Summary
Background
Dengue infection is a major public health problem. During explosive outbreaks, there is sudden surge in demands of platelet products. The present study was carried out in order to review platelet transfusion practices during the epidemic of dengue.
Methods
We retrospectively reviewed the clinical details including the platelet counts and haemorrhagic tendencies of dengue patients as well as the transfusion requirements of diagnosed dengue cases admitted at our centre.
Results
A total of 1,750 random donor platelet and 114 single donor platelet units were transfused to 531 patients. 23.2% platelet transfusions were found to be inappropriate Mean dosage of platelets transfused was 2 × 1011 platelets per patient. A total of 347 (65.3%) patients had bleeding diathesis at the time of presentation. Skin and the oropharynx were the most common bleeding sites. Major bleeding was seen in 119 (34.3%) patients, whereas 228 (65.7%) patients had minor bleeding episodes.
Conclusion
The study emphasises the need for minimising unnecessary transfusions and for using this scarce resource judiciously, which can be achieved by strict adherence to evidence-based transfusion guidelines and regular review of the on-going transfusion practices.
Keywords: Platelet transfusion, Dengue infection, Rational use
Introduction
Dengue infections are a worldwide phenomenon spread throughout the tropical and subtropical zones between 30° N and 40° S. It is caused by the dengue virus which is carried by and introduced into human host by the female Aedes mosquito. The global incidence of dengue has significantly increased over the past decade and is now endemic in many developing countries [1]. South-East Asian countries such as India, Indonesia, Myanmar and Thailand are at the highest risk of dengue accounting for nearly half of the global risk [2]. Factors that have contributed to the dramatic expansion of dengue include population growth, urbanisation, inadequate water management leading to mosquito proliferation sites and convenient global travel [3,4].
Dengue infection encompasses a spectrum of clinical manifestations ranging from mild influenza-like illness to dengue fever (DF) or severe dengue illness. The latter comprise of either plasma leakage, which leads to hypovolemic shock or dengue shock syndrome (DSS) and internal haemorrhage, or other organ failure, including encephalopathy [5,6]. DF and its more serious forms, dengue haemorrhagic fever (DHF) and DSS, account for 50 million dengue infection cases every year, with 500,000 requiring hospitalisation. The mortality rate reported is as high as 2.5% [5].
Thrombocytopenia is a prominent feature in dengue infection. Immunological destruction of platelets is the major cause of thrombocytopenia which, along with platelet dysfunction, contributes to disturbed haemostasis in dengue patients [7]. Teo et al. [3] reported that around 8% dengue-infected patients require transfusion support in form of platelet concentrates (PCs). In India, blood transfusion services are highly fragmented, and only 35% blood units are separated into components [8]. Thus, during explosive outbreaks of dengue when there is sudden surge in demands of platelet products, managing platelet inventory becomes a challenge especially when the transfusion facility also caters for oncology patients who are major consumers of the platelet products. Studies from different parts of India report high percentages of inappropriate transfusions ranging from 13.7-71% in dengue epidemics [9,10,11,12]. The present study was undertaken to assess and analyse the utilisation of platelet products in a tertiary care hospital during the 2013 dengue epidemic. We also studied the site and clinical significance of bleeding by dividing it into major and minor categories.
Material and Methods
This retrospective study was conducted at the main blood bank of the All India Institute of Medical Sciences, New Delhi, during the DF epidemic from August to November 2013. All serologically confirmed dengue cases who received platelet transfusion were included in the study. The diagnosis was established on clinical suspicion and confirmed by laboratory tests like ELISA and dot blot assays of non-structural protein 1 (NS1) or by commercially available immunochromatographic kits for anti-dengue IgM and IgG antibodies. Patients' clinical data and platelet counts were obtained from platelet requisition forms. The guidelines issued by National Vector Borne Disease Control Program (NVBDCP) in India were used as a criterion to assess the appropriateness of platelet transfusion [13] (table 1). Grades of bleeding were evaluated as per criterion listed in table 2.
