Abstract
Despite the potential for patient-reported outcome measures (PROMs) and experience measures (PREMs) to enhance understanding of patient experiences and outcomes they have not, to date, been widely incorporated into renal registry datasets. This report summarizes the main points learned from an ERA-EDTA QUEST-funded consensus meeting on how to routinely collect PROMs and PREMs in renal registries in Europe. In preparation for the meeting, we surveyed all European renal registries to establish current or planned efforts to collect PROMs/PREMs. A systematic review of the literature was performed. Publications reporting barriers and/or facilitators to PROMs/PREMs collection by registries were identified and a narrative synthesis undertaken. A group of renal registry representatives, PROMs/PREMs experts and patient representatives then met to (i) share any experience renal registries in Europe have in this area; (ii) establish how patient-reported data might be collected by understanding how registries currently collect routine data and how patient-reported data is collected in other settings; (iii) harmonize the future collection of patient-reported data by renal registries in Europe by agreeing upon preferred instruments and (iv) to identify the barriers to routine collection of patient-reported data in renal registries in Europe. In total, 23 of the 45 European renal registries responded to the survey. Two reported experience in collecting PROMs and three stated that they were actively exploring ways to do so. The systematic review identified 157 potentially relevant articles of which 9 met the inclusion criteria and were analysed for barriers and facilitators to routine PROM/PREM collection. Thirteen themes were identified and mapped to a three-stage framework around establishing the need, setting up and maintaining the routine collection of PROMs/PREMs. At the consensus meeting some PROMs instruments were agreed for routine renal registry collection (the generic SF-12, the disease-specific KDQOL™-36 and EQ-5D-5L to be able to derive quality-adjusted life years), but further work was felt to be needed before recommending PREMs. Routinely collecting PROMs and PREMs in renal registries is important if we are to better understand what matters to patients but it is likely to be challenging; close international collaboration will be beneficial.
Keywords: patient-reported measures, quality indicators, registry
INTRODUCTION
Established renal failure is a chronic disease with significant associated morbidity. Although it affects a small proportion of the general population (0.06–0.12% in countries across Europe in 2012) (Table A.4.3 in [1]), the quality of life of those affected is markedly lower than for those with most other chronic conditions and cancers [2]. Standard dialysis provides the equivalent of only 10% of kidney function so many patients are chronically tired, depressed and suffer pain; and many of these symptoms go unrecognized [3].
The success of treatments for renal failure has been historically assessed using measures considered important by doctors, such as phosphate level or urea clearance. Although instruments measuring the patient's perspective have been available for decades, their incorporation into routine clinical practice has until recently been slow. A number of patient-reported measures exist:
Patient-reported outcome measures (PROMs) include any metric assessing health, illness or health care benefits from the patient's perspective; in general they take the form of a questionnaire and more specifically a quality of life or symptom questionnaire. In routine clinical practice, PROMs have the potential to highlight relevant symptoms and changes in symptoms, promote patient engagement in their treatment [4] and improve patient outcomes [5]. Summarizing PROM results across individual patients, for example at the level of treatments or hospitals, they could be used to inform a patient's choice of treatment or assess quality of care across different hospitals [6, 7]. The importance of PROMs as end points in clinical trials is also increasingly being recognized [8].
Patient-reported experience measures (PREMs) capture information about the healthcare experience as perceived by the patient. They can refer to a variety of issues, ranging from cleanliness of facilities to information provision, and from timeliness of transport to family members′ access to health professionals [9]. In routine clinical practice, PREMs can be used to improve quality in clinical services [10].
Measures reflecting aspects of patient involvement in their health care, including patient activation [11] and informed and shared decision-making [12].
Dialysis and transplantation are in the almost unique position of having an existing infrastructure of regional, national and international registries for collecting and reporting information on all patients receiving treatment. Quality of care, as measured against nationally and internationally agreed standards, can be publicly reported and compared between centres and between countries. These instruments were usually developed for measurement at the patient level, however, and caution will need to be exercised when comparing differences between centres or countries until more is known about their performance at these levels. To be able to report symptom burden and quality of life-adjusted survival alongside laboratory measures such as haemoglobin, calcium, phosphate and dialysis dose would be a major step forward in reporting what is important to patients. While some components of quality of life have been shown to be modifiable (e.g. the physical component summary score of the SF-36 in the Frequent Haemodialysis Network Short Daily Trial [13]), others reflect the broader social construct in which the patient functions which so could prove to be beyond the influence of the health care provider.
Part of this infrastructure is the ERA-EDTA Registry, which collects data on renal replacement therapy (RRT) via the national and regional renal registries in Europe: individual patient data is available from 31 national and regional registries in 17 countries and aggregated data from a further 14 national registries [1]. The data items collected vary by country but can include demographics, primary renal disease, RRT treatment history, date and cause of death, comorbidities, details of physical examination (e.g. weight, blood pressure), laboratory measurements (e.g. haemoglobin, albumin) and details of certain therapies given. At present, none of the national and regional registries report patient-reported outcome/experience measures to the ERA-EDTA Registry, but it is not certain what data are collected locally. This paper reports the results of a survey to capture existing experiences of European renal registries in PROM and PREM collection and a literature review of the facilitators and barriers to registries routinely collecting PROMs and PREMs. It then presents the discussions and conclusions of an international consensus meeting, funded by the QUEST initiative of the ERA-EDTA, aimed at promoting and harmonizing the routine collection of patient-reported data by European renal registries.
