Mop regulates endosomal localization and recycling of Frizzled. Hrs is ubiquitinated and degraded in the absence of Mop. Mop aids in the maintenance of Ubpy to control the ubiquitin homeostasis of Hrs. Mop and Ubpy can rescue each other. Mop’s function is not required in the cell in the absence of the ubiquitin ligase Cbl.
Abstract
Endosomal trafficking of signaling proteins plays an essential role in cellular homeostasis. The seven-pass transmembrane protein Frizzled (Fz) is a critical component of Wnt signaling. Although Wnt signaling is proposed to be regulated by endosomal trafficking of Fz, the molecular events that enable this regulation are not completely understood. Here we show that the endosomal protein Myopic (Mop) regulates Fz trafficking in the Drosophila wing disk by inhibiting the ubiquitination and degradation of Hrs. Deletion of Mop or Hrs results in endosomal accumulation of Fz and therefore reduced Wnt signaling. The in situ proximity ligation assay revealed a strong association between Mop and Hrs in the Drosophila wing disk. Overexpression of Hrs rescues the trafficking defect caused by mop knockdown. Mop aids in the maintenance of Ubpy, which deubiquitinates (and thus stabilizes) Hrs. In the absence of the ubiquitin ligase Cbl, Mop is dispensable. These findings support a previously unknown role for Mop in endosomal trafficking of Fz in Wnt-receiving cells.
INTRODUCTION
Coordinated protein trafficking, or sorting, is one of the key regulatory mechanisms in the homeostasis of a living cell. Proteins can be transported to the inner space of an organelle (such as endosomes, lysosomes, mitochondria, and intracellular membranes) or secreted into the extracellular space. Protein sorting is tightly controlled inside a cell, and dysregulations are associated with cell death and development disorders. Receptor-mediated endocytosis, which involves selective internalization of integral membrane proteins from the plasma membrane, is one of the most thoroughly understood mechanisms of membrane protein trafficking (Sorkin and Von Zastrow, 2002).
Several signaling receptors undergo endocytic processing, following ligand-directed activation and internalization, which is regulated in diverse ways (Sorkin and Von Zastrow, 2002). Previous models proposed that the primary role of endocytosis is to attenuate signaling by removing receptors from the plasma membrane (Fiore and Gill, 1999; Platta and Stenmark, 2011). However, further studies fueled alternate theories about the role of receptor trafficking in cell signaling. These studies proposed that endocytosis might aid in the recycling of receptors back to the plasma membrane to restore cellular responsiveness to the ligand (Sorkin and Von Zastrow, 2002). Trafficking can also prolong the duration of the receptor-ligand complexes inside the endosomes, thereby enhancing the length of the signaling (Miaczynska et al., 2004; Polo and Di Fiore, 2006).
The net effect of receptor trafficking on signaling pathways thus depends on the final cellular destination of endocytosed receptors. For example, sorting to lysosomes can result in degradation of the receptors. In many cases, lysosomal degradation is facilitated by the direct ubiquitination of receptors, which is one of the major forms of posttranslational modification that modulate protein trafficking, stability, and quality control (Traub and Lukacs, 2007; Clague et al., 2012). Distinct ubiquitin ligase enzyme complexes (E1, E2, and E3) act together to ubiquitinate substrates. Deubiquitinases (DUBs) counter that process by removing the ubiquitin moiety from substrates (Grabbe et al., 2011). Thus the ubiquitination–deubiquitination cycle is a rate-limiting step that regulates the trafficking of internalized receptors (Wilkinson, 2000; Bonifacino and Traub, 2003). The endosome-associated protein hepatocyte growth factor–regulated tyrosine kinase substrate (Hrs) is involved in sorting of ubiquitinated receptor proteins from early endosomes to late endosomes or multivesicular bodies (MVBs) through a series of complexes referred to as endosomal sorting complexes required for transport (ESCRTs; Sorkin and Von Zastrow, 2002; Miaczynska et al., 2004; Polo and Di Fiore, 2006). Several signaling receptors, including the epidermal growth factor, Smoothened, and interleukin 2 receptors, undergo Hrs-dependent sorting to lysosomes for the attenuation of signaling (Komada and Kitamura, 2001; Lloyd et al., 2002; Jékely and Rørth, 2003; Pullan et al., 2006; Fan et al., 2013). Hrs is also regulated by the ubiquitination–deubiquitination machinery (Kato et al., 2000; Marchese et al., 2003; Zhang et al., 2014). Targeting and degradation of Hrs after ubiquitination have been shown to result in impaired trafficking of internalized signaling receptors (Kobayashi et al., 2005; Zhang et al., 2014); however, the regulatory mechanism underlying Hrs ubiquitination–deubiquitination is not well understood.
Along with Dpp, Hedgehog, epidermal growth factor receptor (EGFR), and Notch, the canonical Wnt/Fz pathway is also regulated by the endosomal sorting machinery (Seto and Bellen, 2006; Gagliardi et al., 2008). Different studies have investigated and debated the requirement of endosomal trafficking in the Wnt pathway (Dubois et al., 2001; Piddini et al., 2005; Marois et al., 2006; Rives et al., 2006; Seto and Bellen, 2006; Franch-Marro et al., 2008; Purvanov et al., 2010). In Wnt signal–receiving cells, activation of the Fz and LRP (Arrow in Drosophila) receptors by Wnt induces receptor–ligand internalization (Blitzer and Nusse, 2006; Seto and Bellen, 2006). Endocytosed Fz has different trafficking routes. From the endosomes, it can either recycle back to the cell surface or traffic to the lysosome for its degradation (Chen et al., 2003; Piddini et al., 2005; Blitzer and Nusse, 2006; Rives et al., 2006; Seto and Bellen, 2006; Yamamoto et al., 2006; Purvanov et al., 2010). Thus blocking Fz recycling or promoting its ubiquitination-mediated degradation results in reduced signaling (Lecourtois et al., 2001; Mukai et al., 2012). Although trafficking of Fz is a regulatory step in Wnt signal transduction, the molecular mechanism by which Fz endocytosis and membrane trafficking is regulated is not entirely known.
We and others (Miura et al., 2008; Pradhan-Sundd and Verheyen, 2014) previously showed that the endosomal protein Myopic (Mop; Miura et al., 2008; Huang et al., 2010; Gilbert et al., 2011; Chen et al., 2012) plays an important role in the trafficking of Wnt pathway components in signal-producing cells. In the present work, we show that Mop also regulates the endosomal trafficking and membrane recycling of Fz in Wnt-receiving cells. Colocalization analyses reveal that in the absence of Mop, Fz is reduced at the cell surface and accumulates in early and late endosomes. Such an accumulation of Fz and its reduced plasma membrane localization are also observed in cells deficient in Hrs, and overexpression of Hrs can rescue the trafficking defect caused by the absence of mop. We find that the dramatic reduction of Hrs levels in mop mutants is due to its increased degradation, as blocking lysosomal degradation rescues the level of Hrs protein. Furthermore, mop loss is phenocopied by the absence of the deubiquitinase ubiquitin-specific processing protease Y (Ubpy), which regulates Hrs degradation. Genetic interaction and colocalization studies suggest that Mop helps in the maintenance of Ubpy, which deubiquitinates (and thus stabilizes) Hrs. Finally, in the absence of the ubiquitin ligase Cbl, Mop is no longer required to promote Hrs activity or Wnt/Fz trafficking. Taken together, these results highlight the importance of Mop in endosomal trafficking of Fz by regulating Hrs stability through inhibition of Cbl and recruitment of Ubpy.
RESULTS
Inactivation of Mop leads to Fz accumulation in endosomes
To determine whether Mop played a role in Wnt signaling at the level of receptor localization, we examined Drosophila Fz2 (Dfz2; hereafter Fz) levels after knockdown of mop (Figure 1, A–A′′′). Fz is ubiquitously expressed in wing disks (Figure 1A′). Knocking down mop in flp-out RNA interference (RNAi) clones (mopRNAi, Vienna Drosophila RNAi Center [VDRC] ID 104860) led to a punctate accumulation of Fz (Figure 1A′′′), which appeared proximal to the apical surface of the disks (Figure 1B) and not the basal surface (Figure 1B′). In addition, when we looked at Mop protein levels in mopRNAi (VDRC ID 104860) tissue (Supplemental Figure S1C) by Western blot, we observed a significant reduction, confirming efficient knockdown. To rule out off-target effects of the RNAi line, we examined a second set of RNAi against mop (VDRC ID 14174), which revealed a weaker accumulation of Fz after knockdown (Supplemental Figure S1, A–A′′). Because we observed strong accumulation of Fz along with a reduced level of Mop protein upon knockdown using VDRC line 104860, we used this line for further analysis.
FIGURE 1:
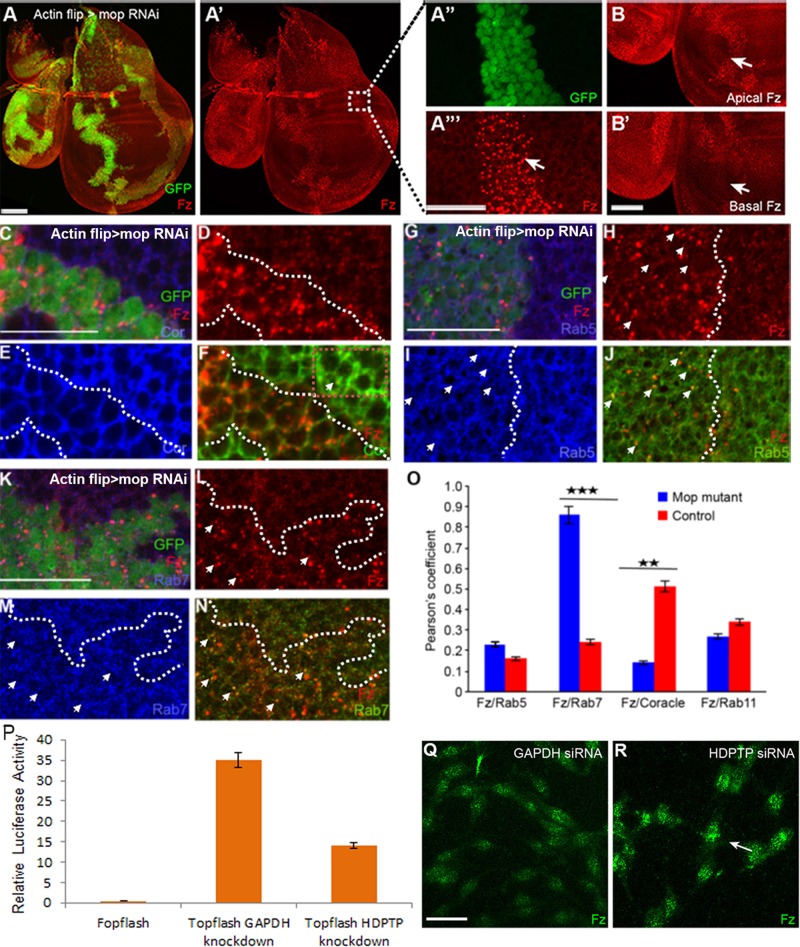
Absence of Mop causes trafficking defects of Fz. (A–A′′′) Knockdown of mop using the flp-out clones causes accumulation of Fz. Clones are marked by the presence of GFP. The boxed region in A′ is zoomed in in A′′ and A′′′. Arrow indicates the punctate localization of Fz. (B, B′) Absence of Mop causes accumulation of Fz protein in the apical surface (B, arrow) as compared with the basal surface (B′, arrow). Colocalization of Fz and Coracle (C–F, O), Fz and Rab5 (G–J, O), and Fz and Rab7 (K–O) in control and mop-knockdown (marked by GFP) tissue. Arrow indicates colocalization of the proteins in each case. Dotted box in F indicates colocalization of Fz and Cor outside the clone. (O) Quantification of colocalization (by measuring Pearson’s coefficient) observed in the conditions shown in F, J, and N. Bar graph shows the increased colocalization of Fz with early and late endosomes in the absence of Mop; p < 0.03 for Fz and Rab5, p < 0.0071 for Fz and Rab7, p < 0.0023 for Fz and Coracle, and p < 0.01 for Fz and Rab11. (P) Knockdown of HDPTP in mouse L cells causes reduced Wnt signaling as measured by Topflash reporter activity. (Q, R) Immunohistochemistry of L cells transfected with GAPDH (Q) and HDPTP siRNA (R). Knockdown of HDPTP causes accumulation of Fz in cells (arrow indicates punctate Fz localization). mop was knocked down using VDRC line 104860. Scale bars, 20 μm.
