Abstract
PURPOSE
We conducted this review to identify published randomized controlled trials (RCTs) of cancer risk assessment tools used in primary care and to determine their impact on clinical utility (clinicians), screening uptake (patients), and psychosocial outcomes (patients).
METHODS
We searched EMBASE, PubMed and the Cochrane databases for RCTs of cancer risk assessment tools in primary care up to May 2014. Only studies set in primary care, with patients eligible for screening, and English-language articles were included.
RESULTS
The review included 11 trials of 7 risk tools. The trials were heterogeneous with respect to type of tool that was used, type(s) of cancer assessed, and outcomes measured. Evidence suggested risk tools improved patient risk perception, knowledge, and screening intentions, but not necessarily screening behavior. Overall, uptake of a tool was greater if initiated by patients, if used by a dedicated clinician, and when combined with decision support. There was no increase in cancer worry. Health promotion messages within the tool had positive effects on behavior change. Trials were limited by low-recruitment uptake, and the heterogeneity of the findings necessitated a narrative review rather than a meta-analysis.
CONCLUSIONS
Risk tools may increase intentions to have cancer screening, but additional interventions at the clinician or health system levels may be needed to increase risk-appropriate cancer screening behavior.
Keywords: cancer screening, risk assessment tools, primary care, practice-based research
INTRODUCTION
Cancer screening programs have been introduced in many countries for breast,1 colorectal,2 and cervical3 cancer. With the growing recognition of the potential harms from population-based cancer screening programs,4 risk-stratified screening is being proposed as a way of reducing harm and focusing on populations at higher risk of cancer. This concept can also be applied to primary preventive measures, especially as the evidence to support chemoprevention for common cancers such as breast and colorectal builds.5,6 If risk-stratified cancer prevention is to be implemented, it requires risk assessment tools that can be used in primary care to identify those most likely to benefit from tailored prevention.7
Cancer risk prediction models, based on epidemiologic data, calculate an individual’s likelihood of developing cancer, identify an individual’s risk of carrying a genetic mutation for a specific cancer (eg, BRCA 1 or BRCA 2), or both.8,9 Newer risk models are beginning to incorporate genomic profiles and environmental exposures,10 a trend that is likely to grow with the movement toward precision medicine.11 Risk assessment tools facilitate the translation of these risk models to estimate an individual’s likelihood of developing different cancers by assessing the combination of risk factors including genetic, environmental12,13 and behavioral12 risk factors. Examples include the US National Cancer Institute (NCI) colorectal cancer risk tool,14 which incorporates the risk model developed by Freedman et al15; the NCI breast cancer risk tool, which applies the Gail breast cancer risk prediction model16; and MelaPRO for assessing risk of melanoma.17
Primary care has an important role in the delivery of cancer screening programs and can increase screening uptake.18 Successful implementation of risk assessment tools into primary care is needed if risk-stratified cancer prevention and the promises of precision medicine are to be achieved.
In this article, we report the first systematic review of randomized controlled trials (RCTs) that have tested cancer risk tools in primary care. The review specifically investigated measures of clinical utility such as clinician referrals and patient cancer screening behaviors, as well as psychosocial outcomes.
METHODS
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Table 1)19 and is registered with Prospero (registration number: CRD42014008892).20
Table 1.
The Systematic Review Question Design According to the PRISMA Guidelines19
| Population | Intervention | Comparison | Outcomes | Study Design |
|---|---|---|---|---|
| Main concept | ||||
| Primary care practitioners Primary care patients |
Cancer risk assessment tool to determine a primary care patient’s individual risk of cancer | Standard clinical care | Clinicians Clinical outcomes including appropriate referral behavior Patterns and accuracy of risk perception Cancer knowledge Frequency of use Acceptability by physicians Confidence of use by clinicians Attitudes to the tool Patients Patient cancer anxiety/worry Acceptability by patients Patient behavior including uptake of secondary referral behavior Adherence to screening recommendations Intention to undergo screening Satisfaction with consultation |
Randomized controlled trials |
| Synonyms/search terms | ||||
| Primary care Primary care clinicians Primary care physicians Family practice General practice GPs Patients |
Risk-assessment tool Clinical tool Risk-prediction tool Decision-support tool Risk-assessment model Computer decision-support tool Adult population Cancer Family history [and synonyms for family] |
Standard care Usual care |
Acceptability Effectiveness Frequency of use Referral data Appropriateness of management Risk accuracy Patient risk perception Psychosocial outcomes Cancer worry Patient behavior |
– |
GP = general practitioner; PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
We searched the PubMed, EMBASE, and Cochrane databases for English-language articles published up to May 2014, focusing on search terms based on the concepts of “risk assessment tools,” “cancer,” “primary care,” and outcomes such as “cancer worry,” “risk perception,” “clinician confidence,” “referral behavior,” and “screening behavior.” Additional articles were identified through citation tracking and reference checking.