SURVEY OF EUROPEAN RENAL REGISTRIES
Prior to the consensus meeting, the organizers contacted all 45 European renal registries (using the ERA-EDTA Registry email contact list) asking for responses to the following questions:
Does your registry currently collect any quality of life or patient satisfaction/experience measures?
If so, what measures do you use and how are these data collected?
If not, have you tried to collect these measures in the past and/or do you have plans to collect these measures in the future?
The survey did not ask about patient involvement questionnaires, patient activation or shared decision making, as these had not been included in the original application for funding. Responses were received from 23 out of 45 registries. Only two registries (Austria and France) reported experience in the collection of PROMs/PREMs and a further three registries reported that they were actively exploring this possibility (Norway, Romania and Sweden). From these responses, there was no obvious consensus on the instruments or methods to use in the collection of PROMs and PREMs, although some barriers to the process were identified. These included low response rates, legal constraints and the burden on staff and patients.
LITERATURE REVIEW AND NARRATIVE SYNTHESIS
A systematic review of the literature was conducted with the primary aim of identifying facilitators and barriers to the routine collection of patient-reported measures by chronic disease registries. Medline and EMBASE databases were searched from 1981 to 2013 to identify papers reporting on the routine collection of patient-reported outcome, experience or quality of life data by chronic disease registries, including both renal and non-renal registries. Full details of the search terms are available in the Supplementary data. Abstracts of papers identified in the search were reviewed and full text versions obtained of those which appeared potentially relevant. Two reviewers (K.B. and F.C.) independently screened the full-text papers for relevancy, and—from the relevant papers—systematically identified any recurring themes across studies.
The search yielded 762 hits, of which 157 papers were deemed to be potentially relevant at the title and abstract screening stage based on pre-defined criteria; 9 papers were finally selected [14–22] and included in the narrative synthesis conducted according to guidelines provided by Popay et al. [23].
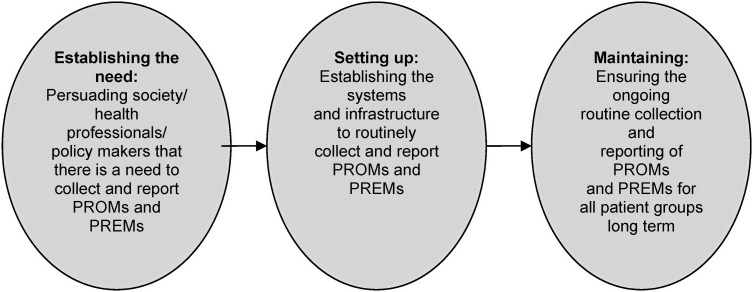
All nine of the selected papers were read by two independent researchers (K.B. and F.C.) and the two most relevant papers used to conduct a preliminary synthesis [20, 21]. Relationships between the data were then explored using all nine studies to develop a broad conceptual model in order to provide an appropriate framework for further exploration of relationships in the data (Figure 1). This framework comprised three stages: establishing the need for, setting-up and maintaining routine PROM/PREM data collection. In total, 13 themes were identified and agreed by two independent researchers (K.B. and F.C.) across the three stages of the conceptual model, each comprising a number of subthemes (Table 1). For each theme that resulted from the thematic analysis, we identified facilitators and barriers to implementation as reported in the selected papers, which we then translated into a series of recommendations (listed in Table 1).
Establishing the need for PROM/PREM data in registries was largely broken down into two main themes: recognizing the importance of PROMs and agreeing that, with all the other data they collect, registries provide a good backdrop for the collection of patient-reported data.
Several of the themes and subthemes were identified as important in setting up PROM/PREM collection by registries. Methodological issues associated with PROMs were identified, and recommendations developed based on the need for PROMs expertise, national and international support, stakeholder involvement (including patient and public involvement) and international standardization. As well as these themes, some specific issues relating to resources and practical aspects were identified.
More exclusive to maintaining a PROM/PREM programme were themes related to ensuring useful, high-impact output and the process of maintaining trust in the data. Several themes crossed the ‘set-up’ and ‘maintenance’ parts of the model, including subthemes under design and evaluation, and technological/information governance.
FIGURE 1:
Framework for narrative synthesis.
Table 1.
Summary of subthemes identified by the narrative synthesis process, organized by theme
| Establishing need |
|
| |
| Set up |
|
| |
| |
| |
| |
| |
| |
| |
| Maintenance |
|
| |
|
A number of these themes, such as the need for stakeholder involvement, PROMs expertise and international standardization, were used when planning the consensus meeting. The results of the review were also presented to the delegates during the preparatory session of the consensus meeting.