The intracellular localization of Fz after knockdown of mop was determined by costaining for Fz and different organelle markers. This was followed by statistical analysis to calculate the Pearson coefficient, which indicates the degree of colocalization (see Materials and Methods for details). We observed reduced accumulation of Fz with the cell surface marker Coracle (Ward et al., 2001) compared with the enriched colocalization seen in wild-type cells (Figure 1, C–F and O). In mop-knockdown disks, Fz strongly colocalized with early (Rab5) (Figure 1, G–J and O) and late endosomal (Rab7) markers (Figure 1, K–N and O). We hypothesized that reduced levels of Mop could interfere with Fz recycling, leading to its increased accumulation in early and late endosomes and reduced levels at the cell surface. Fz and the recycling endosomal marker (Rab11) showed significantly less colocalization than in wild type (Figure 1O), suggesting a recycling defect of Fz in the absence of Mop. Because Arrow, the Fz coreceptor, was normally expressed in mop-knockdown disks (Supplemental Figure S1, B–B′′), the endosomal trafficking of Arrow does not appear to be affected by changes in Mop.
To address whether a role for Mop in Fz recycling and signaling was evolutionarily conserved, we knocked down the closest vertebrate homologue of Mop, His-domain phosphotyrosine phosphatase (HDPTP), in mouse L cells stimulated with Wnt-conditioned media. After HDPTP small interfering RNA (siRNA) treatment, we observed a reduction in expression of the Wnt3A-dependent Topflash reporter compared with a control (glyceraldehyde-3-phosphate dehydrogenase [GAPDH]) knockdown (Figure 1P). In contrast, Fopflash, a mutant form of the reporter, did not show any Wnt activity, confirming the specificity of this assay (Figure 1P). Furthermore, immunohistochemical analyses revealed that, as observed in Drosophila wing disks, vertebrate Fz accumulated in puncta-like structures in L cells after knockdown of HDPTP (Figure 1R). When total Fz protein levels were examined by Western blot, an increase was detected compared with control (Supplemental Figure S1D). These results suggest that, like Mop, HDPTP is required in vertebrate Wnt signal–receiving cells.
To confirm the defect in endosomal localization of Fz in the absence of Mop, we examined the localization of Myc-tagged Fz by immuno–electron microscopy. In control disks, Myc-Fz particles localized to the cell surface (Supplemental Figure S1E) and endosomal structures (Supplemental Figure S1F). Conversely, in mopRNAi disks, Myc-Fz particles accumulated in endolysosomal structures, as identified by their electron-dense membranous structures (Supplemental Figure S1, G and H). When we quantified the accumulation of gold particles in control and mopRNAi disks, we found a twofold-higher endolysosomal accumulation of Fz in mopRNAi disks (Supplemental Figure S1I).
Finally, to verify independently the role of Mop in endosomal trafficking of Fz, we used the loss-of-function allele mopT612. We generated mopT612-homozygous mutant clones in wing disks and stained for endogenous Wg and Fz protein (Supplemental Figure S1, J and K). Mutant cells accumulated both Wg and Fz protein, as with the RNAi lines (Supplemental Figure S1, J and K; Pradhan-Sundd and Verheyen, 2014), and they appeared more punctate inside the clone (Supplemental Figure S1, J′ and K′, arrow). Therefore we conclude that Mop is required for trafficking of Fz and Wg.
Reduction of Hrs causes an accumulation of Fz in endosomes
We and others showed that in the absence of Mop, Hrs levels are reduced (Miura et al., 2008; Pradhan-Sundd and Verheyen, 2014). Because Hrs controls the endosomal sorting of several proteins, we hypothesized that trafficking defects of Fz after knockdown of mop may be due to the absence of Hrs. Contradictory reports on the role of Hrs in Wnt signaling suggest that the function of Hrs is unresolved (Rives et al., 2006; Seto and Bellen, 2006).
To identify the function of Hrs in Wnt-receiving cells in wing disk, we knocked down hrs (hrsRNAi) and assessed Fz levels. The hrs knockdown caused an accumulation of Fz in puncta-like structures (Figure 2B). We ruled out that the phenotype is due to an off-target effect of RNAi by using three different RNAi lines against hrs, which each showed a similar accumulation of Fz in puncta (Supplemental Figure S2, A–A′′). When we looked at Hrs levels in hrs RNAi tissue (Supplemental Figure S2, B–B′′), we observed a significant reduction, confirming efficient knockdown. Rives et al. (2006) showed aberrant Fz accumulation with hrs loss of function. Similarly, while we were preparing the manuscript, a report appeared showing punctate accumulation of Fz in hrs-mutant tissue (Gagliardi et al., 2014). However, mechanistic insight into this phenotype was lacking. To analyze further the trafficking defects of Fz in the absence of Hrs, we examined the intracellular localization of Fz after knockdown of hrs.
FIGURE 2:
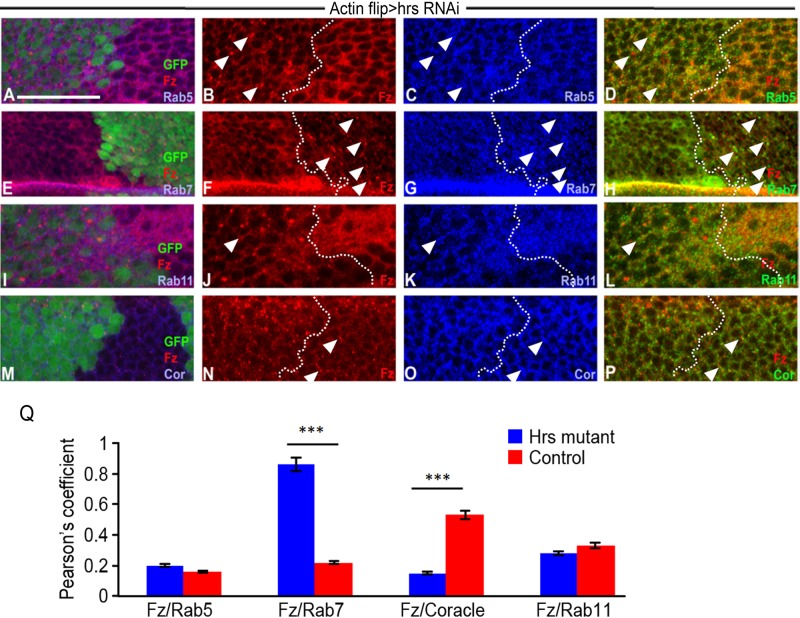
Loss of Hrs causes accumulation of Fz in endosomes. (A, B) Knockdown of hrs using flp-out clones causes accumulation of Fz in puncta (arrowheads). Clones are marked by the presence of GFP. Colocalization of Fz and Rab5 (A–D), Fz and Rab7 (E–H), Fz and Rab11 (I–L), and Fz and Cor (M–P) in control and hrsRNAi-knockdown tissue. Arrowheads indicate colocalization of proteins in each case. (Q) Quantification of colocalization (by measuring Pearson’s coefficient) observed in the conditions shown in D, H, L, and P. Bar graph shows the increased colocalization of Fz with early and late endosomes in the absence of Hrs. p < 0.02 for Fz and Rab5, p < 0.00017 for colocalization of Fz with Rab7, p < 0.001 for Fz and Coracle, and p < 0.028 for Fz and Rab11. Scale bars, 20 μm.
Colocalization analyses with markers of cell organelles, followed by quantification to determine colocalization frequencies, showed increased accumulation of Fz in Rab5-positive (Figure 2, A–D and Q) and Rab7-positive (Figure 2, E–H and Q) endosomes in the absence of Hrs. The localization of Fz in the recycling endosomes (Figure 2, I–L and Q) and at the cell surface (Figure 2, M–P and Q) was significantly reduced after knockdown of hrs, similar to what we observed in mop knockdown. Taken together, these data demonstrate that hrs knockdown phenocopies mop knockdown, including punctate localization of Fz in endosomes and reduced recycling to the cell surface.
Because loss of Mop and Hrs causes punctate accumulation of Fz in the endosomes, we next tested the effect of overexpressing Mop or Hrs on Fz levels in the wing disk. Overexpression of Mop (Supplemental Figure S3, A–C and G) or Hrs (Supplemental Figure S3, D–G) led to increased accumulation of Fz at the cell surface. This suggests that Mop or Hrs might promote the recycling of Fz from the endosomes to the membrane through the recycling endosomes. Recycling endosomes are involved in recycling of receptors, as well as the exosomal release of proteins outside the cell. Of interest, the vertebrate homologues of Hrs and Mop are classified as exosome markers (Deatheragea and Cooksona, 2012; Colombo et al., 2013).
Finally, to determine whether the trafficking defect seen in Mop can be rescued by Hrs, we overexpressed Hrs in the absence of Mop. Because knockdown of mop or hrs causes a robust endosomal trafficking defect of Wg (Miura et al., 2008; Pradhan-Sundd and Verheyen, 2014; see later discussion of Figure 5A), we considered the accumulation of Wg as a positive control for our rescue experiments. Simultaneous overexpression of Hrs in mop-knockdown tissue is able to partially rescue the trafficking defects of Wg (Supplemental Figure S2, D–D′′) caused by the absence of Mop. Fz protein showed similar rescue (Supplemental Figure S2, E–E′′) despite the Fz antibody not being of immunohistochemistry grade (Mukai et al., 2012). This suggests that trafficking defects of Wnt pathway proteins upon mop knockdown are mostly due to the absence of Hrs, and thus overexpression of Hrs can rescue the punctate accumulation of these proteins.
