Abstract
Objective
The aim of the present study was to examine the apoptosis-promoting effects and mechanisms of hematoporphyrin monomethyl ether (HMME)-sonodynamic therapy (SDT) on endometrial cancer cells in vitro.
Methods
Endometrial cancer cell samples were divided into four groups: 1) untreated control group, 2) HMME group, 3) pure ultrasound group, and 4) HMME combined with ultrasound, i.e. SDT group. CCK-8 method was utilized to assess the inhibiting effect of SDT on the proliferation of endometrial cancer cells. Optical microscope and field emission transmission electron microscopy were used to characterize the morphology changes of the cancer cells induced by the treatments. Apoptosis rate, reactive oxygen species (ROS) and mitochondrial membrane potential (MMP) were examined by flow cytometer. Fluorescence intensity measured by laser scanning confocal microscopy was used to explore the variation of intracellular calcium ion (Ca2+) concentration. Apoptosis-related proteins involved in both intrinsic and extrinsic apoptosis signallings were analyzed by western blot.
Results
SDT can effectively induce the apoptosis of endometrial cancer cells. Compared with ultrasound which is known as an effective anti-tumor method, SDT leads to a significant improvement on suppression of cell viability and induction of apoptosis, together with more remarkable modifications on the morphology and substructure in both ultrasound sensitive and resistant endometrial cancer cells. Further studies reveals that SDT promotes ROS production, induces loss of MMP and increases intracellular Ca2+ concentration more efficiently than HMME or ultrasound alone. SDT groups also show a rather high expression of apoptosis-promoting proteins, including Bax, Fas and Fas-L, and a significant low expression of apoptosis-suspending proteins including Bcl-2 and Survivin. Meanwhile, both cleaved caspse-3 and caspase-8 are dramatically enhanced in SDT groups. Multiple pathways has been proposed in the process, including the intrinsic activation by excessive ROS and overloaded Ca2+, silencing survivin gene, and the extrinsic pathway mediated by the death receptor.
Conclusion
Given its considerable effectivity in both ultrasound sensitive and resistant cells, SDT may therefore be a promising therapeutic method for treating endometrial cancers.
Introduction
Endometrial cancer is one of common gynecological malignancies, traditionally treated by surgery and supplemented by chemotherapy and radiotherapy, which unfortunately have a rather low sensitivity to early or metastatic stage of endometrial cancer, normally accompanying with significant side-effects [1–2]. So far, it is still an open issue how early endometrial cancer can be treated while preserving fertility. And there is no report in the literature on the organ retention for patients at an intermediate or advanced stage. Obviously, an efficient approach with low toxicity is paramount.
The inducement of apoptosis is preferred in cancer treatment [3]. It has been demonstrated that ultrasound can cause apoptosis of a big variety of cells, such as myeloid leukemia cells, lymphocytes and sarcoma cells [4–6]. However, the delayed killing effect [7] together with the inefficient efficacy on ultrasound-resistant tumor cells may largely limit its clinic applications. Recently, as a combination of ultrasound and sonosensitizers, sonodynamic therapy (SDT) has become a fascinating non-invasive anti-tumor method with high efficiency and high selectivity in the tumor cells and no obvious influence on adjacent normal tissues [8–10]. Among diverse chemical agents applied in SDT, hematoporphyrin monomethyl ether (HMME) is particular popular as a sonosensitizer due to its optimal optical properties and satisfactory preferential retention in tissue [11]. More importantly, HMME has a much higher uptake rate by tumor than normal tissues [10], and this high selectivity ensures the ultrasound energy to be focused specifically on the cancer cells.
The application of SDT on gynecologic malignancies is quickly emerging. Though a previous study in vitro did show that methylene blue-mediated SDT can significantly increase the reactive oxygen species in ovarian cancer cells and hence induce apoptosis [12], SDT effects on endometrial cancer remain unexplored up to now.
In this study, influence of HMME-SDT on both ultrasound sensitive and resistant endometrial cancer cells has been investigated in vitro. Apoptosis-promoting effects and mechanisms are discussed in the light of available information.
Materials and Methods
2.1. Cell Culture
Human endometrial adenocarcinoma cell lines Ishikawa and HEC-1-a were purchased from Shanghai Bogoo Biotechnology. Co., Ltd. The cells were cultured in a RPMI1640 (Hyclone, Thermo) solution containing 10% fetal calf serum (Hyclone, Thermo) and incubated in a saturated humidity incubator (HF240, Heal Force) at 37°C with 5% CO2. The experiments were carried out when the cells reached the logarithmic growth phase.
2.2. Cell Survival Rate
Cells in the logarithmic growth phase were placed in 0.25% trypsin for digestion and centrifugation and prepared into a single-cell suspension with a concentration of 1×105/ml. The cells were then seeded in 6-well plates. There were four sample groups as follows: 1) untreated control group (control), 2) HMME group (HMME), 3) pure ultrasound group (ultrasound), and 4) ultrasound combined HMME group (SDT). For SDT group, HMME was added one hour before the ultrasound interference, which was 1.0 W/cm2 at 1 MHz for 60 s for Ishikawa and 2.0 W/cm2 at 1 MHz treated 240 s for HEC-1-a. And the final concentration of HMME was 15 μg/ml for Ishikawa and 50 μg/ml for HEC-1-a. A wet sponge was placed between the bottom of culture plate and the ultrasound probe to prevent multiple reflections of ultrasound. After irradiation, the cells were digested and collected immediately, then transferred to 96-well plates after adjusting the concentration, with 100 μl in each well. Two hours later, 10 μl Cell Counting Kit-8 reagent (CCK-8, Dojindo) was added to each well. After one hour of further culture, absorbance at 490 nm was measured using an automatic enzyme mark instrument (Multiskan MK3, Thermo) [13].
