Abstract
Objective To elucidate the role of Gamma Knife radiosurgery (GKRS) in the management of nonfunctioning pituitary adenomas (NFAs).
Materials and Methods A retrospective review of 57 consecutive patients spanning 2000 to 2013 with NFAs was performed. Of 57 patients, 53 patients had recurrent or residual tumors after microsurgical resection. The study population was evaluated clinically and radiographically after GKRS treatment. The median follow-up time was 45.57 months.
Results GKRS in pituitary adenomas showed significant variations in tumor growth control (decreased in 32 patients [56.1%], arrested growth in 21 patients [36.1%], and increased tumor size in 4 patients [7%]). Progression-free survival after GKRS at 3, 7, and 10 years was 100%, 98%, and 90%, respectively. The neurologic signs and symptoms were significantly improved after GKRS (14% versus 107%) compared with pretreated signs and symptoms (p < 0.0001). Five patients (8.8%) required additional treatment.
Conclusion Recent follow-up revealed that GKRS offers a high rate of tumor control and preservation of neurologic functions in both new and recurrent patients with NFAs. Thus GKRS is an effective treatment option for recurrent and residual as well as newly diagnosed patients with NFAs.
Keywords: nonfunctioning pituitary adenomas, Gamma Knife surgery, clinical and radiologic outcome
Introduction
Pituitary adenomas are fairly common benign tumors comprising 10 to 20% of all intracranial tumors. Among these lesions, ∼ 30% are nonfunctioning pituitary adenomas (NFAs).1 Although benign, NFAs are challenging to manage because of difficulties in complete resection and the recurrent behavior of the tumors.2 3 Typically these lesions present due to visual disturbances caused by tumor encroachment on the optic apparatus, or alternatively due to impairment of pituitary hormonal production due to compression of the normal functioning pituitary gland. Management of NFAs varies depending on the specific clinical situation and has recently changed due to the advancement of radioimaging, radiotherapy, surgical techniques, and different medical approaches.4 Transsphenoidal resection is the primary treatment option for NFAs because it offers rapid resolution of tumor mass effect and provides good control of tumor growth and endocrine abnormalities.5 6 However, previous reports suggested that incomplete resection due to tumor infiltration into critical neurovascular structures including cavernous sinus is associated with high long-term recurrence rates.7 8 Furthermore, although additional microsurgical resection is an option in treating recurrent tumors, repeat surgery on the pituitary fossa is associated with higher perioperative and postoperative complications than those encountered during initial resection.9 10 Recently, stereotactic Gamma Knife radiosurgery (GKRS) has emerged as a safe and effective adjunct therapy to microsurgical resection for NFAs.3 11 12 There is relatively little in the literature on the intermediate- and long-term outcomes of Gamma Knife radiosurgery on NFAs, particularly on residual or recurrent tumors. Therefore the outcomes from different treatment modalities would be informative in deciding future treatment policies for NFAs. In the present study, we retrospectively evaluated our experience in the management of patients with newly diagnosed and recurrent or residual NFAs emphasizing long-term tumor growth control rate and complications after GKRS therapy. This article contributes to the literature by evaluating a sizable cohort of patients with NFAs who were treated utilizing GKRs and reporting their subsequent response to treatment.
Materials and Methods
This study was done after approval by the institutional review board at our institution. Information related to clinical history, surgery, neuroimaging, and outcomes of the patients with NFAs between 2000 and 2013 was collected retrospectively by a review of each patient's case notes, follow-up medical record, and radiology reports. We had information on the outcomes of all patients.
Patients and Tumor Characteristics
The median age of the patients in this study was 56 years (range: 18–53 years). Thirty-two (56.1%) were men; 25 (43.9%) were women; 36 (63.2%) were whites, and 21 (32.8%) were African Americans. Overall, 53 patients (93%) had prior resection including 34 cases (59.6%) with residual tumor and 19 cases (33.4%) with recurrent tumor. Pituitary adenomas were extended in the suprasellar region in 20 cases (35%) and in the cavernous sinus region in 27 cases (47%). Table 1 lists other characteristics including Karnofsky Performance Status (KPS) score in all cases with NFAs.