FIGURE 5:
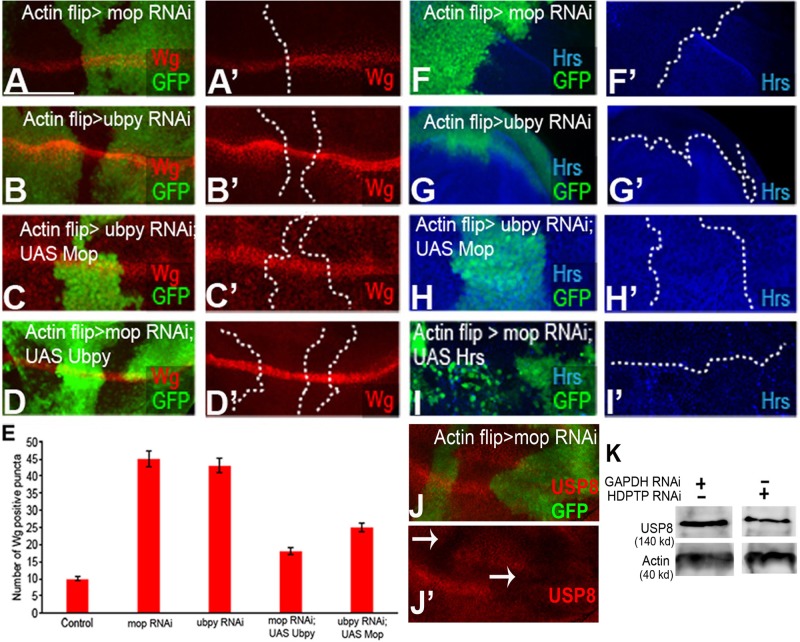
Mop and Ubpy cooperate to protect Hrs from degradation. (A, A′) Knockdown of mop in flipout clones (marked with GFP) causes accumulation of Wg in puncta-like structures. mop was knocked down using VDRC line 104860. (B, B′) Knockdown of ubpy in flipout clones (marked with GFP) produces a similar phenotype to mop knockdown. (C, C′) Overexpression of Mop in ubpy-knockdown clones and (D, D′) Ubpy in mop-knockdown clones can rescue the trafficking defects of Wg. mop was knocked down using VDRC line 104860. (E) Quantification of Wg puncta across the dorsal ventral boundary. (F, F′) Knockdown of mop and ubpy (G, G′) causes almost complete reduction of Hrs. (H, H′) The reduction of Hrs upon ubpy knockdown can be rescued by overexpressing Mop. (I, I′) Overexpression of Hrs in mop knockdown causes accumulation of Hrs in puncta. (J, J′) Knockdown of mop causes reduced expression of Ubpy/USP8. (K) Knockdown of HDPTP causes reduction in vertebrate USP8 protein level in mouse L cells. Scale bars, 20 μm.
Absence of Mop facilitates ubiquitination of Hrs and Fz
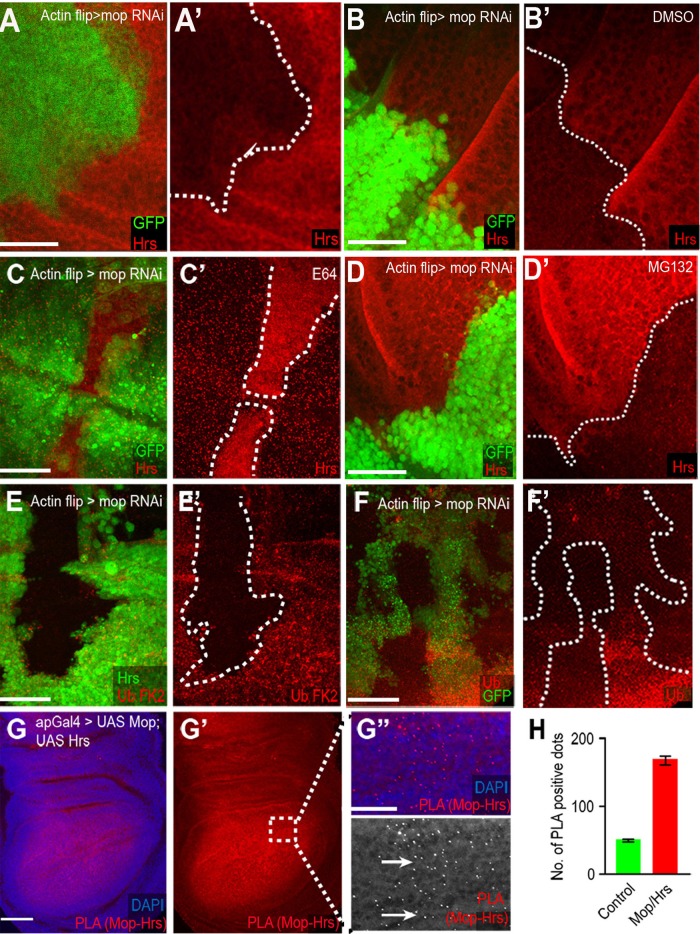
To confirm further that Fz trafficking defects in mopRNAi tissue are due to reduced Hrs levels, we further characterized Mop’s role in regulating Hrs levels. The reduction of Hrs upon mop knockdown (Figure 3, A and A′; Miura et al., 2008; Pradhan-Sundd and Verheyen, 2014) was also seen in other Drosophila disks (Supplemental Figure S2, C–C′′), highlighting Mop’s widespread role in Hrs regulation. To dissect the mode of Hrs degradation, we treated cultured mopRNAi disks with different chemical inhibitors. Blocking lysosomal degradation using E64 (Grinde, 1982) stabilized Hrs in the mop-knockdown disk (Figure 3, C and C′) compared with control (dimethyl sulfoxide [DMSO]) treatment, which caused no change in the reduction of Hrs (Figure 3, B and B′). In contrast, blocking proteasomal degradation by MG132 (Lee, 1998) treatment did not cause any change in Hrs levels in the disk (Figure 3, D and D′). These data prompted us to look at total ubiquitination levels in mop-knockdown disks. Staining mop-knockdown disks with antibodies against monoubiquitinated and polyubiquitinated conjugates (FK2; Figure 3, E and E′), as well as ubiquitins (Figure 3, F and F′), showed a consistent increase. Based on these results, we suggest that in the absence of Mop, Hrs ubiquitination and degradation are accelerated.
FIGURE 3:
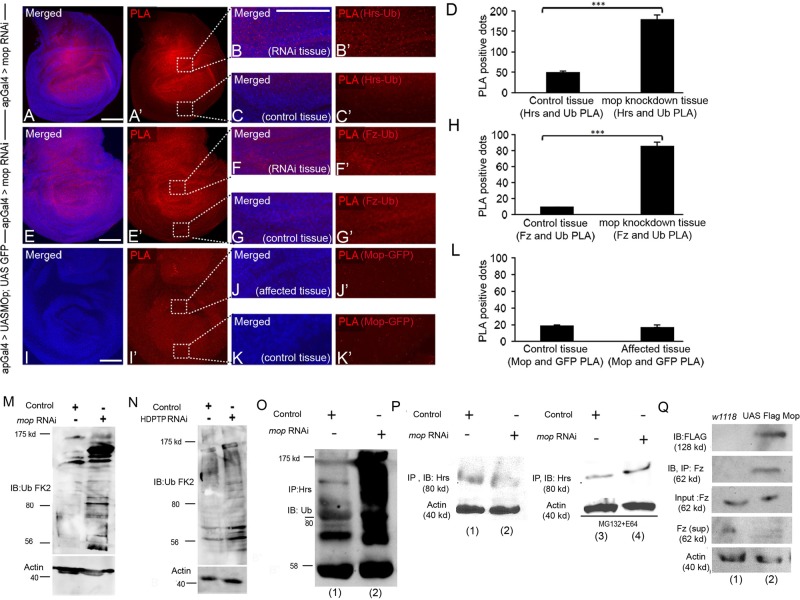
Mop protects Hrs from lysosomal degradation. (A, A′) Hrs is reduced in mopRNAi clones (marked with GFP). (B, B′) mopRNAi disks treated with DMSO. (C, C′) Disruption of lysosomal degradation by E64 treatment prevents Hrs degradation, whereas inhibiting proteasomal degradation by MG132 has no effect (D, D′). (E, E′) Knockdown of mop results in increased ubiquitinated proteins and monoubiquitins and polyubiquitins (F, F′). (G–G′′) PLA between Mop and Hrs shows a strong association between the two (G′′, arrows). (H) Quantification of PLA red dots in control and Mop-Hrs–overexpressing tissue shows an ∼3.5-fold increase in Mop–Hrs association in the affected tissue. mop was knocked down using VDRC line 104860. Scale bars, 20 μm.
To determine whether Mop and Hrs exist in a complex, we used a modified in situ proximity ligation assay (PLA; Söderberg et al., 2006; Jarvius et al., 2007) in the Drosophila wing disk. This assay can detect whether proteins are in close proximity to one another in vivo. After expressing UAS-Mop and UAS-Hrs using apterous Gal4 (ap-Gal4), we observed a strong PLA signal in the disk (seen as red dots), which could be quantified (Figure 3, G, G′, and H). The PLA signal was 3.5 times higher in the ap-Gal4 expression domain in which both proteins were coexpressed (Figure 3, G, G′, and H) compared with the wild-type disk tissue, indicating that Mop and Hrs are in close proximity to each other and thus likely form a complex. When we assessed the association between Mop and cytoplasmic green fluorescent protein (GFP) in a control PLA (Figure 4, I, I′, J, and K), there was no difference in the control versus experimental tissue.
FIGURE 4:
Hrs and Fz show increased association with ubiquitin in the absence of Mop. In situ PLA in Drosophila wing disks expressing (A, A′) mopRNAi in dorsal half of wing pouch using ap-Gal4 to visualize association between Hrs and Ub. Boxed regions in A′ were zoomed in to show affected (B, B′) and control (C, C′) tissue. PLA red dots were more abundant in B and B′ than in C and C′. (D) Quantification of the dots suggests increased complex formation between Hrs and Ub upon mop knockdown (p < 0.003). (E, E′) In situ PLA of Fz and Ub in disks expressing mopRNAi. Boxed regions in E′ were zoomed in to show affected (F, F′) and control (G, G′) tissue. PLA red dots were more abundant in F and F′ than in G and G′. (H) Quantification of the dots indicates increased association between Fz and Ub upon mop knockdown (p < 0.001). (I, I′) In situ PLA of Mop and GFP. Boxed regions in I′ were zoomed in to show affected (J, J′) and control (K, K′) tissue. PLA red dots were not changed in J and J′ compared to K and K′. (L) Quantification of the dots shows no change in red dots in each case (p < 0.19). Scale bars, 20 μm. (M) Knockdown of mop in Drosophila wing disks causes increased levels of ubiquitinated proteins. mop was knocked down using VDRC line 104860. (N) Knockdown of HDPTP causes an increase in ubiquitinated protein levels in mouse L cells. (O) Protein lysates from Drosophila wing disks were assayed for levels of ubiquitin in control and mop-knockdown tissue using MS1096Gal4. Immunoprecipitation assays were performed with anti-Hrs antibodies, and extracts were visualized by Western blotting by using anti-Ub FK2 antibodies. (P) Immunoblotting of Hrs after Hrs immunoprecipitation in control and mop-knockdown tissue under normal conditions (lanes 1 and 2) and after MG132 and E64 treatment (lanes 3 and 4). (Q) UAS Flag Mop was overexpressed in the Drosophila wing disk using MS1096Gal4. CoIP of Mop is seen after immunoprecipitation of Fz (lane 2) compared with control tissue (lane 1).
