Abstract
Ambrosia symbiosis is an obligate, farming-like mutualism between wood-boring beetles and fungi. It evolved at least 11 times and includes many notorious invasive pests. All ambrosia beetles studied to date cultivate ascomycotan fungi: early colonizers of recently killed trees with poor wood digestion. Beetles in the widespread genus Ambrosiodmus, however, colonize decayed wood. We characterized the mycosymbionts of three Ambrosiodmus species using quantitative culturing, high-throughput metabarcoding, and histology. We determined the fungi to be within the Polyporales, closely related to Flavodon flavus. Culture-independent sequencing of Ambrosiodmus minor mycangia revealed a single operational taxonomic unit identical to the sequences from the cultured Flavodon. Histological sectioning confirmed that Ambrosiodmus possessed preoral mycangia containing dimitic hyphae similar to cultured F. cf. flavus. The Ambrosiodmus-Flavodon symbiosis is unique in several aspects: it is the first reported association between an ambrosia beetle and a basidiomycotan fungus; the mycosymbiont grows as hyphae in the mycangia, not as budding pseudo-mycelium; and the mycosymbiont is a white-rot saprophyte rather than an early colonizer: a previously undocumented wood borer niche. Few fungi are capable of turning rotten wood into complete animal nutrition. Several thousand beetle-fungus symbioses remain unstudied and promise unknown and unexpected mycological diversity and enzymatic innovations.
Introduction
Ambrosia beetles (Coleoptera: Curculionidae: Scolytinae and Platypodinae) are a polyphyletic collection of clades within the hyper-diverse bark beetles. Ambrosia beetles have evolved the symbiotic evolutionary mode referred to as fungiculture or fungal farming. The more than 3200 known ambrosia beetle species gain nutrition from fungi grown on abundant yet recalcitrant substrates—dead trees and tree parts. The beetles transport inoculum of specific fungal symbionts from their natal galleries to newly established galleries in a storage organ called a mycangium [1]. Location and morphology of mycangia vary across species and include preoral, mandibular, elytral, mesothoracic, and many others [2], and, in most instances, are phylogenetically highly conserved [1,3]. The only fungal symbionts of ambrosia beetles confirmed thus far have been ascomycotan fungi (Ascomycota: Sordariomycetes) that are transported as budding yeast-like pseudo-mycelium or conidia [4]. In all studied cases, fungi are ecologically “early colonizers”, specialized on extracting nutrients from freshly dead wood but lacking any significant cellulolytic capability [5] and most ambrosial beetle-fungus couples colonize recently killed (dead) trees.
Ambrosia beetles are becoming increasingly economically and ecologically important. Numerous non-native species were inadvertently introduced to new regions via international trade, and in several such cases, formerly harmless beetle species turned into invasive pests [6–8]. In at least four cases, ambrosia beetle-fungus symbionts have triggered acute and ongoing epidemics among susceptible host trees [8–12].
Ambrosia beetles are derived from bark beetles (Coleoptera: Curculionidae: Scolytinae). Bark beetles colonize and consume phloem, a tissue that is more nutrient-rich than wood. Bark beetles, like ambrosia beetles, are also often associated with fungal symbionts, usually ascomycotan and rarely basidiomycotan fungi, and the intensity of association is more variable, ranging from facultative to obligate [13–15]. Beetle-fungus consortia are responsible for spectacular outbreaks, driven either by climate, the pathogenicity of the fungus, or the mass-attacking nature of the beetles [9]. Other insects also engage in symbioses functionally analogous to the ambrosia beetle-fungus symbiosis: attine ants [16], termites [17], ship timber beetles [5], and wood wasps [18]. While most of these use basidiomycotan fungi to gain access to nutrients from plant matter, the fungi are usually either not particularly competitive wood-decomposers or provide only partial nutrition [18]. Though research focused on wood borer-fungus associations has recently increased, it has been almost entirely restricted to a minority of species that happen to be economically important. At the same time, thousands of beetle species remain unstudied, and each new exploration of the understudied taxa revealed interactions with unsuspected and undescribed fungal communities [19,20].
Ambrosiodmus (Fig 1; [21–23]) is a genus of 80 species within the largest group of ambrosia beetles, the Xyleborini (1,200 spp.; Coleoptera: Scolytinae). The genus deserves closer scrutiny for several reasons. While its economic importance has been minimal, it is a diverse and globally distributed genus. Many species are ecologically successful and abundant throughout their range. In the twentieth century, species were increasingly established in non-native habitats via human-aided introductions. In the US alone, the originally Asiatic A. rubricollis Eichhoff [24] has been present for nearly five decades [25]. More recently, A. minor Stebbing [26], a species formerly known only from southern Asia [22], has spread across at least three southeastern states in the US [27,28].
Fig 1. Female Ambrosiodmus lecontei with eggs in plant host.
Note white fungal mycelial garden (arrows) in gallery.
