Abstract
We present a case of suspected linezolid toxicity in a 34-year-old man with sickle cell disease and line-related vancomycin-resistant enterococcal bacteremia and tricuspid valve endocarditis. The patient developed sudden-onset hypoglycemia, lactic acidosis, and acute pancreatitis 11 days after initiation of linezolid. All adverse effects quickly resolved with drug cessation. The pathophysiology underlying this triad of linezolid toxicity is unclear, but may be related to mitochondrial dysfunction.
Linezolid is a mainstay antimicrobial in the management of a number of bacterial infections and is approved by the Food and Drug Administration in the treatment of vancomycin-resistant enterococcus (1). However, despite its proven efficacy, linezolid's use is limited by certain toxicities. Here, we describe a case of linezolid toxicity causing hypoglycemia, lactic acidosis, and acute pancreatitis.
CASE DESCRIPTION
A 34-year-old man with sickle cell disease (SCD), end-stage renal disease, secondary hemochromatosis, multiple prior strokes, seizures, polymicrobial line infections, and line-associated deep vein thromboses presented initially with hypotension and seizure-like activity. He was cachectic and ill-appearing, but was otherwise alert and oriented. Precordial examination disclosed a holosystolic murmur. He had bilateral calf tenderness and a palpable thrill over a left upper extremity fistula. His fistula site was nonwarm and nontender to palpation. His initial white blood cell count was 10.4 × 103 cells/mm3. Blood cultures grew Candida albicans and vancomycin-resistant enterococcus, for which he was treated with amphotericin B 300 mg intravenous daily, linezolid 600 mg intravenous twice daily, micafungin 100 mg intravenous daily, and piperacillin/tazobactam 2.25 g intravenous every 8 hours. Transthoracic echocardiography revealed tricuspid valve endocarditis. He was transferred to the intensive care unit for hypoxemic respiratory failure likely related to septic pulmonary emboli. He was continued on broad-spectrum antimicrobial treatment, and his oxygen requirement improved gradually with supportive management. He did not require intubation.
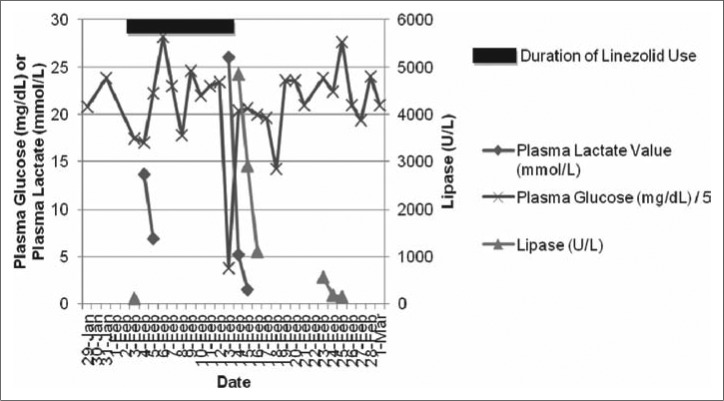
Approximately 11 days after initiation of linezolid, he suddenly became somnolent, withdrawn, and minimally responsive to noxious stimuli. No response to intravenous naloxone was observed. His serum lactate rose acutely to 23.5 mmol/L (most recent value 2.1 mmol/L). Concurrent arterial blood gas sampling revealed a pH of 7.07, a partial pressure of carbon dioxide of 25 mm Hg, and a partial pressure of oxygen of 50 mm Hg with a corresponding serum bicarbonate level of 7 mmol/L. His basic metabolic panel showed that his anion gap was 42 mEq/L. His acid-base status was driven by an anion-gap metabolic acidosis, a metabolic alkalosis (iatrogenic from sodium bicarbonate administration), and a respiratory acidosis (incomplete compensation). His plasma glucose level was 19 mg/dL (most recent value, 101 mg/dL). He was given several amps of D50W with normalization of his plasma glucose with rapid improvement in his mental status. His lactate peaked at 26.0 mmol/L and downtrended to 1.8 mmol/L within 33 hours.
Approximately 24 hours after his initial hypoglycemia and lactic acidosis, he developed sudden-onset epigastric abdominal pain, nausea, and vomiting. His serum lipase level was 3599 U/L (most recent value, 87 U/L). He was treated conservatively for pancreatitis with intravenous narcotics and maintenance fluids. His lipase peaked at 4844 U/L and slowly improved to 147 U/L within 12 days (Figure 1). His abdominal pain gradually improved over the next 2 to 3 days.