On the basis of our findings that Mop protects Hrs from lysosomal degradation (because in the absence of mop, Hrs is rapidly degraded) and that Mop and Hrs likely form a complex in the disk, we tested whether reduction of Mop caused increased ubiquitination of Hrs. Zhang et al. (2014) recently showed that Hrs is controlled through ubiquitin-mediated degradation and that Ubpy counteracts this by deubiquitinating Hrs. mop was knocked down using ap-Gal4 in the dorsal half of the wing disk, and the association between Hrs and Ub was quantified by measuring the PLA spots in the affected and wild-type tissue. Knockdown of mop showed a three-times-higher PLA signal (Figure 4, A–C), indicating a significantly higher level of association between Hrs and Ub and, likely, Hrs ubiquitination in mop-knockdown tissue compared with wild-type tissue (Figure 4D). The low-level PLA signal observed in control tissue is probably the result of the baseline ubiquitination of Hrs. These results show that the absence of Mop results in enhanced proximity of ubiquitin and Hrs, likely through direct modification, as previously observed (Kato et al., 2000; Bishop et al., 2002; Marchese et al., 2003; Zhang et al., 2014).
Along with its well-studied role in regulating protein stability, ubiquitination can also influence the sorting of proteins inside the cell (Hershko and Ciechanover, 1998). Given that we see an endosomal sorting defect of Fz in the absence of Mop or Hrs, we wanted to further analyze that mechanistically. To address the cause of trafficking defects and the punctate localization of Fz after knockdown of mop, we determined whether Fz ubiquitination is also altered in the absence of Mop. Fz is ubiquitinated at multiple sites both in vivo and in vitro (Mukai et al., 2012), which serves to regulate surface expression of Fz in Wnt-receiving cells. Ubiquitination results in reduced localization of Fz at the cell surface, which reduces the magnitude of Wnt signaling (Mukai et al., 2012). Although the mechanism of ubiquitin-mediated degradation of Fz is not completely understood, recent reports demonstrated the role of cell surface transmembrane E3 ubiquitin ligase zinc and ring finger 3 (ZNRF3) and its homologue ring finger 43 (RNF43) in the ubiquitination and degradation of Fz (Hao et al., 2012; Koo et al., 2012).
We observed that loss of mop also caused a similar reduction in cell surface level of Fz (Figure 1, C–F and O). An in situ PLA, followed by quantification, revealed that the association of Fz and Ub was four times greater in mop-knockdown tissue than in wild-type tissue (Figure 4, E–H). No changes in PLA signal were observed when we measured the interaction between Mop and GFP (negative control) in wild-type or Mop-GFP–expressing tissue (Figure 4, H–K). These results strongly suggest that ubiquitination of Fz is also increased in the absence of Mop and furthermore that ubiquitinated Fz localizes to the endosomes, affecting its recycling to the surface. We observed that the PLA signal for Hrs and Ub in mop-knockdown disks was much stronger than that of Fz and Ub. These results suggest that Mop normally promotes the stability of Hrs by preventing its ubiquitination, which results in subsequent events responsible for deubiquitination of Fz.
Furthermore, to analyze the functional conservation of Mop, we compared total ubiquitin levels by Western blot in mop-knockdown disks and mouse L cells in the absence of HDPTP. Knockdown of Mop (Figure 4M) and its vertebrate homologue HDPTP (Figure 4N) led to increased levels of ubiquitinated proteins. Previous studies described the role of HDPTP in regulating Hrs levels (Stefani et al., 2011), and we also found that HDPTP knockdown caused reduced Hrs levels (unpublished data).
To confirm that Hrs reduction after mop knockdown is due to its increased ubiquitination and degradation, we performed coimmunoprecipitation (CoIP) experiments using Drosophila imaginal disk lysates. In the absence of Mop, Hrs was abundantly ubiquitinated as detected by an anti-ubiquitin antibody (Figure 4O, lanes 1 and 2). Furthermore, because reducing Mop function through RNAi results in low Hrs levels (Figure 4P, lanes 1 and 2), in the latter assay the disks were treated with a mixture of E64 and MG132 to block lysosomal and proteasomal degradation, which resulted in more, presumably ubiquitinated, Hrs in mop-knockdown tissue (Figure 4P, lanes 3 and 4). Together our data showed that Mop/HDPTP regulates Hrs levels and its overall ubiquitination in vivo and in vertebrate cells. Absence of Mop causes increased ubiquitination of Hrs, followed by its degradation in the lysosomes.
Finally, because our PLA suggested an increase in ubiquitination of Fz upon mop knockdown, we examined whether Fz and Mop can form a complex in Drosophila wing imaginal disks by IP studies. Comparable amount of lysates of wild-type and FLAG Mop wing disks were used in this study. Of interest, in the wild-type tissue, a clear band of Fz was not seen upon immunoprecipitation (Figure 4Q, lane 1), possibly due to Fz being a membranous protein not present in the lysate fraction or due to low-affinity interaction with the antibody. In contrast, when Flag-tagged Mop was overexpressed in Drosophila wing imaginal disks, a robust association between Fz and Mop was observed upon IP of Fz (Figure 4Q, lane 2), suggesting not only a possible interaction between these two proteins, but also a potential modification to Fz protein (possibly localization or conformation) in the presence of Mop.
Mop recruits Ubpy to promote the deubiquitination of Hrs
On the basis of finding that loss of Mop causes increased association between Hrs and Ub, we speculated that Mop’s role is to maintain ubiquitin homeostasis of Hrs either directly by deubiquitinating Hrs or indirectly by promoting the removal of ubiquitin by recruiting a DUB domain–containing deubiquitinase. Because Mop does not contain a DUB domain (Miura et al., 2008), we tested the latter hypothesis.
Previous studies showed that Hrs is deubiquitinated by Ubpy (Kato et al., 2000; Zhang et al., 2014). Ubpy (or USP8) is a USP-family deubiquitinase (Naviglio et al., 1998), which participates in sorting of ubiquitinated receptors, including receptor tyrosine kinases (Row et al., 2005; Mizuno et al., 2006; Ali et al., 2013). It also regulates the Wnt pathway by suppressing Fz ubiquitination and turnover (Mukai et al., 2012). Loss of ubpy causes a reduction of Fz at the cell surface, resulting in reduced Wnt targets in the wing disk (Mukai et al., 2012). Based on these previous findings, it is tempting to speculate that Mop may provide an as-yet- unknown link between Hrs, Ubpy, and Wnt receptors. To determine whether Mop recruits Ubpy to deubiquitinate Hrs (and thus affect Fz trafficking), we first assessed the phenotype caused by the absence of Ubpy in the wing disk. Knockdown of ubpy caused trafficking defects of Wg (Figure 5, B and B′), mimicking the mop-knockdown phenotype (Figure 5, A and A′; Pradhan-Sundd and Verheyen, 2014). Wg levels were increased in the DV boundary and appeared largely punctate in signal-receiving cells (Figure 5, B and B′). Similarly, as previously reported, Fz levels were reduced at the cell surface and appeared punctate (unpublished data; Mukai et al., 2012; Zhang et al., 2014).
To determine whether Ubpy is affected by the altered expression of Mop or vice versa, we analyzed the genetic interaction between the two genes. Because Wg and Fz both accumulate in puncta-like structures in the Wnt signal–receiving cells upon mop and ubpy knockdown, we selected the Wg antibody for further analyses, since the stain is of higher quality than what can be seen with Fz antibody. As a first step, we overexpressed UAS-Mop in a ubpy-knockdown background and found that it partially rescued the punctate accumulation of Wg in the signal- receiving cells (Figure 5, C, C′, and E). Conversely, when we overexpressed UAS-Ubpy in a mop-knockdown background, we observed a partial rescue of Wg puncta (Figure 5, D, D′, and E). We ruled out that this rescue could be due to titration of Gal4 by the presence of multiple UAS sequences by including a UAS-LacZ in the mop or ubpy knockdown alone (Supplemental Figure S3, H–M). Overexpression of Mop or Ubpy can partially rescue knockdown of the other, and this suggests that Mop and Ubpy might regulate the trafficking of Wnt pathway components using parallel pathways. It is also possible that Mop and Ubpy acts redundantly in the cells to influence the trafficking of Wnt pathway proteins such that the presence of one can compensate for the absence of the other.
To elucidate whether Hrs reduction can also be rescued by overexpressing Mop in ubpyRNAi tissue, we measured Hrs levels. Both mop (Figure 5, F and F′) and ubpy (Figure 5, G and G′) knockdown caused a similar reduction of Hrs. Overexpressing Mop in ubpy knockdown rescued the Hrs level (Figure 5, H and H′) to the level observed in wild-type tissue. However, overexpressing Hrs in mop knockdown led to punctate localization of Hrs (Figure 5, I and I′), resembling the overexpression phenotype of Hrs alone (unpublished data). Together the results suggest that as overexpression of Mop can rescue the reduced Hrs levels seen in ubpy knockdown, Mop might act downstream of Ubpy in regulating Hrs stability. It is also possible that Mop and Ubpy act in parallel or redundant ways to regulate Hrs stability by promoting its deubiquitination.
Next we analyzed the expression pattern and localization of Ubpy in the absence of Mop in the Drosophila wing disk. Although Ubpy is involved in numerous cellular processes, its localization in Drosophila tissues was not well known. Immunofluorescence staining revealed that endogenous Ubpy (USP8) is mostly expressed at the cell surface and also in the endosomes (Supplemental Figure S4, H–I). Of interest, knockdown of mop caused reduced expression of Ubpy (Figure 5, J–J′). We could rescue this reduction by simultaneously overexpressing Flag-Ubpy (Supplemental Figure S3, N–P) in mop-mutant tissue. Moreover, when we quantified the colocalization of Ubpy with markers for different organelles in the wing disk (Rab5 [Supplemental Figure S4, A–A′′], Rab7 [Supplemental Figure S4, B–B′′], Rab11 [Supplemental Figure S4, C–C′′], Coracle [Supplemental Figure S4, D–D′′], and Hrs [Supplemental Figure S4, E–E′′ and F]) in control cells, a considerable amount of Ubpy colocalized with Coracle, Rab11, Rab7, and Hrs. In contrast, when mop was knocked down, Ubpy localization both at the cell membrane and endosomes was significantly reduced (Figure 5J and Supplemental Figure S4G). Furthermore, we found that in vertebrate L cells, total USP8 levels upon HDPTP knockdown were significantly reduced (Figure 5K), suggesting the importance of Mop and its vertebrate homologue in maintaining Ubpy levels.