Table 1. Characteristics of patients and nonfunctional pituitary adenomas.
| Variables | Value |
|---|---|
| Total patients | 57 |
| Age, y | |
| Median | 56 |
| Range | 18–83 |
| Gender (%) | |
| Male | 32 (56.1) |
| Female | 25 (43.9) |
| Ethnicity (%) | |
| White | 36 (63.2) |
| African Americans | 21 (32.8) |
| KPS (median, %) | |
| 100 | 4 (7) |
| 90 | 32 (56.1) |
| 80 | 21 (36.8) |
| Prior surgery (%) | |
| No prior surgery | 4 (7) |
| Residual | 34 (59.6) |
| Recurrence | 19 (33.4) |
| Tumor extension (%) | |
| Suprasellar | 20 (35) |
| Cavernous sinus | 27 (47.3) |
| Infrasphenoid | 12 (21) |
| Treatment policy (%) | |
| GKRS | 4 (7) |
| Prior resection (signs + GKRS) | 53 (93) |
| Median time to GKRS from surgery, d | 373 |
| Median follow-up period, mo | 45.57 |
Abbreviation: GKRS, Gamma Knife radiosurgery; KPS, Karnofsky Performance Status.
Radiosurgical Technique
GKSR was performed using the Leksell stereotactic unit model “C” (Elekta, Stockholm, Sweden) with the automatic positioning system.The Leksell head frame was applied to the patient's head while the patient was under intravenous sedation and local anesthesia. The patient was then transferred to the magnetic resonance imaging (MRI) suite for imaging. High-resolution contrast-enhanced axial images of the brain were obtained in the three-dimensional spoiled gradient recalled sequence. The imaging data were then transferred to the Gamma Knife planning computer via the Ethernet. The Leksell Gamma Plan software v.5.34 was used to perform the dose planning. The median marginal dose to the tumor was 15 Gy (range: 8–20), the median maximum dose to the tumor was 30 Gy (range: 16–40), and the median isodose line was 50% (range: 30–54%). The median number of shots was 10 (range: 5–15), and in all cases the median dose in optical apparatus was ≤8 Gy (Table 2).1 13 During the second GKR, the marginal dose and maximal dose were 18, 14, and 15 Gy, and 36, 28, and 30 Gy, respectively, with an isodose of 50%. The radiation dose in optic apparatus was ≤8 Gy for all three patients.
Table 2. Summary of doses used during Gamma Knife radiosurgery.
| Parameter | Value |
|---|---|
| Median marginal dose, Gy (range) | 15 (12–20) |
| Median maximum dose, Gy (range) | 30 (16–40) |
| Median isodose line, % (range) | 50 (30–54) |
| Median no. of shots (range) | 10 (5–15) |
| Median dose in optic chiasm, Gy | ≤8 |
| Median dose in optic tract, Gy | ≤8 |
| Median dose in optic nerve, Gy | ≤8 |
Follow-up
Preoperative and follow-up data were collected from the study population. If necessary, patients were contacted by telephone to update their outcome status. Neuroimaging studies were performed at 3-month intervals in the first year of the GKRS treatment, at 6-month intervals for the following 2 years, and annually thereafter. The median duration of follow-up was 45.57 months (range: 12–157 months). Median and mean time to GKRS from surgery was 373 and 1093 days, respectively.
Statistical Analysis
Commercially available software, SPSS v.21.0 (SPSS, Inc., Chicago, IL, USA), was used for statistical analysis. Progression-free survival was analyzed using the Kaplan- Meier test. When necessary a chi-square and t test were also used. A p value < 0.05 was considered significant.
