Abstract
Pulmonary tumor thrombotic microangiopathy (PTTM) is a rare cause of rapidly progressive dyspnea in a background of metastatic cancer. Gastric adenocarcinoma is the neoplasm most frequently associated with PTTM. Unfortunately, PTTM is difficult to identify clinically and is most commonly a postmortem diagnosis. We present the case of a woman with no previous diagnosis of cancer who presented with rapidly progressive shortness of breath. She was diagnosed with severe pulmonary arterial hypertension and rapidly succumbed to her illness. A postmortem diagnosis of PTTM was established based on autopsy results.
Pulmonary tumor thrombotic microangiopathy (PTTM) is a rare and usually fatal cause of rapidly progressive dyspnea, caused by malignancy (1, 2). It was first described by von Herbay et al, who identified fibrocellular proliferation of the intima of small pulmonary arterial vessels in patients with metastatic carcinoma (2). This report describes a patient who presented with rapidly progressive dyspnea and pulmonary hypertension and was subsequently found at autopsy to have PTTM from an undiagnosed metastatic gastric adenocarcinoma.
CASE REPORT
A 52-year-old woman was transferred from an outside hospital with a 1.5-month history of minimally productive cough and a 2-week history of progressive dyspnea. She complained of nausea, vomiting, and vague abdominal pain. Her medical history was significant for untreated diabetes and hypertension. Her only medications were famotidine and esomeprazole as needed for gastroesophageal reflux. Review of systems was positive for night sweats, fatigue, a 10-lb weight loss over 6 months, anorexia, and paroxysmal nocturnal dyspnea. Her temperature was 95.3°F; pulse, 80 beats per minute; blood pressure, 151/94 mm Hg; respiratory rate, 20 breaths per minute; and oxygen saturation, 99% on 15 L/minute with a nonrebreather mask. Physical exam revealed an obese woman in moderate respiratory distress, with jugular venous distention, a prominent P2 heart sound, few bilateral basilar crackles with deep inspiration, and mild epigastric tenderness but no palpable masses or ascites.
Abnormal laboratory results included a hemoglobin level of 10.2 g/dL; platelets, 96 K/μL; albumin, 2.9 g/dL; and alkaline phosphatase, 189 U/L. Arterial blood gas analysis demonstrated a pH of 7.40; the patient's partial pressure of carbon dioxide was 41 mm Hg, partial pressure of oxygen in arterial blood, 141 mm Hg, and bicarbonate, 25.1 mmol/L. A transthoracic echocardiogram revealed a dilated right atrium and right ventricle and moderate to severe pulmonary hypertension with an estimated right ventricular systolic pressure of 66 mm Hg and a D-shaped septum. A ventilation-perfusion scan showed a very low probability of a pulmonary embolism, and lower extremity venous Doppler studies were negative. A right heart catheterization demonstrated moderate pulmonary hypertension nonresponsive to inhaled nitric oxide, with a pulmonary artery pressure of 70/30 mm Hg, mean 37 mm Hg, a pulmonary capillary wedge pressure of 6 mm Hg, and a cardiac index of 1.7.
She was started on intravenous epoprostenol with the goal of aggressive titration; however, only minimal increases could be made given her nausea. She continued to deteriorate clinically with worsening respiratory distress. On day 6, she required emergent endotracheal intubation and subsequently sustained a pulseless electrical activity arrest and failed resuscitation attempts.
An autopsy revealed diffuse thickening of the gastric wall with a linitis plastica appearance. Microscopic analysis of the stomach demonstrated poorly differentiated adenocarcinoma with transmural infiltration and extensive invasion of the lymphatic vasculature. Diffuse metastatic lesions were identified. Cardiac examination revealed pericardial effusion, cardiac adiposity, and right ventricular hypertrophy but no myocardial lesions and normal epicardial coronary arteries. The larynx, trachea, and bronchi were nonobstructed. Microscopically, the lungs revealed arteriolar thickening consisting of medial hypertrophy and intimal fibroplasia. Additionally, organizing thrombi with recanalization and anaplastic tumor cells were observed in small pulmonary vessels (Figure 1a). There were no grossly visible metastatic nodules within the lungs. Tumor cells were strongly positive for vascular endothelial growth factor (VEGF) within the vasculature of the gastric wall (Figure 1b).
Figure 1.
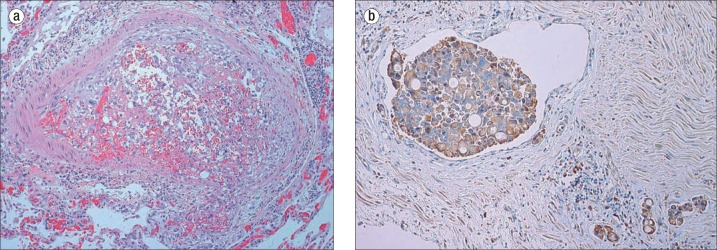
(a) Lung: Organizing intravascular fibrin thrombus containing anaplastic tumor cells similar to the gastric primary tumor (hematoxylin and eosin, 100×). (b) Muscularis propria of stomach: Strongly positive VEGF immunohistochemical staining of intravascular adenocarcinoma (EnVision+ system, 100×).
DISCUSSION
PTTM is characterized by marked thickening of the arterial intima and activation of the coagulation cascade, leading to occlusion of pulmonary arteries and arterioles (2–5). It is strongly associated with carcinomas, most often of gastric origin (1, 2). PTTM is believed to originate when tumor cells metastasize to the pulmonary vasculature, where they activate the coagulation cascade and cause the release of growth factors and inflammatory cytokines leading to the deposition of platelets and fibrin along the intima of the artery (4). The diffuse narrowing of pulmonary arterial vessels accounts for the increased vascular resistance and the frequent diagnosis of idiopathic pulmonary arterial hypertension (6, 7). VEGF is believed to be a primary mediator of this process and is often histologically positive in PTTM; however, it is not pathognomonic and should not be used in isolation for diagnosis (4).