Figure 1.
Select laboratory parameters measured during the 15 days before and after the reported adverse effects of linezolid. The shaded region represents the duration of linezolid intravenous exposure. Plasma glucose is reported in mg/dL divided by 5; plasma lactate is reported in mmol/L; and lipase is reported in U/L.
Linezolid was suspected as the causative agent, and he was transitioned to intravenous daptomycin 6 mg/kg every 48 hours. Upon retrospective chart review, he had experienced a prior episode of elevated lactate, hypoglycemia, and elevated lipase, temporally correlated with linezolid administration (occurring approximately 12 days after initial dosing). In the time between these two episodes, the patient received linezolid on two other occasions (12-hour and 4- to 6-day duration of administrations) without experiencing this triad of adverse effects.
DISCUSSION
Linezolid has an established role in the treatment of serious bacterial infections, including vancomycin-resistant enterococcus (2). Although it is generally well tolerated and can be administered orally, linezolid has important adverse effects, including myelosuppression, peripheral neuropathy, and serotonin syndrome (3, 4). Isolated reports have identified cases of hypoglycemia, lactic acidosis, and acute pancreatitis in linezolid-treated patients. However, to our knowledge, this is the first case of linezolid contributing to hypoglycemia, lactic acidosis, and acute pancreatitis in a single patient.
In prior cases of linezolid-induced hypoglycemia, the patients' median age was 77 years, and the median duration before hypoglycemia was 7 days, with prompt improvement with drug discontinuation (5). Duration of use appears to be a risk factor for development of lactic acidosis, with an average time before onset of 5.5 weeks (6). However, early onset lactic acidosis after a period of hours has also been reported (7). Lactic acidosis occurs across a broad age range and has been described in children as well (8). Pancreatitis has also been reported as a complication of linezolid therapy, but appears to be isolated to the pediatric population (9, 10).
Given the limited cases of linezolid-related adverse effects in adults, the pathophysiology of these phenomena has not been well characterized. As such, it is unclear whether the same mechanisms contribute to their development, especially in a critically ill individual with multiple intervening processes. Differences in severity and presentation of linezolid toxicity may be related to duration of use, repeated exposure, concomitant treatments, and background comorbid disease burden.
However, despite these uncertainties and confounding features, given the temporal clustering of these toxicities and their rapid resolution with drug withdrawal, we propose a unified hypothesis of mitochondrial dysfunction driving the observed linezolid-related adverse effects in our patient. Linezolid, an oxazolidinone antimicrobial, has been shown to interfere with mitochondrial protein synthesis, perhaps due to structural similarities between bacterial and mitochondrial ribosomes (11). A reduction in mitochondria-derived complex IV (12, 13) impairs cellular oxidative phosphorylation and augments glycolysis. Interestingly, patients with SCD have deficient complex V activity (14), which may be additive or synergistic in contributing to mitochondrial dysfunction and production of lactic acidosis. Indeed, decreased complex V activity augments platelet activation, potentiating SCD-induced microcirculatory dysfunction and production of reactive oxygen species (14). In the absence of other clear precipitants, localized ischemia secondary to vasoocclusive phenomena in our patient may have caused pancreatitis. The pathogenesis of linezolid-induced hypoglycemia has previously been related to its nonselective monoamine oxidase inhibitor and dopaminergic properties (15). However, it is plausible that mitochondrial dysfunction may have led to dysregulation of insulin secretion from pancreatic beta-cells, causing systemic hypoglycemia (16). We suggest that high clinical suspicion be maintained for potential linezolid toxicity, particularly in patients receiving high cumulative doses and repetitive exposures.