2.3. Preparation for Characterization of the Cell Morphology and Substructure
The morphology and adherent growth of cells were observed 6h after the four different treatments by using an inverted microscope (Olympus CKX4). The cells were digested in 0.25% trypsin for 90s, washed by phosphate buffered saline, then centrifuged at 2000 r/min for 5min. Removing the supernatant, the cells were fixed between 2.5% glutaraldehyde and 1% osmic acid, dehydrated by ethanol and acetone, embedded in Epon812 epoxy resin, and cut by III ultra-thin slicer. The samples were then double-dyed with uranyl acetate and lead citrate, and then characterized using a field emission transmission electron microscopy (TEM, JEM-2010, JEOL).
2.4. Apoptosis Analysis
6h after the treatments, the cells of the four groups mentioned above were collected and counted, then preserved avoiding light for 15 min following an addition of 10 μl fluorescein isothiocyanate (FITC)-labeled AnnexinV (20μg/ml) for 1×106 cells. Afterwards, 5 μl propidium iodide (PI) (50 μg/ml) was added. After reaction for 5min, 400 μl binding buffer was mixed. The specimens were tested by flow cytometer (BD FACSCanto) immediately.
2.5. Reactive Oxygen Species (ROS) and Mitochondrial Membrane Potential (MMP)
After ultrasonic irradiation, the cells were incubated for 2h. 2’7’-dichlorofluorescein diacetate (DCFH-DA, Beyotime) and Rhodamine123 (Sigma) with a final concentration of 10 μmol/L and 200 nmol/L were added to each sample for the ROS and MMP test. In both cases, the cells were cultured at 37°C with 5% CO2 avoiding light for 30min. Fluorescence intensity was then measured by a flow cytometer at an excitation and emission wavelength of 480 nm and 520 nm for ROS test, and 490 nm and 530 nm for MMP test, respectively.
2.6. Determination of Intracellular Calcium (Ca) Ion Concentration
Endometrial cancer cells of control and SDT group were washed three times by phosphate buffer solution, loaded by Fluo-3/AM (Bio-Rad) with a final concentration of 10 μmol/L at 37°C for 45 min, and then transferred to a special small groove. Fluorescence intensity was tested by a laser scanning confocal microscope (Meridian, Insight-PlusIQ) at an argon laser excitation of 488 nm. The free Ca2+ concentration in cells was calibrated with the equation: [Ca 2+] = K d[(F − F min)/(F max − F)], where K d is 400 nMol, F is the measured relative fluorescence value, F max is the maximum fluorescence value after the addition of 10 μmol/L 4-Br-A23187 high Ca liquid (10 mmol/L K2CaEGTA, 10mmol/L K-mops and 100 mmol/L KCl, Bio-Rad Company), F min is the minimum fluorescence value after the addition of 10 mmol/L 4-Br-A23187 Ca-free solution [14]. This is a relative simple and low-cost method normally applied for intracellular Ca2+ concentration.
2.7. The Expression of Apoptotic-Related Proteins
Apoptosis-related proteins were analyzed 24 h after each treatment by western blot. Briefly, cells were harvested after different treatments. Proteins were separated by the SDS-PAGE gel and then transferred to PVDF membranes, which were firstly blocked with 5% skimmed milk and then incubated with specific antibodies as indicated in the figures at 4°C overnight. The horseradish peroxidase-conjugated secondaries were added afterwards. The expression of proteins was visualized by enhanced chemiluminescence (ECL) system (Thermo Scientific Pierce).
2.8. Statistical Analysis
All experiments were reproduced three times independently in the different cells. The SPSS Version 13.0 for Windows (SPSS Inc., Chicago, Illinois) was used for data analysis. Data are presented as mean ±square deviation (SD). The date was analyzed using unpaired T-test. P<0.05 was considered to be statistically significant.
Results
3.1. Effect of SDT on Survival Rate of the Endometrial Cancer Cells
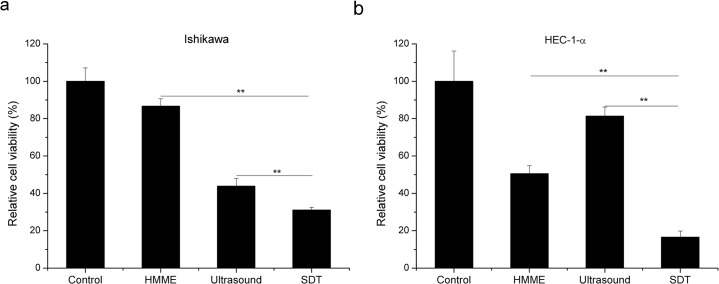
In order to evaluate the effect of SDT on cell viability, a CKK-8 assay was applied to Ishikawa and HEC-1-a cells upon different treatments as indicated in Fig 1. The survival rate is decreased with single treatment of either HMME or ultrasound in both Ishikawa and HEC-1-a cells (Fig 1). Interestingly, cell viability of Ishikawa cells is decreased to 33.99 ± 4.06% with 1 MHz ultrasound (1.0 W/cm2) for 60 s; whilst no obvious growth inhibition is observed in HEC-1-a cells under the same condition (Refer to S1A Fig). Furthermore, it is found that the cell viability of HEC-1-a cells is maintained no less than 81.39 ± 4.83% upon treatment even with stronger ultrasound (2.0 W/cm2) for an extended duration from 60 s to 240 s (S1B Fig). This directly indicates the sensitivity of Ishikawa and resistance of HEC-1-a upon ultrasound treatment. Importantly, as shown in Fig 1, although ultrasound inhibits cell viability of endometrial cancer cells to different extend, the synergy of ultrasound with HMME can consistently and substantially arouse a largely enhanced killing effectivity, i.e. 165% and 115% as that of ultrasound alone in HEC-1-a and Ishikawa cells, respectively.