While the majority of ambrosia fungi and ambrosia beetles depend on newly dead and relatively fresh tree tissues, Ambrosiodmus species appear to be able to colonize wood throughout the process of its decay, including latter stages when xylem is co-colonized by competitive wood-rot fungi. Phylogenetically, Ambrosiodmus is sister to Ambrosiophilus, an unusual genus of ambrosia beetles, many of which have lost their capacity to culture their own fungal gardens and depend on mycoclepty, or fungus theft [1]. Such unique ecological features and phylogenetic position suggest the possibility of association with fungi different than those typically associated with other ambrosia beetles, yet virtually nothing is known about the Ambrosiodmus symbionts. The only report on mycangia and fungal symbionts in Ambrosiodmus examined A. rubricollis [24] and determined the presence of preoral mycangia at the bases of the mandibles behind the labrum [29]. The mycangial content was described as globose conidia and short hyphal fragments, which yielded “white wooly mycelia only” when grown on agar.
To clarify the presence and identity of fungal symbionts of Ambrosiodmus, and to hypothesize upon the ecological habits and success of the genus, we characterized fungi associated with the ambrosia beetle genus and found entirely unexpected symbionts. We focused on three different Ambrosiodmus species: A. lecontei Hopkins [23], A. minor, and A. rubricollis, representing both the Eurasian and American fauna. We have used a comprehensive suite of complementary approaches including quantitative culturing for the main symbiont characterization, culture-independent high-throughput community sequencing for the detection of any potentially unculturable symbionts, anatomical analysis of the beetle for the characterization of the mycangia and their content, and morphological cross-validation by microscopy of the symbiont identity in the mycangia and cultured fungi.
Materials and Methods
Isolation and culturing of fungi from host beetles and galleries
To isolate the dominant fungal symbionts in Ambrosiodmus, three beetle species were sampled: A. lecontei (native to the South-Eastern US), A. rubricollis (native to Japan, but widely established in the US) and A. minor (native to SE Asia and recently established in the SE US). Female specimens of all three species were collected by light-trapping (August 2014 through April 2015) and from moribund-to-rotten wood of Myrica cerifera (A. lecontei), Liquidambar styraciflua (A. rubricollis), and Platanus occidentalis (A. minor), in Gainesville, Florida, USA (29° 37′ 16″ N 82° 22′ 32″ W). Collecting has been carried out on a University property and on a private property of the senior author and did not require permits. Ambrosiodmus are not endangered. Males have not been included in our analyses as all Xyleborini ambrosia beetle males are flightless, rare, and lack mycangia necessary for transporting symbiotic fungi [30]. Live beetles and wood sections were transported to the laboratory in clean containers with a tissue moistened with sterile water. After identification in the laboratory, whole beetles were surface-washed by vortexing for 1 min in 1 mL of sterile distilled water with one small drop (<10 μL) of Tween detergent. Beetle heads were aseptically removed, crushed, and placed in a 500 μL solution of sterile 1X phosphate buffer saline and vortexed for 30 s. Resulting solutions were diluted to 1:100 and 1:1000 concentrations, and ~100 μL of each dilutions were used to inoculate potato dextrose agar (PDA) medium (BD Difco™), which was strengthened with additional agar (5 g/L). Colony forming units (CFUs) within mycangia were estimated for each morphotype by multiplying the number of colonies on a plate by the inverse of the inoculum dilution. To determine symbionts in the ambrosial gardens, fungi were collected from Ambrosiodmus tunnels in Liquidambar styraciflua and Platanus occidentalis using sterile scalpels. Dominant morphotypes were quantified and purified by the methods described above.
DNA extraction, amplification, and sequencing
Fungal DNA was isolated with Extract-N-Amp Plant PCR kits (Sigma-Aldrich Co. LLC.), with the modification of using 3% bovine serum albumin (BSA) in the place of a dilution solution. Sequences of the nuclear internal transcribed spacers ITS1-5.8S-ITS2 (ITS) and nuclear 28S ribosomal DNA (rDNA) regions were amplified with the primer combinations ITS1/ITS4 [31] and LR0R/LR5 [32], respectively, and ExTaq polymerase kits (Clontech-TaKaRa). Amplified products were visualized with SYBR® Green I Stain (Lonza Group Ltd.) on a 1% agarose gel in 1X TAE buffer. Products were purified with ExoSAP-IT (Affymetrix, Inc.) and submitted to the University of Florida Interdisciplinary Center for Biotechnology Research for Sanger sequencing. Chromatograms were assembled and inspected in Geneious (Geneious version 7.1.8).
Phylogenetic analyses
Ribosomal DNA sequences from three representative fungal isolates were aligned to ITS and nuclear 28S rDNA sequences from additional Polyporales and Russulales taxa (S1 Table; [33,34]) in Geneious using the MAFFT alignment tool [35]. A data matrix was deposited in TreeBASE under accession number S17679. The Akaike information criterion (AIC) in jModeltest 0.1.1 [36,37] was used to select a nucleotide substitution model for the dataset. Maximum likelihood (ML) phylogenetic analyses were conducted using the suggested model parameters in GARLI 2.01 [38] and additional AIC recommended settings to determine the best tree topology. Bootstrap support values were calculated in GARLI from 100 search replicates, which were summarized with SumTrees [39]. A Bayesian phylogenetic analysis was conducted using the same AIC recommended model parameters in MrBayes 3.1.2 [40], in which two runs of four chains each were executed simultaneously from 5 000 000 generations, with sampling every 500 generations. The resulting 7501 trees retained after a burn-in of 2500 trees in SumTrees were used to compute Bayesian posterior probabilities (BPP). The new fungal species will be taxonomically described elsewhere.