The patient in the current case, who had no history of cancer, presented with severe dyspnea and was found to have severe pulmonary arterial hypertension in a decompensated state. Previous imaging revealed enlarged thoracic and abdominal lymph nodes along with thickening of the gastric wall and pancreatic head; however, she was not stable enough to undergo further diagnostic testing and deteriorated despite aggressive treatment.
Only a few cases exist in the literature of patients with PTTM diagnosed prior to death (8–12). Miyano et al reported a patient with previously resected gastric cancer in whom PTTM was suggested by elevated serum VEGF and D-dimer levels (8). The diagnosis was confirmed via transbronchial lung biopsy and video-assisted thoracoscopic surgery. Accurate and timely diagnosis of PTTM led to the successful treatment of the patient with corticosteroids, anticoagulation, and chemotherapy (8).
PTTM is often fatal and caused by malignancy. It should be suspected with new pulmonary arterial hypertension or rapidly worsening respiratory failure in the presence or absence of a previous diagnosis of cancer. Early antemortem diagnosis of PTTM is imperative for potential survival, and serum VEGF and D-dimer levels can be used as screening tools in the evaluation of a patient with suspected PTTM. However, a clinical diagnosis of PTTM remains difficult, and there are no controlled studies evaluating the usefulness of medical intervention.
References
- 1.Uruga H, Fujii T, Kurosaki A, Hanada S, Takaya H, Miyamoto A, Morokawa N, Homma S, Kishi K. Pulmonary tumor thrombotic microangiopathy: a clinical analysis of 30 autopsy cases. Intern Med. 2013;52(12):1317–1323. doi: 10.2169/internalmedicine.52.9472. [DOI] [PubMed] [Google Scholar]
- 2.von Herbay A, Illes A, Walderr R, Otto H. Pulmonary tumor thrombotic microangiopathy with pulmonary hypertension. Cancer. 1990;66(3):587–592. doi: 10.1002/1097-0142(19900801)66:3<587::aid-cncr2820660330>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 3.Kinuya K, Yamanouchi K, Terahata S. Diagnosis: pulmonary tumor thrombotic microangiopathy developing cor pulmonale. Ann Nucl Med. 2002;16(3):220. doi: 10.1007/BF02996304. [DOI] [PubMed] [Google Scholar]
- 4.Kuwabara H, Yoshida S, Takasu T, Yuki M, Goto I, Hanafusa T, Shibayama Y. Pulmonary tumor thrombotic microangiopathy caused by gastric cancer. Ann Thorac Med. 2012;7(3):168–169. doi: 10.4103/1817-1737.98853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demirag F, Cakir E, Yazici U, Tastepe I. Pulmonary tumor thrombotic microangiopathy from metastatic epithelioid angiosarcoma. J Thorac Dis. 2013;5(3):E107–E111. doi: 10.3978/j.issn.2072-1439.2012.10.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinckard JK, Wick MR. Tumor-related thrombotic pulmonary microangiopathy: review of pathologic findings and pathophysiologic mechanisms. Ann Diagn Pathol. 2000;4(3):154–157. doi: 10.1016/s1092-9134(00)90038-8. [DOI] [PubMed] [Google Scholar]
- 7.Buser M, Felizeter-Kessler M, Lenggenhager D, Maeder MT. Rapidly progressive pulmonary hypertension in a patient with pulmonary tumor thrombotic microangiopathy. Am J Respir Crit Care Med. 2015;191(6):711–712. doi: 10.1164/rccm.201501-0004IM. [DOI] [PubMed] [Google Scholar]
- 8.Miyano S, Izumi S, Takeda Y, Tokuhara M, Mochizuki M, Matsubara O, Kuwata H, Kobayashi N, Kudo K. Pulmonary tumor thrombotic microangiopathy. J Clin Oncol. 2007;25(5):597–599. doi: 10.1200/JCO.2006.09.0670. [DOI] [PubMed] [Google Scholar]
- 9.Kitamura A, Nishimura N, Jinta T, Suda R, Yamano Y, Ishikawa G, Tomishima Y, Hamaoka T, Suzuki K, Chohnabayashi N. A case of pulmonary tumor thrombotic microangiopathy diagnosed by transbronchial lung biopsy and treated with chemotherapy and long-term oxygen and anticoagulation therapies. Case Rep Pulmonol. 2013;2013:259080. doi: 10.1155/2013/259080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kayatani H, Matsuo K, Ueda Y, Matsushita M, Fujiwara K, Yonei T, Yamadori I, Shigematsu H, Andou A, Sato T. Pulmonary tumor thrombotic microangiopathy diagnosed antemortem and treated with combination chemotherapy. Intern Med. 2012;51(19):2767–2770. doi: 10.2169/internalmedicine.51.7682. [DOI] [PubMed] [Google Scholar]
- 11.Mandaliya R, Farhat S, Uprety D, Balla M, Gandhi A, Goldhahn R, Auerbach H, Christensen C, Reed C, Cohen S. Occult gastric cancer presenting as hypoxia from pulmonary tumor thrombotic microangiopathy. J Gastric Cancer. 2014;14(2):142–146. doi: 10.5230/jgc.2014.14.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujishiro T, Shuto K, Shiratori T, Kono T, Akutsu Y, Uesato M, Hoshino I, Murakami K, Imanishi S, Tochigi T, Yonemori Y, Matsubara H. A case report of pulmonary tumor thrombotic microangiopathy (PTTM) caused by esophageal squamous cell carcinoma. Esophagus. 2013;10(4):247–251. doi: 10.1007/s10388-013-0382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


