Abstract
Bipotent axial stem cells residing in the caudal epiblast during late gastrulation generate neuroectodermal and presomitic mesodermal progeny that coordinate somitogenesis with neural tube formation, but the mechanism that controls these two fates is not fully understood. Retinoic acid (RA) restricts the anterior extent of caudal fibroblast growth factor 8 (Fgf8) expression in both mesoderm and neural plate to control somitogenesis and neurogenesis, however it remains unclear where RA acts to control the spatial expression of caudal Fgf8. Here, we found that mouse Raldh2-/- embryos, lacking RA synthesis and displaying a consistent small somite defect, exhibited abnormal expression of key markers of axial stem cell progeny, with decreased Sox2+ and Sox1+ neuroectodermal progeny and increased Tbx6+ presomitic mesodermal progeny. The Raldh2-/- small somite defect was rescued by treatment with an FGF receptor antagonist. Rdh10 mutants, with a less severe RA synthesis defect, were found to exhibit a small somite defect and anterior expansion of caudal Fgf8 expression only for somites 1–6, with normal somite size and Fgf8 expression thereafter. Rdh10 mutants were found to lack RA activity during the early phase when somites are small, but at the 6-somite stage RA activity was detected in neural plate although not in presomitic mesoderm. Expression of a dominant-negative RA receptor in mesoderm eliminated RA activity in presomitic mesoderm but did not affect somitogenesis. Thus, RA activity in the neural plate is sufficient to prevent anterior expansion of caudal Fgf8 expression associated with a small somite defect. Our studies provide evidence that RA restriction of Fgf8 expression in undifferentiated neural progenitors stimulates neurogenesis while also restricting the anterior extent of the mesodermal Fgf8 mRNA gradient that controls somite size, providing new insight into the mechanism that coordinates somitogenesis with neurogenesis.
Introduction
Knowledge of how stem cells produce differentiated progeny is essential for understanding organogenesis and for realizing the full potential of stem cells as therapeutic agents. In this regard, an understanding of how extrinsic signals, such as retinoic acid (RA) and fibroblast growth factor (FGF), normally regulate stem cell differentiation in vivo is of paramount importance for elucidating effective stem cell treatment regimens that efficiently generate specialized cells. Treatment of stem/progenitor cells in vitro with supraphysiological levels of RA (1–10 micromolar) has for many years been used to induce differentiation in various directions [1,2]. However, little is known about how endogenous RA, normally present at 1–100 nM in various mammalian embryonic or adult tissues [3,4,5,6], controls differentiation of endogenous stem cells in embryos or adults. Thus, knowledge of how endogenous RA controls stem cell populations in vivo is needed to provide guidance on how RA can be used most effectively for therapeutic stem cell treatments.
Recent studies have demonstrated that an endogenous axial (neuromesodermal) stem cell population in vertebrate embryos is an excellent model for investigating signaling mechanisms that normally control stem cell differentiation in vivo [7]. Bipotent axial stem cells expressing T (Brachyury) and Sox2 reside in the caudal lateral epiblast lying on each side of the primitive streak [8,9,10]. Axial stem cells differentiate into either neuroectodermal or presomitic mesodermal progeny in a coordinated manner to generate the neural tube and somites that comprise much of the trunk and tail regions [11,12]. Axial stem cells that enter the primitive streak undergo epithelial-to-mesenchymal transition and differentiate into presomitic mesoderm progenitors expressing Tbx6, while cells that stay in the anterior region of the caudal epiblast epithelial layer differentiate to neural plate expressing high levels of Sox2 as the body axis extends. The fate of axial stem cells during differentiation is determined by the decision to express either Sox2 needed for neural fate or Tbx6 that helps stimulate presomitic mesodermal fate by repressing Sox2 [13]. Consistent with this idea, Tbx6 loss-of-function results in the formation of ectopic neural tubes at the location where somites normally form [14]. Caudal Wnt and FGF signals are required to maintain progenitors (including axial stem cells) that promote body axis extension [8,9,15,16,17,18,19,20]. Wnt and FGF have also been associated with priming of the Sox2 N1-enhancer to allow moderate expression of Sox2 in the caudal epiblast (where axial stem cells reside) which is later up-regulated in neural progeny [13]. However, mechanisms that govern this signaling network in order to determine the correct proportion of axial stem cell fates and proper formation of tissues remain unclear.
RA functions as a ligand for widely-expressed nuclear RA receptors (RARa, RARb, RARg) that bind as RAR/RXR heterodimers to RA response elements (RAREs) near target genes [21]. RA is synthesized by mesodermal progeny of the axial stem cell niche through the actions of retinol dehydrogenase 10 (RDH10) that metabolizes retinol (vitamin A) to retinaldehyde followed by retinaldehyde dehydrogenase 2 (RALDH2; ALDH1A2) that metabolizes retinaldehyde to RA which functions as a ligand for RARs [22,23]. Loss of RA synthesis in avian vitamin A deficient embryos and Raldh2-/- embryos results in loss of posterior neural differentiation, expansion of presomitic mesoderm along the anteroposterior axis, smaller somite size associated with shortening along the anteroposterior axis of the segmented domain, and premature termination of body axis extension [24,25,26,27]. Excess RA also results in axial truncation, but this is normally prevented by caudal expression of Cyp26a1 (encoding a RA-degrading enzyme) that is induced by Brachyury (T) under the control of Wnt and FGF signaling [28,29]. As loss of RA results in ectopic anterior expansion of caudal Fgf8 expression, it has been suggested that RA may control posterior neurogenesis and somitogenesis by antagonizing caudal FGF signaling [24,26,27,30]. Treatment of chick embryos with RA or an RA synthesis inhibitor has been reported to affect not only caudal Fgf8 expression but also the balance of Sox2/Tbx6 expression in caudal progenitors at the tailbud stage during termination of body axis extension [9]. Studies on the mechanism of Fgf8 repression by RA found that during movement of cells from the caudal progenitor zone to the developing trunk, the Fgf8 chromosomal locus within the nucleus becomes situated more peripherally (a location associated with repression), but this shift to the nuclear periphery was not observed in Raldh2-/- embryos [31]. In addition, analysis of transgenes carrying Fgf8 regulatory elements has shown that a conserved upstream RARE functions to restrict Fgf8 expression to the caudal progenitor zone of mouse embryos, showing that RA can directly repress Fgf8 at the transcriptional level [32]. However, it remains unclear in which tissues RA acts to restrict caudal Fgf8 expression.