Fungal community amplicon analysis
One representative female individual of Ambrosiodmus minor, collected 24 November 2014 in Gainesville, FL, USA (29° 37′ 16″ N 82° 22′ 32″ W), was processed for DNA extraction and PCR amplification, as above. Amplification of nuclear 28S rDNA was targeted with primers LR0R [32] and JH-LSU-369rc (5′-CTTCCCTTTCAACAATTTCAC-3′) to yield a ~350–400 base pair (bp) product. We chose nuclear 28S rDNA rather than the more commonly used nuclear ITS rDNA “fungal barcode” because it amplifies better in many Ophiostomatales (Ascomycota: Sordariomycetes) ambrosia fungi while being sufficient for species identification in most Polyporales (previous section). ExTaq polymerase kits (Clontech-TaKaRa) were used for the amplifications. To generate an amplicon library with sequencing adaptors for Illumina MiSeq® sequencing, a double PCR protocol was used [41]. In the first PCR, the target rDNA fragments were amplified using template primers. A 1 μL aliquot of this enriched library was used as the template in the second PCR, in which fusion primers were used. The 5′ fusion primer was composed of the P5 Illumina adapter, spacer, SBS3 sequencing primer, and the template primer. The 3′ fusion primer was composed of the P7 adapter, spacer, a sample-specific 7 bp index, the SBS12 sequencing primer, and the template primer. The amplicon library was purified using the UltraClean purification kit (MoBio), visualized on an agarose gel, quantified using the QuantIT PicoGreen fluorescent dye (Life Technologies), read on an Eppendorf RealPlex qPCR thermal cycler, and diluted to a concentration comparable with other amplicons included in the sequencing run (samples not related to this project). Illumina MiSeq® sequencing was conducted at the Interdisciplinary Center for Biotechnology Research at the University of Florida. Sequence data were processed and evaluated using QIIME 1.7.0 [42]. MiSeq sequences were deposited in NCBI Sequence Read Archive under accession number SRP058537. Operational taxonomic units (OTUs) were clustered at 2% sequence similarity, and each representative sequence was identified by NCBI BLAST queries, specifically by comparison with type strain sequences, when available.
Mycangial presence and content
Eleven females of Ambrosiodmus lecontei from a cryopreserved beetle depository in the Hulcr lab were examined to determine the location of mycangia. Individuals examined were collected 29 November 2010 from redbay trees (Persea borbonia (L.) Spreng.) near Lake Alfred, Polk County, Florida and preserved in cryotubes containing a 95% ethanol solution, which was stored at -80°C. Given the phylogenetic position of Ambrosiodmus within the Xyleborini with preoral mycangia [1] and a previous examination of the genus [29], the beetle heads were targeted for examination. Heads were aseptically removed from the pronotum before sectioning. Once removed, beetle heads were fixed in 10% neutral buffered formalin for 24 h and then soaked in phenol for 6 d to soften the cuticle [8]. Heads were treated with an automated tissue processor to allow desiccation of tissues and infiltration of paraffin and subsequently embedded in paraffin blocks. Heads were orientated with the most anterior end facing downward to permit transverse sectioning and simultaneous bilateral visualization of the mycangia. A Microm HM 325 rotary microtome (Walldorf, Germany) was used to cut 10-μm transverse sections. Selected slides confirmed by immediate viewing were then dried at 60°C for three days, double-stained with Harris-hematoxylin and eosin-phloxine, and examined and photographed using a Nikon Eclipse E600 compound microscope (Nikon Instruments, Melville, New York) equipped with a Nikon Digital Sight DS-Ri1 high-resolution microscope camera and Nikon NIS-Elements BR 3.2 imaging software.
Fungal morphology on agar medium
To document the morphology of the fungal symbionts of Ambrosiodmus, we used six isolates that were recovered from heads of all three Ambrosiodmus species, as well as two isolates recovered from the galleries of A. minor and A. rubricollis (Table 1). Isolates were cultured on PDA medium and incubated at 20°C for three weeks. Hyphae were aseptically removed from the edges of the representative fungal colonies, and examined microscopically with a Nikon Eclipse 55i equipped with a ProgRes® SpeedXT core 3 camera (Jenoptik Optical Systems).
Table 1. Cultures of Flavodon cf. flavus and host information.
| Subculture | Original culture | Host or associated beetle | Isolation source | Estimated CFU |
|---|---|---|---|---|
| 6853 | 6853_white_myce | Ambrosiodmus minor | Head extract | 1500 |
| 6855 | 6855_white_myce | A. minor | Head extract | 1500 |
| 6860 | 6860_sub_white_myce | A. lecontei | Head extract | 4000 |
| 7324 | 7324_white_myce | A. lecontei | Head extract | - |
| 7346 | 7346_white_myce | A. minor | Gallery in Platanus occidentalis | - |
| 7353 | 7326_white_myce | A. rubricollis | Head extract | 30 |
| 7354 | 7341_white_myce | A. rubricollis | Head extract | 100 |
| 7373 | 7325_white_myce | A. rubricollis | Gallery in Liquidambar styraciflua | - |
Results
Isolation and culturing of fungi from host beetles and galleries
Individuals of each of the three Ambrosiodmus species contained a single fungal morphotype in at least two head extract isolation events, and estimated CFUs of the same dominant morphotype from different isolation events ranged from 10 to 4000 (Table 1). Subcultures were cryoarchived in PDA slant vials, immersed in 15% glycerol, and stored at -80°C, and deposited in the Hulcr collection at the University of Florida and at the Forestry & Agricultural Biotechnology Institute (FABI) fungal culture collection (CMW) in Pretoria, Gauteng Province, South Africa.