Results
Tumor Growth Control
Table 3 lists the tumor growth control after GKRS. The average volume of all tumors was 3.7 cm3 (range: 0.43–18.5 cm3). The most recent follow-up showed shrinkage of tumor size in 29 (51%) of the patients, arrested tumor growth in 22 (38.5%) of the patients, and progression in tumor size in 6 (10.5%) of the patients. Control of tumor, defined by a tumor either shrinking or halting growth in response to therapy, was achieved in 90% of cases. The average tumor size in the shrinkage, stable, and progression groups before GKRS was 3.9 (0.43–18.5) cm3, 3.4 (1.26–12.9) cm3, and 3.9 (1.3–11.4) cm3, respectively. Tumor size after GKRS was significantly reduced > 66% (post-GKRS, 1.3 cm3 versus pre-GKRS, 3.9 cm3) of the pretreated tumor size. There was no significant difference in tumor size in growth-arrested tumors (post-GKRS, 3.3 cm3 versus pre-GKRS, 3.4 cm3) after GKRS. There was a significant increase (61%) in tumor size in the progression tumors group (post-GKRS, 6.3 cm3 versus pre-GKRS 3.9 cm3) after GKRS. Median time for shrinkage and progression of the tumor was 43.51 and 73.33 months, respectively. Kaplan-Meier statistical analysis revealed that progression-free survival after GKRS at 3, 5, 7, and 10 years was 100%, 98%, 98%, and 90%, respectively (Fig. 1).
Table 3. MRI follow-up results after Gamma Knife radiosurgery.
| Follow-up | Value |
|---|---|
| Tumor size, cm3 | |
| Before treatment (mean, all tumors) | 3.7 (0.43–18.5) |
| Shrinkage | 3.9 (0.43–18.5) |
| No change | 3.4 (1.26–12.9) |
| Progressed | 3.9 (1.3–11.4) |
| After treatment | |
| Shrinkage | 1.3 (0.2–5.07) |
| No change | 3.3 (1–11.29) |
| Progressed | 6.3 (2.9–18) |
| Mean time to control or progression, mo | |
| Shrinkage | 54.45 (12.1–157) |
| Progression | 71.07 (34.7–102.8) |
| Median time to control or progression, mo | |
| Shrinkage | 43.51 |
| Progressed | 73.33 |
| No. of patients (%) | |
| Shrinkage | 29 (51) |
| No change | 22 (38.5) |
| Progressed | 6 (10.5) |
Fig. 1.
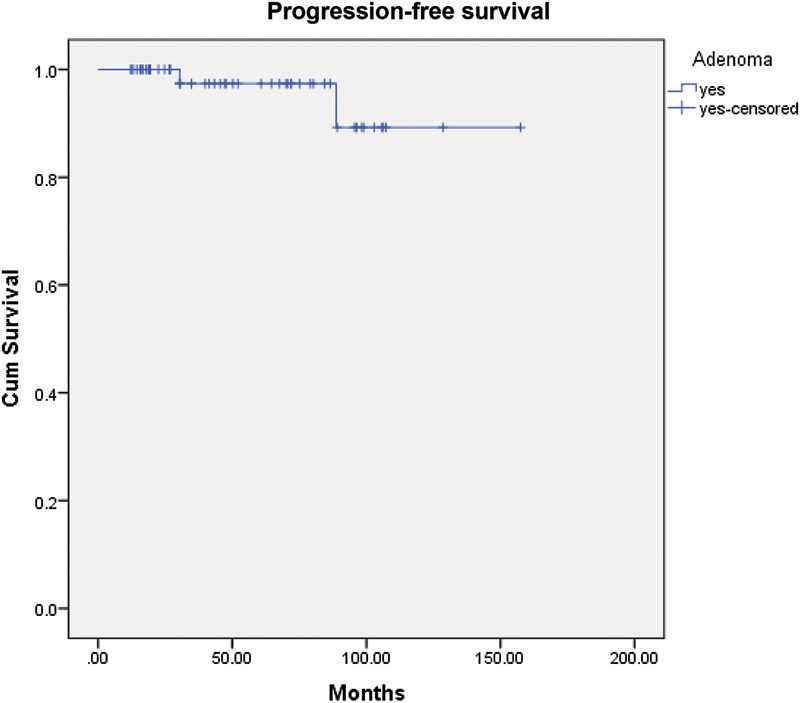
Kaplan-Meier progression-free survival rate in all patients with nonfunctioning pituitary adenomas after Gamma Knife radiosurgery treatment.