CONSENSUS MEETING
In June 2014, experts from across Europe participated in a consensus meeting in Bristol, UK. Invited to the meeting were participants representing all European renal registries with an interest in collecting PROMs and PREMs routinely; experts on PROMs, PREMs and shared decision making; a representative from the National Cancer Registry Ireland with experience in routine PROMs collection among cancer patients, and; patient and carer representatives from a large UK kidney patient charity the National Kidney Federation. The objectives of this meeting were to (i) share any experience renal registries in Europe already have in this area; (ii) establish how patient-reported data might be collected by understanding how registries currently collect routine data and how patient-reported data is collected in other settings; (iii) harmonize the future collection of patient-reported data by renal registries in Europe by agreeing upon preferred instruments and (iv) identify the barriers to routine collection of patient-reported data in renal registries in Europe.
The opening sessions of the meeting comprised the presentation of the results of the narrative synthesis followed by a series of presentations aimed at addressing the first two objectives. Representatives from four of the five renal registries with PROMs/PREMs experience (France [24], Norway, Romania and Sweden) and the National Cancer Registry Ireland shared their experience of PROMs/PREMs data collection. The director of the ERA-EDTA Registry then described the current state of routine data collection in renal registries in Europe; this was followed by presentations from three experts in PROMs, PREMs and shared decision making. Summaries of the three methodological presentations follow.
EXPERT REPORTS
Patient-reported outcome measures—Elizabeth Gibbons, patient-reported outcome measurement group, University of Oxford, UK
PROMs capture patients' perceptions of their health and quality of life, and vary according to factors such as method of completion (e.g. paper-based or electronic) and content (ranging from general items to more disease-specific symptoms). PROMs are useful for research purposes, clinical monitoring and more recent applications include service improvement and national benchmarking (e.g. the National PROMs programme in England for elective procedures). In addition to this established programme, several PROMs pilots are in progress in the UK, looking at long-term conditions (LTCs) in primary care, depression in secondary care, cardiac revascularization and skin cancer.
Several challenges have been identified through such pilot studies including poor response rates and concerns about benchmarking and performance management. However benefits include the value of individual patient monitoring, plus the potential for service improvement.
Selection of a PROM should be informed by a systematic review of the literature reporting psychometric properties and consideration of the practicalities of data collection. In 2009 the PROM group, University of Oxford, was commissioned by the Department of Health in England to review PROMs for chronic kidney disease and established renal failure [25]. Recommendations based on the strength of evidence included the SF-36 [26], EQ-5D-5L [27] and KDQOL™-36 [28] measures.
When selecting a PROM, consideration needs to be given to the purpose of measurement and practicalities of data collection. Complexity of scoring may outweigh the benefits of precision whereas short versions of instruments with narrow focus and simple indices may not provide breadth of information. Data linkage and clinical information systems can support collection and feedback of the data but may be complex to develop and maintain.
Patient-reported experience measures—Dr Sabine van der Veer, Amsterdam Medical Center, the Netherlands
Patient experience has long since been acknowledged as an important dimension of quality of care [29]. Whereas patient satisfaction is the perceived discrepancy between the expected and experienced quality of care, the construct of patient experience attempts to exclude the former element from the equation [30]. However, when translating these two constructs into actual measurement instruments, the distinction between them often becomes less clear.
During the consensus meeting, eight instruments were discussed that were either available in English or on which an English publication was available in PubMed [31–38]. Most included items on a broad spectrum of care delivery aspects [31, 32, 35, 36, 38]; others focussed on capturing experiences with specific aspects like education, or with treatment in general [32, 33, 36]. The number of items varied widely between instruments, ranging from eleven in the Renal Treatment Satisfaction Questionnaire [33] to over 60 in the Scottish Renal Patient Experience Survey [36]. Only two instruments were applicable to any type of RRT [33, 37], while others were designed for one [32, 34] or more dialysis modalities [31, 35, 36, 38].
Almost all instruments were developed with input from patients: for example, focus groups or interviews which identified relevant aspects of care [31–33, 35, 38] or cognitive testing of a preliminary version of the instrument [32, 33, 38]. In most cases, developers evaluated the instruments' internal consistency [33–35, 38, 39]. Some also assessed construct validity by exploring the correlation between patient experience as measured by the instrument and global assessments of satisfaction [34, 38], clinical performance [31] or health-related quality of life [34]. Outside a development context, wide scale use has only been reported for the CHOICE and the CAHPS questionnaire [40, 41].