Table 1.
Guidelines for platelet transfusion in dengue by National Vector Borne Disease Control Programme, 2008 issued by Directorate General of Health Services, Ministry of Health & Family Welfare, Government of India
| Indications | |
|---|---|
| – | In general there is no need to give prophylactic platelets even at <20 × 103/μl |
| – | Prophylactic platelet transfusion may be given at level of <10 × 103/μl in absence of bleeding manifestations |
| – | Prolonged shock; with coagulopathy and abnormal coagulogram* |
| – | In case of systemic massive bleeding, platelet transfusion may be needed in addition to red cell transfusion |
Abnormal bleeding time or deranged PT/aPTT.
Table 2.
Grades of bleeding
| Category | Definition |
|---|---|
| Minor | petechiae, purpura, ecchymosis, oropharyngeal/gum bleeding, epistaxis, vaginal spotting, retinal haemorrhage without visual impairment and any bleeding not requiring red cell transfusion, |
| Major | melaena, haematemesis, haemoptysis, haematuria, haematochezia, abnormal vaginal bleeding, musculoskeletal/soft tissue bleed, CNS bleeding including retinal bleeding with visual impairment, invasive site bleeding or any bleeding requiring red cell transfusion and any debilitating/ fatal bleed |
Statistical Analysis
Data management and analysis was done by SPSS version 19.0 (Chicago, IL, USA).
Results
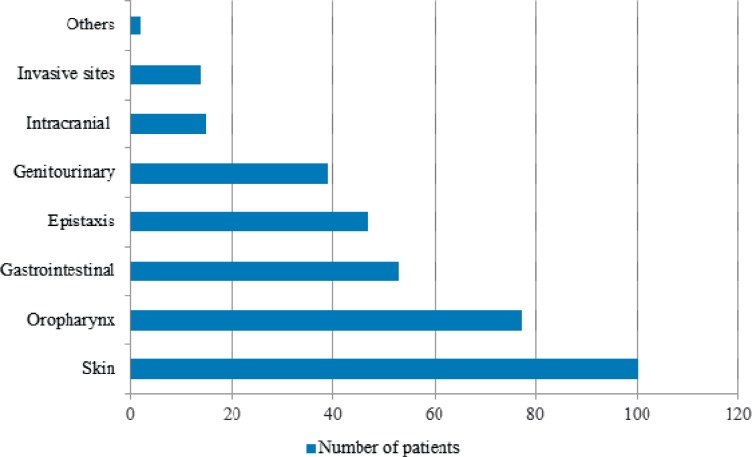
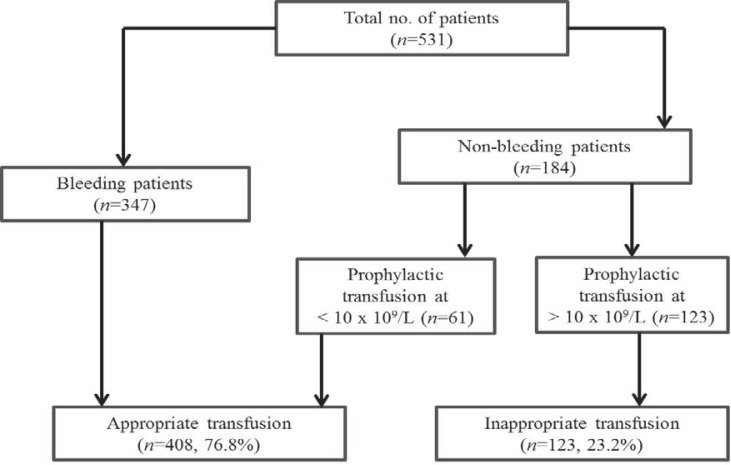
During the study period, a total of 531 diagnosed cases of dengue infection (376 males, 155 females) were admitted and administered platelet transfusions. The median age of the patients was 24 years (range 1-76 years). Age and sex distribution of patients in different age groups is shown in table 3. All patients were categorised as per WHO dengue case classification [14]. We included a fourth category of ‘DF with associated co-morbidities’ to incorporate dengue cases with acute lymphoid leukaemia (n = 9), acute myeloid leukaemia (n = 3), chronic myeloid leukaemia (n = 2), post-organ transplant (n = 3) and thalassaemia (n = 2) (table 4). At presentation, median platelet count was 14 × 109/l (range 6-80 × 109/l). A total of 347 (65.3%) patients had bleeding diathesis at the time of presentation, 272 (78.4%) of whom had platelet count ≤20 × 109/l (table 5). Most of the patients bled into skin (n = 100, 28.8%) followed by oropharyngeal mucosa (n = 77, 22.2%) (fig. 1). Major bleeding was seen in 34.3% (119) patients, whereas 65.7% (228) patients had minor bleeding episodes. A total of 1,750 random donor platelets (RDP) and 114 single donor platelets (SDP) were transfused to 531 patients. Of these 531 patients, 184 received prophylactic platelet transfusions. 