Neurologic Problems
With the GKRS, the total number of neurologic signs and symptoms was significantly improved when compared with the amount of pretreated signs and symptoms (post-GKRS, 8 patients [14%] versus pre-GKRS, 61 patients [107%]). The visual deficits were improved significantly after GKRS (pre-GKRS, 41 patients [71.9%] versus post-GKRS, 5 patients [8.8%]). Neurologic deficits were improved significantly after GKRS (pre-GKRS, 20 patients [35%] versus post-GKRS, 3 patients [5.3%]). The probability of progression-free preservation of vision in patients with NFAs after GKRS therapy at 3, 5, 7, and 10 years was 100%, 100%, 100%, and 85%, respectively (Fig. 2). There was significant improvement of headache after GKRS compared with pre-GKRS (2 [3.5%] versus 28 [49.1%], respectively) (Table 4).
Fig. 2.
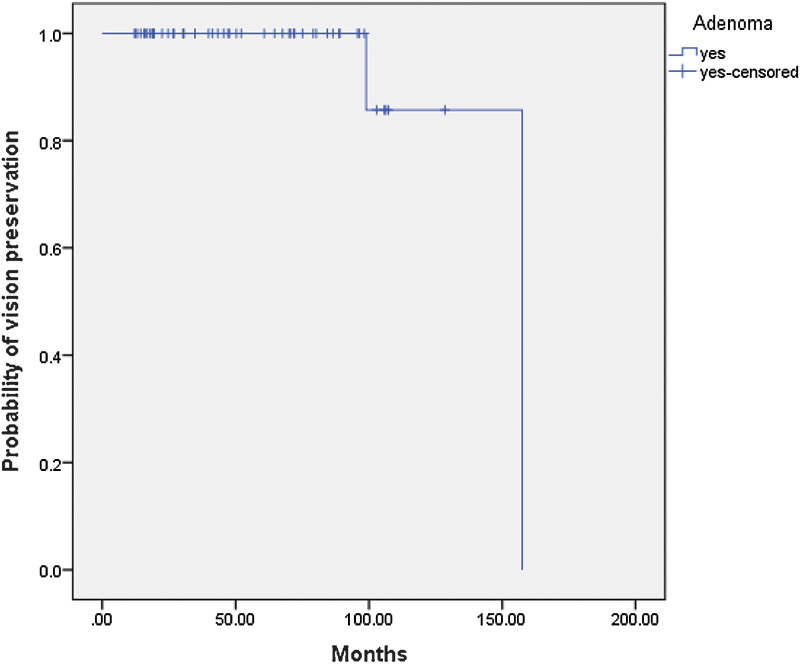
Probability of progression-free preservation of vision in the patients with nonfunctioning pituitary adenomas after Gamma Knife radiosurgery treatment.
Table 4. Clinical outcome after Gamma Knife radiosurgery.
| Clinical features | Pre-GKRS | Post-GKRS | p value |
|---|---|---|---|
| Neurologic problems | |||
| Total visual impairment (%) | 41 (71.9) | 5 (8.8) | < 0.0001 |
| Temporal field defect (%) | 34 (59.7) | 3 (5.3) | |
| Complete visual loss (%) | 7 (12.2) | 2 (3.5) | |
| Neurodeficits (%) | 20 (35) | 3 (5.3) | < 0.0001 |
| Total symptoms (neurologic) (%) | 61 (107) | 8 (14) | < 0.0001 |
| Headache (%) | 28 (49.1) | 2 (3.5) | < 0.0001 |
| Performance status | |||
| KPS score (median), % | 90 | 90 | NS |
Abbreviation: GKRS, Gamma Knife radiosurgery; KPS, Karnofsky Performance Status; NS, not significant.
