Abstract
We report a late presentation of adenovirus-induced renal allograft and bladder infection causing azotemia and hemorrhagic cystitis in a patient 5 years after simultaneous kidney-pancreas transplantation. Adenovirus has been increasingly recognized as a cause of morbidity and mortality in both solid organ and stem cell transplant recipients. We wish to emphasize the importance of early detection, as treatment options involve reduction of immunosuppression, followed by the addition of antiviral agents and supportive care.
Adenovirus causes 5% to 10% of all childhood febrile illnesses, and the immunocompetent host generally endures a mild, self-limited upper respiratory tract infection. Adenovirus then establishes latent infection in the lymphoepithelial tissues (1). In immunocompromised hosts, the clinical manifestations of adenovirus reactivation or de novo infection can range from asymptomatic to fatal. Adenovirus is identified as a late complication of bone marrow transplantation with hemorrhagic cystitis, and it is increasingly recognized as a serious, though rare, adverse complication of solid organ transplantation. We describe a rare late presentation of hemorrhagic cystitis and significant renal allograft dysfunction with ureteral obstruction in a patient who had undergone simultaneous kidney-pancreas transplantation (SKP) nearly 5 years earlier. With adenovirus detected in the urine, blood, and renal allograft, the patient was treated with reduction of immunosuppression, intravenous immunoglobulin, and cidofovir. Renal function did not improve despite treatment. Pancreas function, as in rare case reports, was preserved, suggesting tropism of the adenovirus.
CASE DESCRIPTION
A 45-year-old African American woman had a past medical history of type 1 diabetes mellitus complicated by peripheral neuropathy, retinopathy, and end-stage renal disease. She underwent SKP in February 2009 and received daclizumab induction per protocol. The donor was a 2/6 human leukocyte antigen match, and both donor and recipient were cytomegalovirus positive. The transplant was complicated by cellular rejection of the kidney in March 2009 treated with Solu-Medrol and biopsy-proven pancreas rejection treated with 10 doses of muromonab-CD3. Renal function stabilized with a creatinine of 1.4 mg/dL. Maintenance immunosuppression included azathioprine 100 mg daily, prednisone 5 mg daily, and tacrolimus 4 mg twice daily.
Five years after transplant, the patient presented with a 1-week history of fever, suprapubic pain, and nausea, with the development of hematuria 3 days later. She had presented to an emergency department for presumed urinary tract infection 3 days before admission and was treated with nitrofurantoin. However, she developed worsening hematuria and presented to the transplant clinic. She denied sick contacts and had been compliant with all medications. On examination, she had orthostatic hypotension and fever. She was pale and appeared acutely ill. The left lower quadrant and suprapubic area were tender without rebound or guarding.
Initial laboratory data revealed normal chemistries and blood counts except for blood urea nitrogen 53 mmol/L and creatinine 4.6 mg/dL. Her tacrolimus level was acceptable, at 5.3 ng/mL. Pertinent findings on urinalysis were 3+ protein, nitrite positive, and 3+ blood, with microscopy demonstrating red blood cells too numerous to count, obscuring further results. A renal transplant sonogram showed a normal resistive index and an irregular bladder wall.
Volume resuscitation with intravenous crystalloid and vancomycin and cefepime were initiated. Her serum creatinine improved but hematuria persisted. Cystoscopy revealed hemorrhagic cystitis, a dilated ureter, and hydronephrosis. A 6 French, double-J ureteral stent was placed.
The patient's fever persisted, and her creatinine level increased despite fluids and antibiotics. Results of polymerase chain reaction (PCR) tests for chlamydia, gonorrhea, herpes, BK, and cytomegalovirus were all negative. Blood and urine cultures were sterile. Renal biopsy showed severe interstitial nephritis, necrotizing with granulomas and adenovirus staining in the tubular epithelium (Figure 1). Tubular atrophy and interstitial fibrosis comprised at least 50% of the interstitium. Azathioprine was discontinued and tacrolimus was decreased. Whole-blood PCR for adenovirus revealed 47,100 copies, and urine PCR showed 3,650,000 copies/mL. Intravenous gammaglobulin was started on day 12. Cidofovir 80 mg weekly was given with probenecid on day 15 for 3 weeks when her creatinine level failed to improve. Nine days after initiation of cidofovir, adenovirus was undetectable by blood PCR; 7 days later, it was undetectable by urine PCR (Figure 2).