61 (33.1%) of 184 non-bleeding patients were transfused at a threshold of 10 × 109/l, whereas 123 (66.9%) patients received transfusions at platelet count >10 × 109/l without any associated risk factors for bleeding. Based on quality control parameters of RDPs and SDPs, calculated transfused mean dose was approximately 2 × 1011 platelets per patient (4-5 RDPs).
Table 3.
Age and sex distribution of dengue-affected patients
| Age group, years | Male | Female | Total |
|---|---|---|---|
| <10 | 22 | 18 | 40 |
| 11–20 | 121 | 45 | 166 |
| 21–30 | 137 | 35 | 172 |
| 31–40 | 52 | 28 | 80 |
| 41–50 | 26 | 19 | 45 |
| 51–60 | 10 | 4 | 14 |
| >60 | 8 | 6 | 14 |
| Total | 376 (70.8%) | 155 (29.2%) | 531 (100) |
Table 4.
Categories of dengue infection
| Category | Number | % |
|---|---|---|
| Dengue fever | 457 | 86.0 |
| Dengue fever with associated comorbidities | 19 | 3.6 |
| DHF* | 34 | 6.4 |
| DSS ψ | 21 | 4.0 |
| Total | 531 | 100 |
Dengue haemorrhagic fever.
Dengue shock syndrome
Table 5.
Platelet counts of patients receiving platelet transfusion
| Platelet count × 109/l | Number of patients | ||
|---|---|---|---|
| total | bleeding | non-bleeding | |
| < 10 | 190 | 115 | 75 |
| 11–20 | 248 | 157 | 91 |
| 21–30 | 55 | 47 | 8 |
| 31–40 | 19 | 14 | 5 |
| 41–50 | 9 | 7 | 2 |
| >50 | 10 | 7 | 3 |
| Total | 531 | 347 (65.3%) | 184 (34.7%) |
Fig. 1.
Sites of bleeding.
According to platelet transfusion guidelines in dengue infection prescribed by NVBDCP, 123 (23.2%) of transfusion requests were found to be inappropriate (fig. 2). Our study also showed that in 35 (6.6%) instances requisition forms were incomplete lacking relevant clinical details.
Fig. 2.
Appropriateness of platelet trans- fusion.
Discussion
In South East Asia, dengue epidemics occurring every two to three years are a major health concern. The diagnosis is established on clinical suspicion supported by laboratory tests like ELISA and dot blot assays of NS1 or by commercial serological kits for anti-dengue IgM and IgG antibodies. In the present study, the whole spectrum of dengue infections including DF (89.6%), DHF (6.4%) and DSS (4%) was seen. In a study by Pallavi et al. [9], which included 71 serologically confirmed cases of dengue, who were given platelet transfusion, 65 patients (91.5%) had DF, 4 patients (5.6%) had features of DHF and 2 patients (2.9%) had signs and symptoms of DSS.