Also, in order to understand the mechanism of RA-FGF antagonism in somitogenesis, another important concept to incorporate is the discovery that caudal Fgf8 transcription occurs in caudal undifferentiated cells but not in more mature progenitors lying anteriorly including the anterior presomitic mesoderm and neural plate [33]; Fgf8 exon and intron in situ hybridization probes were used to show that anterior presomitic mesoderm displays Fgf8 mRNA but not Fgf8 hnRNA primary transcripts, whereas caudal undifferentiated cells exhibited both Fgf8 mRNA and hnRNA [33]. Although Fgf8 transcription is turned off in more anterior progenitors, Fgf8 mRNA persists in these cells to establish a caudal-high to anterior-low gradient of Fgf8 mRNA and FGF8 protein activity that enacts a gradient of random cell motility and dictates the somite formation wavefront where FGF8 reaches a low anterior signaling threshold [18,19,33,34].
Here, we use RA deficient mouse embryos to show that RA activity in undifferentiated neural progenitors is sufficient to restrict caudal Fgf8 expression in both neuroectoderm and mesoderm and hence control somite size. Thus, during the process of restricting Fgf8 expression to control neurogenesis in the axial stem cell niche, RA also restricts the anterior extent of the mesodermal Fgf8 mRNA gradient to control somite size. Our studies provide new insight into the molecular network that coordinates somitogenesis with neurogenesis.
Results
RA deficiency in mouse results in abnormal gene expression in the axial stem cell niche
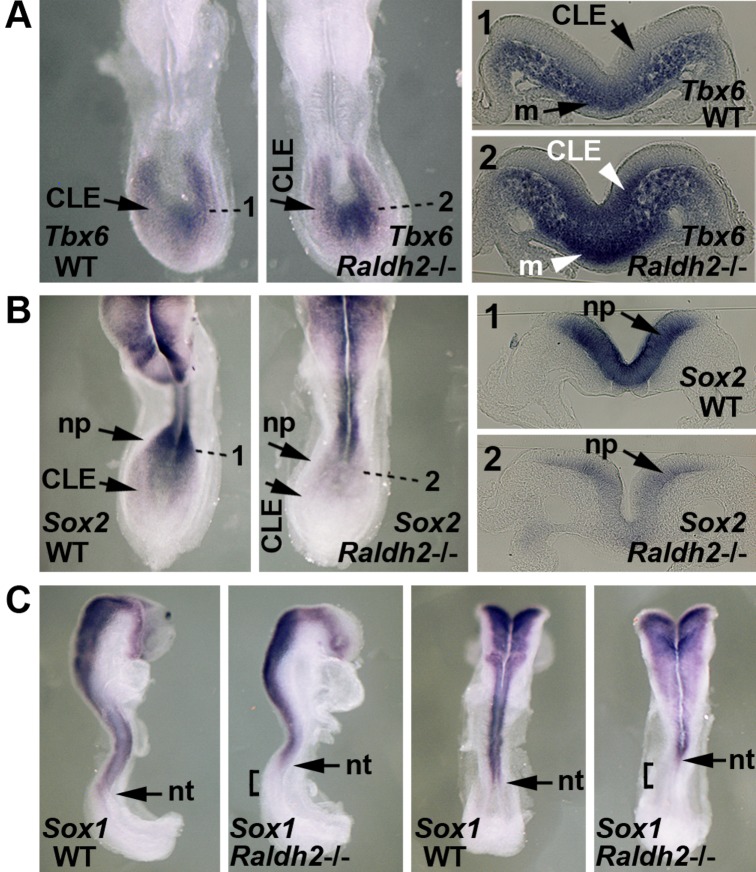
We used genetic loss-of-function studies to explore whether loss of RA synthesis in mouse affects differentiation of axial stem cells needed to generate the body axis. During normal mouse development, Sox2 (needed for neural fate) is expressed at moderate levels in caudal lateral epiblast (CLE) and at high levels in neural plate progeny located just anterior to the CLE in the same epithelial plane. In contrast, Tbx6 (that stimulates presomitic mesodermal fate by repressing Sox2) is expressed in mesodermal progeny that emerge from the primitive streak near the CLE [13]. We found that E8.25 (5–8 somite) Raldh2-/- embryos exhibited ectopic Tbx6 expression encroaching into the CLE and an expanded domain of Tbx6+ presomitic mesoderm suggesting an increased number of presomitic mesodermal progeny. (Fig 1A; n = 3/3). Conversely, Sox2 expression was decreased in the CLE and neural plate (Fig 1B; n = 5/5). This finding is consistent with studies showing that Tbx6 represses Sox2 in the axial stem cell niche [13]. In addition, expression of Sox1 (an early marker of neural progeny) was down-regulated in the posterior-most neural tube but normal more anteriorly, indicating anteriorization of the neural specification boundary, consistent with the lack of Sox2 upregulation that normally defines the neural plate (Fig 1C; n = 3/3). Previous studies reported down-regulation of caudal Sox2 in RA-deficient embryos, but Tbx6 and Sox1 were not examined [35]. Therefore, our additional examination of Tbx6 and Sox1 allows insight into axial stem cell regulation. Together, these observations indicate that endogenous RA signaling is required for normal Sox2/Tbx6 expression in the mouse axial stem cell niche, with abnormal Sox2/Tbx6 expression likely contributing to somite axial defects and loss of posterior neurogenesis observed in RA-deficient embryos.
Fig 1. Loss of RA disrupts expression of axial stem cell niche markers.
Shown is a comparison of E8.25 (5-somite) wild-type (WT) and Raldh2-/- embryos. (A) Tbx6 mRNA; dotted lines indicate transverse sections through whole-mount stained embryos showing that loss of RA results in the appearance of ectopic Tbx6 expression in the caudal lateral epiblast (CLE) and expansion of Tbx6+ presomitic mesoderm along the dorsoventral axis. (B) Sox2 mRNA; dotted lines indicate transverse sections showing that loss of RA down-regulates Sox2 expression in neural plate (np); also, down-regulation in CLE is observed in whole-mount. (C) Sox1 mRNA; the bar shows that loss of RA results in loss of Sox1 expression in posterior neural tube (nt) adjoining the neural plate.