Previous studies demonstrated a punctate localization of Mop close to early endosomes (Miura et al., 2008). When we overexpressed Flag-tagged Mop in the wing disk, it localized in similar puncta-like structures close to the membrane (Supplemental Figure S5B). Localization of Mop appeared to be predominantly endosomal compared with Ubpy (Supplemental Figure S5B), which is highly membranous (Figure 5J and Supplemental Figure S4A′). Flag-Mop colocalized mostly with Rab7 and Rab5, whereas it was least abundant in the recycling endosomes (Supplemental Figure S5, A–D). Consistent with the model in which Mop recruits Ubpy and not vice versa, Flag-Mop localization was unchanged after ubpy knockdown (Supplemental Figure S5, E and F). Taken together, these results suggest that Mop and Ubpy interact to deubiquitinate Hrs and that Mop is important in maintaining Ubpy levels in the cell.
Mop is not essential for the stability of Hrs in the absence of Cbl
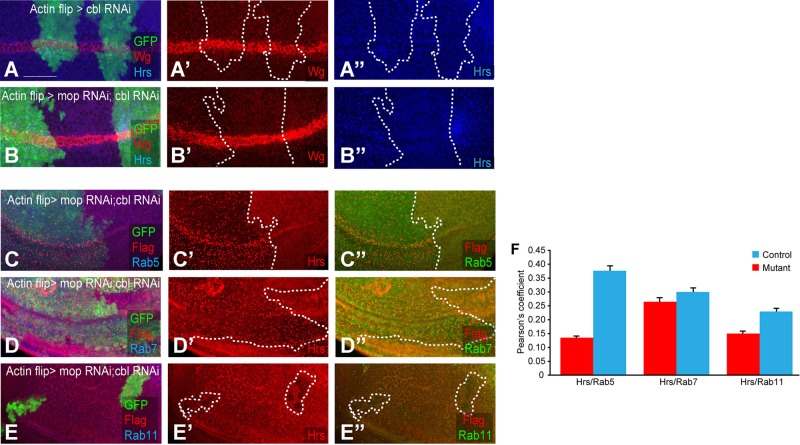
The finding that Mop is involved in maintaining Ubpy levels prompted us to determine whether Mop also functions to regulate Hrs by inhibiting a ubiquitin ligase. We screened a number of ubiquitin ligases that are associated with Hrs ubiquitination (Marchese et al., 2003; Stern et al., 2007; unpublished data). After knockdown of individual ubiquitin ligases, we found that Wg and Fz trafficking is weakly affected by modulation of Cbl (Figure 6, A and A′). Cbl reduction caused a mild accumulation of Wg in puncta. Remarkably, when we knocked down mop and cbl together, the Wg/Fz trafficking defects caused by mop knockdown were rescued (Figure 6B′). Moreover, the Hrs loss in mop knockdown was also modified by knocking down cbl (Figure 6B′′). Hrs looked punctate in cblRNAi; mopRNAi, a phenotype that resembled the Hrs localization in mop clones with E64 treatment (Figure 3, C and C′). Knockdown of cbl alone caused a mild accumulation of Hrs in puncta (Figure 6A′′), and this accumulation was strongly enhanced after simultaneous knockdown of cbl and mop (Figure 6B′′).
FIGURE 6:
In the absence of Cbl, Mop function is dispensable. (A–A′′) Knockdown of cbl in flipout clones (marked with GFP) causes mild accumulation of Wg and Hrs in the wing disk. (B–B′′) cbl; mop double knockdown in clones causes a sharp increase in Hrs puncta (B′′) without affecting Wg trafficking (B′). Colocalization of Hrs and Rab5 (C–C′′), Hrs and Rab7 (D–D′′), and Hrs and Rab11 (E–E′′) in the wing disk after knockdown of cbl in flipout clones (marked with GFP). (F) Quantification of colocalization of Hrs and markers was done by measuring Pearson’s coefficient in control and cbl; mop double-knockdown tissue. Bar graph shows continued localization of Hrs in Rab7-positive late endosomes. p < 0.001 for Hrs and Rab5, p < 0.01 for Hrs and Rab7, and p < 0.002 for Hrs and Rab11. mop was knocked down using VDRC line 104860. Scale bars, 20 μm.
Cbl is an E3 ubiquitin ligase involved in the internalization and degradation of EGF receptors (Hime et al., 1997; Stern et al., 2007). It was also shown to regulate Hrs phosphorylation and ubiquitination (Hime et al., 1997; Swaminathan and Tsygankov, 2006; Stern et al., 2007). Because the simultaneous knockdown of cbl; mop further enhances the phenotype (i.e., accumulation of Hrs in puncta) caused by cbl knockdown alone, Mop seems to inhibit Cbl either by direct interaction or by counteracting Cbl through recruiting Ubpy.
We next conducted colocalization assays of Hrs with different organelle markers in cbl; mop–knockdown disks. Compared to wild-type tissue, in which Hrs predominantly localized to Rab5- and Rab7-positive early and late endosomes (Figure 6, C, D, and F), Hrs puncta colocalized with Rab7-positive late endosomes in cbl; mop–knockdown disks (Figure 6D′′). However, Hrs localization to early endosomes was significantly reduced (Figure 6C′′). Localization of Hrs in recycling endosomes in wild-type and cbl; mop–knockdown disk was almost identical (Figure 6E′′). These results suggest that Hrs is degraded in the absence of Mop and Ubpy (and presence of Cbl). However, removing Cbl in a mop-knockdown background brings Hrs back to the late endosomes by inhibiting its degradation. In the absence of Cbl, Mop function is not required to regulate Hrs.
DISCUSSION
The Bro1 domain–containing protein Mop is known to regulate EGFR, Hippo, and Toll immune signaling and cell migration processes (Miura et al., 2008; Huang et al., 2010; Gilbert et al., 2011). We previously identified Mop in a kinome and phosphatome screen for novel regulators of the Wnt pathway (Swarup et al., 2015). Further characterization in Wnt signal–sending cells revealed a role for Mop in the endosomal trafficking of Wnt affecting its overall secretion (Pradhan-Sundd and Verheyen, 2014). Although Mop’s role in endosomal trafficking was elaborated on by these studies, detailed mechanistic insight into the function of Mop was lacking. Thus it was necessary to determine Mop’s mode of regulation of trafficking of endocytosed receptors. This study described our investigation into the mechanistic role of Mop by investigating trafficking of the Wnt receptor Fz.
Ligand–receptor internalization is tightly controlled in Wnt signaling; however, the molecular events that enable this regulation remain largely unknown. Here we show that Mop regulates signaling in Wnt-receiving cells by influencing the endosomal trafficking of Fz. The defects in Fz localization upon mop knockdown were previously unknown. We show that in the absence of Mop, Fz localizes to Rab5- and Rab7-positive early and late endosomes, whereas cell surface expression of Fz is significantly reduced. Knocking down the vertebrate homologue of Mop, HDPTP, also led to the same phenotype as seen in Mop-deficient cells. Furthermore, hrs knockdown completely mimicked the mop-knockdown phenotype, suggesting a similar role of Hrs in Fz trafficking.
The role of Mop in Hrs regulation
Although it was previously known that mop knockdown caused loss of Hrs (Miura et al., 2008; Pradhan-Sundd and Verheyen, 2014), our immunohistology and biochemical analyses revealed that it is due to Hrs ubiquitination followed by degradation. We first showed that inhibiting lysosomal, but not proteasomal, degradation could rescue the loss of Hrs in mop knockdown. Although there are a few reports of ubiquitin-mediated degradation of Hrs (Kato et al., 2000; Bishop et al., 2002; Marchese et al., 2003; Zhang et al., 2014), the entire cascade of events that drives this process is not completely understood. We identified a previously unknown role for Mop in regulating Hrs stability by suppressing its ubiquitination and degradation.
Thus we postulated that Mop’s role in controlling endocytosis is via regulating the localization and stability of Hrs. Simultaneous overexpression of Hrs in mop-knockdown tissue can rescue the trafficking defects of Wg and Fz, further supporting our hypothesis. Whereas Hrs is known for terminating many signaling pathways by targeting the receptors to the lysosome, its distinct role in recycling of specific G protein–coupled receptors (GPCRs) such as β2‐adrenergic receptor and μ-opioid receptor to the membrane and thereby increasing signaling has also been reported (Hanyaloglu et al., 2005). Given that we see an increased accumulation of Fz in the membrane upon Mop or Hrs overexpression, we propose that recycling of another GPCR-family protein, Fz, requires Hrs and Mop. Hrs initiates the MVB invagination process by binding with phosphatidylinositol 3-phosphate and members of ESCRTI and II (Bache et al., 2003a, b; Petiot et al., 2003; Hanyaloglu et al., 2005). It will be exciting to study how the absence of Mop affects the function of other ESCRTs and their relevance in the Wnt pathway.
The relationship between Mop and Ubpy
In this study, we show that ubpy knockdown mimics the trafficking defects of mop knockdown, suggesting that both Ubpy and Mop function to regulate trafficking of Fz by stabilizing Hrs. A recent article showed the role of Ubpy in Hrs deubiquitination and stability (Zhang et al., 2014). Of interest, overexpression of Ubpy in a mop knockdown background and vice versa rescued the trafficking defects of the internalized Wnt–Fz complex. Furthermore, we show that Ubpy requires Mop to maintain its levels. Together these data suggest that Ubpy and Mop cooperate or act in parallel in the deubiquitination and stabilization of Hrs and that Ubpy requires Mop for its localization and function. In yeast and vertebrates, the closest homologues of Mop perform a similar function by recruiting various deubiquitinases (Doyotte et al., 2008; Stefani et al., 2011), suggesting a conserved role of Mop in recruiting deubiquitinases. We here characterize a previously unknown role of Drosophila Mop in recruiting Ubpy. Future investigation is necessary to understand whether the recruitment of Ubpy is the primary function of Mop or whether the change in Ubpy localization upon mop knockdown is due to an indirect effect caused by the absence of other protein(s).
Ubpy is also known to regulate the Wnt pathway by controlling the deubiquitination (and thus stability) of Fz (Mukai et al., 2012). Given that we see reduced Fz levels at the cell surface after knockdown of mop and ubpy, an increase in Fz-Ub signal with PLA, and immunoprecipitation of Mop with Fz upon Mop overexpression, we suggest that Fz also undergoes ubiquitination in Mop’s absence. This could be due to aberrant localization of Ubpy upon mop knockdown, resulting in its reduced activity. We previously showed that Mop is not a general facilitator of deubiquitination (Pradhan-Sundd and Verheyen, 2014). The levels of Cubitus Interruptus (Ci; a Hedgehog pathway component known to be regulated by ubiquitination; Dai et al., 2003) was unaffected (Pradhan-Sundd and Verheyen, 2014) by mop knockdown, suggesting Mop’s specificity toward Hrs and Fz in controlling the endosomal trafficking of proteins.