Shared decision-making—Dr Hilary Bekker, University of Leeds, UK
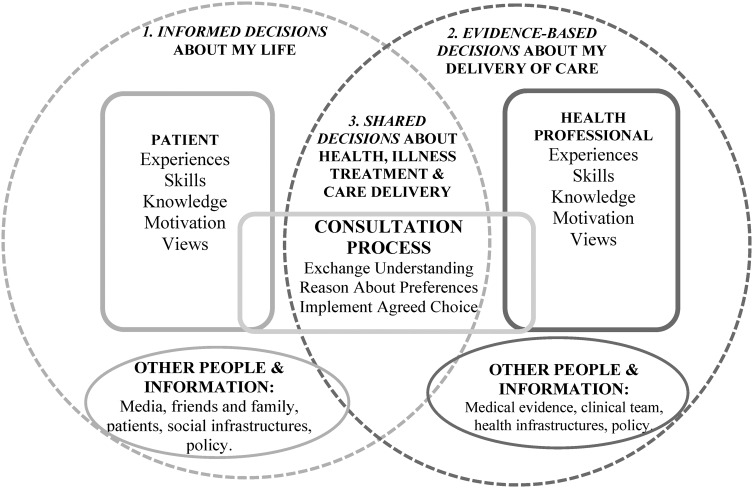
Measures used to assess shared decision making are predominantly self-report questionnaires designed to evaluate a decision support intervention's effectiveness [12, 42–45] and/or screen for decisional outcomes within usual care [46–51]. The substantial number of measures available reflects the complexity of these interventions in terms of their impact on different people within the process of delivering care and components needed to enhance people's active reasoning and engagement with others [52] (Figure 2).
FIGURE 2:
A framework representing informed, evidence-based and shared decisions with people and their healthcare roles.
Decision support intervention types include:
Patient Decision Aids [12, 45] enabling people to make informed decisions (point 1, Figure 2) between options by consideration of accurate information about all options and their consequences without bias, evaluation of this information with their values and making a decision on trade-offs between evaluations [12, 53, 54]. There are several patient-reported informed decision outcome measures [37, 38, 46–50, 55–59]. Alternatively, proxy outcomes may be used to assess an aid's impact by capturing people's knowledge, risk perception, values, involvement, activation, usefulness intervention, value-choice consistency and/or decision quality [12, 45, 60–62].
Professional Decision Support [63] enabling professionals to make evidence-based choices (point 2, Figure 2) by using the best evidence available, in consultation with the patient, to decide upon the option which best suits that patient [64].
Shared Decision-Making Support [65–68] within patient-professional consultations enabling the process of choosing healthcare collaboratively (point 3, Figure 2) by exchanging information, preferences and values about treatments, explicit reasoning about choices and agreeing a choice and implementation plan. Some measures assess patient-reported shared decision making outcomes [48, 69, 70]. Proxy outcomes assess an aid's impact on the professional (e.g. provided option information, elicited values, awareness of patient experience, etc.), [71–73] the patient (e.g. asked questions, provided values, awareness of professional viewpoint, etc.) [61, 62, 74–76] and/or the concordance between patient-professional factors (e.g. SDM-Q-9; decisional conflict) [77–79].
When informed [56, 57, 59] and shared [60] decision outcomes are used in renal services, findings suggest that they are useful service-quality indicators [49, 50, 77, 80]. However, they may respond differently from application in other contexts, as they are not designed for decisions taking place across multiple consultations, health professionals and services, with delayed implementation and chronically ill, elderly and/or often frail patients [80].
REACHING A CONSENSUS
The second part of the meeting addressed the objective of harmonizing the routine collection of patient-reported data by renal registries in Europe by agreeing on preferred instruments and discussing some of the practicalities of this process. Attendees were divided into four groups and asked to discuss which of the PROM/PREM instruments on a list should be employed, how often they should be administered and in what format. Due to time constraints and as they had not been included in the original application for funding it was decided to exclude the measurement of Shared Decision Making from the consensus discussions. Groups were chaired by four of the attendees, who later reported back to the whole meeting. Discussions were also audio-recorded. Participants were also asked to vote on which PROMs/PREMs they thought would be most appropriate based on the evidence outlined throughout the course of the meeting.
PROM instruments. The overwhelming consensus was that any PROMs measurement programme should aim to support improvements in the quality of care for patients. In terms of the measures adopted, there was agreement that the programme should aim to include both generic and disease-specific measures if possible, whilst minimizing the overall length of questionnaires administered. Of all the instruments discussed, the KDQOL™-36 seemed to be preferred by delegates as it offers both generic and disease-specific outcomes. Of the generic instruments, the SF-12 [81] was the most preferred. The importance of capturing patient symptoms was recognized, especially if a generic health-related quality of life instrument was being used, but no preferred symptom burden instrument was agreed on. There was not complete agreement on whether a preference-based measure should be included, but consensus suggested that it would be useful to have a measure that would allow health economic evaluations provided that the length was not prohibitive. The EQ-5D-5L was the preferred instrument for this purpose but it was recognized that SF-6D [82], which could also provide the utility data necessary for calculating quality adjusted life years in health economic evaluations, could be derived from longer SF instruments (Table 2) [82, 83].
PREM instruments. Delegates were much less familiar with the various PREM instruments available. Therefore, although the strengths and weaknesses of the various instruments had been presented to them earlier in the meeting, there was a broad consensus that more work was needed to recommend specific PREMs for broader use.