Defective somitogenesis in RA-deficient embryos is due to ectopic caudal Fgf8 expression
During differentiation of caudal progenitors, FGF signaling becomes gradually down-regulated in both mesodermal and neural progeny due to cessation of Fgf8 transcription and gradual Fgf8 mRNA decay [33]. Down-regulation of FGF signaling in neural plate cells emerging from the caudal epiblast is required for neurogenesis to proceed normally as the body axis extends [31]. FGF signaling stimulates presomitic mesoderm production through Tbx6 activation in the primitive streak [15,18], while a posterior (high) to anterior (low) FGF gradient controls a mesodermal random cell motility gradient required for presomitic mesoderm to differentiate into somites during axial elongation [36]. Thus, our observed alterations in Tbx6/Sox2 expression and somite defects in Raldh2-/- embryos may both be explained by expanded caudal Fgf8 expression that favors Tbx6+ presomitic mesoderm production and maintenance but also disturbs the caudal FGF motility gradient such that the expanded domain of Tbx6+ mesoderm is unable to mature to somite formation due to excess random motility [26,27]. To functionally test the requirement of caudal RA-FGF antagonism for somitogenesis, we cultured wild-type and Raldh2-/- embryos in the presence or absence of 20 micromolar SU5402, an inhibitor of FGF receptor activity [37]. We examined embryos for expression of Uncx [26,27], a marker for somitic mesodermal progeny of axial stem cells downstream of Tbx6 expression in presomitic mesoderm. Embryos collected at E8.25 and cultured for 12 hours consistently extended the body axis by five somites (n = 8/8 wild-type; n = 10/10 Raldh2-/-). In control cultures, the newly-generated somites in Raldh2-/- embryos were reduced in axial length by 65% versus wild-type (Fig 2A and 2B; n = 5/5 for both control and SU5402-treated), recapitulating in vitro the small somite phenotype observed in vivo. SU5402-treated wild-type embryos appeared similar to wild-type controls, indicating that the 20 micromolar concentration used here does not reduce normal FGF signaling enough to affect somitogenesis (Fig 2A; n = 4/4 for both control and SU5402-treated). In SU5402-treated Raldh2-/- embryos, the newly-generated somites were increased in axial length by 55% versus untreated Raldh2-/- controls (Fig 2B; n = 5/5 for both control and SU5402-treated). Rescue of the Raldh2-/- small somite phenotype following reduction of FGF signaling provides evidence that ectopic caudal FGF signaling observed in Raldh2-/- embryos is responsible for the defect.
Fig 2. RA antagonism of caudal FGF signaling rescues somitogenesis defect.
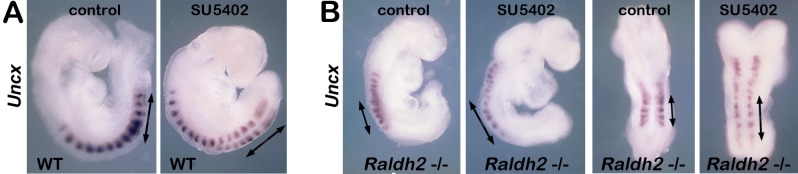
(A-B) Wild-type (WT) and Raldh2-/- embryos at the 6–9 somite stages were cultured for 12 h in the presence of SU5402 (20 micromolar) or DMSO vehicle control, then processed to visualize mRNA for Uncx gene expressed in the posterior domain of each somite [26,27] to monitor somite length along the anteroposterior axis. Somite length was compared by measuring the bars bracketing the caudal-most 5 somites generated in culture.
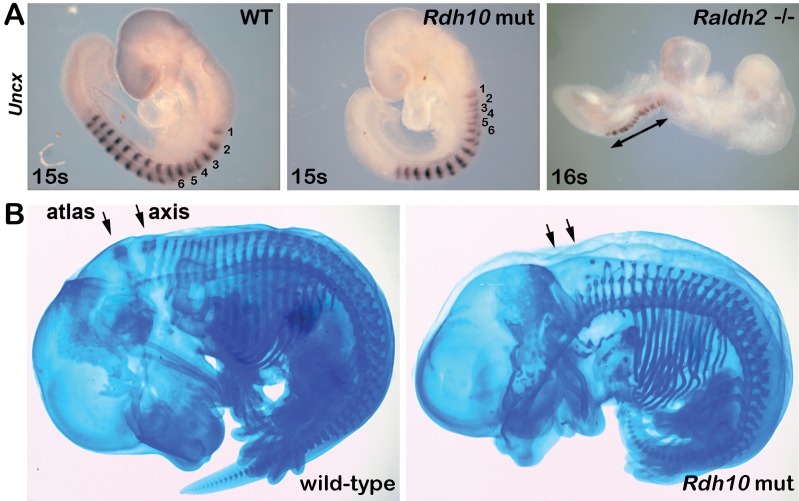
To gain further insight into caudal RA-FGF antagonism during body axis extension, we examined Rdh10 trex/trex mice (Rdh10 mutants) that lack RA activity in somitic mesoderm but retain RA activity in the neural tube and survive longer than Raldh2-/- mice [38]. Rdh10 mutants examined between the 8–15 somite stages exhibited an axial defect in which the first 6 somites were consistently shorter along the anteroposterior axis (axial length between somites 1–6 reduced to 60% of wild-type), whereas somite size returned to normal beginning with somite 7 (Fig 3A; n = 8/8). For comparison, analysis of Raldh2-/- embryos examined between the 8–16 somite stages demonstrated that all somites were smaller along the anteroposterior axis (Fig 3A; n = 11/11). To obtain further insight we examined Rdh10 mutants at later stages; after E9.5, somites 1–4 normally degenerate and contribute to the occipital bone at the base of the skull, whereas somites 5 and 6 form the atlas and axis vertebrae, respectively [39]. Alcian blue staining of vertebrae at E14.5 revealed that Rdh10 mutants lack the prominent dorsal extensions normally observed in the atlas and axis vertebra derived from somites 5 and 6, whereas more posterior vertebrae were comparatively normal (Fig 3B; n = 2/2). Our observation that the first six somites of Rdh10 mutants are small but that somite size returns to normal afterwards provides further evidence that anterior trunk mesoderm is developmentally distinct from the posterior trunk, and may require different genetic and signaling networks for its formation as previously described [40,41,42,43]. Together, these observations demonstrate that Rdh10 mutants undergo an early period where somites appear small, but that somitogenesis appears normalized by the 7-somite stage.
Fig 3. Rdh10 mutants exhibit a small somite defect during early but not late stages.
(A) Uncx mRNA in WT, Rdh10 mutants, and Raldh2-/- embryos at the 15–16 somite stage. Numbers marking the first 6 somites of WT and Rdh10 mutants reveal a temporary shortening of somite size along the anteroposterior axis in the Rdh10 mutant; arrows mark the region displaying a much larger region of small somites in Raldh2-/- embryos. (B) Alcian blue staining of E14.5 wild-type and Rdh10 mutant embryos was performed; arrows indicate that the mutant lacks the atlas and axis vertebrae derived from somites 5 and 6, respectively.
Rdh10 mutants at the 2–3 somite stages exhibited more intense caudal Fgf8 expression in the anterior region of its normal caudal epiblast domain as well as some ectopic expression located anterior to its normal domain (n = 4/4), similar to Raldh2-/- embryos (n = 3/3) (Fig 4A). At both the 6-somite and 8-somite stages, the caudal Fgf8 expression domain in Rdh10 mutants had returned to normal (n = 4/4), whereas Raldh2-/- embryos still exhibited ectopic anterior expansion (n = 4/4) (Fig 4B). These findings show that during early somite stages when Rdh10 mutants exhibit a small somite defect, they cannot properly restrict their caudal Fgf8 expression domain, but caudal Fgf8 expression is normal by the 6-somite stage at which point somite size returns to normal. Together with our observations above, these findings provide evidence that RA controls both axial stem cell differentiation and somitogenesis by restricting caudal Fgf8 expression.
