonance cholangiopancreatography (MRCP) (table 2). For example, MRCP is purely non-invasive in nature but requires that the patient's condition is stable enough to be transported to the radiology department. However, as a first screening modality assessing for gallstone disease, bile duct diameter, and exclusion of other abdominal infectious sources and stone-related complications, transabdominal ultrasound still has its role as a first imaging test in the initial evaluation due to its wide availability, e.g. in the emergency ward, despite its low sensitivity in CBD stone detection ranging from 25 to 63% [16]. Yet, ultrasound has high diagnostic accuracy in the demonstration of biliary dilatation [17]. It has to be kept in mind, however, that a clear-cut definition of biliary dilatation is not available, although the Rome III criteria have proposed a normal bile duct diameter ≤ 8 mm [18]. MRCP has an accuracy of detecting CBD stones surpassing 90%, though, while there is a clinically significant weakness in the detection of small stone diameters [19,20]. EUS is a minimally invasive endoscopic procedure superior to ERC in delineating malignant causes of cholestasis and is at least equal to ERC in terms of stone detection [21,22]. Same-session EUS-guided ERC has also been reported and is a rational approach in prioritizing patients for ERC and, in addition, impacts on ERC strategy by a priori knowledge of the level of obstruction and its underlying cause [23]. As a consequence, overall ERC-related risks of aggravating acute biliary infection are reduced by a minimization of contrast media injection volumes, which might increase intrabiliary pressure levels to an extent high enough to cause reflux of purulent bile contents, and by an avoidance of inadvertent injection into undrainable biliary segments, e.g. in complex hilar strictures [24]. Computed tomography has its clinical value above all in unstable patients with high suspicion of underlying malignancy or suspicion of complicating hepatic abscesses. Aside from patients with severe cholangitis in which delays in biliary drainage due to imaging are inacceptable, it is recommended to conduct EUS if available, alternatively MRCP, prior to ERC with biliary drainage. In a meta-analysis of a pooled data set of 301 patients, the aggregated sensitivities of EUS and MRCP for CBD stone detection were 93 and 85%, whereas their specificities were 96 and 93%, respectively [25].
Table 2.
Relative benefits and drawbacks of different imaging tests in the setting of acute bacterial cholangitis (modified from [4])
| Abdominal ultrasound | EUS | MRCP | CT | ERCP | |
|---|---|---|---|---|---|
| Availability | widely | limited | limited | helical CT rare | available |
| Portability | yes | limited | no | no | limited |
| Invasiveness | no | (minimally) invasive | no | no | invasive |
| Need for sedation | no | yes | some patients | no | yes |
| Sensitivity of stone detection | low | at least as good as ERCP | high | high (best for helical CT) | gold standard in most studies |
| Sensitivity of stricture detection | low | good | best non-invasive method | fair | excellent |
| Sensitivity of tumour detection | low | excellent | good | good | fair |
| Advantages | widely available, non-invasive | excellent for small stones, option for same-session with ERCP | accurate without radiation exposure | widely available and accurate | therapeutic capability |
| Disadvantages | low sensitivity | invasive character, poor delineation of intrahepatic ducts | contraindication in patients with implantable devices, poor detection of small stones, not portable, patient has to be stable | effects on renal function, poor detection of small stones, not portable | invasive, possible worsening of condition |
EUS = Endoscopic ultrasound; MRCP = magnetic resonance cholangiopancreatography; CT = computed tomography; ERCP = endoscopic retrograde cholangiopancreatography.
Severity Assessment and Risk Stratification
There is a wide spectrum of disease courses in acute bacterial cholangitis, ranging from self-limiting to life-threatening with the need to tailor treatment accordingly. An estimated 70% of patients respond to medical treatment comprising supportive and antimicrobial therapy with potentially significant impact on timing and strategy of biliary drainage [26]. In the Tokyo guideline 2013 (TG13), criteria for severity assessment, i.e., the TG07 criteria which have been criticized mostly due to the differentiation of grade I and II severity only by observation, thus making it unsuitable for clinical purposes, have been refined and re-defined in order to allow categorization at initial diagnosis; however, prospective validation is lacking [27].
These revised assessment criteria define acute bacterial cholangitis (table 3) as follows: grade III (severe): presence of organ dysfunction; grade II (moderate): risk of increased severity without early biliary drainage; and grade I (mild).
Table 3.
