Abstract
Background
Immunoglobulin (Ig) G4-associated cholangitis (IAC) is an inflammatory disorder of the biliary tract displaying characteristic features of IgG4-related disease (IgG4-RD): elevation of IgG4 serum levels, infiltration of IgG4+ plasma cells in the affected tissue, and good response to immunosuppressive treatment.
Methods and Results
The clinical presentation of IAC is often misleading, mimicking other diseases of the biliary tract such as cholangiocarcinoma or primary and secondary sclerosing cholangitis. The HISORt criteria form the cornerstone in the diagnosis of IAC, combining histopathological (H), imaging (I), and serological (S) features including serum IgG4, other organ manifestations (O) of IgG4-RD and response to treatment (Rt). The accuracy of the HISORt criteria is limited. Novel diagnostic approaches are under evaluation.
Conclusion
More accurate biomarkers are needed to correctly diagnose IgG4-RD and prevent misdiagnoses and unnecessary therapeutic interventions.
Key Words: IgG4-related systemic disease, Primary sclerosing cholangitis, PSC, Cholangiocarcinoma, CCA
Introduction
Immunoglobulin (Ig) G4-associated cholangitis (IAC) is an inflammatory disease of the biliary tree which is characterized by elevated serum IgG4 levels and infiltration of IgG4+ plasma cells in the bile duct walls [1]. IAC typically shows excellent response to immunosuppressive treatment. However, relapse rates after tapering of immunosuppression are considerable, ranging from 30 to 50% [1,2,3]. IAC falls under the umbrella of IgG4-related disease (IgG4-RD) that can affect various organs sharing the same histopathological features [4,5]. IAC may present independently or together with other manifestations of IgG4-RD, particularly autoimmune pancreatitis (AIP) [1]. Due to the lack of central registries and the use of different diagnostic criteria, the true incidence and prevalence of pancreatobiliary manifestations of IgG4-RD are unknown. IgG4-RD is regarded as a systemic disease with multiple organ manifestations which share common pathogenetic, still enigmatic mechanisms [4,6].
Notably, making the diagnosis of IAC may be challenging. The clinical presentation of IAC can be deceiving and may mimic difficult-to-treat diseases such as pancreatobiliary malignancies, lymphomas, and primary sclerosing cholangitis (PSC) or secondary sclerosing cholangitis (SSC). Accurate diagnostic markers are lacking, and awareness of the disease among physicians is low. Therefore, patients suffer from delayed diagnosis or misdiagnosis as well as erroneous extensive hepatobiliary surgery which has been reported in up to a third of patients prior to diagnosis of IgG4-RD [1]. New biomarkers for the diagnosis and management of IAC are urgently needed.
Here we will discuss patient characteristics and diagnostic criteria that have been developed to aid physicians in the diagnosis of IAC.
Patient Characteristics
Typically, IAC patients are elderly men (85%) [1]. They present with obstructive jaundice, weight loss, and/or abdominal discomfort. Patients may exhibit swelling of other organs such as submandibular and parotid glands with or without impaired function. Inflammation of the pancreas may lead to endocrine and exocrine insufficiency with new-onset diabetes and/or steatorrhea. Retroperitoneal fibrosis may cause ureteral strictures resulting in renal impairment and hydronephrosis.
We recently reported that the majority of two independent cohorts of patients with IgG4-RD consisted of ‘blue-collar workers’ with long-term (>1 year) or lifelong exposure to industrial solvents and gases [7]. Cross-validation of these findings in additional independent cohorts is needed.