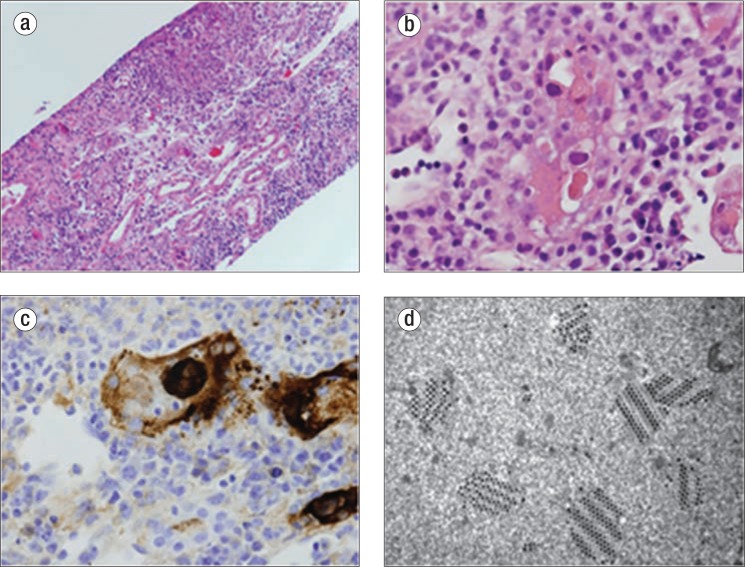
Figure 1.
Renal biopsy. (a) Severe adenovirus interstitial nephritis in a kidney allograft consisting of tubular necrosis and brisk interstitial inflammation, hematoxylin and eosin (H&E) ×200. (b) Smudgy basophilic nuclear inclusion within a necrotic tubule, which is indicative of a viral inclusion, H&E ×400. (c) An immunoperoxidase stain with an antibody to adenovirus revealing positive staining in both the nucleus and cytoplasm of an infected cell, ×400. (d) Electron microscopy crystalline array of virus particles within a tubular epithelial cell, ×42,000.
Figure 2.
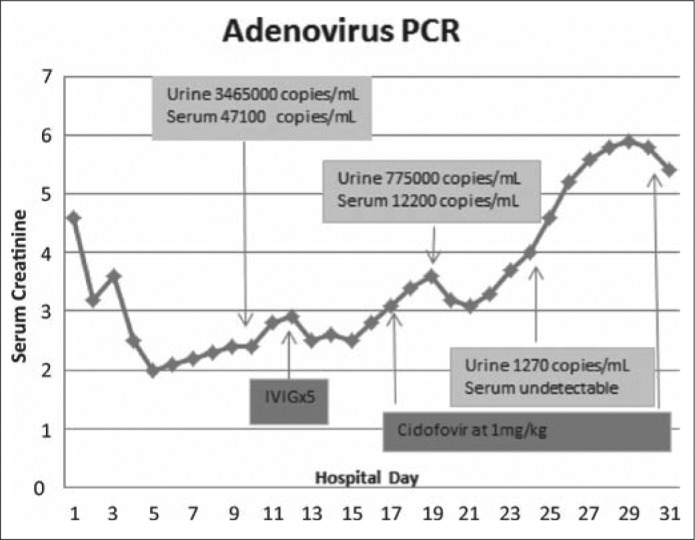
Timeline and clinical course during hospitalization.