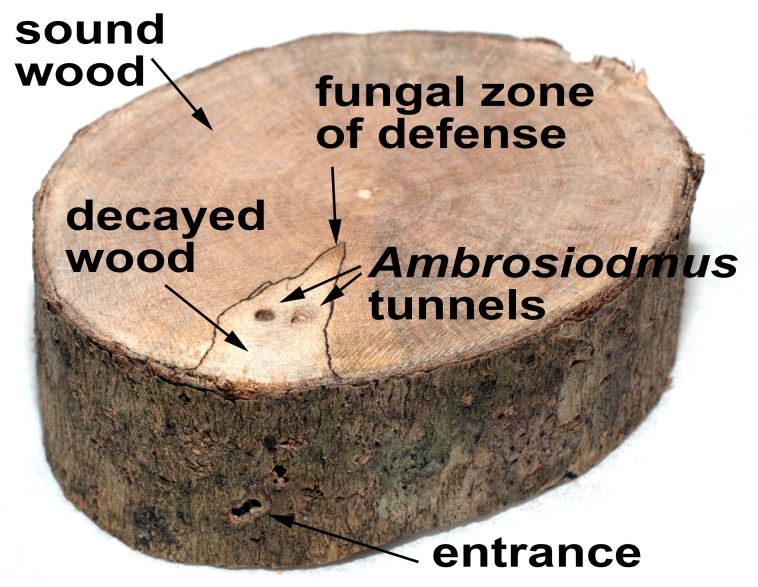
Active Ambrosiodmus galleries were routinely found in decayed wood. The wood adjacent to the Ambrosiodmus gallery was typically soft and spongy, and the area was surrounded by dark lines (Fig 2). These lines are a common result of antagonistic interactions among genetically distinct decay fungi in co-colonized dead wood. Other ambrosia fungi studied thus far do not form such zones of defense.
Fig 2. Wood decay as a result of colonization by the Ambrosiodmus-Flavodon symbiosis.
Phylogenetic analyses
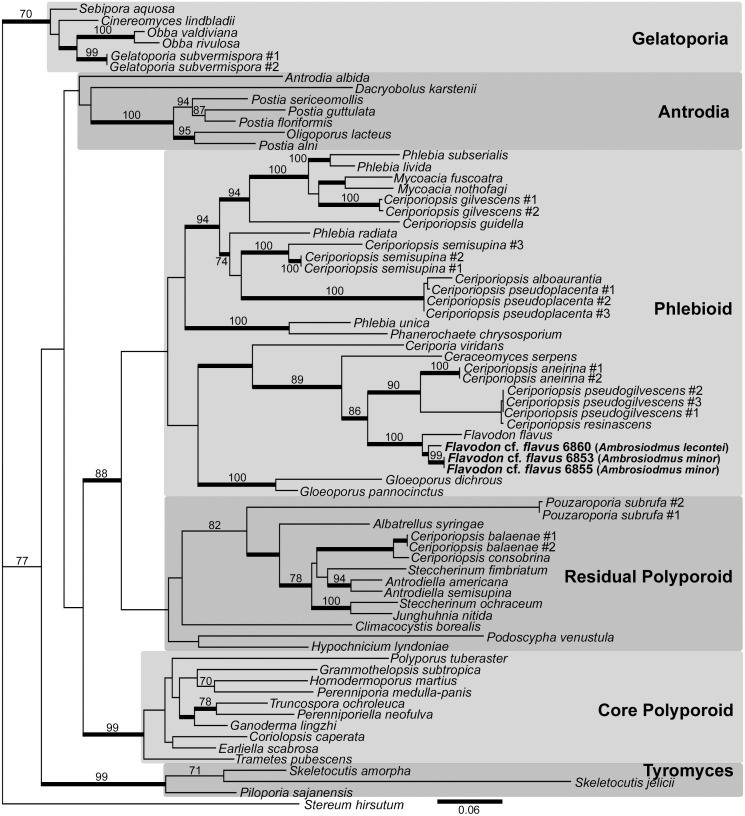
The best ML phylogeny (Fig 3) placed the representative fungal isolates from A. lecontei and A. minor heads in a monophyletic group within the phlebioid clade of the Polyporales [43] with 98% BPP but only 48% ML bootstrap support. These isolates are sister to GenBank accession JN710543 [44], identified as Flavodon flavus (Klotzsch) Ryvarden [45], a placement supported by 100% BPP and bootstrap value. Sequence data from all isolated cultures was deposited in GenBank accessions KR119072–KR119080 and KR871005–KR871009.