Patient groups to be covered. There was a clear consensus that the aim should be to include all patients on RRT in any PROMs/PREMs programme, and that data should be collected on at least an annual basis. The possibility of extending coverage to pre-dialysis patients in the future, when these patients are captured by registries, was discussed. The importance of making patients aware of how the data were being used was emphasized if high response rates were to be achieved from a broad range of patients. Several issues relating to the timing of data collection were discussed, and the general view was that although it may be useful to collect data at specific time points (e.g. in relation to commencement of RRT), this may not be feasible. As regards timings, the patient representative suggested that patients may not wish to complete PROMs/PREMs questionnaires whilst attending for dialysis and PROM experts raised concerns that responses given while on dialysis may be sensitive to the current dialysis experience rather than ‘usual’ health-related quality of life. No clear preference for paper or web-based reporting emerged, but the group felt that data should be collected via unassisted self-reporting. Other issues that featured prominently in discussions were the need to consider the legal, data protection and consent constraints of participating countries, and the possibility of a pilot study to assess feasibility.
Table 2.
Summary of recommendations from consensus discussions for routine renal registry PROM collection
| Consensus | |
|---|---|
| Which instruments | |
| Generic | SF-12 |
| Preference-based | EQ-5D-5L |
| Kidney specific | KDQOL™-36 |
| Practical issues | |
| Who? | All patients on RRT |
| When? | At least annually |
| Preferably not during dialysis | |
| How? | Unassisted self-report |
| No clear preference for paper/web | |
| Other issues | Consider ethics/consent/data protection |
| Conduct initial pilot study | |
There are limitations to this report. There is a scarcity of robust data on measuring and reporting PROMs and PREMs in kidney patients. Further, as mentioned above, the instruments available were generally developed for measurement at the individual patient level, rather than the centre or national level. In some ways these limitations reflect the attempt to reach consensus across European renal registries before instruments and processes become established.
SUMMARY
From the survey of European renal registry representatives and discussions with those attending the meeting there seems to be a widespread acceptance of the need to extend renal registry data collection to capture patient-reported outcomes and experience. It is recognized that this represents a considerable challenge for renal registries and services if all the potential benefits from the information gathered—such as focussing consultations on what matters to patients, having real-life information on quality of life for decision-making, incorporating patient measures into the quality assurance of renal units, research and improving services—are to be realized. Establishing a dialogue between registries around PROMs/PREMs and agreeing on some preferred measures at this stage will hopefully enable the sharing of expertize and experience and stimulate the collection, and facilitate the collection and comparison of patients' outcomes and experiences across Europe in the future.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
ACKNOWLEDGEMENT
This work was funded by the ERA-EDTA Quest initiative.
REFERENCES
- 1.ERA-EDTA Registry. ERA-EDTA Registry 2014 Annual Report. Amsterdam: Department of Medical Informatics, Academic Medical Centre, 2014 [Google Scholar]
- 2.Mittal SK, Ahern L, Flaster E et al. . Self-assessed physical and mental function of haemodialysis patients. Nephrol Dial Transplant 2001; 16: 1387–1394 [DOI] [PubMed] [Google Scholar]
- 3.Murtagh FE, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: a systematic review. Adv Chronic Kidney Dis 2007; 14: 82–99 [DOI] [PubMed] [Google Scholar]
- 4.Greenhalgh J. The applications of PROs in clinical practice: what are they, do they work, and why? Qual Life Res 2009; 18: 115–123 [DOI] [PubMed] [Google Scholar]
- 5.Etkind SN, Daveson BA, Kwok W et al. . Capture, transfer, and feedback of patient-centered outcomes data in palliative care populations: does it make a difference? a systematic review. J Pain Symptom Manage 2015; 49: 611–624 [DOI] [PubMed] [Google Scholar]
- 6.Appleby J. Patient reported outcome measures: how are we feeling today? BMJ 2012; 344: d8191. [DOI] [PubMed] [Google Scholar]
- 7.Black N. Patient reported outcome measures could help transform healthcare. BMJ 2013; 346: f167. [DOI] [PubMed] [Google Scholar]
- 8.Calvert M, Blazeby J, Altman DG et al. . Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA 2013; 309: 814–822 [DOI] [PubMed] [Google Scholar]