The interaction between Mop and Cbl
We also found that in the absence of the E3 protein ubiquitin ligase Cbl, Mop is not required to promote the deubiquitination of Hrs. Cbl has been shown to promote Hrs ubiquitination and contribute to its degradation (Row et al., 2005; Stern et al., 2007). Knockdown of cbl caused a mild accumulation of Hrs and Wg in large endosomal vesicles, suggesting that in the steady state, Cbl is not primarily required to degrade these proteins. Double knockdown of mop and cbl resulted in significant accumulation of Hrs in endosomes. Our colocalization assay revealed that Hrs puncta localized to Rab7-positive late endosomes in cbl; mop–knockdown tissue. Because late endosomes are the ultimate organelle involved in sorting of a protein before its transport to lysosomes for degradation, we propose that simultaneously blocking Cbl and Mop inhibits the degradation of Hrs and leads to its retention in the late endosomes. We suggest that Cbl promotes ubiquitination and degradation of Hrs either by direct interaction or by recruiting other E3 ligases. Mop’s role in this context is to inhibit Cbl, and therefore the reduction of Hrs is rescued in a cbl; mop double knockdown. Although counterintuitive, our results can also explain the findings by Miura et al. (2008) that in the absence of Cbl, Mop is no longer required for photoreceptor differentiation in Drosophila. We propose that this interaction of Mop–Cbl is required not only for the photoreceptor differentiation in the eye disk, but also for global endosomal sorting and Hrs stability. Drosophila Cbl interacts with a vast number of signaling proteins and promotes their ubiquitination. Cbl itself is regulated by phosphorylation, which promotes its association with SH2 domain–containing proteins. It will be interesting to see how Mop counteracts the function of Cbl and whether the localization or phosphorylation of Cbl is influenced by Mop.
The role of the Mop-Hrs-Ubpy nexus in regulating Fz trafficking in Wnt signal–receiving cells
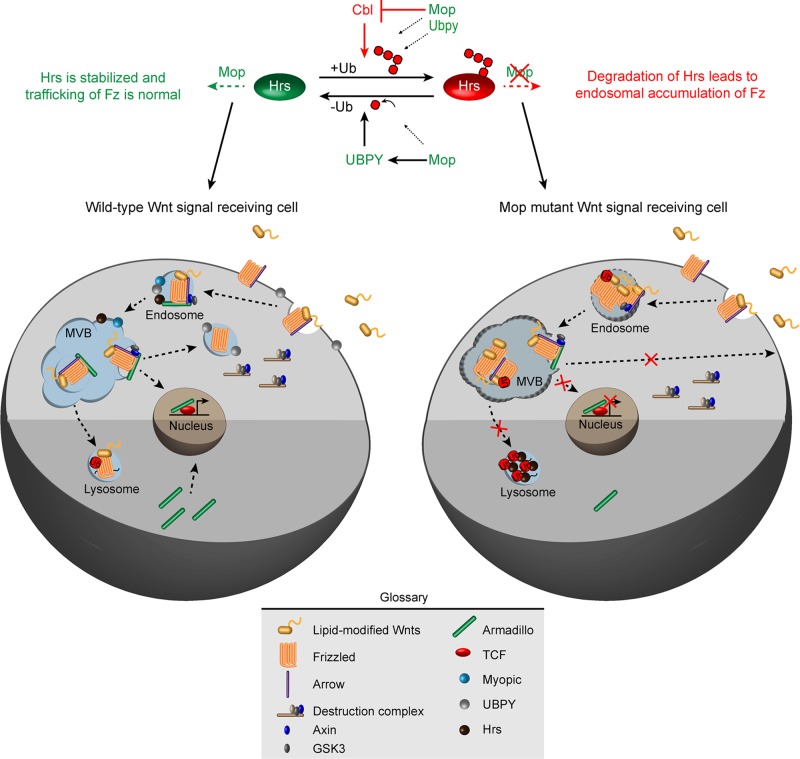
On the basis of our findings, we propose that localization of Hrs to MVB (where it regulates Fz sorting) requires the presence of Mop. Our results suggest a model in which the role of Mop is to facilitate the ubiquitination–deubiquitination homeostasis of Hrs by simultaneously inhibiting Cbl and recruiting Ubpy (Figure 7). Therefore Cbl, Mop, and Ubpy participate in the regulation of Hrs levels in the cell. We propose that Cbl inhibits the function of Ubpy. Overexpression of Mop can possibly suppress Cbl activity, which can in turn rescues the loss of Hrs in ubpy knockdown. Similarly, the overexpression of Ubpy can also rescue the loss of Hrs in mop knockdown by removing the ubiquitin moiety from Hrs. Therefore Ubpy works on Hrs, which is already ubiquitinated, and thus functions after Mop and Cbl (Figure 7). Cells overexpressing Hrs show a punctate accumulation of Hrs protein in the endosomes (and as a result causing mislocalization of endocytosed proteins; Bishop et al., 2002). Here we show that blocking Mop does not modify this phenotype, suggesting that Hrs is downstream of Mop in the endosomal sorting machinery. Taking our results together, we provide the first evidence of a unique interaction between these three proteins in regulating stability and ubiquitination of Hrs, which in turn is responsible for endosomal trafficking of Fz in both Drosophila and vertebrate cells.
FIGURE 7:
A model for Mop function in the Wnt signal–receiving cell. On the basis of our results, we propose that in the absence of Mop, Cbl promotes the degradation of Hrs. Mop’s presence inhibits Cbl and helps to maintain the levels of Ubpy, which deubiquitinate Hrs. Thus the presence of Mop stabilizes Hrs, which promotes Fz trafficking to endosomes. Endocytosed Fz can traffic to either lysosomes (when ubiquitinated) for its degradation or can be recycled back to the membrane for continued interaction with Wnts.
The absence of Hrs causes the accumulation of Fz in endosomes, leading to reduced recycling of Fz to the cell surface. Endosomal Fz in this case can neither signal nor be degraded. Similar effects were also observed for Wnt in the absence of Mop (Pradhan-Sundd and Verheyen, 2014) in the signal-producing and -receiving cells. We suggest that Hrs ubiquitination and endosomal accumulation of Fz occur in a sequential manner. At first, the absence of Mop causes ubiquitination and degradation of Hrs, which then leads to accumulation of ubiquitinated Fz in the endosomes. How the cell maintains this sequence will be the study of future research. Given the fact that we do not see reduction in the expression of other endosomal components upon mop knockdown, Mop might not directly affect the expression of general endocytic components. Signaling networks such as EGFR, Toll, Wnt, and Hippo are especially sensitive to the changes caused by the reduction in Mop. In contrast, many other signaling networks are able to bypass the effect caused by the loss of Mop. Future study may reflect on how different signaling networks act differentially upon the absence of Mop.
In conclusion, endosomal sorting and ubiquitination, followed by lysosomal degradation, are key regulators of the surface expression of Fz in Wnt-receiving cells. In this study, we identify a previously unknown function of Mop in the recycling of Fz by interacting with Hrs, Ubpy, and Cbl. Although the interaction between Mop, Cbl, Ubpy, and Hrs seems to play a role in the recycling of Fz, our present findings do not exclude a role for other unidentified proteins in this molecular cascade. Our findings lay the groundwork for future investigations aimed at understanding the kinetics of the molecular interactions involved in Fz trafficking.
MATERIALS AND METHODS
Fly strains and crosses
Fly strains used in the various crosses were hs-flp; Act>CD2>UAS-GAL4, UAS-GFP/SM6∼TM6 (a gift from Bruce Edgar, University of Heidelberg, Germany), enGal4; UAS GFP (a gift from Konrad Basler, UZH, Zurich, Switzerland), and ap-Gal4/Cyo, MS1096-Gal4, UAS-Gal4, UAS-LacZ and UAS-GFP (obtained from the Bloomington Drosophila Stock Center, Bloomington, IN). The following RNAi lines were obtained from the Vienna Drosophila RNAi Center (Dietzl et al., 2007) and the Bloomington Drosophila Stock Center: mop (VDRC 104860, 14174, Trip 34085), ubpy (VDRC 107623), hrs (Trip 28026, 33900, 34086), and cbl (Trip 27500). Other fly lines used were UAS Hrs (Hugo Bellen, Baylor College of Medicine, Houston,TX), UAS Ubpy, ubpy RNAi (Satoshi Gato, Mitsubishi-Kagaku Institute of Life Sciences, Machida, Japan), UAS Flag Mop on III, UAS Mop on II, UAS Mop on III (Jessica Treisman, New York University), ubpy RNAi, UAS Flag Ubpy (Junzheng Zhang, Cleveland Clinic, Cleveland, OH), UAS Myc-Fz, and UAS Flag-Fz (Bloomington Drosophila Stock Center, Bloomington, IN).
All wild-type flies used were w1118, and all crosses were performed according to standard procedures at 25°C. In genetic interaction assays examining interaction between two UAS transgenes, control crosses were performed with UAS-LacZ to eliminate effects caused by titration of Gal4.
Immunohistochemistry of wing imaginal disk
Third-instar wing imaginal disks were dissected in phosphate-buffered saline (PBS). Antibody staining was performed according to standard protocols (Lee et al., 2009). The following primary antibodies and dilutions were used: 1:100 anti-Wg 4D4, 1:50 anti-Frizzled (Developmental Studies Hybridoma Bank [DSHB], Iowa City, IA), 1:1000 anti–ubiquitinated proteins, 1:1000 monoubiquitinated and polyubiquitinated conjugates FK2 (Enzo Life Sciences), 1:100 Drosophila anti-USP8 (Junzheng Zhang), 1:1000 anti-Arrow (Steven Dinardo, University of Pennsylvania), 1:100 anti-Hrs (Hugo Bellen), 1:1000 anti-Rab5, 1:3000 anti-Rab7, 1:3000 anti-Rab11 (Takeshi Nakamura, Tokyo University, Tokyo, Japan), and 1:5000 anti-Coracle (Richard Fehon, University of Chicago, Chicago, IL). Disks were then washed five times for 5 min each in PBS-Tween 20 and mounted in Vectashield (Vector Laboratories).
E64 and MG132 treatment of wing imaginal disks
In some experiments, third-instar wing disks were dissected and incubated at 25°C for 4 h in complete Drosophila insect cell medium supplemented with 2% heat-inactivated fetal bovine serum (FBS; Invitrogen), supplemented with either lysosomal (E64 [50 μM; Sigma-Aldrich]) or proteasomal (MG132 [50 μM; Sigma-Aldrich, St. Louis, MO]) inhibitors before fixation.