Note: p <0.05 is considered significant.
Endocrine Abnormalities
There was no improvement of preexisting hypopituitarism as well as no change in the continuation of hormonal replacement. Preexisting hypopituitarism was seen in 36.6% of patients including four patients with panhypopituitarism. New or worsening deficit was observed in 11 patients in the following order: growth hormone axis, 3 (5.3%); adrenocorticotropic hormone, 10 (17.5%); thyroid axis, 8 (14%); gonadotrophin, 3 (5.3%); and diabetes insipidus, 1 (1.7%) (Table 5).
Table 5. Complications after Gamma Knife radiosurgery.
| Clinical Features | Number (%) |
|---|---|
| Complications | |
| Hypopituitarism | 11 (19.2) |
| ACTH | 10 (17.5) |
| Thyroid | 8 (14) |
| Gonadotroph | 3 (5.3) |
| GH | 3 (5.3) |
| Panhypopituitarism | 3 (5.3) |
| Diabetes Insipidus | 1 (1.7) |
| Visual deterioration | 2 (3.5) |
| Radiation necrosis | 0 (0) |
| CVA | 0 (0) |
| New CN III palsy | 1 (1.7) |
| Hydrocephalus | 1 (1.7) |
| Additional therapy required | |
| GKRS | 3 (5.3) |
| Resection | 3 (5.3) |
Abbreviations: ACTH, adrenocorticotropic hormone; CN, cranial nerve; CVA, cerebrovascular accident; GH, growth hormone; GKRS, Gamma Knife radiosurgery.
Functional Results
We demonstrated the KPS score in our case series and found no significant improvement in the median KPS score after GKRS compared with pre-GKRS (KPS of 90 versus KPS of 90; not significant). Fifteen patients (25.8%) had no change in the KPS score, and the KPS score of four patients (7%) deteriorated after GKRS (Table 5).
Complications
The complications including new visual problems, radiation necrosis, new cranial nerve palsy, and hydrocephalus in the patients after GKRS treatments are listed in Table 5. One patient (1.7%) experienced hydrocephalus, and two patients (3.5%) experienced deterioration of visual deficits. One patient (1.7%) developed new cranial nerve (CN) III palsy. No patient experienced any radionecrosis or cerebrovascular accident (CVA).
Additional Management
Six patients (10.5%) had progression of tumor growth and underwent secondary GKRS or microsurgical resection after the initial GKRS. In three of these cases, further management was via microsurgical resection of tumor, one of these cases had two subsequent operations for surgical control of tumor growth, and in the other case both surgery and GKRS for residual tumor was used. In the remaining three, the patients were opposed to any further surgical intervention, and as such GKRS was repeated. The patients required GKRS or microsurgical resection after 6.7 years of the initial GKRS treatment as an additional treatment for residual and recurrent NFAs (Table 5).