- 9.Crow R, Gage H, Hampson S et al. . The measurement of satisfaction with healthcare: implications for practice from a systematic review of the literature. Health Technol Assess 2002; 6: 1–244 [DOI] [PubMed] [Google Scholar]
- 10.Haugum M, Danielsen K, Iversen HH et al. . The use of data from national and other large-scale user experience surveys in local quality work: a systematic review. Int J Qual Health Care 2014; 26: 592–605 [DOI] [PubMed] [Google Scholar]
- 11.Hibbard JH, Stockard J, Mahoney ER et al. . Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Ser Res 2004; 39(4 Pt 1): 1005–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bekker H, Thornton JG, Airey CM et al. . Informed Decision Making: an Annotated Bibliography and Systematic Review. Basingstoke, UK: Health Technology Assessment Number 3: NHS R&D, 1999 [PubMed] [Google Scholar]
- 13.Group FHNT, Chertow GM, Levin NW et al. . In-center hemodialysis six times per week versus three times per week. New Engl J Med 2010; 363: 2287–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daly BJ, Douglas SL, Foley H et al. . Psychosocial registry for persons with cancer: a method of facilitating quality of life and symptom research. Psychooncology 2007; 16: 358–364 [DOI] [PubMed] [Google Scholar]
- 15.Pugliatti M, Eskic D, Mikolcic T et al. . Assess, compare and enhance the status of Persons with Multiple Sclerosis (MS) in Europe: a European Register for MS. Acta Neurol Scand 2012; 195: 24–30 [DOI] [PubMed] [Google Scholar]
- 16.Ghatnekar O, Eriksson M, Glader EL. Mapping health outcome measures from a stroke registry to EQ-5D weights. Health Qual Life Outcomes 2013; 11: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norlin JM, Steen CK, Persson U et al. . Analysis of three outcome measures in moderate to severe psoriasis: a registry-based study of 2450 patients. Br J Dermatol 2012; 166: 797–802 [DOI] [PubMed] [Google Scholar]
- 18.Paulsen A, Pedersen AB, Overgaard S et al. . Feasibility of 4 patient-reported outcome measures in a registry setting. Acta Orthop 2012; 83: 321–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van de Poll-Franse LV, Horevoorts N, van Eenbergen M et al. . The Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship registry: scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts. Eur J Cancer 2011; 47: 2188–2194 [DOI] [PubMed] [Google Scholar]
- 20.Thong MS, Mols F, Stein KD et al. . Population-based cancer registries for quality-of-life research: a work-in-progress resource for survivorship studies? Cancer 2013; 119(Suppl 11): 2109–2123 [DOI] [PubMed] [Google Scholar]
- 21.Kennedy L, Craig AM. Global registries for measuring pharmacoeconomic and quality-of-life outcomes: focus on design and data collection, analysis and interpretation. Review 128 refs. PharmacoEconomics 2004; 22: 551–568 [DOI] [PubMed] [Google Scholar]
- 22.Boyce MB, Browne JP, Greenhalgh J. The experiences of professionals with using information from patient-reported outcome measures to improve the quality of healthcare: a systematic review of qualitative research. BMJ Qual Saf 2014; 23: 508–518 [DOI] [PubMed] [Google Scholar]
- 23.Popay J, Roberts H, Sowden A et al. . Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. A Product from the ESRC Methods Programme Lancaster: Lancaster University, 2006 [Google Scholar]
- 24.Gentile S, Beauger D, Speyer E et al. . Factors associated with health-related quality of life in renal transplant recipients: results of a national survey in France. Health Qual Life Outcomes 2013; 11: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibbons E, Fitzpatrick R. A Structured Review of Patient-Reported Outcome Measures for People with Chronic Kidney Disease. Oxford: Department of Public Health, University of Oxford, 2009 [Google Scholar]
- 26.The Short Form-36 version 2 Health Survey. https://www.optum.com/optum-outcomes/what-we-do/health-surveys/sf-36v2-health-survey.html (3 May 2015, date last accessed)
- 27.The EQ-5D-5L. http://www.euroqol.org/eq-5d-products/eq-5d-5l.html (3 May 2015, date last accessed)
- 28.The Kidney Disease Quality of Life-36. http://www.rand.org/content/dam/rand/www/external/health/surveys_tools/kdqol/kdqol36.pdf (3 May 2015, date last accessed)
- 29.Donabedian A. Evaluating the quality of medical care. Milbank Mem Fund Q 1966; 44(Suppl): 166–206 [PubMed] [Google Scholar]
- 30.Sitzia J, Wood N. Patient satisfaction: a review of issues and concepts. Soc Sci Med 1997; 45: 1829–1843 [DOI] [PubMed] [Google Scholar]
- 31.Rubin HR, Jenckes MW, Fink NE et al. . Patient's view of dialysis care: development of a taxonomy and rating of importance of different aspects of care. CHOICE study. Choices for Healthy Outcomes in Caring for ESRD. Am J Kidney Dis 1997; 30: 793–801 [DOI] [PubMed] [Google Scholar]
- 32.US Agency for Healthcare Research and Quality (AHRQ) Centers for Medicare & Medicaid Services (CMS). CAHPS In-Center Hemodialysis Survey English version. Available from https://www.cahps.ahrq.gov/surveys-guidance/ich/, 2006. (3 May 2015, date last accessed)