Cell culture, transfection, transcription assays, and immunohistochemistry
L Wnt-3a cells (Ramanuj Dasgupta) and L cells were cultured at 37°C in DMEM supplemented with 10% FBS (Invitrogen). Cells were transfected 24 h after seeding with siRNA constructs of GAPDH and HDPTP (Cedarlane, Ontario, Canada) by using RNAiMAX transfection reagent (Invitrogen) according to the manufacturer’s instructions. The cells were lysed 48 h after transfection by using lysis buffer (Cell Signaling Technology, Boston, MA) supplemented with protease inhibitors (Roche, Basel, Switzerland). Wnt medium was collected from L Wnt3A cells after 48 h of seeding.
Luciferase assay was performed with a Promega Dual Luciferase Reporter Assay System as per manufacturer’s protocol. Topflash and Fopflash plasmids were used to carry out the experiment. The value shown here are averages of six separate experiments.
Western blot analysis
Protein lysates were prepared from L cells according to standard procedures (Swarup and Verheyen, 2011). Denatured proteins were separated on 10% SDS–PAGE gels and transferred to nitrocellulose membranes. Membranes were blocked using 2% bovine serum albumin (BSA) in 0.1% Triton X-100 in Tris-buffered saline (TBST) for 1 h at room temperature and incubated overnight at 4°C using primary antibodies (diluted in TBST). The following primary antibodies were used: anti-tubulin (1:500), anti-actin (1:500; Sigma-Aldrich), anti-Fz (1:500; Santa Cruz Biotechnology, Dallas, TX), anti-ubiquitins (1:300; Enzo Life Sciences), and anti-USP8 (1:500; Abcam, Eugene, OR). Membranes were washed five times for 5 min each in TBST before being probed with horseradish peroxidase–conjugated secondary antibodies (1:5000 diluted in TBST; Santa Cruz Biotechnology) for 1.5 h at room temperature. Membranes were washed three times for 10 min each in TBST and visualized using the Enhanced Chemiluminescence System (GE Healthcare, Pittsburgh, PA). For CoIP assay, 50 disks of control and MS1096>mop RNAi and MS1096>UAS FLAG Mop were dissected in insect medium and lysed in lysis buffer. In addition, to measure ubiquitination, 50 μM MG132 and E64 was added to inhibit proteasomal and lysososmal degradation. Proteins were immunoprecipitated using protein G beads, followed by SDS–PAGE/Western blotting. Antibodies used were anti-Hrs (1:1000), anti-ubiquitin (1:1000), anti–ubiquitinylated conjugates FK2 (1:1000), and anti-Fz (1:500; 1C11; DSHB).
Proximity ligation assay
The PLA was performed with Duolink PLA Kit. Drosophila wing disks were dissected in PBS and washed in PBS with 0.1% Triton X-100 for 10 min (three times) and blocked with 1% BSA for 1 h. The disks were then incubated with primary antibodies overnight at 4°C, followed by wash with PBT for 10 min (three times). The remaining process was done in the dark. The disks were then incubated for 1 h at 37°C with PLA probes diluted in 1% BSA, followed by ligation at 37°C for 30 min and amplification at 37°C for 100 min. After the final wash, the disks were mounted in Duolink mounting media with 4′,6-diamidino-2-phenylindole.
Immuno–electron microscopy
For the analysis, third-instar larvae of MS1096-Gal4> UAS Fz2-Myc and MS1096-Gal4>mopRNAi; UAS Fz2-Myc were dissected and immediately fixed with 4% paraformaldehyde and 0.1% glutaraldehyde in PBS (pH 7.4) for 4–6 h. After washing in 0.1 M PBS three times for 15 min each, the disks were dehydrated in graded series of alcohol (in PBS) for 15 min each (30, 50, 70, 90, 3× 100%, 15 min each). The disks were then infiltrated with LR White resin in two 1-h changes and embedded in LR White resin. Thin sections were cut with a Reichert Ultracut E microtome and imaged with a JEM1011 transmission electron microscope (images were taken with the generous help of Donna Stolz’s lab, University of Pittsburgh, Pittsburgh, PA). To quantitate the localization of Myc-Fz in control and affected tissue, 20 disks of mop and 10 of control disks were observed.
Image analysis
Images were taken with a Nikon A1R laser scanning confocal microscope and processed using Photoshop CS3 (Adobe, San Jose, CA). For measurements of colocalization between two proteins, images were analyzed with NLS software (Nikon Elements 3.10). Colocalization was measured by finding the Pearson coefficient of six different wing disks. Pearson’s coefficient represents intensity correlation of all nonzero pixels that overlay in images of two channels.
Statistical analysis of data
Statistical significance of colocalization of proteins was calculated by unpaired Student’s t test. Error bars represent SE.
Supplementary Material
Acknowledgments
We thank the following individuals and stock centers for reagents and fly strains: Jessica Treisman, Satoshi Goto, Hugo Bellen, Junzheng Zhang, Takeshi Nakamura, Ramanuj Dasgupta, Bruce Edgar, Kenneth Moberg, Richard Fehon, Steven Dinardo, the Developmental Studies Hybridoma Bank, the Bloomington Drosophila Stock Center, the Drosophila Genetic Resource Center, the Harvard RNAi Facility, the Vienna Drosophila RNAi Center, and NIG Japan. Many thanks go to Nick Harden, Prithu Sundd, and Vilaiwan Fernandes for insightful comments on the study. We thank Brooke McCartney and her lab and Prithu Sundd and his lab for help in completing revisions to this study. This work was supported by an operating grant from the Canadian Institutes of Health Research.
Abbreviations used:
- Fz
Frizzled
- Hrs
hepatocyte growth factor–regulated tyrosine kinase substrate
- Mop
Myopic
- Ubpy
ubiquitin-specific processing protease Y.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.3329) on July 29, 2015.
REFERENCES
- Ali N, Zhang L, Taylor S, Mironov A, Urbé S, Woodman P. Recruitment of UBPY and ESCRT exchange drive hd-ptp-dependent sorting of egfr to the mvb. Curr Biol. 2013;23:453–461. doi: 10.1016/j.cub.2013.02.033. [DOI] [PubMed] [Google Scholar]
- Bache KG, Brech A, Mehlum A, Stenmark H. Hrs regulates multivesicular body formation via ESCRT recruitment to endosomes. J Cell Biol. 2003a;162:435–442. doi: 10.1083/jcb.200302131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bache KG, Raiborg C, Mehlum A, Stenmark H. STAM and Hrs are subunits of a multivalent ubiquitin-binding complex on early endosomes. J Biol Chem. 2003b;278:12513–12521. doi: 10.1074/jbc.M210843200. [DOI] [PubMed] [Google Scholar]
- Bishop N, Horman A, Woodman P. Mammalian class E vps proteins recognize ubiquitin and act in the removal of endosomal protein-ubiquitin conjugates. J Cell Biol. 2002;157:91–101. doi: 10.1083/jcb.200112080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer JT, Nusse R. A critical role for endocytosis in Wnt signaling. BMC Cell Biol. 2006;7:28. doi: 10.1186/1471-2121-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Chen D-Y, Li M-Y, Wu S-Y, Lin Y-L, Tsai S-P, Lai P-L, Lin Y-T, Kyo J-C, Ming T, Chen G-C. The Bro1-domain-containing protein Myopic/HDPTP coordinates with Rab4 to regulate cell adhesion and migration. J Cell Sci. 2012;125:4841–4852. doi: 10.1242/jcs.108597. [DOI] [PubMed] [Google Scholar]
- Chen W, ten Berge D, Brown J, Ahn S, Hu LA, Miller WE, Caron MG, Barrack LS, Nusse R, Lefkowitz RJ. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science. 2003;301:1391–1394. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- Clague MJ, Liu H, Urbé S. Governance of endocytic trafficking and signaling by reversible ubiquitylation. Dev Cell. 2012;23:457–467. doi: 10.1016/j.devcel.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moitra LF, Thery C, Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126:5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- Dai P, Akimaru H, Ishii S. A Hedgehog-responsive region in the Drosophila wing disc is defined by Debra-mediated ubiquitination and lysosomal degradation of Ci. Dev Cell. 2003;4:917–928. doi: 10.1016/s1534-5807(03)00158-8. [DOI] [PubMed] [Google Scholar]
- Deatheragea BL, Cooksona BT. Membrane vesicle release in bacteria, eukaryotes, and archaea: A conserved yet underappreciated aspect of microbial life. Infect Immun. 2012;80:1948–1957. doi: 10.1128/IAI.06014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su K-C, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Doyotte A, Mironov A, McKenzie E, Woodman P. The Bro1-related protein HD-PTP/PTPN23 is required for endosomal cargo sorting and multivesicular body morphogenesis. Proc Natl Acad Sci USA. 2008;105:6308–6313. doi: 10.1073/pnas.0707601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois L, Lecourtois M, Alexandre C, Hirst E, Vincent JP. Regulated endocytic routing modulates wingless signaling in Drosophila embryos. Cell. 2001;105:613–624. doi: 10.1016/s0092-8674(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Fan J, Jiang K, Liu Y, Jia J. Hrs promotes ubiquitination and mediates endosomal trafficking of smoothened in Drosophila Hedgehog signaling. PLoS One. 2013;8:e79021. doi: 10.1371/journal.pone.0079021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore PPD, Gill GN. Endocytosis and mitogenic signaling. Curr Opin Cell Biol. 1999;11:483–488. doi: 10.1016/s0955-0674(99)80069-6. [DOI] [PubMed] [Google Scholar]
- Franch-Marro X, Wendler F, Guidato S, Griffith J, Baena-Lopez A, Itasaki N, Maurice MM, Vincent J-P. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat Cell Biol. 2008;10:170–177. doi: 10.1038/ncb1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi M, Hernandez A, McGough IJ, Vincent JP. Inhibitors of endocytosis prevent Wnt/Wingless signalling by reducing the level of basal β-catenin/Armadillo. J Cell Sci. 2014;127:4918–4926. doi: 10.1242/jcs.155424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi M, Piddini E, Vincent J-P. Endocytosis: a positive or a negative influence on Wnt signalling? Traffic. 2008;9:1–9. doi: 10.1111/j.1600-0854.2007.00662.x. [DOI] [PubMed] [Google Scholar]
- Gilbert MM, Tipping M, Veraksa A, Moberg KH. A screen for conditional growth suppressor genes identifies the Drosophila homolog of HD-PTP as a regulator of the oncoprotein Yorkie. Dev Cell. 2011;20:700–712. doi: 10.1016/j.devcel.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabbe C, Husnjak K, Dikic I. The spatial and temporal organization of ubiquitin networks. Nat Rev Mol Cell Biol. 2011;12:295–307. doi: 10.1038/nrm3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinde B. Selective inhibition of lysosomal protein degradation by the thiol proteinase inhibitors E-64, Ep-459 and Ep-457 in isolated rat hepatocytes. Biochim Biophys Acta. 1982;701:328–333. doi: 10.1016/0167-4838(82)90235-7. [DOI] [PubMed] [Google Scholar]