TG13 severity assessment criteria for acute bacterial cholangitis (modified from [64])
| Grade III (severe) defined by onset of organ dysfunction in at least one of these organ systems: |
| 1. Cardiovascular: hypotension requiring dopamine ≥ 5 μg/kg per min or any dose of norepinephrine |
| 2. Neurological: disturbance of consciousness |
| 3. Respiratory: PaO2/FiO2 ratio < 300 |
| 4. Renal: oliguria, serum creatinine > 2.0 mg/dl |
| 5. Hepatic: PT-INR > 1.5 |
| 6. Haematological: platelet count < 100,000/mm3 |
|
Grade II (moderate) defined by any two of the following: |
| 1. Abnormal WBC count: (>12,000/mm3; <4,000/mm3) |
| 2. High fever: ≥39°C |
| 3. Age: ≥75 years old |
| 4. Hyperbilirubinaemia: total bilirubin ≥ 5 mg/dl |
| 5. Hypoalbuminaemia: lower limit of normal value × 0.7 |
|
Grade I (mild) does not meet the criteria for grade III and grade II at diagnosis |
Treatment Algorithm
Treatment is directed at the two main pathophysiologic components of acute cholangitis, i.e. biliary infection and obstruction; therefore, both systemic antibiotic treatment and biliary drainage remain the mainstay treatment options. In addition, appropriate supportive care has to be administered, in more severe cases in an intensive care setting with the option of providing adequate organ support if necessary.
Hospitalization is usually considered necessary even for patients with mild acute cholangitis, although an occasional patient, e.g. with recurrent episodes of minor cholangitis bouts after bilioenteric surgery, might be deemed suitable for outpatient management. Otherwise, mild or moderate cholangitis patients are treated in the general medical ward, while patients with severe disease indicators and/or significant comorbid conditions are to be admitted to the intensive care unit.
General Treatment Aspects and Supportive Care
As soon as diagnosis and intravenous access are established, aggressive fluid and electrolyte replacement as well as, if appropriate, intravenous analgesics are to be administered to correct volume depletion and restitute blood pressure levels.
Antibiotic Treatment
In the presence of regular biliary tract anatomy, bile fluid is sterile but might become infected by bacteria via the ampulla, e.g. after stone passage, sphincterotomy, and/or stent placement, or via the portal route with bacterial translocation through hepatic sinusoids and the space of Disse [28,29]. Thus, most pathogens relevant to cholangitis initiation and perpetuation are derived from gastrointestinal microbiota including Gram-negative enteric bacteria and enterococci.
Administration of antibiotic agents should be initiated empirically as early as possible in any patient with a clinical suspicion of cholangitis, i.e., <1 h if there are signs of septic shock (as outlined in the Surviving Sepsis Campaign guidelines [30]), otherwise <4 h for definitive diagnostic studies, and in any event before drainage procedures are performed. There are several aspects to be considered in the selection of an appropriate antibiotic agent such as the potentially infecting bacteria, the severity of the disease and comorbidities, allergies, local susceptibility patterns, and the patient's history of antibiotic usage.
The Tokyo guideline working group has issued expert opinion-based recommendations concerning antibiotic usage in acute bacterial cholangitis [31]. The type and duration of antibiotic treatment is dictated by disease severity, clinical response, and microbial test results [32]. In community-acquired cases, empirical therapy with penicillin/β-lactamase inhibitor combinations, e.g. ampicillin/sulbactam (grade I, 2-3 days), or antipseudomonal active piperacillin/tazobactam (grade II + III, 5-7 days) represents an adequate regimen [31]. Third- or fourth-generation cephalosporins represent alternative broad-spectrum agents in more severe cases. In grade III community-acquired cholangitis within an intensive care setting inclusion of vancomycin is prudent for Enterococcus spp. coverage until culture results are available. Fluoroquinolones (only < grade III) with or without metronidazole for anaerobic coverage (strongly recommended after bilioenteric surgery with high probability of Clostridium and Bacteroides spp.) or carbapenems are alternative considerations once first-choice regimens prove clinically ineffective. In such circumstances, the adequacy of biliary drainage should be critically reconsidered as well. The importance of the quality of biliary drainage is highlighted by another study demonstrating that, in the setting of successful ERC drainage (‘source control’), the clinical results were the same after 3 versus 5 days of antibiotic treatment [33]. By contrast, in the presence of residual stones or ongoing biliary obstruction antimicrobial treatment should be extended until the resolution of the anatomical alteration. However, in cases with documented bacteraemia with endocarditis-prone Gram-positive cocci such as Enterococcus spp. or Streptococcus spp. continuation of antimicrobial therapy over 2 weeks appears reasonable, albeit there are no well-designed randomized controlled trials to support such a procedure.