- 33.Barendse SM, Speight J, Bradley C. The Renal Treatment Satisfaction Questionnaire (RTSQ): a measure of satisfaction with treatment for chronic kidney failure. Am J Kidney Dis 2005; 45: 572–579 [DOI] [PubMed] [Google Scholar]
- 34.Kirchgessner J, Perera-Chang M, Klinkner G et al. . Satisfaction with care in peritoneal dialysis patients. Kidney Int 2006; 70: 1325–1331 [DOI] [PubMed] [Google Scholar]
- 35.Wasserfallen J-B, Moinat M, Halabi G et al. . Satisfaction of patients on chronic haemodialysis and peritoneal dialysis. Swiss Med Wkly 2006; 136: 210–217 [DOI] [PubMed] [Google Scholar]
- 36.NHS Quality Improvement Scotland. Scottish Renal Patient Experience Survey. Dialysis report. Your service—your views, 2010
- 37.Fadem SZ, Walker DR, Abbott G et al. . Satisfaction with renal replacement therapy and education: the American Association of Kidney Patients survey. Clin J Am Soc Nephrol 2011; 6: 605–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van der Veer SN, Jager KJ, Visserman E et al. . Development and validation of the consumer quality index instrument to measure the experience and priority of chronic dialysis patients. Nephrol Dial Transplant 2012; 27: 3284–3291 [DOI] [PubMed] [Google Scholar]
- 39.Wood R, Paoli CJ, Hays RD et al. . Evaluation of the consumer assessment of healthcare providers and systems in-center hemodialysis survey. Clin J Am Soc Nephrol 2014; 9: 1099–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmer SC, de Berardis G, Craig JC et al. . Patient satisfaction with in-centre haemodialysis care: an international survey. BMJ Open 2014; 4: e005020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paddison CM, Elliott MN, Haviland AM et al. . Experiences of care among Medicare beneficiaries with ESRD: Medicare Consumer Assessment of Healthcare Providers and Systems (CAHPS) survey results. Am J Kidney Dis 2013; 61: 440–449 [DOI] [PubMed] [Google Scholar]
- 42.Sepucha KR, Borkhoff CM, Lally J et al. . Establishing the effectiveness of patient decision aids: key constructs and measurement instruments. BMC Med Inform Decis Mak 2013; 13(Suppl 2): S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scholl I, Koelewijn-van Loon M, Sepucha K et al. . Measurement of shared decision making—a review of instruments. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2011; 105: 313–324 [DOI] [PubMed] [Google Scholar]
- 44.Patel D, on behalf of The Evidence Centre. Helping People Share Decision Making. Helping People Share Decision Making, 2012
- 45.Stacey D, Bennett CL, Barry MJ et al. . Decision aids for people facing health treatment or screening decisions, 2011
- 46.Legare F, Kearing S, Clay K et al. . Are you SURE?: assessing patient decisional conflict with a 4-item screening test. Can Fam Physician 2010; 56: e308–e314 [PMC free article] [PubMed] [Google Scholar]
- 47.Ferron Parayre A, Labrecque M, Rousseau M et al. . Validation of SURE, a four-item clinical checklist for detecting decisional conflict in patients. Med Decis Making 2014; 34: 54–62 [DOI] [PubMed] [Google Scholar]
- 48.Bekker HL, Nye A, Walker E et al. . SHARED: Patient Experience of Shared Decision Making Measure. 2012. https://www.gem-measures.org/public/MeasureDetail.aspx?mid=1517&cat=2 (3 May 2015, date last accessed)
- 49.Nye A, on behalf of the NHS Advancing Quality Alliance. Your Health, Your Decision. A report from the creating a receptive culture shared decision making project. Right Care, NHS, 2013. http://arma.uk.net/wp-content/uploads/2013/05/Your-Health-Your-Decision-Evaluation-Report.pdf (3 May 2015, date last accessed) [Google Scholar]
- 50.Durand M-A, Bekker HL, Casula A et al. . Measuring Shared Decision-Making in Chronic Kidney Care. Service Evaluation Report The UK Renal Registry in collaboration with the NHS Institute, 2013 [Google Scholar]
- 51.King E, Taylor J, Williams R et al. . The MAGIC Programme: Evaluation. An Independent Evaluation of the MAGIC (Making Good Decisions in Collaboration) Improvement Programme. London, UK: The Health Foundation, 2013 [Google Scholar]
- 52.Craig P, Dieppe P, Macintyre S et al. . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008; 337: a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bekker HL. The loss of reason in patient decision aid research: do checklists damage the quality of informed choice interventions? Patient Educ Couns 2010; 78: 357–364 [DOI] [PubMed] [Google Scholar]
- 54.Bekker HL, Winterbottom AE, Butow P et al. . Do personal stories make patient decision aids more effective? A critical review of theory and evidence. BMC Med Inform Decis Mak 2013; 13(Suppl 2): S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brehaut JC, O'Connor AM, Wood TJ et al. . Validation of a decision regret scale. Med Decis Making 2003; 23: 281–292 [DOI] [PubMed] [Google Scholar]
- 56.O'Connor AM. Validation of a Decisional Conflict Scale. Med Decis Making 2010; 15: 25–30 [DOI] [PubMed] [Google Scholar]
- 57.Graham I, O'Connor AM. Preparation for Decision Making Scale—User manual, 2010 [DOI] [PubMed]