Fig 4. Rdh10 mutants exhibit ectopic caudal Fgf8 expression at early but not late stages.
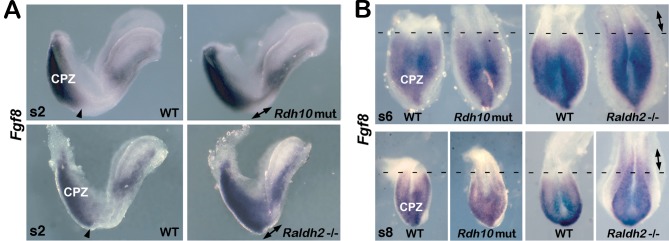
(A) Fgf8 mRNA in 2-somite (2s) embryos. In WT, arrowheads mark the normal anterior border for caudal Fgf8 expression. In mutants, arrows mark regions of expanded Fgf8 expression within and anterior to its normal caudal domain. (B) Fgf8 mRNA in the caudal region (anterior side up) at stages somite-6 (s6) and somite-8 (s8). Dotted lines mark the normal anterior border for caudal Fgf8 expression in WT. Arrows mark regions of expanded Fgf8 expression anterior to its normal caudal domain seen for Raldh2-/- embryos but not Rdh10 mutants. CPZ, caudal progenitor zone.
RA signaling in undifferentiated neural progenitors is sufficient to control somitogenesis
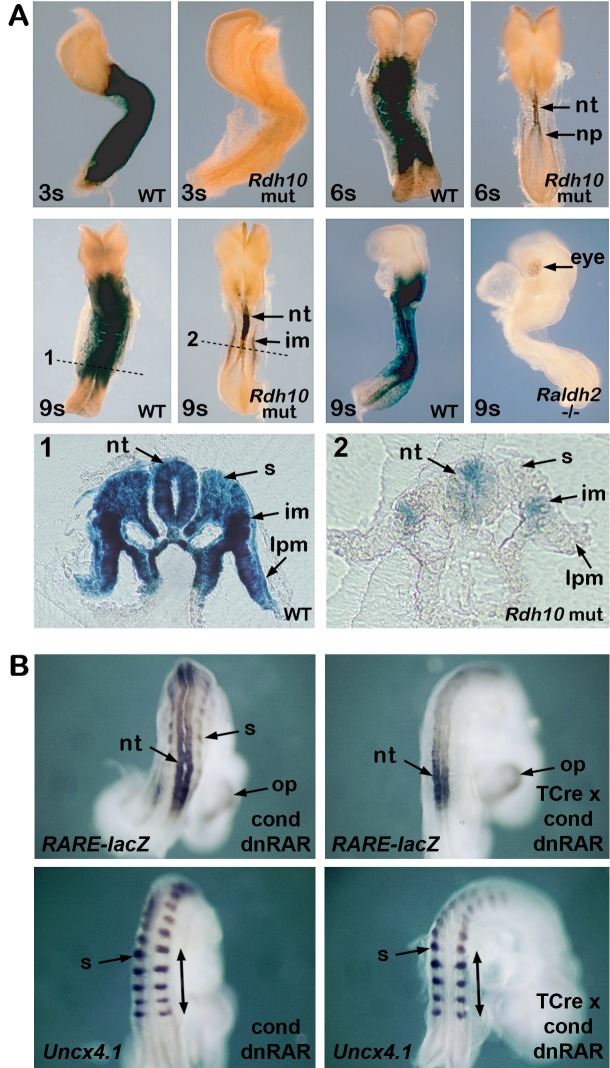
Fgf8 transcription is detected in caudal undifferentiated cells but not in anterior presomitic mesoderm or neural plate [33]. Our previous studies on Raldh2-/- embryos suggested that RA acts in the neural plate and node to control somite bilateral symmetry [27]. However, other studies suggested that RA functions in the presomitic mesoderm to control somitogenesis [44]. To help resolve this controversy, we further examined Rdh10 mutants which lack mesodermal RA activity but retain neural RA activity at E9.5 [38]. We determined in more detail the onset and location of RA activity in Rdh10 mutants using RARE-lacZ RA-reporter mice [45]. RARE-lacZ is a very sensitive RA-reporter as it is induced by 0.25 nM RA, (near the K d values reported for RA binding to RARs) as shown previously [46]. We found that RARE-lacZ expression initiated late and in a restricted location in Rdh10 mutants. At the 1–4 somite stages, RARE-lacZ expression was not detected in Rdh10 mutants, showing that Rdh10 is required to provide retinaldehyde for RA production during these early stages (Fig 5A; n = 0/4). At the 6-somite stage, Rdh10 mutants exhibited RARE-lacZ expression in the posterior neuroectoderm extending caudally to the neural plate, but not in other tissues (Fig 5A; n = 4/4). RARE-lacZ expression in neuroectoderm but not somitic mesoderm was also observed in Rdh10-/- null embryos [47], showing that neural RA activity is not due to residual activity of mutant RDH10 protein in Rdh10 trex/trex embryos but instead is due to another retinol-metabolizing enzyme of unknown identity that contributes to neural RA synthesis beginning at the 6-somite stage (S1 Fig). This unknown activity is not provided by Cyp1b1, encoding a P450 enzyme that was previously suggested to contribute to neural RA synthesis [48], as Rdh10-/-;Cyp1b1-/- double mutants carrying RARE-lacZ still exhibited neuroectodermal RA activity and developed similarly to Rdh10 mutants, plus they exhibited lacZ signals in the telencephalon and diencephalon at E10.5 similar to wild-type and Rdh10 mutants (S1 Fig). At the 9-somite stage, Rdh10 mutants still exhibited RARE-lacZ expression in posterior neuroectoderm but not in presomitic or somitic mesoderm (Fig 5A; n = 2/2). For comparison, Raldh2-/- embryos at 9–10 somites were devoid of RARE-lacZ expression except for weak expression in the eye (Fig 5A; n = 3/3). Together, our observations suggest that the transient small somite phenotype observed in Rdh10 mutants from the 1–6 somite stages is due to a complete lack of early RA synthesis, and that late-occurring RA activity first seen at the 6-somite stage in the neural plate is sufficient to establish normal somitogenesis.
Fig 5. RA signaling in neural plate is sufficient to control somitogenesis.