Eligibility Criteria
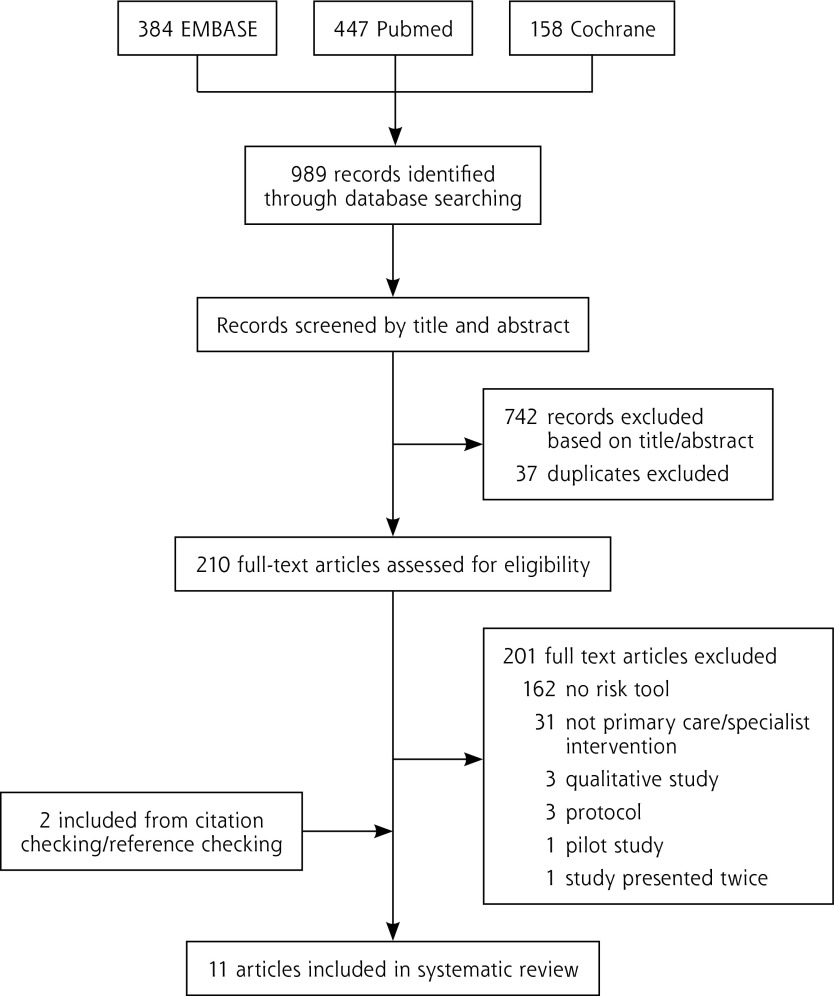
Studies were ineligible if they involved tools that did not estimate cancer risk, assessed prognostic tools for patients with an existing cancer diagnosis, were not implemented in a primary care setting, or did not evaluate the tool using RCTs (Figure 1).
Figure 1.
Article selection for inclusion.
The populations studied included primary care clinicians (general practitioners, family physicians, and community medicine clinicians) and patients of primary care clinicians, as long as they were adults without an existing (known) cancer diagnosis (Table 1).
Study Appraisal and Synthesis Methods
The primary author (J.W.) assessed all citation abstracts, which were reviewed by a second author (M.P.). Two other researchers (P.P.C.C., S.L.) assessed full-text articles. Data were extracted and studies were critically appraised for bias by 3 reviewers (J.W., P.P.C.C., S.L.) using the Cochrane Collaboration’s tool for assessing risk of bias21 (Table 2).22–31
Table 2.
Characteristics of Trials of Cancer Risk Assessment Tools in Primary Care (N = 11)
| Author, Year, Risk Tool, Setting | Disease(s) | Sample | Study Design | Intervention(s) | Overall Risk of Biasa |
|---|---|---|---|---|---|
| Schroy et al22 2011 Your Disease Risk United States |
CRC | 665 patients (223 combined intervention; 212 decision aid alone; 231 control) 50 clinicians (47 general internists; 3 nurse practitioners) 2 clinics |
RCT (3 groups) Patients randomized before routine visit with primary care clinician |
Control: usual care and generic lifestyle change advice for disease prevention Intervention 1: decision aid for CRC screening Intervention 2: decision aid for CRC screening plus CRC personalized risk assessment |
Low/unclear |
| Schroy et al23 2012 Your Disease Risk United States |
CRC | 825 patients (280 combined intervention; 269 decision aid alone; 276 control) 61 clinicians (47 general internists; 11 family physicians; 3 nurse practitioners) 2 clinics |
RCT (3 groups) Patients randomized before routine visit with primary care clinician |
Control: usual care and generic lifestyle change advice for disease prevention Intervention 1: decision aid for CRC screening Intervention 2: decision aid for CRC screening plus CRC personalized risk assessment |
Low/unclear |
| Rubinstein et al24 2011 Family Healthware Impact Trial (1) United States |
CRC, BC, and OC,b heart disease, stroke, and diabetes | 3,283 patients (2,077 intervention; 1,206 control) 41 clinics (23 intervention; 18 control) |
Cluster RCT Cluster randomization at clinic level |
Control: standard print messages about screening and lifestyle choices recommended for general health Intervention: patient self-completed risk assessment using the Family Healthware risk assessment tool; personalized risk prevention messages tailored to familial risk |
Unclear |
| Ruffin et al25 2011 Family Healthware Impact Trial (2) United States |
CRC, BC, and OC,b heart disease, stroke, and diabetes | 3,344 patients (2,105 intervention; 1,239 control) 41 clinics (23 intervention; 18 control) |
Cluster RCT Cluster randomization at clinic level |
Control: standard print messages about screening and lifestyle choices recommended for general health Intervention: patient self-completed risk assessment using the Family Healthware risk assessment tool; personalized risk prevention messages tailored to familial risk |
Unclear |
| Wang et al26 2012 Family Healthware Impact Trial (3) United States |
CRC, BC, and OC,b heart disease, stroke, and diabetes | 3,344 patients (2,105 intervention; 1,239 control) 41 clinics (23 intervention; 18 control) |
Cluster RCT Cluster randomization at clinic level |
Control: standard print messages about screening and lifestyle choices recommended for general health Intervention: patient self-completed risk assessment using the Family Healthware risk assessment tool; personalized risk prevention messages tailored to familial risk |
Unclear |
| Emery et al27 2007 GRAIDS Trial England |
CRC, BC, and OCb | 240 patients received GRAIDS intervention; 84 referred to cancer genetics clinic from control practices 45 clinics (23 intervention; 22 control) |