Discussion
Microsurgical resection is the first line of treatment for most NFAs including nonfunctional and hypersecreting adenomas. However, previous reports suggested that only 46 to 75% of the tumors involving cavernous sinus or suprasellar region achieved complete resection.14 15 In addition, recurrence rate after subtotal and total resection of NFAs is 50% and 22%, respectively.16 17 Furthermore, recurrent or residual tumors are associated with a higher rate of morbidity over the lifetime of patients with NFAs.3 Therefore microsurgical resection does not always allow complete resolution for NFAs, and an effective adjuvant therapy is often required. Historically, external-beam radiation therapy (EBRT) was used to treat recurrent or residual tumors.18 Despite enhanced tumor control with EBRT, this treatment may be associated with delayed optic neuropathy, long-term hypopituitarism, stroke, and cancer.19 20 21 However, GKRS delivers a high single procedural radiation dose to a target volume of tumor and provides various beneficial effects including an excellent rate of local tumor growth control, shorter hospital stay, lower cost, lower mortality and morbidity, and minimum invasiveness.10
Recent reports related to large GKRS series for NFAs demonstrated 87 to 97% tumor growth control and 42 to 78% tumor regression.3 10 12 13 22 23 The present study revealed that the overall tumor control rate was 90% and that the progression-free survival rate at 3, 7, and 10 years was 100%, 98%, and 90%, respectively, which was very consistent with earlier reports. Overall, 29 patients (51%) showed a tumor volume reduction by > 66% and 22 (38.5%) were stable. Six patients (10.5%) showed tumor recurrence at a median time of progression of 73.33 months after GKRS. Recent evidence suggested that GKRS as a primary treatment approach had 87 to 100% control on tumor growth with acceptable risks.1 10 23 In the current study, of 57 patients, 4 patients with advanced age, small tumor size (mean: 2 cm3) with no mass effect, significant surgical risks, and patient's choice of treatment underwent GKRS as a primary treatment. Our data demonstrated that one patient had shrinkage of tumor, and three patients were stable without visual deterioration after GKRS at a median follow-up period of 36 months. Both the tumor shrinkage and the stability are consistent with other reports in the literature.1 10 23
Outcome studies from literature review of previous reports showed low risk of neurologic deterioration after GKRS in the patients with NFAs.10 24 It is well known that the optic apparatus is the most radiosensitive intracranial structure. Optimal radiation dosing at optic apparatus is one of the major concerns in neurosurgery. When pituitary adenomas present with visual loss, the usual preference is to decompress the optic chiasm via transsphenoidal resection. Following decompression, a limited dose of GKRS is often safe to treat recurrent pituitary adenoma close to the optic apparatus or cavernous sinus.25 Earlier studies suggested that ≤10 Gy was a relatively safe dose for optic apparatus.26 27 28 29 In addition, research evidence also suggested that a dose of 8 Gy was safe for single-session radiosurgery in patients with pituitary adenoma.30 31 El-Shehaby et al reported that of 12 patients with a visual defect, 9 (75%) showed improvement of visual impairment in their series.29 As reported in earlier publications, neurologic complications including visual impairment or cranial neuropathy were also observed in our study. In this study, 63% of patients showed the improvement of visual field defect after GKRS, which was comparable with the findings of earlier reports.27 29
In our institution, we have allocated 8 Gy as the maximum tolerance for the optic apparatus and have kept the dose below this level in all cases (Table 6). Moreover, we used the Leksell Gamma Plan software v.5.34 and MRI images to perform the dose planning before GKRS. In addition, with recent advancement in MRI resolution and spatial accuracy, now it is safe to treat lesions involving the optic nerves and chiasm, and we are able to reverse visual loss in selected cases. Therefore, there was a low chance of radiation-related visual field damage after GKRS in this series.26 27 29 In contrast, research evidence also showed the lower rate of vision improvement after GKRS.26 30 The dose used at the optic apparatus in the earlier series was high (≥ 10 Gy), the number of patients imaged with computed tomography or MRI was not specified, and the maximum dose used for the optic apparatus was planned using overlaying two-dimensional isodose curves on the images. These factors might have contributed to the lower rate of vision improvement in the earlier series.26 32 33
Table 6. Details of the patients with visual defects after Gamma Knife radiosurgery.