(A) RA activity was visualized in embryos carrying the RARE-lacZ RA-reporter transgene using staining for beta-galactosidase activity. At the 3-somite (3s) stage RARE-lacZ expression is observed in a wild-type (WT) embryo but not in an Rdh10 mutant. At the 6s stage Rdh10 mutants exhibit RARE-lacZ expression in posterior neural tube (nt) and neural plate (np). At the 9s stage Rdh10 mutants exhibit RARE-lacZ expression in posterior neuroectoderm and intermediate mesoderm (im); (1–2) transverse sections through the regions marked with dotted lines in 9s embryos; lpm, lateral plate mesoderm; s, somitic mesoderm. At the 9s stage Raldh2-/- embryos exhibit no RARE-lacZ expression except for weak expression in the eye. (B) Shown are mouse embryos at E8.5 carrying the conditional dominant-negative RAR construct RAR403 (cond dnRAR) or embryos that carry both RAR403 and TCre expressed in mesoderm (TCre x dnRAR). RARE-lacZ expression detected by lacZ in situ hybridization (to monitor RA activity) shows that TCre-activation of RAR403 prevents RA activity in somitic mesoderm (s) but not neural tube (nt) or optic cup (op). Uncx expression shows that loss of mesodermal RA activity does not affect somite size (bars mark last 5 somites generated).
As an independent means of testing whether RA activity in mesoderm is required for normal somitogenesis, we utilized RAR403 mice carrying a dominant-negative RAR (dnRAR) expressed conditionally using Cre/lox technology [49]. When crossed with TCre mice that express Cre only in mesoderm prior to E9.0 [50] and with RARE-lacZ, we found that RARE-lacZ expression in E8.5 embryos was absent in mesoderm but still present in neuroectoderm (n = 3/3) and that somitogenesis remained normal (n = 3/3) (Fig 5B). The reciprocal experiment to selectively eliminate RA activity in neural plate but not mesoderm was attempted by crossing RAR403 dnRAR mice with Sox2Cre [51] since Sox2 is expressed higher in neural plate than caudal epiblast. However, the resultant embryos did not display a clear reduction in neural RARE-lacZ (while a reduction was evident in the mesoderm) and somitogenesis as well as overall embryonic development appeared normal (S2 Fig). This experiment would require a different Cre expressed specifically in neural plate, but it is possible that the dnRAR may not fully repress neural RA signaling (by outcompeting endogenous RARs) due to the observation that mouse RARb is expressed much higher in the neural tube than the somites [52,53]. However, our observations here with Rdh10 mutants and dnRAR/TCre embryos combined with the previous observations that Fgf8 is transcribed in caudal undifferentiated cells but not anterior presomitic mesoderm [33] and that RA can repress caudal Fgf8 transcription via a RARE [32], suggests that RA transcriptional activity in undifferentiated neural progenitors is sufficient to control the Fgf8 mRNA gradient for somitogenesis, although we cannot completely rule out a redundant post-transcriptional role for RA in regulating Fgf8 mRNA in the presomitic mesoderm.
Discussion
The studies reported here provide important new insight into how extrinsic signals coordinate somitogenesis with neurogenesis. Previous studies demonstrated that axial stem cell differentiation normally results in a balance between progeny that express Sox2 (required for neuroectodermal fate) and progeny that express Tbx6 (required for mesodermal fate), and that the mechanism requires indirect repression of Sox2 by Tbx6 [13]. Also, expression of Sox2 or Sox3 in the caudal lateral epiblast is required to prevent excessive production of mesodermal progeny from axial stem cells [54]. Activation of Tbx6 in the axial stem cell niche is controlled by caudal FGF and Wnt signaling through a signaling loop in which FGF and Wnt positively activate each other [8,9,15,16,17,18,19]. In mouse, those studies revealed that FGF receptor 1 and caudal Fgf8 expression are required to maintain caudal expression of Wnt3a, which in turn is required to maintain caudal Fgf8 expression, with this feedback loop being required for Tbx6 expression during mesoderm induction. Our studies here support and further extend previous studies showing that RA is required to restrict the anterior extent of caudal Fgf8 expression in chick and mouse [24,26,27,30] and another study in chick embryos showing that RA effects caudal Sox2/Tbx6 expression at the tailbud stage when body axis extension is terminated [9]. Our Raldh2, Rdh10, and dnRAR/TCre genetic studies in mouse support a model in which progeny of the axial stem cell population produce a diffusible RA signal that acts in undifferentiated neural progenitors to restrict the anterior extent of caudal Fgf8 expression in order to control a gene regulatory network underlying both neurogenesis and somitogenesis (Fig 6). As a result of this ability to restrict Fgf8 expression, RA signaling functions as a rheostat to dial in the correct amount of caudal FGF signaling needed to coordinate somitogenesis with neurogenesis. This mechanism ensures that generation of presomitic mesodermal progeny is restricted and that mesodermal motility is appropriately graded to allow controlled differentiation into somites, while normal differentiation of neural progeny (i.e. Sox1 activation) is also permitted. Our studies thus reveal that the function of RA during body axis extension is that of a progeny-generated signal regulating differentiation of its parent stem cell population.
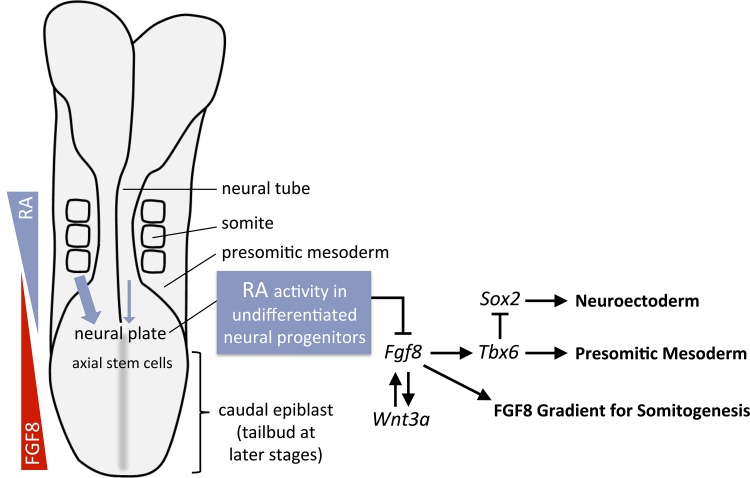
Fig 6. RA control of Fgf8 coordinates somitogenesis with neurogenesis.
Previous studies have shown that RA pushes posterior undifferentiated cells towards differentiation [24,25,26,27]. Our studies support a model in which RA generated by either presomitic mesoderm (high amount—thick blue arrow) or posterior neuroectoderm (low amount—thin blue arrow) functions in undifferentiated neural progenitors to control both neurogenesis and somitogenesis by restricting Fgf8 expression. RA participates in a gene regulatory network in which activation of Tbx6 in the axial stem cell niche is dependent on signaling controlled by Fgf8 and Wnt3a, with Wnt3a and Fgf8 participating in an autoregulatory loop. RA restricts Fgf8 expression to provide the correct amount of both Tbx6 and Sox2 expression, with Sox2 being repressed by Tbx6 during generation of mesodermal progeny. By limiting Fgf8 expression in undifferentiated neural progenitors, RA also establishes the anterior boundary of the Fgf8 mRNA gradient in presomitic mesoderm that controls somitogenesis.