Fig 3. Best ML tree from GARLI analysis of combined nuclear ITS and 28S rDNA datasets.
Flavodon cf. flavus isolates, with host beetle in parentheses, within phlebioid clade of Polyporales. Values at nodes indicate ≥70% ML bootstrap support, and thickened branches indicate ≥95% Bayesian posterior probability support values.
Fungal community amplicon analysis
Sequencing of the fungal community 28S amplicon from Ambrosiodmus minor mycangia resulted in 355,749 sequence reads. There was a single dominant OTU, which represented 94% of the mycangial community (151345 of 160489 reads) after the removal of non-fungal sequences and OTUs corresponding to <1% of all reads. A BLAST query of the representative sequence of this OTU (5′ end of the nuclear 28S rDNA) in GenBank resulted in 26 accessions with 99% similarity, two of which were submitted as Flavodon flavus (accessions KP012792 and JN710543). When compared to the sequences obtained from cultured isolates, the dominant OTU was 99.48% identical (386/388 nucleotides).
Examination of mycangia
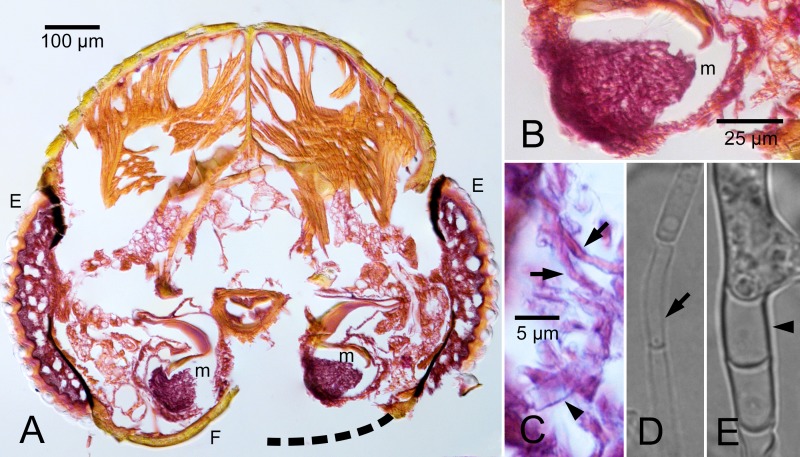
Serial transverse sections from the heads of all female A. lecontei confirmed the presence of one pair of preoral mycangia at the base of the mandibles in nine of 11 beetles examined (Fig 4A and 4B). Two remaining beetle heads could not be visualized on account of sub-optimal orientation during paraffin embedding. Mycangial content was a tightly packed mass of hyphae of varying diameters (Fig 4C). No conidia or budding pseudo-mycelium were observed.
Fig 4. Transverse cross sections of adult female Ambrosiodmus lecontei heads showing position of mycangia within mouthparts.
(A) Section showing entire head with paired mycangia behind the mandibles, with dashed line representing missing exoskeletal structure. (B) Compacted fungal hyphae constituting mycangial inoculum. (C) Loose hyphae from mycangial packet, with both small (arrows) and larger (arrowhead) hyphae, likely representing dimitic generative and skeletal hyphae, respectively. (D& E) Flavodon cf. flavus isolate 6860_sub_white_myce with dimitic generative (D) and skeletal (E) hyphae. Key: E, eye; F, frontal edge (accidentally damaged); m, mycangium.
Fungal morphology
On PDA medium, isolates 6853_white_myce, 6855_white_myce, and 6860_sub_white_myce produced dimitic hyphae, clearly of two diameters, but also with some variation in size. Smaller, generative hyphae (Fig 4D) were present at colony margins but were rapidly obscured by larger, skeletal hyphae (Fig 4E). No reproductive structures and no clamp connections were observed.
Discussion
All analyses indicated that Ambrosiodmus species carry in their mycangia, and culture in their galleries, ambrosial basidiomycotan symbionts closely related to Flavodon flavus (Basidiomycota: Polyporales). The condition of the host trees in which the galleries of Ambrosiodmus were collected, and the structure of the wood surrounding the beetle gallery, indicate that the fungal symbiont is a true wood-degrading saprophyte (Fig 2). There are very few known cases in which a fungus converts degraded lignocellulose into a complete diet for its animal symbiont. Consequently, the metabolic aspects of the Ambrosiodmus-Flavodon symbiosis deserves further scrutiny.
The majority of the ambrosial beetle-fungus symbioses investigated thus far involve ascomycotan fungi, typically from the orders Ophiostomatales or Microascales. These relatives of plant pathogens are excellent at extracting simple nutrients from freshly dead tree tissue, but their cellulolytic capacity is limited [5]. Consequently, most ambrosia insect groups are early colonizers of dying or freshly dead trees, and are unable to utilize older wood colonized by wood-rot basidiomycotan fungi, whose enzymatic machinery for lignocellulose degradation is superior. A few basidiomycetous mutualists of wood boring insects exist. Amylostereum (Russulales), associated with Siricidae wood wasps, is a true saprotroph, but it does not supply the entire nutrition to its host [14]. Several phloem-feeding bark beetles and attine ants require basidiomycete mutualists for their development, but those fungi are not competitive wood-decay saprophytes [14,46]. A recent report of a basidiomycete fungus (Antrodia) from an unrelated ambrosia beetle Anisandrus dispar [13] may not refer to a nutritional symbiont, which is likely Ambrosiella hartigii [12,47]. The only other case in which a cellulolytic saprotroph appears to provide a complete nutrition is the Termitomyces mutualist of fungus-farming termites [48].