- Hanyaloglu AC, McCullagh E, von Zastrow M. Essential role of Hrs in a recycling mechanism mediating functional resensitization of cell signaling. EMBO J. 2005;24:2265–2283. doi: 10.1038/sj.emboj.7600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao H-X, Xie Y, Zhang Y, Charlat O, Oster E, Avello M, Cong F. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 2012;485:195–200. doi: 10.1038/nature11019. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Hime GR, Dhungat MP, Ng A, Bowtell DD. D-Cbl, the Drosophila homologue of the c-Cbl proto-oncogene, interacts with the Drosophila EGF receptor in vivo, despite lacking C-terminal adaptor binding sites. Oncogene. 1997;14:2709–2719. doi: 10.1038/sj.onc.1201223. [DOI] [PubMed] [Google Scholar]
- Huang H-R, Chen ZJ, Kunes S, Chang G-D, Maniatis T. Endocytic pathway is required for Drosophila Toll innate immune signaling. Proc Natl Acad Sci USA. 2010;107:8322–8327. doi: 10.1073/pnas.1004031107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvius M, Paulsson J, Weibrecht I, Leuchowius K-J, Andersson A-C, Wählby C, Söderberg O. In situ detection of phosphorylated platelet-derived growth factor receptor beta using a generalized proximity ligation method. Mol Cell Proteomics. 2007;6:1500–1509. doi: 10.1074/mcp.M700166-MCP200. [DOI] [PubMed] [Google Scholar]
- Jékely G, Rørth P. Hrs mediates downregulation of multiple signalling receptors in Drosophila. EMBO Rep. 2003;4:1163–1168. doi: 10.1038/sj.embor.7400019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Miyazawa K, Kitamura N. A deubiquitinating enzyme UBPY interacts with the Src homology 3 domain of Hrs-binding protein via a novel binding motif PX(V/I)(D/N)RXXKP. J Biol Chem. 2000;275:37481–37487. doi: 10.1074/jbc.M007251200. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Tanaka N, Asao H, Miura S, Kyuuma M, Semura K, Sugamura K. Hrs, a mammalian master molecule in vesicular transport and protein sorting, suppresses the degradation of ESCRT proteins signal transducing adaptor molecule 1 and 2. J Biol Chem. 2005;280:10468–10477. doi: 10.1074/jbc.M409969200. [DOI] [PubMed] [Google Scholar]
- Komada M, Kitamura N. Hrs and hbp: possible regulators of endocytosis and exocytosis. Biochem Biophys Res Commun. 2001;281:1065–1069. doi: 10.1006/bbrc.2001.4441. [DOI] [PubMed] [Google Scholar]
- Koo B-K, Spit M, Jordens I, Low TY, Stange DE, van de Wetering M. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488:665–669. doi: 10.1038/nature11308. [DOI] [PubMed] [Google Scholar]
- Lecourtois M, Alexandre C, Dubois L, Vincent JP. Wingless capture by Frizzled and Frizzled2 in Drosophila embryos. Dev Biol. 2001;235:467–475. doi: 10.1006/dbio.2001.0320. [DOI] [PubMed] [Google Scholar]
- Lee DH. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397-403. doi: 10.1016/s0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- Lee W, Andrews BC, Faust M, Walldorf U, Verheyen EM. Hipk is an essential protein that promotes Notch signal transduction in the Drosophila eye by inhibition of the global co-repressor Groucho. Dev Biol. 2009;325:263–272. doi: 10.1016/j.ydbio.2008.10.029. [DOI] [PubMed] [Google Scholar]
- Lloyd TE, Atkinson R, Wu MN, Zhou Y, Pennetta G, Bellen HJ. Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell. 2002;108:261–269. doi: 10.1016/s0092-8674(02)00611-6. [DOI] [PubMed] [Google Scholar]
- Marchese A, Raiborg C, Santini F, Keen JH, Stenmark H, Benovic JL. The E3 ubiquitin ligase AIP4 mediates ubiquitination and sorting of the G protein-coupled receptor CXCR4. Dev Cell. 2003;5:709–722. doi: 10.1016/s1534-5807(03)00321-6. [DOI] [PubMed] [Google Scholar]
- Marois E, Mahmoud A, Eaton S. The endocytic pathway and formation of the Wingless morphogen gradient. Development. 2006;133:307–317. doi: 10.1242/dev.02197. [DOI] [PubMed] [Google Scholar]
- Miaczynska M, Pelkmans L, Zerial M. Not just a sink: endosomes in control of signal transduction. Curr Opin Cell Biol. 2004;16:400–406. doi: 10.1016/j.ceb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Miura GI, Roignant J-Y, Wassef M, Treisman JE. Myopic acts in the endocytic pathway to enhance signaling by the Drosophila EGF receptor. Development. 2008;135:1913–1922. doi: 10.1242/dev.017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno E, Kobayashi K, Yamamoto A, Kitamura N, Komada M. A deubiquitinating enzyme UBPY regulates the level of protein ubiquitination on endosomes. Traffic. 2006;7:1017–1031. doi: 10.1111/j.1600-0854.2006.00452.x. [DOI] [PubMed] [Google Scholar]
- Mukai A, Yamamoto-Hino M, Komada M, Okano H, Goto S. Balanced ubiquitination determines cellular responsiveness to extracellular stimuli. Cell Mol Life Sci. 2012;69:4007–4016. doi: 10.1007/s00018-012-1084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naviglio S, Matteucci C, Matoskova B, Nagase T, Nomura N, Di Fiore PP, Draetta GF. UBPY: A growth-regulated human ubiquitin isopeptidase. EMBO J. 1998;17:3241–3250. doi: 10.1093/emboj/17.12.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petiot A, Fauré J, Stenmark H, Gruenberg J. PI3P signaling regulates receptor sorting but not transport in the endosomal pathway. J Cell Biol. 2003;162:971–979. doi: 10.1083/jcb.200303018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piddini E, Marshall F, Dubois L, Hirst E, Vincent J-P. Arrow (LRP6) and Frizzled2 cooperate to degrade Wingless in Drosophila imaginal discs. Development. 2005;132:5479–5489. doi: 10.1242/dev.02145. [DOI] [PubMed] [Google Scholar]
- Platta HW, Stenmark H. Endocytosis and signaling. Curr Opin Cell Biol. 2011;23:393–403. doi: 10.1016/j.ceb.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Polo S, Di Fiore PP. Endocytosis conducts the cell signaling orchestra. Cell. 2006;124:897–900. doi: 10.1016/j.cell.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Pradhan-Sundd T, Verheyen EM. The role of Bro1-domain-containing protein Myopic in endosomal trafficking of Wnt/Wingless. Dev Biol. 2014;392:93–107. doi: 10.1016/j.ydbio.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Pullan L, Mullapudi S, Huang Z, Baldwin PR, Chin C, Sun W, Tsujimoto S, Kolodziez SJ, Stoops JK, Lee JC, et al. The endosome-associated protein Hrs is hexameric and controls cargo sorting as a “master molecule.”. Structure. 2006;14:661–671. doi: 10.1016/j.str.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Purvanov V, Koval A, Katanaev VL. A direct and functional interaction between Go and Rab5 during G protein-coupled receptor signaling. Sci Signal. 2010;3:ra65. doi: 10.1126/scisignal.2000877. [DOI] [PubMed] [Google Scholar]
- Rives AF, Rochlin KM, Wehrli M, Schwartz SL, DiNardo S. Endocytic trafficking of Wingless and its receptors, Arrow and DFrizzled-2, in the Drosophila wing. Dev Biol. 2006;293:268–283. doi: 10.1016/j.ydbio.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Row PE, Clague MJ, Urbé S. Growth factors induce differential phosphorylation profiles of the Hrs-STAM complex: a common node in signalling networks with signal-specific properties. Biochem J. 2005;389:629–636. doi: 10.1042/BJ20050067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto ES, Bellen HJ. Internalization is required for proper Wingless signaling in Drosophila melanogaster. J Cell Biol. 2006;173:95–106. doi: 10.1083/jcb.200510123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderberg O, Gullberg M, Jarvius M, Ridderstråle K, Leuchowius K-J, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson L-G, et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- Sorkin A, Von Zastrow M. Signal transduction and endocytosis: close encounters of many kinds. Nat Rev Mol Cell Biol. 2002;3:600–614. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- Stefani F, Zhang L, Taylor S, Donovan J, Rollinson S, Doyotte A, Woodman P. UBAP1 is a component of an endosome-specific ESCRT-I complex that is essential for MVB sorting. Curr Biol. 2011;21:1245–1250. doi: 10.1016/j.cub.2011.06.028. [DOI] [PubMed] [Google Scholar]
- Stern KA, Visser Smit GD, Place TL, Winistorfer S, Piper RC, Lill NL. Epidermal growth factor receptor fate is controlled by Hrs tyrosine phosphorylation sites that regulate Hrs degradation. Mol Cell Biol. 2007;27:888–898. doi: 10.1128/MCB.02356-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan G, Tsygankov AY. The Cbl family proteins: ring leaders in regulation of cell signaling. J Cell Physiol. 2006;209:21–43. doi: 10.1002/jcp.20694. [DOI] [PubMed] [Google Scholar]
- Swarup S, Pradhan-Sundd T, Verheyen EM. Genome-wide identification of phospho-regulators of Wnt signaling in Drosophila. Development. 2015;142:1502–1515. doi: 10.1242/dev.116715. [DOI] [PubMed] [Google Scholar]
- Swarup S, Verheyen EM. Drosophila homeodomain-interacting protein kinase inhibits the Skp1-Cul1-F-box E3 ligase complex to dually promote Wingless and Hedgehog signaling. Proc Natl Acad Sci USA. 2011;108:9887–9892. doi: 10.1073/pnas.1017548108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub LM, Lukacs GL. Decoding ubiquitin sorting signals for clathrin-dependent endocytosis by CLASPs. J Cell Sci. 2007;120:543–553. doi: 10.1242/jcs.03385. [DOI] [PubMed] [Google Scholar]
- Ward RE, IV, Schweizer L, Lamb RS, Fehon RG. The protein 4.1, ezrin, radixin, moesin (FERM) domain of drosophila coracle, a cytoplasmic component of the septate junction, provides functions essential for embryonic development and imaginal cell proliferation. Genetics. 2001;159:219–228. doi: 10.1093/genetics/159.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson KD. Ubiquitination and deubiquitination: targeting of proteins for degradation by the proteasome. Semin Cell Dev Biol. 2000;11:141–148. doi: 10.1006/scdb.2000.0164. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Komekado H, Kikuchi A. Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of beta-catenin. Dev Cell. 2006;11:213–223. doi: 10.1016/j.devcel.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Zhang J, Du J, Lei C, Liu M, Zhu AJ. Ubpy controls the stability of the ESCRT-0 subunit Hrs in development. Development. 2014;141:1473–1479. doi: 10.1242/dev.099564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.