- 58.Holmes-Rovner M, Kroll J, Schmitt N et al. . Patient satisfaction with health care decisions: the satisfaction with decision scale. Med Decis Making 1996; 16: 58–64 [DOI] [PubMed] [Google Scholar]
- 59.O'Connor AM. User Manual—Stage of Decision Making, 2003. http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Stage_Decision_making.pdf (3 May 2015, date last accessed)
- 60.Bekker HL, Legare F, Stacey D et al. . Is anxiety a suitable measure of decision aid effectiveness: a systematic review? Patient Educ Couns 2003; 50: 255–262 [DOI] [PubMed] [Google Scholar]
- 61.Mavis B, Holmes Rovner M, Jorgenson S et al. . Patient participation in clinical encounters: a systematic review to identify self-report measures. Health Expect 2014; doi:10.1111/hex.12186. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sepucha KR, Scholl I. Measuring shared decision making: a review of constructs, measures, and opportunities for cardiovascular care. Circ Cardiovasc Qual Outcomes 2014; 7: 620–626 [DOI] [PubMed] [Google Scholar]
- 63.Hunink M, Glasziou P, Siegel J et al. . Decision Making in Health and Medicine: Integrating Evidence and Values. Cambridge, UK: Cambridge University Press, 2001 [Google Scholar]
- 64.Gray JAM. Evidence-based Healthcare: How to Make Health Policy and Management Decisions. London, UK: London; 1997 [Google Scholar]
- 65.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sc Med 1997; 44: 681–692 [DOI] [PubMed] [Google Scholar]
- 66.Coulter A. Partnerships with patients: the pros and cons of shared clinical decision-making. J Health Serv Res Policy 1997; 2: 112–121 [DOI] [PubMed] [Google Scholar]
- 67.Stacey D, Legare F, Pouliot S et al. . Shared decision making models to inform an interprofessional perspective on decision making: a theory analysis. Patient Educ Couns 2010; 80: 164–172 [DOI] [PubMed] [Google Scholar]
- 68.Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns 2006; 60: 301–312 [DOI] [PubMed] [Google Scholar]
- 69.Lerman CE, Brody DS, Caputo GC et al. . Patients’ perceived involvement in care scale: relationship to attitudes about illness and medical care. J Gen Intern Med 1990; 5: 29–33 [DOI] [PubMed] [Google Scholar]
- 70.Clayman ML, Makoul G, Harper MM et al. . Development of a shared decision making coding system for analysis of patient-healthcare provider encounters. Patient Educ Couns 2012; 88: 367–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elwyn G, Barr PJ, Grande SW et al. . Developing CollaboRATE: a fast and frugal patient-reported measure of shared decision making in clinical encounters. Patient Educ Couns 2013; 93: 102–107 [DOI] [PubMed] [Google Scholar]
- 72.Elwyn G, Hutchings H, Edwards A et al. . The OPTION scale: measuring the extent that clinicians involve patients in decision-making tasks. Health Expect 2005; 8: 34–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kriston L, Scholl I, Holzel L et al. . The 9-item Shared Decision Making Questionnaire (SDM-Q-9). Development and psychometric properties in a primary care sample. Patient Educ Couns 2010; 80: 94–99 [DOI] [PubMed] [Google Scholar]
- 74.De Silva D, on behalf of The Evidence Centre. Helping Measure Person-Centred Care. London, UK: The Health Foundation, 2014 [Google Scholar]
- 75.Graham C, MacCormick S. Overarching questions for patient surveys: development report for the Care Quality Commission (CQC). National Patient Survey Co-ordination Centre Picker Institute Europe, 2012 [Google Scholar]
- 76.Hibbard J, Gilburt H. Supporting People to Manage Their Health: An Introduction to Patient Activation. London, UK: The Kings Fund, 2014 [Google Scholar]
- 77.Fish M, Coyne E, Ferraro A. The utility of the decisional conflict tool in a predialysis clinical (Abstract). In: British Renal Conference Manchester, 2013 [Google Scholar]
- 78.Kasper J, Heesen C, Kopke S et al. . Patients’ and observers’ perceptions of involvement differ. Validation study on inter-relating measures for shared decision making. PloS one 2011; 6: e26255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Legare F, Leblanc A, Robitaille H et al. . The decisional conflict scale: moving from the individual to the dyad level. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2012; 106: 247–252 [DOI] [PubMed] [Google Scholar]
- 80.Winterbottom AE, Gavaruzzi T, Mooney A et al. . Patient Acceptability of the Yorkshire Dialysis Decision Aid (YoDDA) disseminated within predialysis services: a non-randomised controlled study. 2014; (submitted for publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.The Short Form-12 version 2 Health Survey. https://www.optum.com/optum-outcomes/what-we-do/health-surveys/sf-12v2-health-survey.html (3 May 2015, date last accessed)
- 82.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002; 21: 271–292 [DOI] [PubMed] [Google Scholar]
- 83.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care 2004; 42: 851–859 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.