However, her serum creatinine level rose again despite resolution of viremia. A second kidney biopsy obtained on day 25 showed a marked reduction in adenovirus-positive cells compared with the previous biopsy. However, a dense interstitial plasma cell and lymphocytic infiltrate remained with persistent tubular atrophy and interstitial fibrosis. Solu-Medrol was added and changed to 40 mg prednisone daily on discharge. Her discharge creatinine level was 5.4 mg/dL.
Prednisone was tapered to 10 mg daily over 1 month, azathioprine was not restarted, and adenovirus remained undetectable by blood PCR. Three-month follow-up biopsy showed 60% interstitial fibrosis and tubular atrophy and acute tubular necrosis with resolution of active infection (Figure 3). One year after admission, her creatinine level was 3.4 mg/dL, with a glomerular filtration rate of 18 mL/min on Glofil testing. The patient has been listed for another kidney transplant.
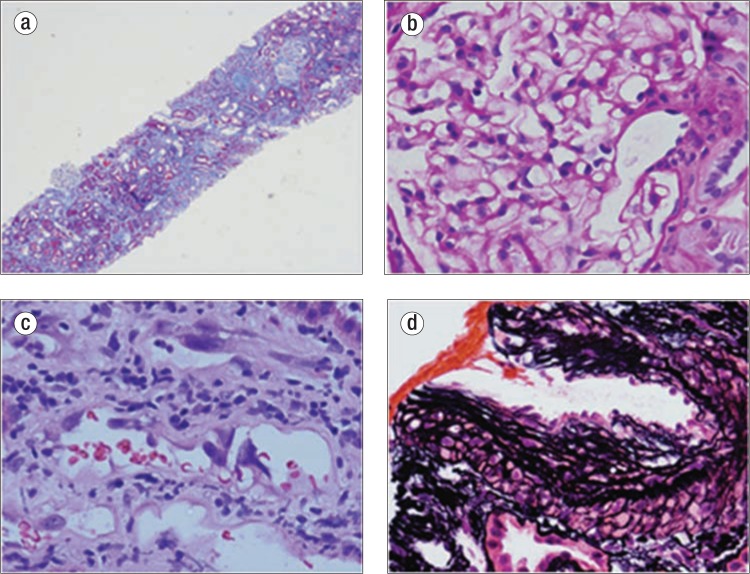
Figure 3.
Biopsy. (a) Severe tubular atrophy and interstitial fibrosis (60%), Trichrome ×40. (b) Normal glomerulus, periodic acid-Schiff ×400. (c) Regenerative changes within tubules, H&E ×400. (d) Severe arteriosclerosis, Silver ×400.
DISCUSSION
Adenovirus is a nonenveloped, double-stranded DNA virus transmitted by inhalation, direct conjunctival inoculation, fecal-oral introduction, or direct exposure to infected blood (2). The adenoviruses are associated with a wide variety of clinical symptoms determined largely by their specific immunologic type and subgroup (3). Importantly, adenovirus can become latent and then reactivate asymptomatically. This characteristic makes it difficult to attribute specific symptoms and pathology to the virus itself. In fact, 6.5% of adult renal transplant recipients may have asymptomatic adenovirus viremia during the first posttransplant year when immunosuppression is highest, but graft infection occurs in <0.5% of these patients (4, 5). Further, in a review of 37 cases of adenovirus renal allograft infection, near complete recovery of allograft function was reported in all despite severe changes on biopsy (6).
Adenovirus type 11 is the most common serotype causing hemorrhagic cystitis and renal graft infection, but other types have also been implicated (3, 7–10). The urinary tract predilection of this virus is noteworthy; our patient had no signs of pancreas rejection or viral infection, with euglycemia and normal pancreatic enzyme levels. Pancreatic grafts have also been spared in two other reported cases of adenovirus infection in SKP recipients (11, 12).
Adenovirus nephritis and cystitis presenting as hemorrhagic cystitis, dysuria, and renal dysfunction are well recognized in bone marrow transplant recipients, and there is increasing awareness of adenovirus infection causing similar symptoms in renal transplant patients (6). However, adenovirus infection has rarely been reported in SKP recipients (11, 12). Presentation beyond 1 year after SKP is extremely rare, as only one reported case of adenovirus infection 18 months after transplantation has been reported (12).