Our study showed that the majority of dengue cases were between 11 and 30 years old. In contrast to our study, Pallavi et al. [9] noted higher percentages of dengue cases among children with the largest proportion in the age group of less than 10 years, while Ayyub et al. [15] and Lye et al. [16] noted preponderance in the age group of 20-40 years which was similar to our study. Male preponderance (Male-to-female ratio 2.4:1) in the present study was similar to that reported in other studies [17,18,19]. In the present study, among the 531 patients who received the platelet transfusions, 347 patients (65.3%) presented with haemorrhagic manifestations. In a similar study, Kumar et al. [10] reported only 31.3% actively bleeding patients. This difference may be attributed to better reporting of bleeding status in our patient population as we have a lesser number of incomplete transfusion request forms. The most common bleeding site in our study was skin followed by oropharynx. In a similar study in a Thai population, Wiwanitkit [20] reported skin bleeding as most common bleeding manifestation in dengue infection.
In our study, 1,750 RDPs and 114 SDPs were transfused to 531 patients which translates into a mean dose of approximately 2 × 1011 platelets (4-5 RDP units) transfused to each patient. Kumar et al. [10] reported transfusion of 431 units of PCs to 208 patients (2 units/patient) during the 1996 dengue epidemic. The higher amounts of platelet transfusions in our study could be attributed to larger number of bleeding patients (272, 51.2%) with platelet count <20 × 109/l in our study who were appropriate candidates for platelet transfusion. Causes of bleeding other than dengue were ruled out before initiation of platelet transfusion in all patients.
Thrombocytopenia is a common denominator in most dengue infections which is primarily immune-mediated. Prophylactic platelet transfusion may aggravate thrombocytopenia by triggering an immune response. Thus, prophylactic transfusion for dengue infection is an irrational and inappropriate intervention. Besides, the short life span of transfused platelets would result only in a transient non-sustained elevation of the platelet count [21]. Of 531 patients, 184 received prophylactic platelet transfusions; 61 (33.1%) of these non-bleeding patients were transfused at a threshold of 10 × 109/l, whereas 123 (66.9%) patients received transfusions at platelet count >10 × 109/l without any associated risk factors for bleeding. According to NVBDCP guidelines, 123 (23.2%) platelet transfusions in our study were inappropriate. This group also included patients of ‘DF with associated co-morbidities’. Prophylactic platelet transfusion in these patients was merely administered due to increased anxiety among both the patient and treating physician regarding the worsening of pre-existing haematological disease. There are few studies elucidating the impact of dengue virus infection in haematology and immunosuppressed patients. A study by Sharma et al. [22] noted that there was no excessive bleeding, prolonged stay in the hospital or relapse of underlying haematological disease in haematology patients; and an immunocompromised state did not seem to alter the clinical impact of dengue infection in patients with pre-existing haematological disease. In a case report of dengue virus infection in a patient with chronic myeloid leukaemia, Chamnanchanunt et al. [23] stated that there is complete recovery from dengue infection without complication albeit platelet recovery was delayed.
Various studies from different parts of India also report high percentages of inappropriate prophylactic transfusions in dengue epidemics [9,10,11,12]. With each outbreak of dengue, there is a considerable increase in demand of platelets. Ahluwalia et al. [24] aptly described this condition as ‘dengue panic syndrome’. In this complex syndrome patients as well as healthcare professionals tend to ‘chase’ platelet counts in dengue patients who are otherwise completely asymptomatic and improving. The most untoward effect of this syndrome is sudden surge in platelet transfusion requisitions based only on platelet counts. In our study 23.2% of the platelet transfusions were deemed as inappropriate. Chaudhary et al. [12] showed that 21.5% patients received unnecessary prophylactic transfusions.