Biochemical Tests
Serum liver tests show a cholestatic pattern with often marked elevation of alkaline phosphatase (ALP) and γ-GT and only mildly increased ALT and AST. Serum bilirubin may be increased. Serum IgG4 levels are elevated (>140 mg/dl) in up to 75-80% of affected patients [1,8,9]; however, this marker shows merely limited sensitivity and specificity when only modestly elevated (<4× upper limit of normal (ULN)) [8] as 10-15% of patients with pancreatobiliary cancers and PSC may also exhibit increased IgG4 levels [8,9]. The tumor marker CA 19-9 is not able to distinguish pancreatobiliary malignancies from IAC since levels of >1,000 IU/ml (ULN = 37 IU/ml) may be observed in IAC. Thus far, no disease-specific serum markers have been identified but are awaited in the near future [10].
Radiographic Features
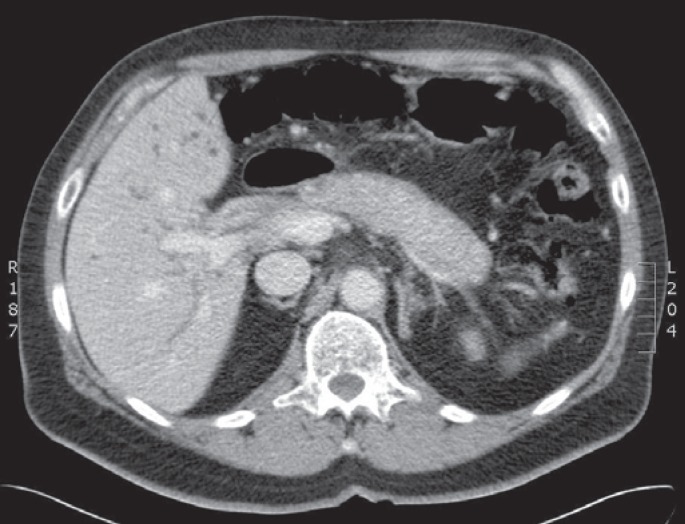
Cholangiography (endoscopic retrograde cholangiography or magnetic resonance pancreatocholangiography) and computed tomography with intravenous contrast may show strictures in the biliary tree with thickening and enhancement of the bile duct wall, dilatation of the upstream bile ducts, and sometimes a soft-tissue mass suspect for malignancy [11]. Depending on the localization of the stenosis and soft-tissue mass, IAC is easily misinterpreted as intra- or extrahepatic cholangiocarcinoma or pancreatic carcinoma (fig. 1). Also, locoregional lymphadenopathy may be present. Even vascular invasion, which is associated with malignancy [12], may be seen in IAC. Sometimes multifocal strictures of intra- and extrahepatic bile ducts with a beaded or pruned-tree appearance are seen, mimicking PSC. However, PSC normally presents earlier in life than IAC (range 20-40 years of age). No radiographic features can clearly distinguish between these different entities [13]. However, extrabiliary involvement, especially of the pancreas, is highly suggestive of IAC. Characteristic features are a diffusely enlarged sausage-shaped pancreas, minimal peripancreatic fat stranding, a hypoattenuating rim (‘halo’), and irregular narrowing of the pancreatic duct with an attenuated wall (fig. 2) [14]. However, AIP may also present as a (multi-)focal mass in the head and/or tail of the pancreas with focal pancreatic duct stricture(s).
Fig. 1.