References
- 1.Birmingham MC, Rayner CR, Meagher AK, Flavin SM, Batts DH, Schentag JJ. Linezolid for the treatment of multidrug-resistant, gram-positive infections: experience from a compassionate-use program. Clin Infect Dis. 2003;36(2):159–168. doi: 10.1086/345744. [DOI] [PubMed] [Google Scholar]
- 2.Patel R, Gallagher JC. Vancomycin-resistant enterococcal bacteremia pharmacotherapy. Ann Pharmacother. 2015;49(1):69–85. doi: 10.1177/1060028014556879. [DOI] [PubMed] [Google Scholar]
- 3.Attassi K, Hershberger E, Alam R, Zervos MJ. Thrombocytopenia associated with linezolid therapy. Clin Infect Dis. 2002;34(5):695–698. doi: 10.1086/338403. [DOI] [PubMed] [Google Scholar]
- 4.Taylor JJ, Wilson JW, Estes LL. Linezolid and serotonergic drug interactions: a retrospective survey. Clin Infect Dis. 2006;43(2):180–187. doi: 10.1086/504809. [DOI] [PubMed] [Google Scholar]
- 5.Viswanathan P, Iarikov D, Wassel R, Davidson A, Nambiar S. Hypoglycemia in patients treated with linezolid. Clin Infect Dis. 2014;59(8):e93–e95. doi: 10.1093/cid/ciu487. [DOI] [PubMed] [Google Scholar]
- 6.Im JH, Baek JH, Kwon HY, Lee JS. Incidence and risk factors of linezolid-induced lactic acidosis. Int J Infect Dis. 2015. pp. 3147–3152. [DOI] [PubMed]
- 7.Contou D, Fichet J, Grimaldi D, Cariou A. Early life-threatening lactic acidosis following a single infusion of linezolid. Int J Antimicrob Agents. 2011;38(1):84–85. doi: 10.1016/j.ijantimicag.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 8.Su E, Crowley K, Carcillo JA, Michaels MG. Linezolid and lactic acidosis: a role for lactate monitoring with long-term linezolid use in children. Pediatr Infect Dis J. 2011;30(9):804–806. doi: 10.1097/INF.0b013e3182186035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garazzino S, Krzysztofiak A, Esposito S, Castagnola E, Plebani A, Galli L, Cellini M, Lipreri R, Scolfaro C, Bertaina C, Calitri C, Bozzola E, Lancella L, Quondamcarlo A, Bosis S, Pugni L, Losurdo G, Soresina A, De Gaudio M, Mariotti I, Mancini L, Gabiano C, Tovo PA. Use of linezolid in infants and children: a retrospective multicentre study of the Italian Society for Paediatric Infectious Diseases. J Antimicrob Chemother. 2011;66(10):2393–2397. doi: 10.1093/jac/dkr285. [DOI] [PubMed] [Google Scholar]
- 10.Rose PC, Hallbauer UM, Seddon JA, Hesseling AC, Schaaf HS. Linezolid-containing regimens for the treatment of drug-resistant tuberculosis in South African children. Int J Tuberc Lung Dis. 2012;16(12):1588–1593. doi: 10.5588/ijtld.12.0322. [DOI] [PubMed] [Google Scholar]
- 11.Velez JC, Janech MG. A case of lactic acidosis induced by linezolid. Nat Rev Nephrol. 2010;6(4):236–242. doi: 10.1038/nrneph.2010.20. [DOI] [PubMed] [Google Scholar]
- 12.Garrabou G, Soriano A, Lopez S, Guallar JP, Giralt M, Villarroya F, Martinez JA, Casademont J, Cardellach F, Mensa J, Miro O. Reversible inhibition of mitochondrial protein synthesis during linezolid-related hyperlactatemia. Antimicrob Agents Chemother. 2007;51(3):962–967. doi: 10.1128/AAC.01190-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soriano A, Miro O, Mensa J. Mitochondrial toxicity associated with linezolid. N Engl J Med. 2005;353(21):2305–2306. doi: 10.1056/NEJM200511243532123. [DOI] [PubMed] [Google Scholar]
- 14.Cardenes N, Corey C, Geary L, Jain S, Zharikov S, Barge S, Novelli EM, Shiva S. Platelet bioenergetic screen in sickle cell patients reveals mitochondrial complex V inhibition, which contributes to platelet activation. Blood. 2014;123(18):2864–2872. doi: 10.1182/blood-2013-09-529420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bodnar T, Starr K, Halter JB. Linezolid-associated hypoglycemia in a 64-year-old man with type 2 diabetes. Am J Geriatr Pharmacother. 2011;9(1):88–92. doi: 10.1016/j.amjopharm.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Michelakis ED. Mitochondrial medicine: a new era in medicine opens new windows and brings new challenges. Circulation. 2008;117(19):2431–2434. doi: 10.1161/CIRCULATIONAHA.108.775163. [DOI] [PubMed] [Google Scholar]