This inappropriate usage causes shortage of platelets resulting in ‘rationing’ of platelet products in order to meet demands of all admitted patients. Consequently, the ‘true’ DHF/DSS patients might not receive optimum dose of platelets intended, in spite of the best efforts by transfusion facility. Another important problem highlighted in our study was incomplete platelet request forms lacking relevant clinical data. In 35 (6.6%) instances, requisition forms were incomplete in regard to platelet count. Kumar et al. [10] reported 51.9% incomplete forms in which platelet counts were unavailable. Unfortunately, post-transfusion platelet count was not available in our study; thus we could not calculate corrected count increment and percent platelet recovery for platelet transfusions so as to document clinical efficacy of transfusions.
In developing countries like India, where the component separation facilities are limited, optimal usage of platelet products is of paramount importance, especially during periods of increased demands such as dengue epidemics. There is a need for minimising unnecessary transfusions and for using this scarce resource judiciously, which can be achieved by strict adherence to guidelines and regular review of the on-going transfusion practices. Constitution of hospital transfusion committee and close coordination between transfusion facility and physicians ordering blood components will ensure optimal utilisation of scarce blood resources. Educating the clinicians about evidence-based transfusion practices will help in reducing the inappropriate transfusions and decrease the strain on transfusion facilities especially at the time of epidemics.
Disclosure Statement
The authors have no conflict of interests to declare.
References
- 1.Sinha N, Gupta N, Jhamb R, Gulati S, Kulkarni Ajit V. The 2006 dengue outbreak in Delhi, India. J Commun Dis. 2008;40:243–248. [PubMed] [Google Scholar]
- 2.Nimmannitya S. Dengue and dengue haemorrhagic fever. In: Cook GC, Zumla AI, editors. Manson's Tropical Diseases. 22nd ed. London: Saunders; 2009. pp. 753–762. [Google Scholar]
- 3.Teo D, Ng LC, Lam S. Is dengue a threat to the blood supply? Transfus Med. 2009;19:66–77. doi: 10.1111/j.1365-3148.2009.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guzmán MG, Kouri G. Dengue: an update. The Lancet Infect Dis. 2002;2:33–42. doi: 10.1016/s1473-3099(01)00171-2. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization, the Special Programme for Research and Training in Tropical Diseases Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva, World Health Organization, 2009. www.who.int/tdr/publications/documents/dengue-diagnosis.pdf (last accessed December 29, 2014).
- 6.Balmaseda A, Hammond SN, Perez L, Tellez Y, Saborio SI, Mercado JC, Cuadra R, Rocha J, Perez MA, Silva S, Rocha C, Harris E. Serotype-specific differences in clinical manifestations of dengue. Am J Trop Med Hyg. 2006;74:449–456. [PubMed] [Google Scholar]
- 7.Mitrakul C, Poshyachinda M, Futrakul P, Sangkawibha N, Ahandrik S. Hemostatic and platelet kinetic studies in dengue hemorrhagic fever. Am J Trop Med Hyg. 1977;26:975–984. doi: 10.4269/ajtmh.1977.26.975. [DOI] [PubMed] [Google Scholar]
- 8.Choudhury N. Blood transfusion in borderless South Asia. Asian J Transfus Sci. 2011;5:117–120. doi: 10.4103/0973-6247.83234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pallavi P, Ganesh CK, Jayashree K, Manjunath GV. Unfurling the rationale use of platelet transfusion in dengue fever. Indian J Hematol Blood Transfus. 2011;27:70–74. doi: 10.1007/s12288-011-0059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar ND, Tomar V, Singh B, Kela K. Platelet transfusion practice during dengue fever epidemic. Indian J Pathol Microbiol. 2000;43:55–60. [PubMed] [Google Scholar]
- 11.Makroo RN, Raina V, Kumar P, Kanth RK. Role of platelet transfusion in the management of dengue patients in a tertiary care hospital. Asian J Transfus Sci. 2007;1:4–7. doi: 10.4103/0973-6247.28065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaudhary R, Khetan D, Sinha S, Sinha P, Sonker A, Pandey P, Das SS, Agarwal P, Ray V. Transfusion support to dengue patients in a hospital based blood transfusion service in North India. Transfus Apher Sci. 2006;35:239–244. doi: 10.1016/j.transci.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Directorate of National Vector Borne Disease Control Programme Guidelines for Clinical Case Management of Dengue Fever/ Dengue Hemorrhagic Fever/ Dengue Shock Syndrome. Delhi, Directorate General of Health Services, Ministry of Health & Family Welfare, Government of India, 2008. www.pcmcindia.gov.in/pdf/dengue/Clinical%20Guidelines%20of%20DF,DHF%20&%20DSS.pdf (last accessed December 29, 2014).