Our findings suggest a mechanism for how loss of RA in Raldh2-/- embryos results in smaller somites, an expanded region of presomitic mesoderm, and ultimately premature cessation of body axis elongation [25,26,27]. We propose that ectopic Fgf8 expression observed in Raldh2-/- embryos not only disturbs the caudal-high motility gradient needed for normal somitogenesis and body axis extension [36], but also expands Tbx6 expression in the axial stem cell niche that may inhibit somite formation as this promotes a migratory presomitic mesodermal fate that must be efficiently down-regulated to form somites [55]; thus fewer cells contribute to somites resulting in smaller somites accompanied by expansion of unsegmented presomitic mesoderm caudally. In addition, we propose that lower Sox2 expression in the caudal epiblast and neural plate of Raldh2-/- embryos may be due to ectopic expression of Tbx6 in the caudal epiblast that may repress Sox2, leading to loss of axial stem cell maintenance. This general failure to coordinately form mature body axis tissues, born out of an imbalance in the axial stem cell niche due to excessive FGF signaling, may explain premature cessation of body axis elongation in RA-deficient embryos.
Previous studies have shown that Fgf8 transcription is detected caudally in undifferentiated cells but not in anterior presomitic mesoderm or neural plate; after presomitic mesoderm cells exit the caudal undifferentiated cell population and turn off Fgf8 transcription, they retain Fgf8 mRNA which persists for some time and forms a caudal-high gradient of Fgf8 mRNA that in presomitic mesoderm terminates anteriorly near the somite formation boundary [33]. Additionally, our previous studies have demonstrated that RA can repress caudal Fgf8 expression directly at the transcriptional level [7]. As our studies here show that RA activity in undifferentiated neural progenitors is sufficient to properly regulate caudal Fgf8 expression and somite size, RA is not required to act in presomitic mesoderm to control the anteroposterior positioning of the mesodermal Fgf8 mRNA gradient. Our conclusions are thus in contrast to previous studies in Xenopus suggesting that RA functions in presomitic mesoderm to control somitogenesis [44]; those studies showed that RA treatment can induce anterior presomitic mesodermal expression of Xenopus Mesp2, a gene required to generate the somite formation boundary, and it was reported that the Xenopus Mesp2 gene has a RARE. However, later studies in mouse Raldh2-/- embryos showed that endogenous RA is not required for expression of Mesp2 [27], demonstrating that results of RA treatment do not always correlate with endogenous RA function. Also, as vertebrate genomes contain more than 10,000 RAREs, many of which may be non-functional [21], it is possible that the genomic RARE located near Xenopus Mesp2 may not be required in vivo although it can function in a transgenic assay. Likewise, although studies on the Fgf8 RARE in transgenic mice suggest that RA can directly repress caudal Fgf8 [32], further studies will be needed to determine whether RA directly represses transcription of the genomic Fgf8 locus solely through this RARE or whether another molecular mechanism also exists to restrict caudal Fgf8 expression.
The knowledge gained by our studies provides new mechanistic insight into the molecular networks controlling robust differentiation of stem/progenitor cells into specialized cells. Awareness of how diffusible RA and FGF signals normally interact in vivo to control stem/progenitor cell differentiation will aid efforts to generate differentiated cells useful in therapeutic applications.
Materials and Methods
Mouse mutants and transgenes
Rdh10 trex/trex mice (Rdh10 mutants) were generated via ethylnitrosourea mutagenesis as previously described [38]. Rdh10 mutant embryos were identified by DNA sequencing of a PCR product overlapping the mutation from analysis of yolk sac DNA [38]. Rdh10 mutants were mated with RARE-lacZ RA-reporter transgenic mice [45], and the offspring were employed throughout the study; Rdh10-/- knockout embryos were described previously [47]. Raldh2-/- mice carrying RARE-lacZ have been previously described [27]. TCre mice were described previously [50]. RAR403 mice carrying a dominant-negative RAR (dnRAR) [49] were obtained from Dr. Shanthini Sockanathan (Johns Hopkins University, Baltimore, MD, USA). Cyp1b1-/- mice [56] were provided by Dr. Simon John (Jackson Laboratory, Bar Harbor, ME, USA). Sox2Cre mice [51] were purchased from The Jackson Laboratory [Tg(Sox2-cre)1Amc/J, stock # 004783]. Genotyping of each strain was performed by PCR analysis of yolk sac DNA. All mouse studies conformed to the regulatory standards adopted by the Institutional Animal Care and Use Committee at the Sanford Burnham Prebys Medical Discovery Institute which approved this study under Animal Welfare Assurance Number A3053-01 (permit #13–003).
In vitro culture of mouse embryos
Wild-type, Rdh10 mutant, and Raldh2-/- embryos at the 6–9 somite stages were cultured for 12 hr in serum-free (retinoid-free) DMEM/F-12 culture media (Gibco-Life Technologies) in Millicell culture plate inserts (Millipore) at 37°C in 5% CO2; treatments included 20 micromolar SU 5402 (Tocris Bioscience) or an equal amount of dimethylsulfoxide vehicle (control). After culture, embryos were stained with X-Gal for 18 hr or processed for in situ hybridization.
Detection of mRNA, retinoic acid, and cartilage
Detection of mRNA and tissue sectioning was performed by whole mount in situ hybridization as previously described [27]. RA activity was detected in mouse embryos carrying the RARE-lacZ transgene by staining 18 hr for beta-galactosidase activity [45]. Alcian blue staining of cartilage was performed as previously described [38].
Supporting Information
Wild type, Cyp1b1-/-, Rdh10-/-, and Rdh10-/-;Cyp1b1-/- double mutants carrying the RARE-lacZ RA-reporter transgene and stained for beta-galactosidase activity at E10.5.
(TIF)
Lateral views (upper panels) and dorsal views (lower panels) of E8.5 embryos carrying the RARE-lacZ RA-reporter transgene and the dnRAR transgene, or additionally carrying Sox2Cre. Embryos were stained for beta-galactosidase activity; np, neural plate.