Fig 1. Effect of SDT on the survival rate of endometrial cancer cells, assessed by the CCK-8 method.
CCK-8 assay was used to evaluate the cell viabilities in control, HMME, ultrasound and SDT groups in Ishikawa (a) and HEC-1-a (b) cells. HMME or ultrasound shows an obvious inhibitory effect on cell survival. SDT inhibits cell viability more effectively than either single treatment. Data are presented as the mean ± SD (n = 3), **P < 0.01.
3.2. Effect of SDT on the Morphology and Substructure of the Endometrial Cancer Cells
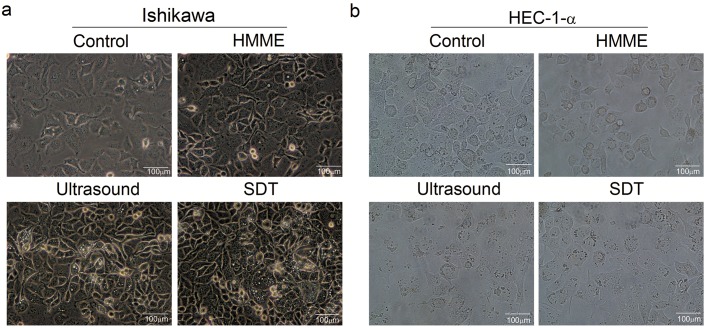
Seen from the images in Fig 2, for the control and HMME groups, most cells are grown adherently and uniformly into a ‘paving-stone’ structure, showing clear contour, good transparency and vitality; while cells of the ultrasound and SDT group are poorly transparent, not firmly attached or even suspended, and the morphology of part cells becomes circular.
Fig 2. Effect of SDT on the morphology of the endometrial cancer cells.
The morphology and adherent growth of Ishikawa (a) and HEC-1-a (b) cells were observed 6h after the four different treatments by using an inverted optical microscope (Olympus CKX4). Control groups show adherently grown cells with clear contour and good transparency; ultrasound and SDT group show poorly transparent cells, which are not firmly attached or even suspended. The magnification of all images is 40 times, scale bar = 100μm.
More details can be explored from the TEM images in Fig 3. It is found that the cells of the control and HMME group possess intact cell and nuclear membranes, clear organelle structures and complete mitochondrial structures without obvious modification. With respect to Ultrasound group, the cell nucleus chromatin is obviously coiled or congealed and marginalized; the mitochondria become swollen, deformed and vacuolar, and the lysosome is increased, which are all indications towards cell apoptosis. For SDT group, typical phenomena of apoptosis can already be observed from the cells, where the nuclei are under obvious pyknosis into a heterogeneous block structure, showing small apoptotic bodies, enlarged perinuclear gaps and nuclear pores, expanded Golgi apparatus and endoplasmic reticulum under degranulation, as well as blurry or vanished mitochondrial cristae. Some cells even show accumulated glycogen granule in their cytoplasm with a decreased cell volume.
Fig 3. Effect of SDT on the substructure of the endometrial cancer cells.
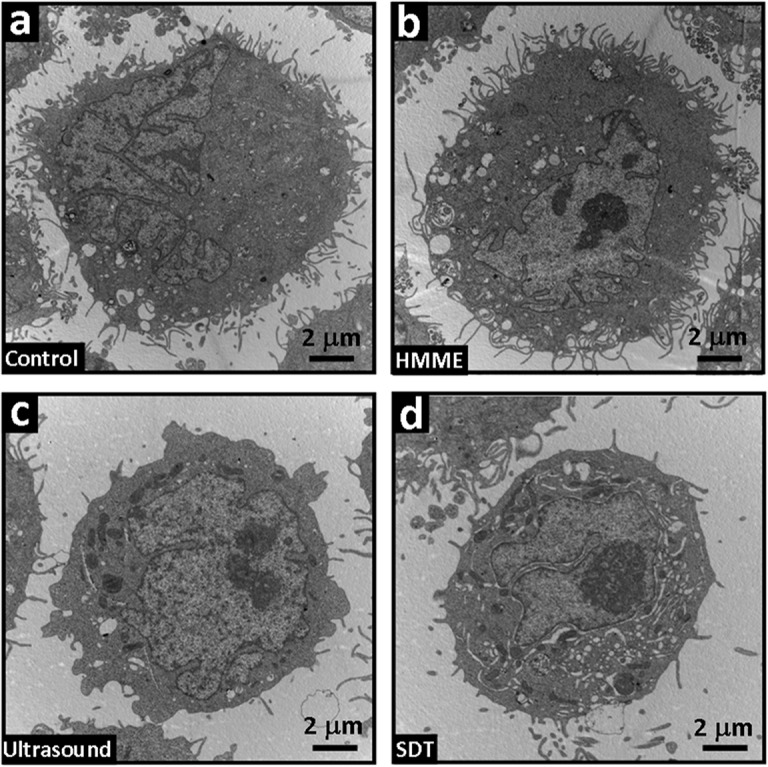
The substructure of the four group cells was characterized by transmission electron microscopy (TEM, JEM-2010, JEOL). (a) control and (b) HMME group, showing intact cell membrane and nuclear membrane, clear organelle structure and complete mitochondrial structure without obvious modification; (c) ultrasound group, showing tendency towards cell apoptosis, including coiled or congealed and marginalized cell nucleus chromatin, deformed and vacuolar mitochondria and increased lysosome; (d) SDT group, showing typical phenomena of apoptosis, where the nuclei are under obvious pyknosis into a heterogeneous block structure, with small apoptotic bodies, enlarged perinuclear gap and nuclear pore, expanded Golgi apparatus and endoplasmic reticulum under degranulation, as well as blurry or vanished mitochondrial cristae. The scale bar in all images is 2 μm.