| Age, y | Sex | Margin dose | Maximum dose | Optic apparatus | Visual defect |
|---|---|---|---|---|---|
| 42 | F | 18 | 36 | 8 | Temporal field defect |
| 64 | F | 16 | 32 | 8 | Right-sided vision loss |
| 59 | M | 14 | 28 | 8 | Temporal field defect |
| 47 | M | 14 | 28 | 8 | Right-sided vision loss |
| 62 | M | 12.5 | 25 | 8 | Temporal field defect |
In the present study, 8.8% of patients experienced some degree of preexisting visual impairment comparable with earlier studies.3 34 35 36 In this series, among the patients with visual improvement after GKRS, 26 patients had shrinkage of tumors (pre-GKRS, 3.2 cm3 versus post-GKRS,1.5 cm3), 7 patients had small and stable tumors with a slight decrease of size (pre-GKRS,1.7 cm3 versus post-GKRS, 1.5 cm3). Therefore shrinkage of the tumors and stable smaller tumor size might have contributed to improvement of vision in our series. Three patients experienced temporal field visual defects, and two patients experienced complete visual loss in this series. These could be due to tumor (mean size: 6.3 cm3) mediated compression of the optic apparatus, postoperative complications, or GKRS-related adverse effects.13 New cranial neuropathy was observed in one patient (1.7%) in this study, which is also consistent with a previous report.10 The patient had transient CN III palsy and improved with steroid treatment. This could be due to tumor mass effect or a radiation-related adverse effect because this patient had a large tumor (18 cm3).13
Another objective of the pituitary adenomas treatment is to assess the endocrine functions in affected patients. Hypopituitarism is the most common complication of radiosurgery and has been observed in 0 to 72% of patients after GKRS. The current findings demonstrated that 19.2% of patients experienced hypopituitarism after GKRS, which is very consistent with earlier reports.3 Although lower in occurrence, panhypopituitarism is also observed after GKRS therapy.3 This study demonstrated panhypopituitarism in three patients (5.3%) after GKRS. The current study is a partial agreement with the earlier reports with deficiency of thyroid (14%), cortisol (17.5%), and gonadotropin (5.3%), and these hormonal deficiencies were well maintained with levothyroxine, hydrocortisone, and androgen, respectively.3 37 Median marginal dose is one of the predictors for hypopituitarism after Gamma Knife surgery in patients with NFAs.
A prior extensive series showed an overall 30% of hypopituitarism after Gamma Knife surgery.38 However, in that series, patients with a lower marginal dose (12–16 Gy) experienced only 11 to 13% of hypopituitarism. Additionally, patients who were prescribed > 20 Gy of radiation showed 33 to 35.5% of hypopituitarism after Gamma Knife surgery.38 In the present series, only two patients were prescribed 20 Gy, 8 patients received 18 Gy, and 47 patients received 12 to 16 Gy of radiation dose and among them 1 (50%), 3 (37.5%), and 7 (14.8%), respectively, experienced hypopituitarism. Moreover, in this series the prescribed median marginal dose was 15 Gy, and overall 19.2% of patients experienced hypopituitarism. Similarly, a recent multicenter-based series showed that 21% patients experienced hypopituitarism after GKRS using a median radiation dose of 16 Gy.3 Therefore, a lower marginal dose is a possible reason for the lower rate of hypopituitarism in this series.
Another component of calculating the effects of GKRS on pituitary adenomas is identifying the performance status in the study population. Our study demonstrated that there was no significant difference in KPS scores (median KPS: 90 versus 90) after GKRS.
Although this study did not show serious complications including, radionecrosis, CVA, and neoplasia, hydrocephalus was found in one patient. This finding partially agrees with previous reports.3 Lastly, our current study revealed that six patients (10.5%) required additional treatment including GKRS and resection after the initial GKRS due to progression of the tumors and worsening of signs and symptoms. Ideally, the patients who had undergone repeat GKRS should have had a transsphenoidal resection of the tumor, but they were not interested in any operative intervention.
Limitations
This study is limited as a retrospective design and lacked a true control group. Given the good tumor growth control, good overall progression-free survival rate, possible preservation of neurologic functions, and lesser number of complications, GKRS is an important treatment option for patients with NFAs. In addition, GKRS can also be a good treatment option for patients with recurrent or residual NFAs to avoid repeated resections along with craniotomy-related complications. Further randomized controlled studies of a large volume of patients with NFAs are required to accomplish a good comparison of treatment modalities.