Cluster RCT Cluster randomization at clinic level |
Control: 45-minute presentation to all GPs in practice on cancer genetics and copy of referral guidelines for cancer genetics clinic Intervention: 45-minute presentation on cancer genetics to all GPs in practice and copy of referral guidelines for cancer genetics clinic; 1–2 “lead clinicians” per practice trained to use web-based GRAIDS risk assessment tool for OC, CRC, and BC |
Low |
| Campbell et al28 1997 Health risk survey Australia |
Cervical cancer | 679 female patients (354 intervention; 325 control) 2 clinics |
RCT Randomization at patient level |
Control: patient self-completed health risk survey Intervention: patient self-completed health risk survey and was given summary including eligibility for cervical screening and date of last Pap test |
Low/unclear |
| Wilson et al29 2006 Risk assessment checklist Scotland |
BC | 346 clinicians (230 intervention; 116 control) 86 clinics (57 intervention; 29 control) |
Cluster RCT (2:1) Randomization at clinic level |
Control: standard Scottish guidelines to assess risk for referral to cancer genetics sent to GPs Intervention: multifaceted intervention including risk assessment checklist for CRC, BC, and OC; information about cancer genetics; patient information booklets; web links cancer/genetics; e-mail link to cancer genetics services; referral letter proforma; education sessions about cancer genetics |
Low |
| Emmons et al30 2004 Harvard Colorectal Cancer Risk Assessment Tool United States |
CRC | 353 patients (134 absolute risk only; 146 absolute plus relative risk; 73 control) 2 clinics |
RCT Randomization at patient level |
All participants used the Harvard Colorectal Cancer Risk Assessment Tool Control: patients received passive risk communication without risk presentation Intervention: patient risk tool providing 4 different combinations of presentations of risk: (1) absolute and relative risk, (2) absolute risk only, (3) absolute and relative risk with the ability to manipulate the risk input to change the output, and (4) same as for (3) but absolute risk only |
Low |
| Weinstein et al31 2004 Harvard Colorectal Cancer Risk Assessment Tool United States |
CRC | 353 patients (134 absolute risk only; 146 absolute plus relative risk; 73 control) 2 clinics |
RCT Randomization at patient level |
All participants used the Harvard Colorectal Cancer Risk Assessment Tool Control: patients received passive risk communication without risk presentation Intervention: patient risk tool providing 4 different combinations of presentations of risk: (1) absolute and relative risk, (2) absolute risk only, (3) absolute and relative risk with the ability to manipulate the risk input to change the output, and (4) same as for (3) but absolute risk only |
Low |
| Holloway et al22 2003 Risk assessment scale Wales |
Cervical cancer | 1,890 female patients (772 intervention; 1,118 control) 29 clinics (15 intervention; 14 control) |
RCT Randomization at clinic level |
Control: no risk assessment Intervention: practice nurse risk communication package including a paper-based risk assessment scale based on level of education, current smoking status, number of years of oral contraceptive use, and number of sexual partners ever33 |
Low |
BC = breast cancer; CRC = colorectal cancer; GP = general practitioner; GRAIDS = Genetic Risk Assessment on the Internet with Decision Support; OC = ovarian cancer; Pap = Papanicolaou; RCT = randomized controlled trial.
Bias assessed using the Cochrane Collaboration risk of bias based on: (1) sequence generation; (2) allocation concealment; (3) blinding of participants, personnel, and outcome assessors; (4) assessment of incomplete outcome data; (5) selective outcome reporting; (6) “other” sources of bias not listed. Low risk of bias = low risk of bias across all domains. Unclear risk of bias = unclear risk of bias for 1 or more key domains. High risk of bias = high risk of bias for 1 or more domains.
These trials assessed patients’ risk for BRCA mutation rather than specifically discussing ovarian cancer screening.
The heterogeneity of interventions and outcomes precluded any meta-analysis of the data. The review provides a narrative synthesis of the data.