Nuclear 28S rDNA sequences of fungal isolates derived from A. minor differed from those of fungi cultured from A. lecontei and A. rubricollis by a single transitional pyrimidine mutation. Additionally, the ITS rDNA sequences from A. minor samples were identical to each other, but only 94% similar to A. lecontei samples (S1 Table). Ambrosiodmus minor was introduced to the US recently, compared to the long established A. rubricollis and the native A. lecontei. Taken together, these comparisons suggest the association of specific fungal strains with the established US beetle species and with the most recent immigrant beetle species.
All the isolates are approximately equally related to sequences reportedly derived from Flavodon flavus (Fig 3). Given the 100% supported monophyly and the matching hyphal morphology, we refer to our isolates as Flavodon cf. flavus until further analyses support their differentiation. Flavodon flavus is a free-living wood saprophyte described from northern Europe, a location far outside of the distribution of any Ambrosiodmus [44]. This may indicate that the fungal genetic markers have not yet diverged from a free-living relative, or that the association is asymmetrical, in which beetles depend on the fungus but the fungus may still be capable of an aposymbiotic lifestyle. Most symbioses between insects and basidiomycotan fungi are similarly assymetrical; this includes the Siricidae wood wasps and the bark beetles mentioned above, and also some fungus growing termites and some fungus growing attine ants [49].
We report a novel form of symbiont dispersal mode in ambrosia fungi: non-budding hyphal growth (Fig 4A and 4B). The A. lecontei mycangia appeared to be packed with hyphae of varying diameters (Fig 4C), corroborating the presence of a dimitic basidiomycotan fungus. The fungus Flavodon flavus was described as dimitic and lacking clamp connections [45], and this description conformed with the hyphae we observed in mycangia (Fig 4C) and from isolate 6860_sub_white_myce from A. lecontei (Fig 4D and 4E) that we molecularly characterized as F. cf. flavus (Fig 3).
The Ambrosiodmus lecontei mycangia are almost certainly homologous with the usual preoral mycangia in some Xyleborini ambrosia beetles; thus it appears that the unusual fungus transmission mode (hyphae) is a feature of the new fungal symbiont, not of the beetle carriers. Note that we use the term “preoral” for xyleborine mycangia that were traditionally called “mandibular”; the term “mandibular” is preoccupied by the true mandibular mycangia in Dendroctonus [2].
From the MiSeq analysis of fungal symbiont rDNA amplicons and sequences from cultured isolates, we posit that Flavodon cf. flavus, or a complex of cryptic species, makes up the overwhelming majority of the fungal community. It therefore seems likely that the beetle uses the fungus as its primary food source on a host plant. By an association with a saprophytic basidiomycotan fungus, it may be that the beetle genus Ambrosiodmus has gained access to a ubiquitous resource (dead and decaying wood) previously unavailable to any other bark or ambrosia beetle. Further study is needed to confirm this hypothesis. The approaches described above serve as a model for rapid and robust exploration and characterization of this frontier of symbiology. Given the number of ambrosia beetle and fungus species worldwide and the increasing research focus on them, we should expect more discoveries of such interactions and evolutionary innovations.
Supporting Information
(DOCX)
Acknowledgments
We thank Chang-lin Zhao and Bao-kai Cui from the Beijing Forestry University for sharing molecular data. We thank Andrew J. Johnson at the University of Florida for plant identification. Lukasz Stelinski and Chris Gibbard provided several specimens. We thank the Pathology Laboratory for Translational Medicine at West Virginia University’s School of Medicine for their assistance in histological preparations.
Data Availability
MiSeq sequences were deposited in NCBI Sequence Read Archive accession SRP058537. Sanger sequence data were deposited in GenBank accessions KR119072–KR119080 and KR871005–KR871009.
Funding Statement
This research was funded by USDA-FS-SRS Coop agreement 14-CA-11330130-032, USDA-FS-FHP Coop agreement 12-CA-11420004-042, USDA Farm Bill agreement 14-8130-0377-CA, NSF DEB 1256968, the West Virginia Agricultural Experiment Station, and a University of Florida Opportunity Seed fund.