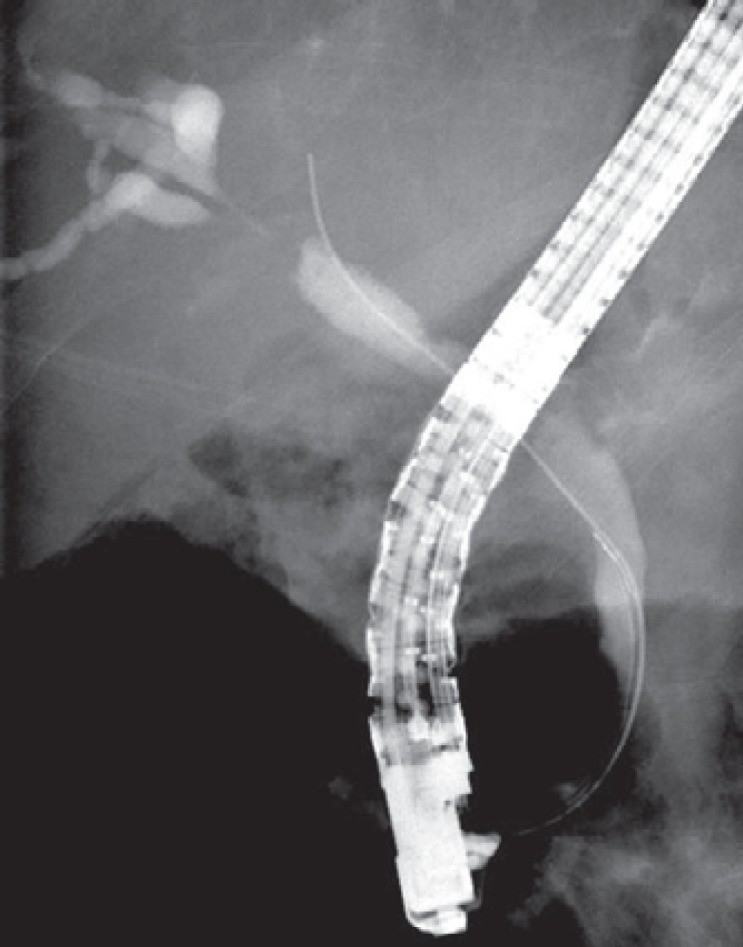
A 69-year-old painter presented with obstructive jaundice and weight loss. Dilated intrahepatic bile ducts were observed on CT scan. Endoscopic retrograde cholangiopancreatography showed a hilar stenosis suspect for a Klatskin tumor Bismuth type IIIa. Serum IgG4 was 1,440 mg/dl (normal <140 mg/dl). The stenosis dissolved completely after treatment with prednisolone (20 mg/day).
Fig. 2.
A 58-year-old warehouse man of a truck manufacturing company presented with weight loss, jaundice, and abdominal pain. Serum IgG4 was 166 mg/dl. A CT scan revealed a sausage-shaped pancreas, which normalized after prednisolone treatment (20 mg/day).
Histopathological Findings
Bile duct biopsies typically reveal a combination of a lymphoplasmacytic infiltrate consisting of high numbers of IgG4+ plasma/B cells (>10/per high power field (hpf)), storiform fibrosis, and obliterative phlebitis [5]. The diagnostic accuracy of the number of IgG4+ cells greatly depends on the chosen cut-off value and varies among tissues. In a case-control study on endoscopic biopsies of the ampulla of Vater and bile duct wall, a threshold of 10 IgG4+ cells/hpf yielded limited sensitivity but reasonable specificity (bile duct biopsies: 52% sensitivity and 89% specificity; ampulla of Vater biopsy: 52% sensitivity and 96% specificity) [15]. A cut-off of 20 IgG4+ cells/hpf increased specificity – at the cost of even lower sensitivities.
Also, the diagnostic value of brush cytology during cholangiography or stent (re-)placement, used for the diagnosis of pancreatobiliary malignancies, is poor with sensitivity rates of 50% [16]. Besides, prolonged stenting of the biliary tract may induce inflammation and cholangiocyte proliferation, making it hard to distinguish between benign and malignant cells and leading to false-positive results.