RESULTS
Study Selection
Our database searches identified 989 studies. After title and abstract review, and removal of 37 duplicates, 210 full-text articles were assessed for eligibility. Eleven articles reporting trials of 7 risk tools were included (Table 1 and Figure 1).
Study Characteristics
The review included trials of risk assessment tools that were either completed by clinicians with patients or self-completed by patients. Risk tools included web-based risk tools, paper-based risk checklists, and multifaceted interventions involving patient resources, clinician education, or both. The studies included a wide range of outcomes and cancers (Table 2).
The trials varied in their design, including the unit of randomization (clinic or patient) and the population testing the intervention (clinician or patient). Of the 11 studies, 3 randomized by clinic and trialed a clinician-targeted intervention,27,29,32 3 randomized by clinic and trialed a patient-targeted intervention,24–26 and 5 randomized by patient and trialed a patient-targeted intervention.22,23,28,30,31 One study randomized patients by clinic days to reduce potential contamination.28 We examined the unit of randomization as a possible source of heterogeneity of the results and found no clear trends (Table 3).
Table 3.
Results of Trials of Cancer Risk Assessment Tools in Primary Care
| Outcomes Evaluated | Author, Year | Randomization Unit | Results |
|---|---|---|---|
| Patients | |||
| Risk perception | Wang et al26 2012 | Clinic | In patients who underestimated their CRC risk, the intervention increased accuracy of risk perception (intervention 17% vs control 10%, P = .05). There was no increase in accuracy of risk perception between groups in women who underestimated their risk for BC (intervention 18% vs control 14%, P = .4) or OC (intervention 8% vs control 13%, P = .4). |
| Emery et al27 2007 | Clinic | There was no difference in mean risk perception between patients referred from intervention vs control practices. Nonsignificant trend seen toward more accurate risk perception at the point of referral in intervention patients, with fewer overestimating their risk of cancer (OR = 1.50; 95% CI, 0.62–3.67; P = .36). | |
| Holloway et al32 2003 | Clinic | There was no change in risk perception of cervical cancer between groups (OR = 1.07; 95% CI, 0.85–1.35). | |
| Emmons et al30 Weinstein et al31 2004 |
Patient | Accuracy of risk perception increased if risk was presented as combined relative and absolute risks or as absolute risk only vs control (for both people who overestimated and who underestimated their isk preintervention). | |
| Screening intentiona | Holloway et al32 2003 | Clinic | Women at intervention clinics were more likely to intend to reduce their screening interval for cervical screening in line with national guidelines (intervention 44% vs control 61%; OR = 0.51; 95% CI, 0.41–0.64; P <.001). |
| Schroy et al22 2011 | Patient | Mean intention scores to schedule a CRC screening test were higher for both intervention groups vs the control group: intervention group 1: DA (mean = 4.4; SD = 1.0); intervention group 2: DA+YDR (mean = 4.3; SD = 1.0); control group (mean = 3.9; SD = 1.4) (P <.001). Mean intention scores to complete a CRC screening test were higher for both intervention groups vs the control: intervention group 1: DA (mean = 4.3; SD = 1.0); intervention group 2: DA+YDR (mean = 4.3; SD = 1.0); control group (mean = 3.9; SD = 1.3) (P <.001). |
|
| Schroy et al23 2012 | Patient | Booking a screening test: DA group was more likely to book a CRC screening test than control group at 1 month (69.1% vs 60.5%, P <.035); 3 months (71.8% vs 62.3%, P = .019); 6 months (77.0% vs 65.2%, P = .002); and 12 months (80.7% vs 71.4%, P = .011). DA group was more likely than DA+YDR group to book a CRC screening test at 1 month (69.1% vs 60.4%, P <.031); 6 months (77.0% vs 67.1%, P <.010); and 12 months (80.7% vs 73.6%, P = .048). |
|
| Screening adherenceb | Rubinstein et al24 2011 | Clinic | CRC screening increased in both groups over time: intervention, from 76% to 84%, and control, from 77% to 84% (P = .95). BC screening increased in both groups over time: intervention, from 73% to 82%, and control, from 78% to 85% (P = .82). No difference between intervention and control groups in screening adherence for CRC, BC, or OC (P >.09) after 6 months. |
| Holloway et al32 2003 | Clinic | No difference in actual cervical screening intervals and consistency with guidelines between groups at 5 years: intervention 5%, control 7% (OR = 0.61; 95% CI, 0.36–1.03; P = .063). | |
| Schroy et al23 2012 | Patient | Completing a CRC screening test: DA group was more likely than control group to complete test (43.1% vs 4.8%, P = .046) |
|
| Campbell et al28 1997 | Patient | No difference in cervical screening in women identified as being “underscreened” (P >.05). | |