- 14.World Health Organization . Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. 2nd ed. Geneva: World Health Organization; 1997. [Google Scholar]
- 15.Ayyub M, Khazindar AM, Lubbad EH, Barlas S, Alfi AY, Al-Ukayli S. Characteristics of dengue fever in a large public hospital, Jeddah, Saudi Arabia. J Ayub Med Coll Abbottabad. 2006;18:9–13. [PubMed] [Google Scholar]
- 16.Lye DC, Lee VJ, Sun Y, Leo YS. Lack of efficacy of prophylactic platelet transfusion for severe thrombocytopenia in adults with acute uncomplicated dengue infection. Clin Infect Dis. 2009;48:1262–1265. doi: 10.1086/597773. [DOI] [PubMed] [Google Scholar]
- 17.Hammond SN, Balmaseda A, Perez L, Tellez Y, Saborio SI, Mercado JC, Videa E, Rodriguez Y, Perez MA, Cuadra R, Solano S, Rocha J, Idiaquez W, Gonzalez A, Harris E. Differences in dengue severity in infants, children, and adults in a 3-year hospital-based study in Nicaragua. Am J Trop Med Hyg. 2005;73:1063–1070. [PubMed] [Google Scholar]
- 18.Bandyopadhyay B, Bhattacharyya I, Adhikary S, Konar J, Dawar N, Sarkar J, Mondal S, Singh Chauhan M, Bhattacharya N, Chakravarty A, Biswas A, Saha B. A comprehensive study on the 2012 dengue fever outbreak in Kolkata, India. ISRN Virol. 2013;2013:5. [Google Scholar]
- 19.Saqib MA, Rafique I, Bashir S, Salam AA. A retrospective analysis of dengue fever case management and frequency of co-morbidities associated with deaths. BMC Res Notes. 2014;7:205. doi: 10.1186/1756-0500-7-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiwanitkit V. Bleeding and other presentations in Thai patients with dengue infection. Clin Appl Thromb Hemost. 2004;10:397–398. doi: 10.1177/107602960401000414. [DOI] [PubMed] [Google Scholar]
- 21.Sellahewa KH. Management dilemmas in the treatment of dengue fever. Dengue Bull. 2008;32:8. [Google Scholar]
- 22.Sharma SK, Seth T, Mishra P, Gupta N, Agrawal N, Broor S, Mahapatra M, Saxena R. Clinical profile of dengue infection in patients with hematological diseases. Mediterr J Hematol Infect Dis. 2011;3:e2011039. doi: 10.4084/MJHID.2011.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chamnanchanunt S, Thungthong P, Nakhakes C, Suwanban T, Iam-arunthai K. Dengue virus infection in a patient with chronic myeloid leukemia. Southeast Asian J Trop Med Public Health. 2012;43:900–903. [PubMed] [Google Scholar]
- 24.Ahluwalia G, Sharma SK. Dengue: Current trends and challenges – an Indian perspective. J Assoc Physicians India. 2004;52:561–563. [PubMed] [Google Scholar]