(TIF)
Acknowledgments
We thank the Sanford Burnham Prebys Animal Resources Core Facility for conducting timed-matings to generate mouse embryos.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by National Institutes of Health grants GM062848 (GD) and DE016082 (PT) (www.nih.gov), and the Stowers Institute for Medical Research (PT) (www.stowers.org). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gudas LJ, Wagner JA (2011) Retinoids regulate stem cell differentiation. J Cell Physiol 226: 322–330. 10.1002/jcp.22417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rochette-Egly C (2015) Retinoic acid signaling and mouse embryonic stem cell differentiation: Cross talk between genomic and non-genomic effects of RA. Biochimica et Biophysica Acta 1851: 66–75. 10.1016/j.bbalip.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 3. Kurlandsky SB, Gamble MV, Ramakrishnan R, Blaner WS (1995) Plasma delivery of retinoic acid to tissues in the rat. J Biol Chem 270: 17850–17857. [DOI] [PubMed] [Google Scholar]
- 4. Horton C, Maden M (1995) Endogenous distribution of retinoids during normal development and teratogenesis in the mouse embryo. Dev Dyn 202: 312–323. [DOI] [PubMed] [Google Scholar]
- 5. Mic FA, Molotkov A, Benbrook DM, Duester G (2003) Retinoid activation of retinoic acid receptor but not retinoid X receptor is sufficient to rescue lethal defect in retinoic acid synthesis. Proc Natl Acad Sci USA 100: 7135–7140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sheikh BN, Downer NL, Kueh AJ, Thomas T, Voss AK (2014) Excessive versus Physiologically Relevant Levels of Retinoic Acid in Embryonic Stem Cell Differentiation. Stem Cells 32: 1451–1458. 10.1002/stem.1604 [DOI] [PubMed] [Google Scholar]
- 7. Wilson V, Olivera-Martinez I, Storey KG (2009) Stem cells, signals and vertebrate body axis extension. Development 136: 1591–1604. 10.1242/dev.021246 [DOI] [PubMed] [Google Scholar]
- 8. Martin BL, Kimelman D (2012) Canonical Wnt Signaling Dynamically Controls Multiple Stem Cell Fate Decisions during Vertebrate Body Formation. Dev Cell 22: 223–232. 10.1016/j.devcel.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Olivera-Martinez I, Harada H, Halley PA, Storey KG (2012) Loss of FGF-Dependent Mesoderm Identity and Rise of Endogenous Retinoid Signalling Determine Cessation of Body Axis Elongation. Plos Biology 10: e1001415 10.1371/journal.pbio.1001415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tsakiridis A, Huang Y, Blin G, Skylaki S, Wymeersch F, Osorno R, et al. (2014) Distinct Wnt-driven primitive streak-like populations reflect in vivo lineage precursors. Development 141: 1209–1221. 10.1242/dev.101014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tzouanacou E, Wegener A, Wymeersch FJ, Wilson V, Nicolas JF (2009) Redefining the progression of lineage segregations during mammalian embryogenesis by clonal analysis. Dev Cell 17: 365–376. 10.1016/j.devcel.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 12. Martin BL, Kimelman D (2009) Wnt signaling and the evolution of embryonic posterior development. Curr Biol 19: R215–219. 10.1016/j.cub.2009.01.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takemoto T, Uchikawa M, Yoshida M, Bell DM, Lovell-Badge R, Papaioannou VE, et al. (2011) Tbx6-dependent Sox2 regulation determines neural or mesodermal fate in axial stem cells. Nature 470: 394–398. 10.1038/nature09729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chapman DL, Papaioannou VE (1998) Three neural tubes in mouse embryos with mutations in the T-box gene Tbx6. Nature 391: 695–697. [DOI] [PubMed] [Google Scholar]
- 15. Ciruna B, Rossant J (2001) FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev Cell 1: 37–49. [DOI] [PubMed] [Google Scholar]
- 16. Aulehla A, Wehrle C, Brand-Saberi B, Kemler R, Gossler A, Kanzler B, et al. (2003) Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev Cell 4: 395–406. [DOI] [PubMed] [Google Scholar]
- 17. Dunty WC Jr., Biris KK, Chalamalasetty RB, Taketo MM, Lewandoski M, Yamaguchi TP (2008) Wnt3a/beta-catenin signaling controls posterior body development by coordinating mesoderm formation and segmentation. Development 135: 85–94. [DOI] [PubMed] [Google Scholar]
- 18. Naiche LA, Holder N, Lewandoski M (2011) FGF4 and FGF8 comprise the wavefront activity that controls somitogenesis. Proc Natl Acad Sci USA 108: 4018–4023. 10.1073/pnas.1007417108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boulet AM, Capecchi MR (2012) Signaling by FGF4 and FGF8 is required for axial elongation of the mouse embryo. Dev Biol 371: 235–245. 10.1016/j.ydbio.2012.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jurberg AD, Aires R, Novoa A, Rowland JE, Mallo M (2014) Compartment-dependent activities of Wnt3a/beta-catenin signaling during vertebrate axial extension. Dev Biol 394: 253–263. 10.1016/j.ydbio.2014.08.012 [DOI] [PubMed] [Google Scholar]
- 21. Cunningham TJ, Duester G (2015) Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nature Rev Mol Cell Bio 16: 110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duester G (2008) Retinoic acid synthesis and signaling during early organogenesis. Cell 134: 921–931. 10.1016/j.cell.2008.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Niederreither K, Dolle P (2008) Retinoic acid in development: towards an integrated view. Nature Rev Genet 9: 541–553. 10.1038/nrg2340 [DOI] [PubMed] [Google Scholar]
- 24. Diez del Corral R, Olivera-Martinez I, Goriely A, Gale E, Maden M, Storey K (2003) Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron 40: 65–79. [DOI] [PubMed] [Google Scholar]
- 25. Niederreither K, Subbarayan V, Dollé P, Chambon P (1999) Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nature Genet 21: 444–448. [DOI] [PubMed] [Google Scholar]
- 26. Vermot J, Llamas JG, Fraulob V, Niederreither K, Chambon P, Dollé P (2005) Retinoic acid controls the bilateral symmetry of somite formation in the mouse embryo. Science 308: 563–566. [DOI] [PubMed] [Google Scholar]
- 27. Sirbu IO, Duester G (2006) Retinoic acid signaling in node ectoderm and posterior neural plate directs left-right patterning of somitic mesoderm. Nature Cell Biol 8: 271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martin BL, Kimelman D (2010) Brachyury establishes the embryonic mesodermal progenitor niche. Genes Dev 24: 2778–2783. 10.1101/gad.1962910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wahl MB, Deng C, Lewandoski M, Pourquie O (2007) FGF signaling acts upstream of the NOTCH and WNT signaling pathways to control segmentation clock oscillations in mouse somitogenesis. Development 134: 4033–4041. [DOI] [PubMed] [Google Scholar]