| Behavior change | Ruffin et al25 2011 | Clinic | Intervention group was more likely than control group to increase daily fruit and vegetable intake from ≤5 servings to ≥5 servings (OR = 1.29; 95% CI, 1.05–1.58) and to increase physical activity to 5–6 times/week for ≥30 minutes per day (OR = 1.47; 95% CI, 1.08–1.98). |
| Anxiety/worry | Emery et al27 2007 | Clinic | Cancer worry was lower in patients referred from intervention practices vs from control practices: mean difference = −1.44 (95% CI, −2.64 to 0.23; P = .02). |
| Holloway et al32 2003 | Clinic | Women at intervention practices were less likely to be “fearful of cervical cancer” (OR = 0.66; 95% CI, 0.47–0.93; P = .019), “concerned about chances of serious problems with a smear in the future” (OR = 0.70; 95% CI, 0.51–0.95; P = .026), and “anxious about a recent smear test” (OR = 0.81; 95% CI, 0.66–0.98; P = .036). No differences seen between women at intervention vs control practices in “concern about their smear result” (OR = 0.75; 95% CI, 0.45–1.24; P = .25). |
|
| Emmons et al30 Weinstein et al31 2004 | Patient | 33% of all participants in the study had less cancer worry and 17% had more cancer worry after using the Harvard CRC Risk Tool (comparative data between groups not reported). | |
| Knowledge | Emery et al27 2007 | Clinic | There was a nonsignificant increase in cancer knowledge in patients referred from intervention practices vs from control practices: BC knowledge mean difference = 0.11 (95% CI, −1.05 to 1.27) and CRC knowledge mean difference = 0.64 (95% CI, −1.01 to 2.29). |
| Wilson et al29 2006 | Clinic | No difference seen in patient knowledge between groups for items “Stress is a major cause of BC” (23% vs 23%, P = .98); “Having one close relative with BC always increases your risk considerably” (88% vs 91%, P = .71); and “Minor injury to the breast can cause BC” (20% vs 23%, P = .78). | |
| Holloway et al32 2003 | Clinic | 85% of women at control practices incorrectly agreed that “cervical cancer is among the top 4 female cancers in the UK” compared with 22% of women at intervention practices (OR = 0.05; 95% CI, 0.02–0.11; P <.0001). | |
| Schroy et al22 2011 | Patient | DA groups and DA+YDR group both had increased knowledge scores vs control: intervention group 1 (DA): mean = 3.2; SD = 2.6; intervention group 2 (DA+YDR): mean = 3.0; SD = 2.5; control: mean = 0.8; SD = 2.2 (P <.001). No differences seen in knowledge scores between DA and DA+YDR groups. |
|
| Satisfaction | Schroy et al22 2011 | Patient satisfaction was higher for DA or DA+YDR vs control: intervention group 1 (DA): mean = 50.7; SD = 6.2; intervention group 2 (DA+YDR): mean = 50.5; SD = 6.2; control group: mean = 46.7; SD = 7.9 (P <.001). Satisfaction did not differ between DA and DA+YDR groups. | |
| Clinicians | |||
| Appropriate screening and/or referral | Emery et al27 2007 | Clinic | Increase seen in referral rate to cancer genetics clinic in intervention practices; mean difference = 3.0 referrals per 10,000 patients per practice per year (95% CI, 1.2–4.8; P = .002). Referrals from intervention practices were more likely to be consistent with referral guidelines and therefore “appropriate” vs control practice referrals (OR = 5.2; 95% CI, 1.7–15.8; P = .006). |
| Wilson et al29 2006 | Clinic | No difference seen between groups in appropriateness of referrals: intervention 58%, control 48% (RR = 1.18; 95% CI, 0.88–1.37). | |
| Clinician confidence | Emery et al27 2007 | Clinic | Clinicians’ confidence in managing people with a family history of cancer increased in intervention practices vs control practices (P <.0001). |
| Wilson et al29 2006 | Clinic | No change seen in clinician confidence between groups for the following about BC risk: “taking appropriate family history” (60% vs 61%, P = .93); “knowing which patients need to be referred” (40% vs 33%, P = .27); “reassuring low-risk patients” (57% vs 52%, P = .46); and “being able to answer questions” (23% vs 22%, P = .77). |
AM = adjusted mean; BC = breast cancer; CRC = colorectal cancer; DA = decision aid; OC = ovarian cancer; OR = odds ratio; RR = risk ratio; YDR = Your Disease Risk.
Participant has the intention to schedule or order a screening test.
Participant has completed a screening test.
Recruitment proportions of eligible study participants varied from very low (14% to 25%)24–26,30,31 to very high (93% to 95%).28,32 Contacting eligible patients by mail and following them up with a telephone call yielded a low recruitment. 24–26,30,31 More successful recruiting (93% to 95% of eligible participants) was achieved with a dedicated research assistant or practice nurse recruiting eligible patients in the primary care waiting room before their appointment.28,32 Similarly, when the intervention was delivered by a practice nurse, 75% of patients completed a risk assessment,28 but when clinicians were required to complete training, engagement was low (12% of intervention general practitioners attended).29
Outcomes
Study outcomes are shown in Table 3 and discussed in detail below.