3.3. Effect of SDT on ROS Generation and MMP Reduction
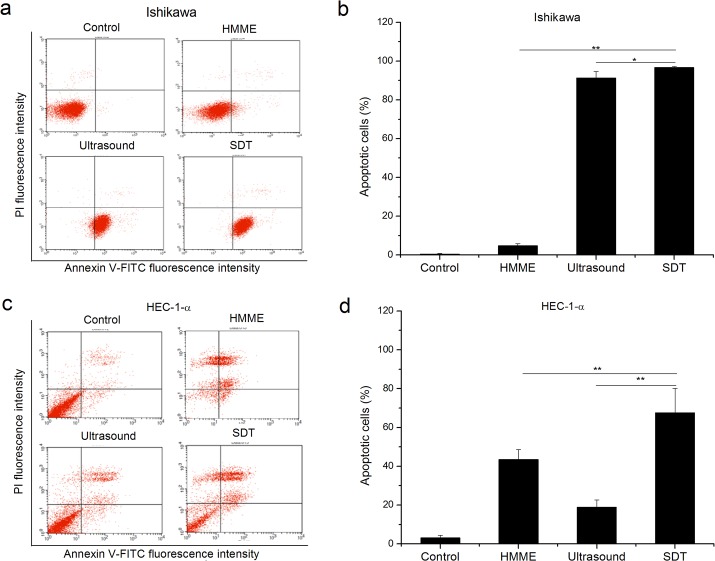
As shown in Fig 4, the apoptotic rate of SDT, ultrasound, HMME and the control group is 96.66±0.45%, 91.21±3.44%, 4.75±1.10% and 0.42±0.35%, respectively, in Ishikawa cells and 67.54±12.65%, 18.88±3.73%, 43.50±5.02% and 3.09±1.37%, respectively, in HEC-1-a cells. The induced apoptosis is inversely correlated with the cell viability determined by CCK-8 assay in the four different groups (S1 Fig and Fig 4), suggesting that apoptosis is one of major mechanisms that attribute to the low cell viability upon treatments. The apoptosis rates in SDT groups are significantly increased compared with either HMME or ultrasound treatment alone in both Ishikawa cells (p<0.05, Fig 4B) and HEC-1-a cells (p<0.01, Fig 4D). Nevertheless, SDT mainly promotes early apoptosis in Ishikawa cells (Fig 4A), while later apoptosis in HEC-1-a case (Fig 4C).
Fig 4. Analysis of apoptosis rate of endometrial cancer cells using flow cytometer.
The apoptosis levels are much higher in SDT groups than the control, HMME or ultrasound groups in Ishikawa (a and b) and HEC-1-a (c and d) cells. Representative FACS profiles were shown in a and c. Histograms present mean ± SD of three independent experiments (b and d, * P < 0.05; ** P < 0.01.)
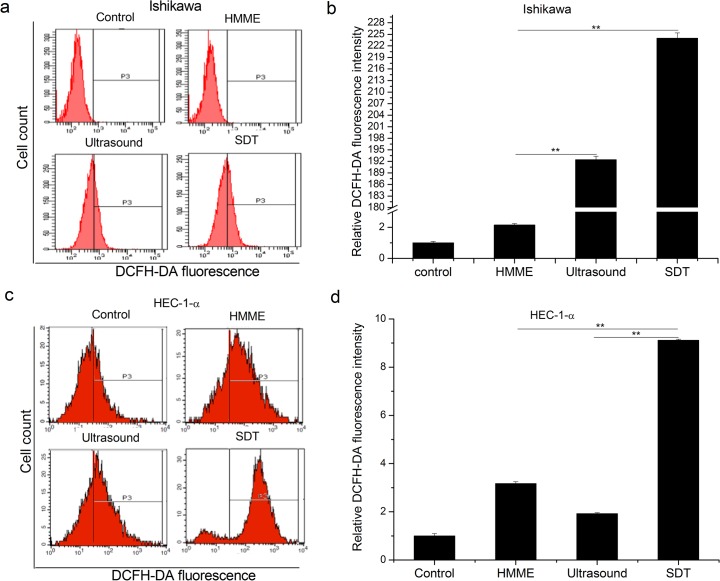
Intracellular ROS are important mediators of apoptosis [15]. To understand whether SDT induced apoptosis is due to ROS production, ROS levels was visualized by DCF-A, a probe that fluoresces when oxidized. It is found that among four different groups, SDT can most significantly increase the ROS levels in both Ishikawa and HEC-1-a cells (p<0.01, Fig 5). Compared with ultrasound, the ROS generation rate of SDT groups are increased up to 1.48-fold in Ishikawa cells (p<0.01) and 4.7-fold in HEC-1-a cells (p<0.01), respectively.