By contrast, healthcare-associated cholangitis is complicated by a higher rate of polymicrobial infections and/or infections with resistant organisms including Pseudomonas, methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant Enterococci (VRE). Therefore, empirical treatment of such infections should address Pseudomonas species and ESBL(extended-spectrum β-lactamase)-producing Gram-negative organisms [34]. Vancomycin is recommended in the presence of known colonization with Gram-positive bacteria such as MRSA and/or Enterococcus spp. or when these multidrug-resistant organisms are of concern. VRE should be addressed by linezolid or daptomycin in individuals with known colonization, vancomycin pretreatment, or if these organisms are common in the community. There appear to be aetiology-specific peculiarities to be considered as well, e.g. the notoriously high rate of Candida and Enterococcus isolates in the rising population of sclerosing cholangitis in critically ill patients (SC-CIP) (see also article by Kirchner and Ruemmele [35] in this issue) [36,37].
If results of blood and biliary cultures become available, empirically initiated broad-spectrum antibiotic regimens should be changed to narrow-spectrum agents and, if feasible, to an oral route of administration. Concerning bile cultures, which have been reported to be positive in the range of 59-93%, the updated TG13 guideline recommends acquisition of bile samples for microbial testing at the beginning of any drainage procedure [38]. By contrast, the rate of positive blood cultures in the cholangitis population is about 21-71%, with most of these bacteraemic isolates generating no cardiac vegetations on normal valves or miliary abscesses. Since the results of blood cultures usually do not affect clinical management and outcomes, routine blood cultures remain a matter of controversy. Biliary penetration of a given antimicrobial agent has traditionally influenced the selection of the agents. However, there are laboratory and clinical data showing that secretion of antimicrobial agents into bile plummets to low to zero levels in the setting of biliary obstruction, thus calling into question the theoretical benefits of biliary secreted over non-secreted compounds [39,40].
Endoscopic Treatment
In the era before effective biliary drainage procedures (and more potent antibiotic agents) became available, the mortality of acute cholangitis approached 100% with conservative treatment only (by now declined to 2.7-10% [41]), highlighting the need for the removal of biliary obstruction as the source of ongoing infection in acute bacterial cholangitis. Biliary drainage can be achieved by a multitude of ways, e.g. ERC, percutaneous transhepatic cholangiography, EUS-guided drainage, or surgical drainage, with the third being the procedure of choice whenever feasible. There are various endoscopic transpapillary options available, including biliary stent or nasobiliary drain placement above the obstruction site ± sphincterotomy, all of which with their appropriate indications corresponding to disease severity and clinical context [42]. Stenting has an equal effectiveness as nasobiliary drainage; however, it is associated with improved patient comfort, while the nasobiliary tube has the potential advantage of repeated bile aspiration for microbiologic analysis, flushings, and cholangiographic evaluation [43]. Routine sphincterotomy with the added risks of haemorrhage and perforation is not universally indicated for stents <10 French and/or nasobiliary drainage, representing the preferred approach in patients with septic and/or therapeutic coagulopathy. In patients where the precise levels of obstruction are not unequivocally identified by limited cholangiography, a stent ≥110 mm (usually 10 French unless tight hilar strictures are present) relieves biliary obstruction proximal to the left or right intrahepatic duct – bilateral stenting is usually not necessary except if both sides have been contaminated by contrast media [44].
Nevertheless, the diagnostic yield of ERC in the detection of CBD stones has been reported to be as high as 95% [45]. However, this implies that small stones may be missed, and another set of studies investigating the diagnostic power of stone detection based on cholangiogram only reported false-negative rates in up to 13% of cases [46]. Therefore, regarding a technical perspective, early radiographs after careful and slow contrast injection appear mandatory to avoid contrast media overfilling (as well as inadvertent proximal stone advancement). Contrast injection may be preceded by aspiration of bile cultures and placing a hydrophilic guide wire deep along the fluoroscopically anticipated course of the bile duct in order to allow drainage of opacified parts of the biliary system at the end of the procedure. By contrast, cholangiography can be avoided altogether in an occasional patient with advanced cholangitis, with the level and nature of biliary obstruction being determined by EUS or MRCP [47]. Overall, endoscopic sphincterotomy and stone extraction have been reported to be successful in more than 90%, with adverse event rates close to 5% and mortality rates <1% [48]. After failure of primary wire-guided biliary cannulation, precut (e.g. needle-knife) sphincterotomy, percutaneous transhepatic drainage procedure, or a combined percutaneous/endoscopic approach may become necessary; parallel to the increase in technical complexity, however, the complication rates for these more advanced techniques are much higher than for standard procedures [49]. In few centres, EUS-guided biliary drainage has been introduced as a viable alternative after failed ERC access [50,51]. This approach requires further standardization and clinical trial validation, though.