Adenovirus can be detected using viral culture, antigen detection, molecular diagnosis, and/or histopathology with electron microscopy (3). No individual diagnostic test identifies all adenovirus subtypes, and the sensitivity and specificity of these tests are variable. Of note, adenovirus can shed from the renal tubular epithelium, upper respiratory tract, or gastrointestinal tract. Thus, the detection of adenovirus at local sites does not necessarily confirm viremia. Therefore, nucleic acid amplification and PCR technologies have become increasingly important diagnostic tools. PCR permits both identification of the virus and quantification of viral replicative activity that can be used to monitor the therapeutic response (13, 14). Rising blood and urine adenovirus PCR levels correlate with hemorrhagic cystitis, although there is no clear threshold for diagnosis.
Histology remains the gold standard for the diagnosis of invasive adenovirus infection. Infected tissue typically shows cytopathic inclusions. Necrotizing interstitial nephritis with granuloma formation and prominent macrophage recruitment are also characteristic of renal adenovirus infection (15). The virus can be confirmed by histochemical staining, or electron microscopy can identify crystalline arrays of adenovirus particles in tubule cell nuclei and cytoplasm (15, 16). The histologic appearance and histochemical staining can provide a definitive diagnosis of adenovirus, even when cultures and PCR are negative (17).
At this time, there is no definitive treatment protocol for adenovirus infection in solid organ transplant recipients. Therefore, the management of adenovirus infection in these patients is largely based on the management and treatment of adenovirus infection in patients after hematopoietic stem cell transplantation. The 2011 European guidelines for diagnosis and treatment of adenovirus infection in leukemia and stem cell transplantation recommend a multipronged approach (18). The key management strategies are reduction of immunosuppression and supportive care (19, 20). As in our patient, adenovirus infection of the kidney and bladder can lead to ureteral obstruction, which was reversed with stent placement.
Treatment of patients with systemic disease using antiviral agents has gained recent favor (3, 21). Cidofovir has demonstrated good virologic response, although the specifics of drug dosing and regimen are variable in reported cases (22, 23). There have been no randomized trials of cidofovir in infected solid organ transplant patients. However, studies in hematopoietic stem cell transplant recipients suggest a benefit (18, 24, 25). An important side effect of cidofovir is nephrotoxicity, which also can manifest as acute tubular necrosis. Cidofovir therapy in our patient reduced the viral load but may have caused the subsequent increase in serum creatinine. Polyclonal immunoglobulin has been used as monotherapy after reducing immunosuppressive agents and is thought to improve passive antiviral activity (12, 15, 26).
Management for our patient consisted of reduction of immunosuppression followed by intravenous immunoglobulin and cidofovir weekly for 3 weeks. Because of prominent granulomatous interstitial nephritis on the biopsy, the patient was given a steroid taper as well. A successful steroid taper has been reported in a collection of case reports (6, 27).