Fig 5. Effect of SDT on the ROS generation in endometrial cancer cells.
ROS levels are significantly enhanced in SDT groups when compared with the control, HMME, and ultrasound groups in Ishikawa (a and b) and HEC-1-a (c and d) cells. Representative FACS profiles were shown in a and c. Histograms present mean ± SD of three independent experiments (b and d, ** P < 0.01.)
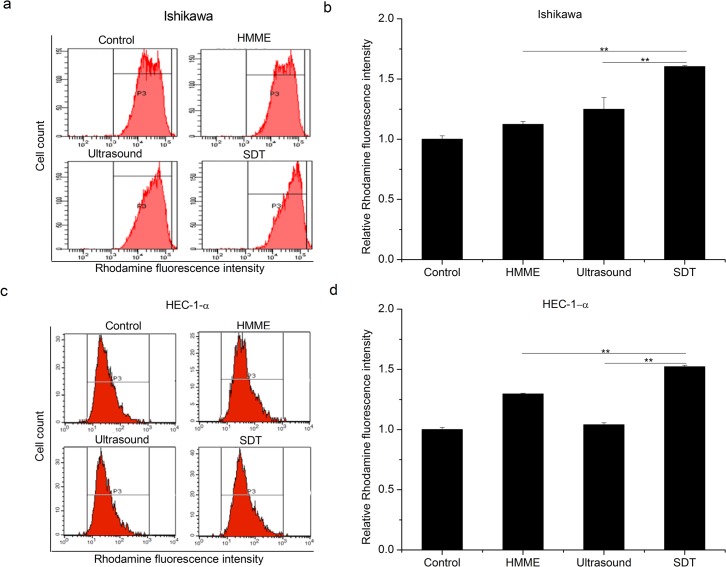
Enhanced ROS can induce changes of MMP, which further promotes apoptosis progression [16]. The mitochondrial membrane potential was thus measured from cells treated with Rhodamine. As shown in Fig 6, a slight decrease of MMP is observed in HMME and ultrasound groups for both Ishikawa and HEC-1-a cells. Importantly, loss of MMP becomes much more evident in SDT group compared with the mono-treatment groups in both cell lines involved. (p<0.01)
Fig 6. Effect of SDT on the MMP reduction in endometrial cancer cells.
Ishikawa (a and b) and HEC-1-a (c and d) were subjected to four different treatments: control, HMME, ultrasound and SDT. Loss of MMP was more evident in SDT group than the other groups. Representative FACS profiles were shown in a and c. Histograms present mean ± SD of three independent experiments (b and d, ** P < 0.01.)
3.4. Effect of SDT on Intracellular Ca Ion Concentration
As indicated in Fig 7 and Table 1, the Ca ion concentration in Ishikawa endometrial cells for the control, HMME, ultrasound and SDT group is of 92.533 nmol, 204.539 nmol, 2991.485 nmol and 3455.750 nmol respectively. Apparently, intracellular Ca2+ concentration by ultrasound treatment can be increased to 32.3 times in comparison to that of the control group, and SDT can induce a further increase of the concentration into about 37.3 times.
Fig 7. Effect of SDT on intracellular Ca ion concentration.

Fluorescence intensity of Ishikawa endometrial cancer cells 1 h after the treatments was tested by a laser scanning confocal microscope (Meridian, Insight-PlusIQ) at an argon laser excitation of 488 nm. (a) Control and (b) SDT group, showing the intracellular calcium ion concentration in SDT is much higher than that in Control group.
Table 1. The concentration of Ca2+ (nmol/l) in Ishikawa endometrial cancer cells.
| Group | N | Minimum value | Maximum value | |
|---|---|---|---|---|
| Control | 10 | 74.498 | 115.592 | 92.533 ±12.868 |
| HMME | 10 | 152.289 | 293.428 | 204.539±46.172 |
| Ultrasound | 10 | 1352.247 | 5131.403 | 2991.485±1116.140 |
| SDT* | 10 | 1466.053 | 7542.348 | 3455.750±2142.808 |
* p<0.05
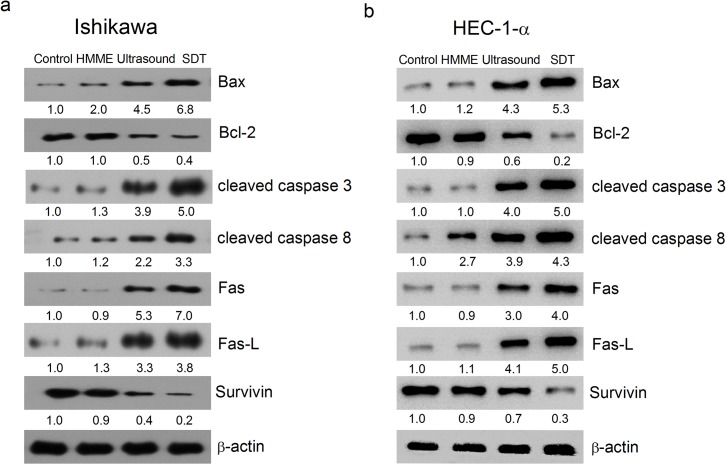
3.5. Effect of SDT on the Expression of Apoptotic-Related Proteins
In order to understand the molecular mechanisms of SDT-induced apoptosis, the activation of caspase-3 and caspase-8 was first detected by western blotting after different treatments. As shown in Fig 8, both cleaved caspase-3 and caspase-8 are significantly enhanced in SDT groups, suggesting both casapses are activated upon the treatment. In addition, the anti-apoptotic protein survivin and Bcl-2 are decreased and the pro-apoptotic Bax is increased with SDT. Furthermore, death receptor pathway markers, such as Fas and Fas-L, are also enhanced by SDT (Fig 8). These data together suggested that both intrinsic and extrinsic pathways have been involved in SDT-induced apoptosis.