Bile duct clearance from stones is usually performed with balloon extractor catheters and/or wire baskets. Especially in a clinical setting of acute biliary tract infection, however, larger stones may become impacted and, thus, more resistant to successful removal. Traditionally, fragmentation of large biliary stones that might become entrapped in impacted baskets requires the performance of mechanical lithotripsy or direct cholangioscopy with electrohydraulic lithotripsy [52,53,54,55]. If complete duct clearance fails in the initial procedure or in the presence of grade III cholangitis, biliary decompression should include placement of a stent or nasobiliary drain in the first place. Especially in patients with severe cholangitis, it is recommended that the initial procedure should be limited to stenting in order to minimize the risk of worsening sepsis and time under conscious sedation and/or anaesthesia. After resolution of the critical episode, complete stone removal should be performed, in more complex cases also by means of direct cholangioscopy, with intraductal lithotripsy being reported to clear difficult extrahepatic biliary stones in 83-100% of patients [56,57,58,59].
After resolution of the acute illness has been achieved, a definitive treatment for the cause of acute cholangitis to reduce the risk of future biliary complications, for the most part in the form of short-term elective laparoscopic cholecystectomy, has to be performed [60]. Endoscopic sphincterotomy and stone extraction without subsequent cholecystectomy may be appropriate in select surgical high-risk patients with significant comorbid conditions. However, biliary symptoms recur twice as commonly and mortality is significantly increased in patients whose gallbladder remains in situ, with a 5-year risk of biliary complications resulting in cholecystectomy in as much as 15% [61,62,63].
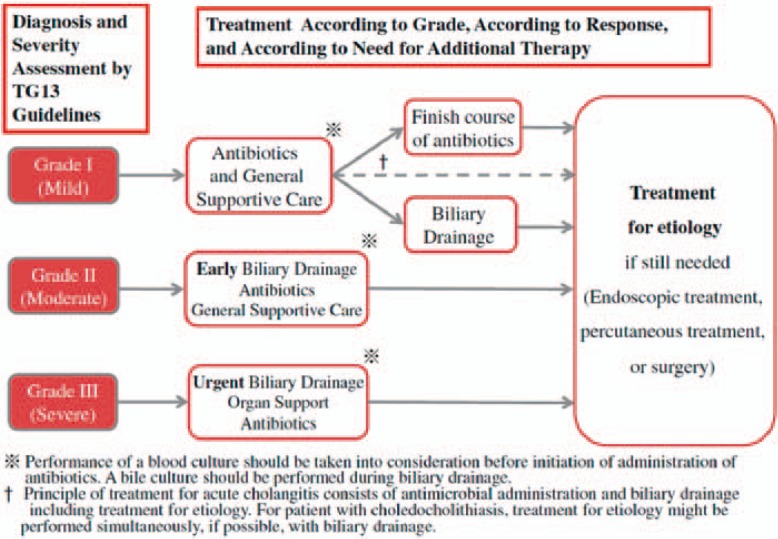
Figure 2 summarizes the clinical management of acute bacterial cholangitis as recommended by the TG13 guidelines.
Fig. 2.
Clinical TG13 flowchart for the management of acute cholangitis (reprinted with permission from [65]).
Conclusions
Clinical diagnosis should be based on clinical findings and laboratory (inflammatory and cholestasis) markers as well as imaging studies to detect biliary dilation and its aetiology. Initiation of broad-spectrum antibiotics addressing the typical Gram-negative enteric bacteria spectrum and (early) biliary drainage including ERC with bile duct clearance and/or biliary stenting are the therapeutic mainstay options. Elective laparoscopic cholecystectomy in case of a gallbladder in situ is generally recommended after the resolution of cholangitis.
Disclosure Statement
The authors declare that there are no conflicts of interest.