References
- 1.Ison MG. Adenovirus infections in transplant recipients. Clin Infect Dis. 2006;43(3):331–339. doi: 10.1086/505498. [DOI] [PubMed] [Google Scholar]
- 2.Ortiz M, Troncoso P, Rabagliati R, Jara A. Hemorrhagic cystitis secondary to adenovirus infection in a kidney transplant recipient: case report. Transplant Proc. 2009;41(6):2685–2687. doi: 10.1016/j.transproceed.2009.06.074. [DOI] [PubMed] [Google Scholar]
- 3.Ison MG, Green M. AST Infectious Diseases Community of Practice. Adenovirus in solid organ transplant recipients. Am J Transplant. 2009;9(Suppl 4):S161–165. doi: 10.1111/j.1600-6143.2009.02907.x. [DOI] [PubMed] [Google Scholar]
- 4.Humar A, Kumar D, Mazzulli T, Razonable RR, Moussa G, Paya CV, Covington E, Alecock E, Pescovitz MD PD16000 Study Group. A surveillance study of adenovirus infection in adult solid organ transplant recipients. Am J Transplant. 2005;5(10):2555–2559. doi: 10.1111/j.1600-6143.2005.01033.x. [DOI] [PubMed] [Google Scholar]
- 5.Singh HK, Nickeleit V. Kidney disease caused by viral infections. Curr Diag Pathol. 2004;10(1):11–21. [Google Scholar]
- 6.Hofland CA, Eron LJ. Hemorrhagic adenovirus cystitis after renal transplantation. Transplant Proc. 2004;36(10):3025–3027. doi: 10.1016/j.transproceed.2004.10.090. [DOI] [PubMed] [Google Scholar]
- 7.Shields AF, Hackman RC, Fife KH, Corey L, Meyers JD. Adenovirus infections in patients undergoing bone-marrow transplantation. N Engl J Med. 1985;312(9):529–533. doi: 10.1056/NEJM198502283120901. [DOI] [PubMed] [Google Scholar]
- 8.Harnett GB, Bucens MR, Clay SJ, Saker BM. Acute hemorrhagic cystitis caused by adenovirus type II in a recipient of a transplanted kidney. Med J Aust. 1982;1(13):565–567. [PubMed] [Google Scholar]
- 9.Asim M, Chong-Lopez A, Nickeleit V. Adenovirus infection of a renal allograft. Am J Kidney Dis. 2003;41(3):696–701. doi: 10.1053/ajkd.2003.50133. [DOI] [PubMed] [Google Scholar]
- 10.Hierholzer JC. Adenoviruses in the immunosuppressed host. Clin Microbiol Rev. 1992;5(3):262–272. doi: 10.1128/cmr.5.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathur SC, Squiers EC, Tatum AH, Szmalc FS, Daucher JW, Welker DM, Shanley PF. Adenovirus infection of the renal allograft with sparing of pancreas graft function in the recipient of a combined kidney-pancreas transplant. Transplantation. 1998;65(1):138–141. doi: 10.1097/00007890-199801150-00027. [DOI] [PubMed] [Google Scholar]
- 12.Emovon OE, Lin A, Howell DN, Afzal F, Baillie M, Rogers J, Baliga PK, Chavin K, Nickeleit V, Rajagapalan PR, Self S. Refractory adenovirus infection after simultaneous kidney-pancreas transplantation: successful treatment with intravenous ribavirin and pooled human intravenous immunoglobulin. Nephrol Dial Transplant. 2003;18:2436–2438. doi: 10.1093/ndt/gfg365. [DOI] [PubMed] [Google Scholar]
- 13.Seidemann K, Heim A, Pfister ED, Köditz H, Beilken A, Sander A, Melter M, Sykora KW, Sasse M, Wessel A. Monitoring of adenovirus infection in pediatric transplant recipients by quantitative PCR: report of six cases and review of the literature. Am J Transplant. 2004;4(12):2102–2108. doi: 10.1111/j.1600-6143.2004.00631.x. [DOI] [PubMed] [Google Scholar]
- 14.Leruez-Ville M, Minard V, Lacaille F, Buzyn A, Abachin E, Blanche S, Freymuth F, Rouzioux C. Real-time blood plasma polymerase chain reaction for management of disseminated adenovirus infection. Clin Infect Dis. 2004;38(1):45–52. doi: 10.1086/380450. [DOI] [PubMed] [Google Scholar]