Fig 8. Effect of SDT on the expression of apoptotic-related proteins by western blot.
Ishikawa (a) and HEC-1-a (b) were subjected to four different treatments: control, HMME, ultrasound and SDT. Apoptosis related markers were detected by Western blotting with specific antibodies as indicated. β-actin was used a loading control.
Discussion
Although ultrasound has been considered as an effective treatment for cancers, unsatisfactory killing effects have been suggested by HEC-1-a cells in this study and also by previous work [7]. Importantly, SDT not only acts efficiently on ultrasounds sensitive Ishikawa cells, and also the ultrasounds resistant HEC-1-a cells, indicating that SDT may have applications on wider range of cancer cells. The synergistic pro-apoptosis effect by the combination of HMME and ultrasound in endometrial cancers may be attributed to two factors, i.e. the high efficiency of HMME as a sonosensitizer and the killing effect of ultrasound.
SDT normally has two killing modes on tumor cells, i.e. necrosis and apoptosis [17]. The dominant apoptotic-like change of cell morphology and substructure shown in the inverted microscope and TEM images points out that apoptosis is the major death manner of endometrial cancer cells in the present case. Based on our experimental results, possible mechanisms of SDT on apoptosis of endometrial cancer cells are proposed as following:
(1) Loss of MMP by ROS generation and Bcl-2 family alternation may be one of SDT-induced apoptosis pathways.
It is revealed that ROS in cells treated by SDT is most excessive among all samples. Under normal conditions, the cells themselves can delete continuously small molecules generated during aerobic respiration, including singlet oxygen [18,19]. However, at an imbalanced state, intracellular ROS can be accumulated, damage mitochondrial membrane and reduce the membrane potential [20–22]. This is consistent with the experimental results, where the most significant ROS generation rate and MMP reduction rate were observed simultaneously in SDT groups. In addition, mitochondrial membrane potential is also subjected to Bcl-2 family members’ regulation. In this study, Bcl-2 family member Bax is increased and Bcl-2 is decreased in SDT groups. The increased Bax may thus form Bax/Bax homodimers or antagonizes Bcl-2’s anti-apoptosis effect through forming Bax/Bcl-2 heterodimers at outer membrane of mitochondria [23]. Such changes can then increase membrane permeability so that apoptosis-promoting factors, such as cytochrome c, will release from mitochondria to the cytosol, where they trigger caspase cascades and eventually lead to apoptosis of endometrial cancer cells. Therefore, we conclude that excessive ROS and changes of Bcl-2 family members in SDT may activate the intrinsic apoptosis pathway by affecting mitochondria membrane potentials.
(2) Overloaded Ca ions in cells may be another major intrinsic factor for the cell apoptosis in SDT.
The overload of Ca ions involves various apoptosis processes, in particular the upstream regulation ones. Ca ions are normally stored in endoplasmic reticulum. The endoplasmic reticulum stress due to excessive ROS [14] can increase Ca2+ storage capacity of cells as well as the release speed of Ca2+ into the cells. As demonstrated by the present confocal microscope results, Ca2+ overload in cells is rather severe for SDT group. Since the release of cytochrome c has a positive feedback on the Ca2+ concentration [24–26], the Ca2+ overload in SDT can be another stimulant for the large release of cytochrome c, which then activates Caspase-3 and induce cell apoptosis as mentioned above.
(3) SDT may effectively silence surviving gene.
Recently, it has been demonstrated that the survivin expression rate in endometrial carcinoma tissue is (100%) higher than that in longer endometrial hyperplasia (73%) [27], implying that survivin may be closely associated to malignant transformation of endometrium. More importantly, earlier work has pointed out that the survival protein survivin can strongly inhibit apoptosis stimulated by diverse factors [28]. Silencing survivin may therefore break the protection and favor the apoptosis of cancer cells. In this work, SDT has been demonstrated to be an effective way to suppress this gene, leading to an extraordinarily weakened expression of survivin and more effective activation of caspase-8 and caspase-3 compared with the three other groups.
(4) Extrinsic pathway may assist the intrinsic factors for apoptosis in SDT.
The presence of Fas/Fas-l is a sign of the death receptor-mediated extrinsic apoptosis pathway where caspase-8 acts as an important initiation caspase and caspase-3 is the executive caspase. Our results show that the death receptor proteins Fas/Fas-l significantly increased in SDT groups and both caspase-8 and caspase-3 are activated with the same treatment, which directly indicates the activation of extrinsic apoptotic pathways in response to SDT.