References
- 1.Lammert F, Miquel JF. Gallstone disease: from genes to evidence-based therapy. J Hepatol. 2008;48(suppl 1):S124–135. doi: 10.1016/j.jhep.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Neuhaus H, Feussner H, Ungeheuer A, Hoffmann W, Siewert JR, Classen M. Prospective evaluation of the use of endoscopic retrograde cholangiography prior to laparoscopic cholecystectomy. Endoscopy. 1992;24:745–749. doi: 10.1055/s-2007-1010576. [DOI] [PubMed] [Google Scholar]
- 3.Friedman GD. Natural history of asymptomatic and symptomatic gallstones. Am J Surg. 1993;165:399–404. doi: 10.1016/s0002-9610(05)80930-4. [DOI] [PubMed] [Google Scholar]
- 4.Lee JG. Diagnosis and management of acute cholangitis. Nat Rev Gastroenterol Hepatol. 2009;6:533–541. doi: 10.1038/nrgastro.2009.126. [DOI] [PubMed] [Google Scholar]
- 5.Parks RW, Clements WD, Smye MG, Pope C, Rowlands BJ, Diamond T. Intestinal barrier dysfunction in clinical and experimental obstructive jaundice and its reversal by internal biliary drainage. Br J Surg. 1996;83:1345–1349. doi: 10.1002/bjs.1800831007. [DOI] [PubMed] [Google Scholar]
- 6.Huang T, Bass JA, Williams RD. The significance of biliary pressure in cholangitis. Arch Surg. 1969;98:629–632. doi: 10.1001/archsurg.1969.01340110121014. [DOI] [PubMed] [Google Scholar]
- 7.Csendes A, Sepulveda A, Burdiles P, Braghetto I, Bastias J, Schutte H, Diaz JC, Yarmuch J, Maluenda F. Common bile duct pressure in patients with common bile duct stones with or without acute suppurative cholangitis. Arch Surg. 1988;123:697–699. doi: 10.1001/archsurg.1988.01400300039005. [DOI] [PubMed] [Google Scholar]
- 8.Swidsinski A, Lee SP. The role of bacteria in gallstone pathogenesis. Front Biosci. 2001;6:E93–103. doi: 10.2741/swidsinski. [DOI] [PubMed] [Google Scholar]
- 9.Kumar R, Sharma BC, Singh J, Sarin SK. Endoscopic biliary drainage for severe acute cholangitis in biliary obstruction as a result of malignant and benign diseases. J Gastroenterol Hepatol. 2004;19:994–997. doi: 10.1111/j.1440-1746.2004.03415.x. [DOI] [PubMed] [Google Scholar]
- 10.Landau O, Kott I, Deutsch AA, Stelman E, Reiss R. Multifactorial analysis of septic bile and septic complications in biliary surgery. World J Surg. 1992;16:962–964. doi: 10.1007/BF02067003. discussion 964-965. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds BM, Dargan EL. Acute obstructive cholangitis; a distinct clinical syndrome. Ann Surg. 1959;150:299–303. doi: 10.1097/00000658-195908000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Csendes A, Diaz JC, Burdiles P, Maluenda F, Morales E. Risk factors and classification of acute suppurative cholangitis. Br J Surg. 1992;79:655–658. doi: 10.1002/bjs.1800790720. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal N, Sharma BC, Sarin SK. Endoscopic management of acute cholangitis in elderly patients. World J Gastroenterol. 2006;12:6551–6555. doi: 10.3748/wjg.v12.i40.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saharia PC, Cameron JL. Clinical management of acute cholangitis. Surg Gynecol Obstet. 1976;142:369–372. [PubMed] [Google Scholar]
- 15.Wada K, Takada T, Kawarada Y, et al. Diagnostic criteria and severity assessment of acute cholangitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg. 2007;14:52–58. doi: 10.1007/s00534-006-1156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Einstein DM, Lapin SA, Ralls PW, Halls JM. The insensitivity of sonography in the detection of choledocholithiasis. AJR Am J Roentgenol. 1984;142:725–728. doi: 10.2214/ajr.142.4.725. [DOI] [PubMed] [Google Scholar]
- 17.Taylor KJ, Rosenfield AT, Spiro HM. Diagnostic accuracy of gray scale ultrasonography for the jaundiced patient. A report of 275 cases. Arch Intern Med. 1979;139:60–63. [PubMed] [Google Scholar]
- 18.Behar J, Corazziari E, Guelrud M, Hogan W, Sherman S, Toouli J. Functional gallbladder and sphincter of oddi disorders. Gastroenterology. 2006;130:1498–1509. doi: 10.1053/j.gastro.2005.11.063. [DOI] [PubMed] [Google Scholar]
- 19.Demartines N, Eisner L, Schnabel K, Fried R, Zuber M, Harder F. Evaluation of magnetic resonance cholangiography in the management of bile duct stones. Arch Surg. 2000;135:148–152. doi: 10.1001/archsurg.135.2.148. [DOI] [PubMed] [Google Scholar]