- 15.Storsley L, Gibson I. Adenovirus interstitial nephritis and rejection in an allograft. J Am Soc Nephrol. 2011;22(8):1423–1427. doi: 10.1681/ASN.2010090941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Randhawa P. Adenovirus nephritis: a pathologic perspective. Am J Kid Dis. 2012. Available at http://ajkdblog.org/2012/04/05/adenovirus-nephritis-a-pathologic-perspective/
- 17.Lim A, Parsons S, Ierino F. Adenovirus tubulointerstitial nephritis presenting as a renal allograft space occupying lesion. Am J Transplant. 2005;5(8):2062–2066. doi: 10.1111/j.1600-6143.2005.00945.x. [DOI] [PubMed] [Google Scholar]
- 18.Matthes-Martin S, Feuchtinger T, Shaw PJ, Engelhard D, Hirsch HH, Cordonnier C, Ljungman P. Fourth European Conference on Infections in Leukemia. European guidelines for diagnosis and treatment of adenovirus infection in leukemia and stem cell transplantation: summary of ECIL-4. Transplant Infect Dis. 2012;14(6):555–563. doi: 10.1111/tid.12022. [DOI] [PubMed] [Google Scholar]
- 19.Sujeet K, Vasudev B, Desai P. Acute kidney injury requiring dialysis secondary to adenovirus nephritis in renal transplant recipient. Transpl Infect Dis. 2011;13(2):174–177. doi: 10.1111/j.1399-3062.2010.00577.x. [DOI] [PubMed] [Google Scholar]
- 20.Mori K, Yoshihara T, Nishimura Y, Uchida M, Katsura K, Kawase Y, Hatano I, Ishida H, Chiyonobu T, Kasubuchi Y, Morimoto A, Teramura T, Imashuku S. Acute renal failure due to adenovirus-associated obstructive uropathy and necrotizing tubulointerstitial nephritis in a bone marrow transplant recipient. Bone Marrow Transplant. 2003;31(12):1173–1176. doi: 10.1038/sj.bmt.1704077. [DOI] [PubMed] [Google Scholar]
- 21.Lenaerts L, Naesens L. Antiviral therapy for adenovirus infections. Antiviral Res. 2006;71(2–3):172–180. doi: 10.1016/j.antiviral.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Refaat M, McNamara D, Teuteberg J, Kormos R, McCurry K, Shullo M, Toyoda Y, Bermudez C. Successful cidofovir treatment in an adult heart transplant recipient with severe adenovirus pneumonia. J Heart Lung Transplant. 2008;27(6):699–700. doi: 10.1016/j.healun.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Neofytos D, Ojha A, Mookerjee B, Wagner J, Filicko J, Ferber A, Dessain S, Grosso D, Brunner J, Fomenberg N, Flomenberg P. Treatment of adenovirus disease in stem cell transplant recipients with cidofovir. Biol Blood Marrow Transplant. 2007;13(1):74–81. doi: 10.1016/j.bbmt.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 24.Ljungman P, Ribaud P, Eyrich M, Matthes-Martin S, Einsele H, Bleakley M, Machaczka M, Bierings M, Bosi A, Gratecos N, Cordonnier C. Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Cidofovir for adenovirus infections after allogeneic hematopoietic stem cell transplantation: a survey by the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2003;31(6):481–486. doi: 10.1038/sj.bmt.1703798. [DOI] [PubMed] [Google Scholar]
- 25.Keddis M, Leung N, Herrmann S, El-Zoghby Z, Sethi S. Adenovirus-induced interstitial nephritis following umbilical cord blood transplant for chronic lymphocytic leukemia. Am J Kidney Dis. 2012;59(6):886–890. doi: 10.1053/j.ajkd.2011.10.056. [DOI] [PubMed] [Google Scholar]
- 26.Chakrabarti S, Mautner V, Osman H, Collingham KE, Fegan CD, Klapper PE, Moss PA, Milligan DW. Adenovirus infections following allogeneic stem cell transplantation: incidence and outcome in relation to graft manipulation, immunosuppression, and immune recovery. Blood. 2002;100(5):1619–1627. doi: 10.1182/blood-2002-02-0377. [DOI] [PubMed] [Google Scholar]
- 27.Joss N, Morris S, Young B, Geddes C. Granulomatous interstitial nephritis. Clin J Am Soc Nephrol. 2007;2(2):222–230. doi: 10.2215/CJN.01790506. [DOI] [PubMed] [Google Scholar]