- 30. Vermot J, Pourquié O (2005) Retinoic acid coordinates somitogenesis and left-right patterning in vertebrate embryos. Nature 435: 215–220. [DOI] [PubMed] [Google Scholar]
- 31. Patel NS, Rhinn M, Semprich CI, Halley PA, Dolle P, Bickmore WA, et al. (2013) FGF Signalling Regulates Chromatin Organisation during Neural Differentiation via Mechanisms that Can Be Uncoupled from Transcription. PLoS Genetics 9: e1003614 10.1371/journal.pgen.1003614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kumar S, Duester G (2014) Retinoic acid controls body axis extension by directly repressing Fgf8 transcription. Development 141: 2972–2977. 10.1242/dev.112367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dubrulle J, Pourquié O (2004) fgf8 mRNA decay establishes a gradient that couples axial elongation to patterning in the vertebrate embryo. Nature 427: 419–422. [DOI] [PubMed] [Google Scholar]
- 34. Yu SR, Burkhardt M, Nowak M, Ries J, Petrasek Z, Scholpp S, et al. (2009) Fgf8 morphogen gradient forms by a source-sink mechanism with freely diffusing molecules. Nature 461: 533–536. 10.1038/nature08391 [DOI] [PubMed] [Google Scholar]
- 35. Ribes V, Le Roux I, Rhinn M, Schuhbaur B, Dolle P (2009) Early mouse caudal development relies on crosstalk between retinoic acid, Shh and Fgf signalling pathways. Development 136: 665–676. 10.1242/dev.016204 [DOI] [PubMed] [Google Scholar]
- 36. Benazeraf B, Francois P, Baker RE, Denans N, Little CD, Pourquie O (2010) A random cell motility gradient downstream of FGF controls elongation of an amniote embryo. Nature 466: 248–252. 10.1038/nature09151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mohammadi M, McMahon G, Sun L, Tang C, Hirth P, Yeh BK, et al. (1997) Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science 276: 955–960. [DOI] [PubMed] [Google Scholar]
- 38. Sandell LL, Sanderson BW, Moiseyev G, Johnson T, Mushegian A, Young K, et al. (2007) RDH10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes Dev 21: 1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kaufman MH, Bard JBL (1999) The Anatomical Basis of Mouse Development. San Diego: Academic Press; 103–104 p. [Google Scholar]
- 40. Herrmann BG, Labeit S, Poustka A, King TR, Lehrach H (1990) Cloning of the T gene required in mesoderm formation in the mouse. Nature 343: 617–622. [DOI] [PubMed] [Google Scholar]
- 41. Yamaguchi TP, Takada S, Yoshikawa Y, Wu N, McMahon AP (1999) T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes Dev 13: 3185–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Szeto DP, Kimelman D (2006) The regulation of mesodermal progenitor cell commitment to somitogenesis subdivides the zebrafish body musculature into distinct domains. Genes Dev 20: 1923–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shifley ET, Vanhorn KM, Perez-Balaguer A, Franklin JD, Weinstein M, Cole SE (2008) Oscillatory lunatic fringe activity is crucial for segmentation of the anterior but not posterior skeleton. Development 135: 899–908. 10.1242/dev.006742 [DOI] [PubMed] [Google Scholar]
- 44. Moreno TA, Kintner C (2004) Regulation of segmental patterning by retinoic acid signaling during Xenopus somitogenesis. Dev Cell 6: 205–218. [DOI] [PubMed] [Google Scholar]
- 45. Rossant J, Zirngibl R, Cado D, Shago M, Giguère V (1991) Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev 5: 1333–1344. [DOI] [PubMed] [Google Scholar]
- 46. Cunningham TJ, Zhao X, Sandell LL, Evans SM, Trainor PA, Duester G (2013) Antagonism between retinoic acid and fibroblast growth factor signaling during limb development. Cell Reports 3: 1503–1511. 10.1016/j.celrep.2013.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chatzi C, Cunningham TJ, Duester G (2013) Investigation of retinoic acid function during embryonic brain development using retinaldehyde-rescued Rdh10 knockout mice. Dev Dyn 242: 1056–1065. 10.1002/dvdy.23999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chambers D, Wilson L, Maden M, Lumsden A (2007) RALDH-independent generation of retinoic acid during vertebrate embryogenesis by CYP1B1. Development 134: 1369–1383. [DOI] [PubMed] [Google Scholar]
- 49. Rajaii F, Bitzer ZT, Xu Q, Sockanathan S (2008) Expression of the dominant negative retinoid receptor, RAR403, alters telencephalic progenitor proliferation, survival, and cell fate specification. Dev Biol 316: 371–382. 10.1016/j.ydbio.2008.01.041 [DOI] [PubMed] [Google Scholar]
- 50. Perantoni AO, Timofeeva O, Naillat F, Richman C, Pajni-Underwood S, Wilson C, et al. (2005) Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development 132: 3859–3871. [DOI] [PubMed] [Google Scholar]
- 51. Hayashi S, Lewis P, Pevny L, McMahon AP (2002) Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Gene Expr Patterns 2: 93–97. [DOI] [PubMed] [Google Scholar]
- 52. Mendelsohn C, Ruberte E, LeMeur M, Morriss-Kay G, Chambon P (1991) Developmental analysis of the retinoic acid-inducible RAR-beta2 promoter in transgenic animals. Development 113: 723–734. [DOI] [PubMed] [Google Scholar]
- 53. de The H, Marchio A, Tiollais P, Dejean A (1989) Differential expression and ligand regulation of the retinoic acid receptor alpha and beta genes. EMBO Journal 8: 429–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yoshida M, Uchikawa M, Rizzoti K, Lovell-Badge R, Takemoto T, Kondoh H (2014) Regulation of mesodermal precursor production by low-level expression of B1 Sox genes in the caudal lateral epiblast. Mechanisms of Development 132: 59–68. 10.1016/j.mod.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 55. Oginuma M, Niwa Y, Chapman DL, Saga Y (2008) Mesp2 and Tbx6 cooperatively create periodic patterns coupled with the clock machinery during mouse somitogenesis. Development 135: 2555–2562. 10.1242/dev.019877 [DOI] [PubMed] [Google Scholar]
- 56. Buters JT, Sakai S, Richter T, Pineau T, Alexander DL, Savas U, et al. (1999) Cytochrome P450 CYP1B1 determines susceptibility to 7, 12-dimethylbenz[a]anthracene-induced lymphomas. Proc Natl Acad Sci USA 96: 1977–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Wild type, Cyp1b1-/-, Rdh10-/-, and Rdh10-/-;Cyp1b1-/- double mutants carrying the RARE-lacZ RA-reporter transgene and stained for beta-galactosidase activity at E10.5.
(TIF)
Lateral views (upper panels) and dorsal views (lower panels) of E8.5 embryos carrying the RARE-lacZ RA-reporter transgene and the dnRAR transgene, or additionally carrying Sox2Cre. Embryos were stained for beta-galactosidase activity; np, neural plate.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.