- 20.Soto JA, Barish MA, Alvarez O, Medina S. Detection of choledocholithiasis with MR cholangiography: comparison of three-dimensional fast spin-echo and single- and multisection half-fourier rapid acquisition with relaxation enhancement sequences. Radiology. 2000;215:737–745. doi: 10.1148/radiology.215.3.r00ma12737. [DOI] [PubMed] [Google Scholar]
- 21.Amouyal P, Amouyal G, Levy P, Tuzet S, Palazzo L, Vilgrain V, Gayet B, Belghiti J, Fekete F, Bernades P. Diagnosis of choledocholithiasis by endoscopic ultrasonography. Gastroenterology. 1994;106:1062–1067. doi: 10.1016/0016-5085(94)90768-4. [DOI] [PubMed] [Google Scholar]
- 22.Sgouros SN, Bergele C. Endoscopic ultrasonography versus other diagnostic modalities in the diagnosis of choledocholithiasis. Dig Dis Sci. 2006;51:2280–2286. doi: 10.1007/s10620-006-9218-x. [DOI] [PubMed] [Google Scholar]
- 23.Rocca R, De Angelis C, Castellino F, Masoero G, Daperno M, Sostegni R, Rigazio C, Crocella L, Lavagna A, Ercole E, Pera A. EUS diagnosis and simultaneous endoscopic retrograde cholangiography treatment of common bile duct stones by using an oblique-viewing echoendoscope. Gastrointest Endosc. 2006;63:479–484. doi: 10.1016/j.gie.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 24.Ozden I, Tekant Y, Bilge O, Acarli K, Alper A, Emre A, Rozanes I, Ozsut H, Ariogul O. Endoscopic and radiologic interventions as the leading causes of severe cholangitis in a tertiary referral center. Am J Surg. 2005;189:702–706. doi: 10.1016/j.amjsurg.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Verma D, Kapadia A, Eisen GM, Adler DG. EUS vs MRCP for detection of choledocholithiasis. Gastrointest Endosc. 2006;64:248–254. doi: 10.1016/j.gie.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 26.Attasaranya S, Fogel EL, Lehman GA. Choledocholithiasis, ascending cholangitis, and gallstone pancreatitis. Med Clin North Am. 2008;92:925–960. doi: 10.1016/j.mcna.2008.03.001. x. [DOI] [PubMed] [Google Scholar]
- 27.Nishino T, Hamano T, Mitsunaga Y, Shirato I, Shirato M, Tagata T, Shimada M, Yoshida S, Mitsunaga A. Clinical evaluation of the Tokyo guidelines 2013 for severity assessment of acute cholangitis. J Hepatobiliary Pancreat Sci. 2014;21:841–849. doi: 10.1002/jhbp.189. [DOI] [PubMed] [Google Scholar]
- 28.Sung JY, Shaffer EA, Olson ME, Leung JW, Lam K, Costerton JW. Bacterial invasion of the biliary system by way of the portal-venous system. Hepatology. 1991;14:313–317. [PubMed] [Google Scholar]
- 29.Sung JY, Leung JW, Shaffer EA, Lam K, Olson ME, Costerton JW. Ascending infection of the biliary tract after surgical sphincterotomy and biliary stenting. J Gastroenterol Hepatol. 1992;7:240–245. doi: 10.1111/j.1440-1746.1992.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 30.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka A, Takada T, Kawarada Y, et al. Antimicrobial therapy for acute cholangitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg. 2007;14:59–67. doi: 10.1007/s00534-006-1157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stallmach A, Hagel S, Bruns T, Pletz M, Eckmann C. Empirical antibiotic therapy in intra-abdominal infections: cases and evidence-based therapeutic recommendations (Article in German) Z Gastroenterol. 2013;51:1069–1081. doi: 10.1055/s-0033-1335043. [DOI] [PubMed] [Google Scholar]
- 33.van Lent AU, Bartelsman JF, Tytgat GN, Speelman P, Prins JM. Duration of antibiotic therapy for cholangitis after successful endoscopic drainage of the biliary tract. Gastrointest Endosc. 2002;55:518–522. doi: 10.1067/mge.2002.122334. [DOI] [PubMed] [Google Scholar]
- 34.Sung YK, Lee JK, Lee KH, Lee KT, Kang CI. The clinical epidemiology and outcomes of bacteremic biliary tract infections caused by antimicrobial-resistant pathogens. Am J Gastroenterol. 2012;107:473–483. doi: 10.1038/ajg.2011.387. [DOI] [PubMed] [Google Scholar]