In summary, SDT can significantly inhibit the proliferation of endometrial cancer cells, much more effective than HMME or ultrasound alone in both ultrasound sensitive and resistant cells. The apoptosis induced by HMME-SDT involves multiple pathways, including intrinsic apoptotic pathway activated by ROS generation, MMP reduction and Ca2+ overload, effective silence of survivin gene, together with Fas/Fas-l-mediated extrinsic apoptosis pathway. Considering the remarkable effectivity in both ultrasound sensitive and resistant cells, HMME-SDT may therefore have opened up a new rehabilitation road for patients who cannot survive from surgery, radiotherapy or chemotherapy, and who require preserving organs and even reproductive functions.
Supporting Information
(a) Ishikawa and HEC-1-a cells were treated with ultrasound (1 MHz) at the intensity of 1.0 W/cm2 for 60 s and then subjected to a CKK-8 assay. Ishikawa is more sensitive to ultrasound treatment than HEC-1-a. Data are presented as the mean ± SD (n = 3), **P < 0.01. (b) Ultrasound resistant HEC-1-a cells were treated with ultrasound at an increased intensity of 2.0 W/cm2 for 0 s, 60 s, 120 s, and 240 s, respectively. A slight and time-dependent cell viability inhibition is observed with the treatments. Data are presented as the mean ± SD (n = 3).
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was financially supported by the National Natural Science Foundation of China (Grant No. 30872650); Program for New Century Excellent Talents in University of China (Grant No. NCET-09-0131); MY acknowledges financial support from the Recruitment Program of Global Young Experts, China.
References
- 1. Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366(9484):491–505. 10.1016/S0140-6736(05)67063-8 . [DOI] [PubMed] [Google Scholar]
- 2. Dellinger TH, Monk BJ. Systemic therapy for recurrent endometrial cancer: a review of North American trials. Expert Rev Anticanc. 2009;9(7):905–16. 10.1586/Era.09.54 . [DOI] [PubMed] [Google Scholar]
- 3. Sun SY, Hail N, Lotan R. Apoptosis as a novel target for cancer chemoprevention. J Natl Cancer I. 2004;96(9):662–72. 10.1093/jnci/djh123 . [DOI] [PubMed] [Google Scholar]
- 4. Ashush H, Rozenszajn LA, Blass M, Barda-Saad M, Azimov D, Radnay J, et al. Apoptosis induction of human myeloid leukemic cells by ultrasound exposure. Cancer Res. 2000;60(4):1014–20. . [PubMed] [Google Scholar]
- 5. Ando H, Feril LB, Kondo T, Tabuchi Y, Ogawa R, Zhao QL, et al. An echo-contrast agent, Levovist, lowers the ultrasound intensity required to induce apoptosis of human leukemia cells. Cancer Lett. 2006;242(1):37–45. 10.1016/j.canlet.2005.10.032 . [DOI] [PubMed] [Google Scholar]
- 6. Tang W, Liu QH, Wang XB, Wang P, Zhang J, Cao B. Potential mechanism in sonodynamic therapy and focused ultrasound induced apoptosis in sarcoma 180 cells in vitro. Ultrasonics. 2009;49(8):786–93. 10.1016/j.ultras.2009.06.002 . [DOI] [PubMed] [Google Scholar]
- 7. Rosenthal I, Sostaric JZ, Riesz P. Sonodynamic therapy—a review of the synergistic effects of drugs and ultrasound. Ultrason Sonochem. 2004;11(6):349–63. 10.1016/j.ultsonch.2004.03.004 . [DOI] [PubMed] [Google Scholar]
- 8. Yumita N, Nishigaki R, Umemura K, Umemura S. Hematoporphyrin as a Sensitizer of Cell-Damaging Effect of Ultrasound. Jpn J Cancer Res. 1989;80(3):219–22. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Furusawa Y, Fujiwara Y, Campbell P, Zhao QL, Ogawa R, Hassan MA, et al. DNA Double-Strand Breaks Induced by Cavitational Mechanical Effects of Ultrasound in Cancer Cell Lines. Plos One. 2012;7(1). ARTN e29012 10.1371/journal.pone.0029012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen HJ, Zhou XB, Gao Y, Zheng BY, Tang FX, Huang JD. Recent progress in development of new sonosensitizers for sonodynamic cancer therapy. Drug Discov Today. 2014;19(4):502–9. 10.1016/j.drudis.2014.01.010 . [DOI] [PubMed] [Google Scholar]
- 11. Lei TC, Glazner GF, Duffy M, Scherrer L, Pendyala S, Li BH, et al. Optical properties of hematoporphyrin monomethyl ether (HMME), a PDT photosensitizer. Photodiagn Photodyn. 2012;9(3):232–42. 10.1016/j.pdpdt.2012.01.003 . [DOI] [PubMed] [Google Scholar]
- 12. Ding XM, Xu QZ, Liu FG, Zhou PK, Gu Y, Zeng J, et al. Hematoporphyrin monomethyl ether photodynamic damage on HeLa cells by means of reactive oxygen species production and cytosolic free calcium concentration elevation. Cancer Lett. 2004;216(1):43–54. doi: 10.1016/j.canlet.2004.07. 005 . [DOI] [PubMed] [Google Scholar]
- 13. Wang HY, Kwok DTK, Wang W, Wu ZW, Tong LP, Zhang YM, et al. Osteoblast behavior on polytetrafluoroethylene modified by long pulse, high frequency oxygen plasma immersion ion implantation. Biomaterials. 2010;31(3):413–9. 10.1016/j.biomaterials.2009.09.066 . [DOI] [PubMed] [Google Scholar]