References
- 1. Hulcr J, Cognato AI. Repeated evolution of crop theft in fungus-farming ambrosia beetles. Evolution (N Y). 2010;64:3205–3212. [DOI] [PubMed] [Google Scholar]
- 2. Six DL. Bark beetle-fungus symbioses In: Bourtzis K, Miller TA, editors. Insect symbiosis. New York, New York: CRC Press; 2003. pp. 97–114. [Google Scholar]
- 3. Batra LR. Ecology of ambrosia fungi and their dissemination by beetles. Trans Kansas Acad Sci. 1963;66(2):213–236. [Google Scholar]
- 4. Beaver RA. Insect-fungus relationship in the bark and ambrosia beetles In: Wilding N, Collins NM, Hammond PM, Webber JF, editors. Insect-fungus interactions. Elsevier Ltd.; 1989. pp. 121–143. [Google Scholar]
- 5. De Fine Licht HH, Biedermann PHW. Patterns of functional enzyme activity in fungus farming ambrosia beetles. Front Zool; 2012;9:13 10.1186/1742-9994-9-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hulcr J, Dunn R. The sudden emergence of pathogenicity in insect-fungus symbioses threatens naive forest ecosystems. Proc R Soc B. 2011;278:2866–2873. 10.1098/rspb.2011.1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ranger CM, Reding ME, Persad AB, Herms DA. Ability of stress-related volatiles to attract and induce attacks by Xylosandrus germanus and other ambrosia beetles (Coleoptera: Curculinonidae: Scolytinae). Agric For Entomol. 2010;12:177–185. [Google Scholar]
- 8. Fraedrich SW, Harrington TC, Rabaglia RJ, Ulyshen MD, Mayfield AE III, Hanula JL, et al. A fungal symbiont of the redbay ambrosia beetle causes a lethal wilt in redbay and other Lauraceae in the southeastern United States. Plant Dis. 2008;92:215–224. [DOI] [PubMed] [Google Scholar]
- 9. Freeman S, Sharon M, Maymon M, Mendel Z, Protasov A, Aoki T, et al. Fusarium euwallaceae sp. nov.—a symbiotic fungus of Euwallacea sp., an invasive ambrosia beetle in Israel and California. Mycologia. 2013;105:1595–1606. 10.3852/13-066 [DOI] [PubMed] [Google Scholar]
- 10. Hijii N, Kajimura H, Urano T, Kinuura H, Itami H. The mass mortality of oak trees induced by Platypus quercivorus (Murayama) and Platypus calamus Blandford (Coleoptera: Platypodidae): the density and spatial distribution of attack by the beetles. J Japanese For Soc. 1991;73:471–476. [Google Scholar]
- 11. Hong KJ, Kwon YD, Park SW, Lyu DP. Platypus koryoensis (Murayama) (Platypodidae: Coleoptera), the vector of oak wilt disease. Korean J Appl Entomol. 2006;45:113–117. [Google Scholar]
- 12. Happ GM, Happ CM, French JRJ. Ultrastructure of the mesonotal mycangium of an ambrosia beetle, Xyleborus dispar (F.) (Coleoptera: Scolytidae). Int J Insect Morphol Embryol. 1976;5:381–391. [Google Scholar]
- 13. Hsiau PTW, Harrington TC. Phylogenetics and adaptations of basidiomycetous fungi fed upon by bark beetles (Coleoptera: Scolytidae). Symbiosis. 2003;34:111–131. [Google Scholar]
- 14. Harrington TC. Ecology and evolution of mycophagous bark beetles and their fungal partners In: Vega FE, Blackwell M, editors. Insect-fungal associations: ecology and evolution. Oxford University Press; 2005. pp. 257–291. [Google Scholar]
- 15. Vega FE, Hofstetter RW, editors. Bark beetles: biology and ecology of native and invasive species. San Diego, California: Academic Press; 2015. 640 p. [Google Scholar]
- 16. Schultz TR, Brady SG. Major evolutionary transitions in ant agriculture. Proc Natl Acad Sci U S A. 2008;105:5435–5440. 10.1073/pnas.0711024105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aanen DK, Eggleton P, Rouland-Lefèvre C, Guldberg-Frøslev T, Rosendahl S, Boomsma JJ. The evolution of fungus-growing termites and their mutualistic fungal symbionts. Proc Natl Acad Sci U S A. 2002;99:14887–14892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Talbot PHB. The Sirex-Amylostereum-Pinus association. Annu Rev Phytopathol. 1977;15:41–54. [Google Scholar]
- 19. Kolařík M, Hulcr J. Mycobiota associated with the ambrosia beetle Scolytodes unipunctatus (Coleoptera: Curculionidae, Scolytinae). Mycol Res. 2009;113:44–60. 10.1016/j.mycres.2008.08.003 [DOI] [PubMed] [Google Scholar]
- 20. Kasson MT, O’Donnell K, Rooney A, Sink S, Ploetz R, Ploetz JN, et al. An inordinate fondness for Fusarium: phylogenetic diversity of fusaria cultivated by ambrosia beetles in the genus Euwallacea on avocado and other plant hosts. Fungal Genet Biol. 2013;56:147–157. 10.1016/j.fgb.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 21. Wood SL. The bark and ambrosia beetles of North and Central America (Coleoptera: Scolytidae), a taxonomic monograph. Gt Basin Nat Mem. 1982;6:1–1356. [Google Scholar]
- 22. Wood SL, Bright DE Jr.. A catalog of Scolytidae and Platypodidae (Coleoptera), Part 2. Taxonomic Index. Gt Basin Nat Mem. 1992;13:1–1553. [Google Scholar]
- 23.Hopkins AD. Report No. 99: Classification of the Cryphalinae, with descriptions of new genera and species. 1915.