- 35.Kirchner GI, Ruemmele P. Update on sclerosing cholangitis in critically ill patients. Viszeralmedizin. 2015;31:178–184. doi: 10.1159/000431031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirchner GI, Scherer MN, Obed A, Ruemmele P, Wiest R, Froh M, Loss M, Schlitt HJ, Scholmerich J, Gelbmann CM. Outcome of patients with ischemic-like cholangiopathy with secondary sclerosing cholangitis after liver transplantation. Scand J Gastroenterol. 2011;46:471–478. doi: 10.3109/00365521.2010.537683. [DOI] [PubMed] [Google Scholar]
- 37.Voigtlander T, Negm AA, Schneider AS, Strassburg CP, Manns MP, Wedemeyer J, Lankisch TO. Secondary sclerosing cholangitis in critically ill patients: model of end-stage liver disease score and renal function predict outcome. Endoscopy. 2012;44:1055–1058. doi: 10.1055/s-0032-1325733. [DOI] [PubMed] [Google Scholar]
- 38.Gomi H, Solomkin JS, Takada T, Strasberg SM, Pitt HA, Yoshida M, Kusachi S, Mayumi T, Miura F, Kiriyama S, Yokoe M, Kimura Y, Higuchi R, Windsor JA, Dervenis C, Liau KH, Kim MH. TG13 antimicrobial therapy for acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2013;20:60–70. doi: 10.1007/s00534-012-0572-0. [DOI] [PubMed] [Google Scholar]
- 39.van den Hazel SJ, Speelman P, Tytgat GN, Dankert J, van Leeuwen DJ. Role of antibiotics in the treatment and prevention of acute and recurrent cholangitis. Clin Infect Dis. 1994;19:279–286. doi: 10.1093/clinids/19.2.279. [DOI] [PubMed] [Google Scholar]
- 40.Leung JW, Chan RC, Cheung SW, Sung JY, Chung SC, French GL. The effect of obstruction on the biliary excretion of cefoperazone and ceftazidime. J Antimicrob Chemother. 1990;25:399–406. doi: 10.1093/jac/25.3.399. [DOI] [PubMed] [Google Scholar]
- 41.Kimura Y, Takada T, Strasberg SM, et al. TG13 current terminology, etiology, and epidemiology of acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2013;20:8–23. doi: 10.1007/s00534-012-0564-0. [DOI] [PubMed] [Google Scholar]
- 42.Mosler P. Diagnosis and management of acute cholangitis. Curr Gastroenterol Rep. 2011;13:166–172. doi: 10.1007/s11894-010-0171-7. [DOI] [PubMed] [Google Scholar]
- 43.Lee DW, Chan AC, Lam YH, Ng EK, Lau JY, Law BK, Lai CW, Sung JJ, Chung SC. Biliary decompression by nasobiliary catheter or biliary stent in acute suppurative cholangitis: a prospective randomized trial. Gastrointest Endosc. 2002;56:361–365. doi: 10.1016/s0016-5107(02)70039-4. [DOI] [PubMed] [Google Scholar]
- 44.De Palma GD, Galloro G, Siciliano S, Iovino P, Catanzano C. Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: results of a prospective, randomized, and controlled study. Gastrointest Endosc. 2001;53:547–553. doi: 10.1067/mge.2001.113381. [DOI] [PubMed] [Google Scholar]
- 45.NIH state-of-the-science statement on endoscopic retrograde cholangiopancreatography (ERCP) for diagnosis and therapy. NIH Consens State Sci Statements. 2002;19:1–26. [PubMed] [Google Scholar]
- 46.ASGE Standards of Practice Committee. Chathadi KV, Chandrasekhara V, Acosta RD, et al. The role of ERCP in benign diseases of the biliary tract. Gastrointest Endosc. 2015;81:795–803. doi: 10.1016/j.gie.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 47.Hintze RE, Abou-Rebyeh H, Adler A, Veltzke-Schlieker W, Felix R, Wiedenmann B. Magnetic resonance cholangiopancreatography-guided unilateral endoscopic stent placement for Klatskin tumors. Gastrointest Endosc. 2001;53:40–46. doi: 10.1067/mge.2001.111388. [DOI] [PubMed] [Google Scholar]
- 48.Carr-Locke DL. Therapeutic role of ERCP in the management of suspected common bile duct stones. Gastrointest Endosc. 2002;56:S170–174. doi: 10.1067/mge.2002.129024. [DOI] [PubMed] [Google Scholar]
- 49.Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, Fennerty MB, Ryan ME, Shaw MJ, Lande JD, Pheley AM. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909–918. doi: 10.1056/NEJM199609263351301. [DOI] [PubMed] [Google Scholar]
- 50.Sarkaria S, Sundararajan S, Kahaleh M. Endoscopic ultrasonographic access and drainage of the common bile duct. Gastrointest Endosc Clin N Am. 2013;23:435–452. doi: 10.1016/j.giec.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 51.Park do H, Jeong SU, Lee BU, Lee SS, Seo DW, Lee SK, Kim MH. Prospective evaluation of a treatment algorithm with enhanced guidewire manipulation protocol for EUS-guided biliary drainage after failed ERCP (with video) Gastrointest Endosc. 2013;78:91–101. doi: 10.1016/j.gie.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 52.Leung JW, Tu R. Mechanical lithotripsy for large bile duct stones. Gastrointest Endosc. 2004;59:688–690. doi: 10.1016/s0016-5107(04)00174-9. [DOI] [PubMed] [Google Scholar]