Accuracy of Patient Risk Perception
Overall, there was limited evidence that risk assessment tools altered patients’ risk perception, except in specific subgroups. For example, in the Family Healthware Impact trial,26 there was a significant increase in accuracy of risk perception in those patients who underestimated their risk of colorectal cancer at baseline, but not in women who underestimated their risk of breast cancer. The trial of the Harvard Colorectal Cancer Risk Assessment Tool specifically tested different risk presentation formats. In people who either underestimated or overestimated their risk at baseline, accuracy of risk perception was improved by either absolute risk alone or absolute risk plus relative risk formats, compared with the control patients.26,30,31 The Genetic Risk Assessment on the Internet with Decision Support (GRAIDS) trial and a trial assessing cervical cancer risk found no significant differences in risk accuracy for colorectal cancer,27 breast cancer,27 or cervical cancer.32
Patient Behaviors
Four trials explored screening behavior outcomes, including screening intentions, patient booking/planning a screening test, and patient completing a screening test.
Schroy et al22 tested a pair of interventions. Intervention 1 was a shared decision-making tool, and intervention 2 was a combined shared decision-making tool plus a risk assessment tool (Your Disease Risk), comparing them both with usual care. Immediately postintervention, intentions to order a screening test and intentions to complete a screening test were higher in both intervention groups relative to the control group (P <.001) with no difference between the 2 interventions. During the 12-month follow-up, participants using the decision aid alone (intervention 1) were more likely at every time point to book a test than the control group, and similarly, the decision aid increased the likelihood of completing a screening test when used alone, but not when combined with risk assessment.23
In the Family Healthware Impact trial, there was an increase in colorectal cancer and breast cancer screening in both groups, but no difference in screening rates between the intervention and control groups at 6 months. Of further note, the relatively high rates of cancer screening at baseline in both groups suggested a ceiling effect.24
Holloway et al32 trialed the effect of a risk tool on reducing time intervals between cervical screening, which, at the time of the trial in the United Kingdom, was recommended every 5 years. In the short term, women in the intervention group intended to have screening less frequently, but at 5 years of follow-up, there was no significant difference.
In contrast, Campbell et al28 tested a risk tool with women in primary care in Australia to identify underscreened women and encourage them to have risk-based cervical screening. At 6 months of follow-up, women in the intervention group were no more likely to have had a cervical screening test than those in the control group.
The Family Healthware Impact trial also assessed impact on lifestyle behaviors.25 The risk tool provided age-specific and sex-specific health messages to participants based on their family history of heart disease, stroke, diabetes, colorectal cancer, breast cancer, and ovarian cancer. After 6 months, participants in the intervention group were significantly more likely to have increased their daily fruit and vegetable intake, and their physical activity.
Patient Cancer Worry
None of the 3 trials that measured cancer-related anxiety found any increase after risk assessment. The GRAIDS trial recruited patients who had discussed concerns about their familial cancer risk with their general practitioner.27 Patients referred to cancer genetics services from practices that used the GRAIDS tool had a lower cancer worry than patients referred from the control practices. In the cervical screening trial of Holloway et al,32 women receiving the intervention were less likely to be “fearful” of cervical cancer, less “concerned about chances of serious problems with a smear in the future,” and less “anxious about a recent smear test.”
In the trial of the Harvard Cancer Risk Assessment and Communication Tool, 33% of participants reported feeling less worried about getting colorectal cancer, but 17% reported increased worry about the disease after using the tool.30,31 These associations were seen regardless of whether the risk was presented as absolute risk, relative risk, or combined risk. There were no comparable control data in this trial for cancer worry.
Patient Knowledge
Patient knowledge was measured by understanding of population cancer risk, causes of cancer, and screening guidelines. Schroy et al22 found that both intervention groups had improvements in their knowledge of colorectal cancer screening guidelines, rationale, and goals. Women in the cervical screening trial had a greater understanding of screening guidelines and, in particular, screening intervals recommended for cervical screening as a result of the intervention.32 Wilson et al29 found no differences in patient knowledge between groups despite patient and clinician education.
Patient Satisfaction
Only 1 study measured patient satisfaction. In this study, the use of a decision aid with or without a risk tool improved patient satisfaction with making screening decisions compared with the control condition.22
Appropriate Clinician Referrals, Screening, or Both
Two trials from the United Kingdom looked at the effect of risk tools on “appropriateness of referrals” to cancer genetics services by comparing them against local referral guidelines.27,29 In the GRAIDS trial, risk assessment increased the proportion of appropriate referrals when compared with local guidelines that were implemented with the GRAIDS tool.29 Although there was an increase in appropriate referrals based on the local guidelines, the actual proportion of patients found to be at high risk was no different after more detailed assessment at the genetics clinic. This finding suggests a lack of specificity of the referral guideline that is likely to be implemented more systematically using a risk tool.