- 24.Eichhoff WJ. Felicien Chapuis et W Eichhoff, Scolytides recueillis au Japan par M C Lewis. Soc Entomol Belgique, Ann. 1875;18195–18203.
- 25. Bright DE Jr.. Review of the Tribe Xyleborini in America north of Mexico (Coleoptera: Scolytidae). Can Entomol. 1968;100:1288–1323. [Google Scholar]
- 26. Stebbing EP. On some undescribed Scolytidae of economic importance from the Indian Region, II. Indian For Mem For Zool Ser. 1909;1:13–32. [Google Scholar]
- 27. Seltzer JL, Schiefer TL, Brown RL. Results from the Regional Identification Center of the USDA-APHIS (Eastern Region) at the Mississippi Entomological Museum for the 2012 and 2013 Wood Boring Beetle Surveys, including new state and county records for Alabama and Mississippi. Midsouth Entomol. 2013;6:119–133. [Google Scholar]
- 28. Halbert SE. Entomology section. Tri-ology Tech Rep Entomol Nematol Pathol. 2011;50:6–9. [Google Scholar]
- 29. Tagaki K. The storage organ of symbiotic fungus in the ambrosia beetle Xyleborus rubricollis Eichhoff (Coleoptera: Scolytidae). Japanese Soc Appl Entomol Zool. 1967;2:168–170. [Google Scholar]
- 30. Kirkendall LR, Biedermann P, Jordal BH. Evolution and diversity of bark and ambrosia beetles In: Vega FE, Hofstetter RW, editors. Bark beetles: biology and ecology of native and invasive species. San Diego, California: Academic Press; 2015. pp. 85–156. [Google Scholar]
- 31. White TJ, Bruns TD, Lee SB, Taylor JW. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. San Diego, California: Academic Press; 1990. pp. 315–22. [Google Scholar]
- 32. Vilgalys R, Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol. 1990;172:4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tomšovský M, Menkis A, Vasaitis R. Phylogenetic relationships in European Ceriporiopsis species inferred from nuclear and mitochondrial ribosomal DNA sequences. Fungal Biol. 2010;114(4):350–358. 10.1016/j.funbio.2010.02.004 [DOI] [PubMed] [Google Scholar]
- 34. Zhao C, Cui B. Phylogeny and taxonomy of Ceriporiopsis (Polyporales) with descriptions of two new species from southern China. Phytotaxa. 2014;16:17–28. [Google Scholar]
- 35. Katoh K, Misawa K, Kum K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. [DOI] [PubMed] [Google Scholar]
- 37. Posada D. jModeltest: phylogenetic model averaging. Mol Biol Evol. 2008;25:1253–1256. 10.1093/molbev/msn083 [DOI] [PubMed] [Google Scholar]
- 38. Zwickl DJ. Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. The University of Texas at Austin; 2006. [Google Scholar]
- 39. Sukumaran J, Holder MT. DendroPy: a python library for phylogenetic computing. Bioinformatics. 2010;26:1569–1571. 10.1093/bioinformatics/btq228 [DOI] [PubMed] [Google Scholar]
- 40. Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. [DOI] [PubMed] [Google Scholar]
- 41. Kostovcik M, Bateman CC, Kolařík M, Stelinski LL, Jordal BH, Hulcr J. The ambrosia symbiosis is specific in some species and promiscuous in others: evidence from community pyrosequencing. ISME J. 2014;1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costella EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Binder M, Justo A, Riley R, Salamov A, Lopez-Giraldez F, Sjökvist E, et al. Phylogenetic and phylogenomic overview of the Polyporales. Mycologia. 2013;105:1350–1373. 10.3852/13-003 [DOI] [PubMed] [Google Scholar]
- 44. Miettinen O, Larsson E, Sjökvist E, Larsson KH. Comprehensive taxon sampling reveals unaccounted diversity and morphological plasticity in a group of dimitic polypores (Polyporales, Basidiomycota). Cladistics. 2012;28:251–270. [DOI] [PubMed] [Google Scholar]
- 45. Ryvarden L. New genera in the Polyporaceae. Nor J Bot. 1973;20:1–5. [Google Scholar]
- 46. Mueller UG, Schultz TR, Currie CR, Adams RMM, Malloch D. The origin of the attine ant-fungus mutualism. Q Rev Biol. 2001;76:169–197. [DOI] [PubMed] [Google Scholar]
- 47. Batra LR. Ambrosia fungi: a taxonomic revision, and nutritional studies of some species. Mycologia. 1967;59:976–1017. [Google Scholar]
- 48. Hyodo F, Inoue T, Azuma JI, Tayasu I, Abe T. Role of the mutualistic fungus in lignin degradation in the fungus-growing termite Macrotermes gilvus (Isoptera; Macrotermitinae). Soil Biol Biochem. 2000;32:653–658. [Google Scholar]
- 49. Mueller UG, Gerardo NM, Aanen DK, Six DL, Schultz TR. The evolution of agriculture in insects. Annu Rev Ecol Evol Syst. 2005;36:563–595. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
MiSeq sequences were deposited in NCBI Sequence Read Archive accession SRP058537. Sanger sequence data were deposited in GenBank accessions KR119072–KR119080 and KR871005–KR871009.