Diagnostic Criteria
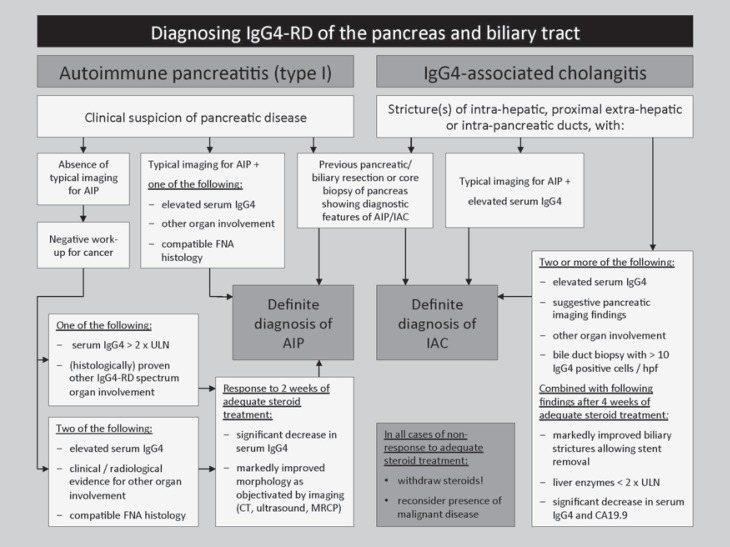
Several diagnostic consensus criteria have been developed in the past decade, including the HISORt (Histology, Imaging, Serology, Other organ involvement, and Response to therapy) criteria [1,17] and international diagnostic consensus criteria (ICDC) [18], as no single biomarker has proven to be accurate in diagnosing IAC. These combine histopathological, radiographic, and laboratory characteristics with responsiveness to immunosuppressive treatment and multi-organ involvement. A diagnostic flow diagram summarizing the widely used HISORt criteria is shown in figure 3. The Boston criteria describe characteristic histopathological features [5].
Fig. 3.
HISORt criteria for the diagnosis of AIP and IAC (histology, imaging, serology, other organ involvement, and response to therapy) (modified from Alderlieste et al. [20] and Hubers et al. [6]. FNA = Fine-needle aspiration; IgG4-RD = IgG4-related disease; CT = computed tomography; MRI = magnetic resonance imaging; MRCP = magnetic resonance cholangiopancreatography; ULN = upper limit of normal.
New Diagnostic Markers
Recently, clonally expanded IgG4+ B cells were found in the blood and affected tissues of patients with IAC by novel next-generation sequencing techniques; however, these were not present in healthy and disease control patients. Besides, these dominant B clones disappeared upon successful steroid treatment [10]. In confirmation of these findings, high numbers of circulating plasmablasts detected by flow cytometry were described to be specific for several organ manifestations of IgG4-related disease [19]. Prospective trials must be conducted to evaluate whether these new biomarkers are suitable for clinical use.
Disclosure Statement
UB is listed as co-inventor on a patent held by the Academic Medical Center in Amsterdam: ‘Method for diagnosing IgG4-related diseases’, application number PCT/EP2013/070384, publication number WO/2014/049177.
Acknowledgements
The work of the authors on IgG4-RD was made possible by a grant support from the German Crohn Colitis Society (section PSC) and the American PSC Partners patient organization (to UB).