- 53.Attila T, May GR, Kortan P. Nonsurgical management of an impacted mechanical lithotriptor with fractured traction wires: endoscopic intracorporeal electrohydraulic shock wave lithotripsy followed by extra-endoscopic mechanical lithotripsy. Can J Gastroenterol. 2008;22:699–702. doi: 10.1155/2008/798527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trikudanathan G, Arain MA, Attam R, Freeman ML. Advances in the endoscopic management of common bile duct stones. Nat Rev Gastroenterol Hepatol. 2014;11:535–544. doi: 10.1038/nrgastro.2014.76. [DOI] [PubMed] [Google Scholar]
- 55.Maydeo A, Kwek BE, Bhandari S, Bapat M, Dhir V. Single-operator cholangioscopy-guided laser lithotripsy in patients with difficult biliary and pancreatic ductal stones (with videos) Gastrointest Endosc. 2011;74:1308–1314. doi: 10.1016/j.gie.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 56.Piraka C, Shah RJ, Awadallah NS, Langer DA, Chen YK. Transpapillary cholangioscopy-directed lithotripsy in patients with difficult bile duct stones. Clin Gastroenterol Hepatol. 2007;5:1333–1338. doi: 10.1016/j.cgh.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 57.Arya N, Nelles SE, Haber GB, Kim YI, Kortan PK. Electrohydraulic lithotripsy in 111 patients: a safe and effective therapy for difficult bile duct stones. Am J Gastroenterol. 2004;99:2330–2334. doi: 10.1111/j.1572-0241.2004.40251.x. [DOI] [PubMed] [Google Scholar]
- 58.Farrell JJ, Bounds BC, Al-Shalabi S, Jacobson BC, Brugge WR, Schapiro RH, Kelsey PB. Single-operator duodenoscope-assisted cholangioscopy is an effective alternative in the management of choledocholithiasis not removed by conventional methods, including mechanical lithotripsy. Endoscopy. 2005;37:542–547. doi: 10.1055/s-2005-861306. [DOI] [PubMed] [Google Scholar]
- 59.Binmoeller KF, Bruckner M, Thonke F, Soehendra N. Treatment of difficult bile duct stones using mechanical, electrohydraulic and extracorporeal shock wave lithotripsy. Endoscopy. 1993;25:201–206. doi: 10.1055/s-2007-1010293. [DOI] [PubMed] [Google Scholar]
- 60.Boerma D, Rauws EA, Keulemans YC, Janssen IM, Bolwerk CJ, Timmer R, Boerma EJ, Obertop H, Huibregtse K, Gouma DJ. Wait-and-see policy or laparoscopic cholecystectomy after endoscopic sphincterotomy for bile-duct stones: a randomised trial. Lancet. 2002;360:761–765. doi: 10.1016/S0140-6736(02)09896-3. [DOI] [PubMed] [Google Scholar]
- 61.Pereira-Lima JC, Jakobs R, Winter UH, Benz C, Martin WR, Adamek HE, Riemann JF. Long-term results (7 to 10 years) of endoscopic papillotomy for choledocholithiasis. Multivariate analysis of prognostic factors for the recurrence of biliary symptoms. Gastrointest Endosc. 1998;48:457–464. doi: 10.1016/s0016-5107(98)70085-9. [DOI] [PubMed] [Google Scholar]
- 62.Yi SY. Recurrence of biliary symptoms after endoscopic sphincterotomy for choledocholithiasis in patients with gall bladder stones. J Gastroenterol Hepatol. 2000;15:661–664. doi: 10.1046/j.1440-1746.2000.02192.x. [DOI] [PubMed] [Google Scholar]
- 63.Lau JY, Leow CK, Fung TM, Suen BY, Yu LM, Lai PB, Lam YH, Ng EK, Lau WY, Chung SS, Sung JJ. Cholecystectomy or gallbladder in situ after endoscopic sphincterotomy and bile duct stone removal in Chinese patients. Gastroenterology. 2006;130:96–103. doi: 10.1053/j.gastro.2005.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kiriyama S, Takada T, Strasberg SM, et al. TG13 guidelines for diagnosis and severity grading of acute cholangitis (with videos) J Hepatobiliary Pancreat Sci. 2013;20:24–34. doi: 10.1007/s00534-012-0561-3. [DOI] [PubMed] [Google Scholar]
- 65.Miura F, Takada T, Strasberg SM, et al. TG13 flowchart for the management of acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2013;20:47–54. doi: 10.1007/s00534-012-0563-1. [DOI] [PubMed] [Google Scholar]