Clinician Confidence
In the GRAIDS trial, clinicians’ confidence in assessing patients’ family history of cancer was increased.27 In contrast, in the Scottish trial, no differences were observed in clinician confidence about family history risk assessment and referral.29
DISCUSSION
This systematic review identified only 11 articles reporting trials of 7 cancer risk assessment tools in primary care. Overall, this sample represents a relatively small evidence base, especially in the context of the growing number of cancer risk tools available online. The findings suggest potentially beneficial effects of cancer risk assessment tools in terms of improving accuracy of patient risk perception and knowledge, intentions to have cancer screening, and changes in diet and physical activity, without causing an increase in cancer-specific anxiety. Effects on actual cancer-screening behaviors are less clear. Cancer risk assessment tools may also improve clinician confidence and appropriateness of referrals to cancer genetics services, although the evidence for this benefit is somewhat contradictory from only 2 trials. Risk tools were more successful when they were initiated by patient who were concerned about their family history (of cancer),27 were used by a dedicated clinician,27,32 included health promotion messages,25 and included decision support within the tool.23 Interventions were less successful when tested in trials that involved a passive system for using the risk assessment tool.29
There are some important caveats. The trials included in this review were heterogeneous in terms of the precise nature of the intervention, the unit of randomization, how they were implemented, and the health care setting in which they were studied. Furthermore, some of the populations in which the tools were used were selected toward a group who had existing concerns about their risk, especially about their family history. For example, the relatively low recruitment rates in the US Family Healthware Impact trial probably were associated with response bias toward a well-educated sample with relatively high baseline rates of cancer screening. Additional methodologic weaknesses in some studies included small sample sizes and therefore potentially underpowered trials,28,30,31 poor recruitment rates22–24,26,29–31 lack of clinician engagement in the intervention,29 and patient-reported outcomes that may be influenced by social desirability bias.28 The unit of randomization was not a clear source of heterogeneity despite greater risk of contamination in the patient-randomized trials.
Previous systematic reviews have examined the effect of patient-oriented decision aids in screening33 and also communication of risks in screening programs.34 Our review differs in terms of the nature of the interventions and the populations studied, although the findings are consistent: communication of risks is associated with increased intention to screen, and patient-oriented decision aids can increase knowledge. Two of the included studies examined different methods of communicating risk. The way risks were presented across all trials varied, and none complied with current perceived best practice in presenting risk information as recommended by the International Patient Decisions Aid Standards.35,36
If we are to move toward risk-stratified cancer screening, primary care clinicians will require simple tools to implement validated risk models, which are likely to incorporate genomic as well as lifestyle factors. As the GRAIDS trial demonstrated, risk tools are only as effective as the underlying risk model. Ideally, tools will be able to present absolute risks and the predicted effects of behavior change or chemoprevention on an individual’s risk of cancer. Importantly, they need to be designed to present evidence in ways that highlight the risks of overscreening people at average or low risk as well as the benefit of screening in populations who are most likely to benefit.37 Most of the trials to date have focused on a single cancer or those for which predictive genetic testing was relevant. Validated risk prediction models, however, exist for many common cancers that could ideally be incorporated into a single tool.
In conclusion, despite the existence of many cancer risk assessment tools, there is relatively limited evidence from RCTs of their effectiveness, especially in terms of their impact on risk-appropriate cancer screening behaviors. Risk tools may increase actual intentions to have cancer screening, but additional interventions at the clinician or system level may be needed to increase screening behavior. The results support the use of dedicated staff to maximize implementation of the intervention. The incorporation of health economic evaluation to determine the most cost-effective approaches to delivering risk-stratified cancer screening in primary care, and the potential added cost-benefit of genomic profiling within these trials, will be important outcomes to measure in future trials.
Footnotes
Conflicts of interest: authors report none.
Funding support: This work was supported by funding from the Victorian Comprehensive Cancer Centre, and the National Health and Medical Research Council of Australia (APP1042021).
References
- 1.Australian Government Department of Health. BreastScreen Australia. http://www.cancerscreening.gov.au/internet/screening/publishing.nsf/Content/breast-screening-1 Accessed Aug 21, 2014.
- 2.Australian Government Department of Health. National Bowel Cancer Screening Program. http://www.cancerscreening.gov.au/internet/screening/publishing.nsf/Content/bowel-screening-1 Accessed Jul 27, 2014.
- 3.Australian Government Department of Health. National Cervical Screening Program. http://www.cancerscreening.gov.au/internet/screening/publishing.nsf/Content/cervical-screening-1 Accessed Aug 21, 2014.
- 4.Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380(9855):1778–1786. [DOI] [PubMed] [Google Scholar]
- 5.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377(9759):31–41. [DOI] [PubMed] [Google Scholar]
- 6.Cuzick J, DeCensi A, Arun B, et al. Preventive therapy for breast cancer: a consensus statement. Lancet Oncol. 2011;12(5):496–503. [DOI] [PubMed] [Google Scholar]
- 7.Burton H, Chowdhury S, Dent T, Hall A, Pashayan N, Pharoah P. Public health implications from COGS and potential for risk stratification and screening. Nat Genet. 2013;45(4):349–351. [DOI] [PubMed] [Google Scholar]
- 8.Marcus J, Page D, Watson P, Narod S, Lenoir G, Lynch H. BRCA1 and BRCA2 hereditary breast carcinoma phenotypes. Cancer. 1997;80(S3):543–556. [DOI] [PubMed] [Google Scholar]
- 9.Aaltonen L, Johns L, Järvinen H, Mecklin J-P, Houlston R. Explaining the familial colorectal cancer risk associated with mismatch repair (MMR)-deficient and MMR-stable tumors. Clin Cancer Res. 2007;13(1):356–361. [DOI] [PubMed] [Google Scholar]
- 10.Yarnall JM, Crouch DJ, Lewis CM. Incorporating non-genetic risk factors and behavioural modifications into risk prediction models for colorectal cancer. Cancer Epidemiol. 2013;37(3):324–329. [DOI] [PubMed] [Google Scholar]
- 11.Khoury MJ, Gwinn ML, Glasgow RE, Kramer BS. A population approach to precision medicine. Am J Prev Med. 2012;42(6):639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. [DOI] [PubMed] [Google Scholar]
- 13.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. [DOI] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services. National Cancer Institute. Colorectal Cancer Risk Tool. http://www.cancer.gov/colorectalcancerrisk/tool.aspx Accessed Aug 21, 2014.