References
- 1.Ghazale A, Chari ST, Zhang L, et al. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008;134:706–715. doi: 10.1053/j.gastro.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Sandanayake NS, Church NI, Chapman MH, et al. Presentation and management of post-treatment relapse in autoimmune pancreatitis/immunoglobulin G4-associated cholangitis. Clin Gastroenterol Hepatol. 2009;7:1089–1096. doi: 10.1016/j.cgh.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 3.Hart PA, Kamisawa T, Brugge WR, et al. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut. 2013;62:1771–1776. doi: 10.1136/gutjnl-2012-303617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2014;6736:1–12. doi: 10.1016/S0140-6736(14)60720-0. [DOI] [PubMed] [Google Scholar]
- 5.Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25:1181–1192. doi: 10.1038/modpathol.2012.72. [DOI] [PubMed] [Google Scholar]
- 6.Hubers LM, Maillette de Buy Wenniger LJ, Doorenspleet ME, et al. IgG4-associated cholangitis: a comprehensive review. Clin Rev Allergy Immunol. 2015;48:198–206. doi: 10.1007/s12016-014-8430-2. [DOI] [PubMed] [Google Scholar]
- 7.Maillette de Buy Wenniger LJ, Culver EL, Beuers U. Exposure to occupational antigens might predispose to IgG4-related disease. Hepatology. 2014;60:1–4. doi: 10.1002/hep.26999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oseini AM, Chaiteerakij R, Shire AM, et al. Utility of serum immunoglobulin G4 in distinguishing immunoglobulin G4-associated cholangitis from cholangiocarcinoma. Hepatology. 2011;54:940–948. doi: 10.1002/hep.24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boonstra K, Culver EL, Maillette de Buy Wenniger L, et al. Serum immunoglobulin G4 and immunoglobulin G1 for distinguishing immunoglobulin G4-associated cholangitis from primary sclerosing cholangitis. Hepatology. 2014;59:1954–1963. doi: 10.1002/hep.26977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maillette de Buy Wenniger LJ, Doorenspleet ME, Klarenbeek PL, et al. Immunoglobulin G4+ clones identified by next-generation sequencing dominate the B cell receptor repertoire in immunoglobulin G4 associated cholangitis. Hepatology. 2013;57:2390–2398. doi: 10.1002/hep.26232. [DOI] [PubMed] [Google Scholar]
- 11.Vlachou PA, Khalili K, Jang HJ, Fischer S, Hirschfield GM, Kim TK. Autoimmune pancreatitis and extrapancreatic manifestations of IgG4-related sclerosing disease. Radiographics. 2011;31:1379–1402. doi: 10.1148/rg.315105735. [DOI] [PubMed] [Google Scholar]
- 12.Kloek JJ, van Delden OM, Erdogan D, et al. Differentiation of malignant and benign proximal bile duct strictures: the diagnostic dilemma. World J Gastroenterol. 2008;14:5032–5038. doi: 10.3748/wjg.14.5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalaitzakis E, Levy M, Kamisawa T, et al. Endoscopic retrograde cholangiography does not reliably distinguish IgG4-associated cholangitis from primary sclerosing cholangitis or cholangiocarcinoma. Clin Gastroenterol Hepatol. 2011;9:800–803.e2. doi: 10.1016/j.cgh.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahani D V, Sainani NI, Deshpande V, et al. Autoimmune pancreatitis: disease evolution, staging, response assessment, and CT features that predict response to corticosteroid therapy. Radiology. 2009;250:118–129. doi: 10.1148/radiol.2493080279. [DOI] [PubMed] [Google Scholar]
- 15.Kawakami H, Zen Y, Kuwatani M, et al. IgG4-related sclerosing cholangitis and autoimmune pancreatitis: histological assessment of biopsies from Vater's ampulla and the bile duct. J Gastroenterol Hepatol. 2010;25:1648–1655. doi: 10.1111/j.1440-1746.2010.06346.x. [DOI] [PubMed] [Google Scholar]
- 16.Navaneethan U, Njei B, Lourdusamy V, et al. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc. 2015;81:168–176. doi: 10.1016/j.gie.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chari ST, Takahashi N, Levy MJ, et al. A diagnostic strategy to distinguish autoimmune pancreatitis from pancreatic cancer. Clin Gastroenterol Hepatol. 2009;7:1097–1103. doi: 10.1016/j.cgh.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Shimosegawa T, Chari ST, Frulloni L, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352–358. doi: 10.1097/MPA.0b013e3182142fd2. [DOI] [PubMed] [Google Scholar]
- 19.Wallace ZS, Mattoo H, Carruthers M, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis. 2014;74:190–195. doi: 10.1136/annrheumdis-2014-205233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alderlieste YA, Elzen van den BDJ, Rauws EAJ, Beuers U. Immunoglobulin G4-associated cholangitis: one variant of immunoglobulin G4-related systemic disease. Digestion. 2009;79:220–228. doi: 10.1159/000213364. [DOI] [PubMed] [Google Scholar]