- 14. Hammadi M, Oulidi A, Gackiere F, Katsogiannou M, Slomianny C, Roudbaraki M, et al. Modulation of ER stress and apoptosis by endoplasmic reticulum calcium leak via translocon during unfolded protein response: involvement of GRP78. Faseb J. 2013;27(4):1600–9. 10.1096/fj.12-218875 . [DOI] [PubMed] [Google Scholar]
- 15. Masgras I, Carrera S, de Verdier PJ, Brennan P, Majid A, Makhtar W, et al. Reactive Oxygen Species and Mitochondrial Sensitivity to Oxidative Stress Determine Induction of Cancer Cell Death by p21. J Biol Chem. 2012;287(13):9845–54. 10.1074/jbc.M111.250357 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang L, Zhou LW, Du J, Li MX, Qian CY, Cheng Y, et al. Induction of Apoptosis in Human Multiple Myeloma Cell Lines by Ebselen via Enhancing the Endogenous Reactive Oxygen Species Production. Biomed Res Int. 2014. Artn 696107 10.1155/2014/696107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wood AKW, Sehgal CM. A Review of Low-Intensity Ultrasound for Cancer Therapy. Ultrasound Med Biol. 2015;41(4):905–28. 10.1016/j.ultrasmedbio.2014.11.019 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li YC, Lin HJ, Yang JH, Yang JS, Ho HC, Chang SJ, et al. Baicalein-Induced Apoptosis via Endoplasmic Reticulum Stress Through Elevations of Reactive Oxygen Species and Mitochondria Dependent Pathway in Mouse-Rat Hybrid Retina Ganglion Cells (N18). Neurochem Res. 2009;34(3):418–29. 10.1007/s11064-008-9799-1 . [DOI] [PubMed] [Google Scholar]
- 19. Tang W, Liu QH, Zhang J, Cao B, Zhao P, Qin XF. In vitro activation of mitochondria-caspase signaling pathway in sonodynamic therapy-induced apoptosis in sarcoma 180 cells. Ultrasonics. 2010;50(6):567–76. 10.1016/j.ultras.2009.12.001 . [DOI] [PubMed] [Google Scholar]
- 20. Zhou YJ, Zhang SP, Liu CW, Cai YQ. The protection of selenium on ROS mediated-apoptosis by mitochondria dysfunction in cadmium-induced LLC-PK(1) cells. Toxicology in vitro: an international journal published in association with BIBRA. 2009;23(2):288–94. 10.1016/j.tiv. 2008.12.009. . [DOI] [PubMed] [Google Scholar]
- 21. Vyssokikh MY, Zorova L, Zorov D, Heimlich G, Jurgensmeier JM, Brdiczka D. Bax releases cytochrome c preferentially from a complex between porin and adenine nucleotide translocator. Hexokinase activity suppresses this effect. Mol Biol Rep. 2002;29(1–2):93–6. 10.1023/A:1020383108620 . [DOI] [PubMed] [Google Scholar]
- 22. Wang B, Van Veldhoven PP, Brees C, Rubio N, Nordgren M, Apanasets O, et al. Mitochondria are targets for peroxisome-derived oxidative stress in cultured mammalian cells. Free Radical Bio Med. 2013;65:882–94. 10.1016/j.freeradbiomed.2013.08.173 . [DOI] [PubMed] [Google Scholar]
- 23. Breckenridge DG, Xue D. Regulation of mitochondrial membrane permeabilization by BCL-2 family proteins and caspases. Current opinion in cell biology. 2004;16(6):647–52. 10.1016/j.ceb.2004.09.009 . [DOI] [PubMed] [Google Scholar]
- 24. Zhivotovsky B, Orrenius S. Calcium and cell death mechanisms: A perspective from the cell death community. Cell Calcium. 2011;50(3):211–21. 10.1016/j.ceca.2011.03.003 . [DOI] [PubMed] [Google Scholar]
- 25. Kumar B, Kumar A, Ghosh S, Pandey BN, Mishra KP, Hazra B. Diospyrin derivative, an anticancer quinonoid, regulates apoptosis at endoplasmic reticulum as well as mitochondria by modulating cytosolic calcium in human breast carcinoma cells. Biochem Bioph Res Co. 2012;417(2):903–9. 10.1016/j.bbrc.2011.12.072 . [DOI] [PubMed] [Google Scholar]
- 26. Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–6. 10.1038/35037710 . [DOI] [PubMed] [Google Scholar]
- 27. Tarkowski R, Jurczak-Jamroz W, Kotarski J. Expression of survivin and cell apoptosis in endometrial cancer and hyperplastic endometrium. International Journal of Gynecological Cancer. 2003;13:72. [Google Scholar]
- 28. Guan HT, Xue XH, Dai ZJ, Wang XJ, Li A, Qin ZY. Down-regulation of survivin expression by small interfering RNA induces pancreatic cancer cell apoptosis and enhances its radiosensitivity. World J Gastroentero. 2006;12(18):2901–7. . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) Ishikawa and HEC-1-a cells were treated with ultrasound (1 MHz) at the intensity of 1.0 W/cm2 for 60 s and then subjected to a CKK-8 assay. Ishikawa is more sensitive to ultrasound treatment than HEC-1-a. Data are presented as the mean ± SD (n = 3), **P < 0.01. (b) Ultrasound resistant HEC-1-a cells were treated with ultrasound at an increased intensity of 2.0 W/cm2 for 0 s, 60 s, 120 s, and 240 s, respectively. A slight and time-dependent cell viability inhibition is observed with the treatments. Data are presented as the mean ± SD (n = 3).
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.