- 15.Freedman AN, Seminara D, Gail MH, et al. Cancer risk prediction models: a workshop on development, evaluation, and application. J Natl Cancer Inst. 2005;97(10):715–723. [DOI] [PubMed] [Google Scholar]
- 16.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–1886. [DOI] [PubMed] [Google Scholar]
- 17.Wang W, Niendorf KB, Patel D, et al. Estimating CDKN2A carrier probability and personalizing cancer risk assessments in hereditary melanoma using MelaPRO. Cancer Res. 2010;70(2):552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emery JD, Shaw K, Williams B, et al. The role of primary care in early detection and follow-up of cancer. Nat Rev Clin Oncol. 2014;11(1):38–48. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269, W64. [DOI] [PubMed] [Google Scholar]
- 20.Walker J, Pirotta M, Licqurish S, Chiang PP-C, Emery J. Systematic review of the clinical use of risk assessment tools in primary care for cancer screening. 2014. http://www.crd.york.ac.uk/PROSPERO Accessed Dec 2, 2013.
- 21.The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. London, England: The Cochrane Collaboration and John Wiley & Sons Ltd; 2008. [Google Scholar]
- 22.Schroy PC, III, Emmons K, Peters E, et al. The impact of a novel computer-based decision aid on shared decision making for colorectal cancer screening: a randomized trial. Med Decis Making. 2011; 31(1):93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schroy PC, III, Emmons KM, Peters E, et al. Aid-assisted decision making and colorectal cancer screening: a randomized controlled trial. Am J Prev Med. 2012;43(6):573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubinstein WS, Acheson LS, O’Neill SM, et al. ; Family Healthware Impact Trial (FHITr) Group. Clinical utility of family history for cancer screening and referral in primary care: a report from the Family Healthware Impact Trial. Genet Med. 2011;13(11):956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruffin MT, IV, Nease DE, Jr, Sen A, et al. ; Family History Impact Trial (FHITr) Group. Effect of preventive messages tailored to family history on health behaviors: the Family Healthware Impact Trial. Ann Fam Med. 2011;9(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C, Sen A, Ruffin MT, IV, et al. ; Family Healthware™ Impact Trial (FHITr) Group. Family history assessment: impact on disease risk perceptions. Am J Prev Med. 2012;43(4):392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emery J, Morris H, Goodchild R, et al. The GRAIDS Trial: a cluster randomised controlled trial of computer decision support for the management of familial cancer risk in primary care. Br J Cancer. 2007;97(4):486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell E, Peterkin D, Abbott R, Rogers J. Encouraging under-screened women to have cervical cancer screening: the effectiveness of a computer strategy. Prev Med. 1997;26(6):801–807. [DOI] [PubMed] [Google Scholar]
- 29.Wilson BJ, Torrance N, Mollison J, et al. Cluster randomized trial of a multifaceted primary care decision-support intervention for inherited breast cancer risk. Fam Pract. 2006;23(5):537–544. [DOI] [PubMed] [Google Scholar]
- 30.Emmons KM, Wong M, Puleo E, Weinstein N, Fletcher R, Colditz G. Tailored computer-based cancer risk communication: correcting colorectal cancer risk perception. J Health Commun. 2004; 9(2):127–141. [DOI] [PubMed] [Google Scholar]
- 31.Weinstein ND, Atwood K, Puleo E, Fletcher R, Colditz G, Emmons K. Colon cancer: risk perceptions and risk communication. J Health Commun. 2004;9(1):53–65. [DOI] [PubMed] [Google Scholar]
- 32.Holloway RM, Wilkinson C, Peters TJ, et al. Cluster-randomised trial of risk communication to enhance informed uptake of cervical screening. Br J Gen Pract. 2003;53(493):620–625. [PMC free article] [PubMed] [Google Scholar]
- 33.Stacey D, Légaré F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;1:CD001431. [DOI] [PubMed] [Google Scholar]
- 34.Edwards A, Unigwe S, Elwyn G, Hood K. Effects of communicating individual risks in screening programmes: Cochrane systematic review. BMJ. 2003;327(7417):703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spiegelhalter DJ. Understanding uncertainty. Ann Fam Med. 2008; 6(3):196–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trevena LJ, Zikmund-Fisher BJ, Edwards A, et al. Presenting quantitative information about decision outcomes: a risk communication primer for patient decision aid developers. BMC Med Inform Decis Mak. 2013;13(Suppl 2):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ait Ouakrim D, Boussioutas A, Lockett T, et al. Screening practices of unaffected people at familial risk of colorectal cancer. Cancer Prev Res (Phila). 2012;5(2):240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]