References
- 1.Lee C C, Kano H, Yang H C. et al. Initial Gamma Knife radiosurgery for nonfunctioning pituitary adenomas. J Neurosurg. 2014;120(3):647–654. doi: 10.3171/2013.11.JNS131757. [DOI] [PubMed] [Google Scholar]
- 2.Laws E R Jr, Vance M L. Radiosurgery for pituitary tumors and craniopharyngiomas. Neurosurg Clin N Am. 1999;10(2):327–336. [PubMed] [Google Scholar]
- 3.Sheehan J P, Starke R M, Mathieu D. et al. Gamma Knife radiosurgery for the management of nonfunctioning pituitary adenomas: a multicenter study. J Neurosurg. 2013;119(2):446–456. doi: 10.3171/2013.3.JNS12766. [DOI] [PubMed] [Google Scholar]
- 4.Rim C H, Yang D S, Park Y J, Yoon W S, Lee J A, Kim C Y. Radiotherapy for pituitary adenomas: long-term outcome and complications. Radiat Oncol J. 2011;29(3):156–163. doi: 10.3857/roj.2011.29.3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner H E, Stratton I M, Byrne J V, Adams C B, Wass J A. Audit of selected patients with nonfunctioning pituitary adenomas treated without irradiation—a follow-up study. Clin Endocrinol (Oxf) 1999;51(3):281–284. doi: 10.1046/j.1365-2265.1999.00865.x. [DOI] [PubMed] [Google Scholar]
- 6.Chang E F, Sughrue M E, Zada G, Wilson C B, Blevins L S Jr, Kunwar S. Long term outcome following repeat transsphenoidal surgery for recurrent endocrine-inactive pituitary adenomas. Pituitary. 2010;13(3):223–229. doi: 10.1007/s11102-010-0221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gittoes N J, Bates A S, Tse W. et al. Radiotherapy for non-function pituitary tumours. Clin Endocrinol (Oxf) 1998;48(3):331–337. doi: 10.1046/j.1365-2265.1998.00393.x. [DOI] [PubMed] [Google Scholar]
- 8.Marcou Y, Plowman P N. Stereotactic radiosurgery for pituitary adenomas. Trends Endocrinol Metab. 2000;11(4):132–137. doi: 10.1016/s1043-2760(00)00242-3. [DOI] [PubMed] [Google Scholar]
- 9.Laws E R Jr, Fode N C, Redmond M J. Transsphenoidal surgery following unsuccessful prior therapy. An assessment of benefits and risks in 158 patients. J Neurosurg. 1985;63(6):823–829. doi: 10.3171/jns.1985.63.6.0823. [DOI] [PubMed] [Google Scholar]
- 10.Park K J, Kano H, Parry P V. et al. Long-term outcomes after gamma knife stereotactic radiosurgery for nonfunctional pituitary adenomas. Neurosurgery. 2011;69(6):1188–1199. doi: 10.1227/NEU.0b013e318222afed. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi M, Chernov M, Tamura N. et al. Gamma Knife robotic microradiosurgery of pituitary adenomas invading the cavernous sinus: treatment concept and results in 89 cases. J Neurooncol. 2010;98(2):185–194. doi: 10.1007/s11060-010-0172-2. [DOI] [PubMed] [Google Scholar]
- 12.Pollock B E, Cochran J, Natt N. et al. Gamma knife radiosurgery for patients with nonfunctioning pituitary adenomas: results from a 15-year experience. Int J Radiat Oncol Biol Phys. 2008;70(5):1325–1329. doi: 10.1016/j.ijrobp.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Sheehan J P, Niranjan A, Sheehan J M. et al. Stereotactic radiosurgery for pituitary adenomas: an intermediate review of its safety, efficacy, and role in the neurosurgical treatment armamentarium. J Neurosurg. 2005;102(4):678–691. doi: 10.3171/jns.2005.102.4.0678. [DOI] [PubMed] [Google Scholar]
- 14.Mortini P Losa M Barzaghi R Boari N Giovanelli M Results of transsphenoidal surgery in a large series of patients with pituitary adenoma Neurosurgery 20055661222–1233.; discussion 1233 [DOI] [PubMed] [Google Scholar]
- 15.Kurosaki M Lüdecke D K Flitsch J Saeger W Surgical treatment of clinically nonsecreting pituitary adenomas in elderly patients Neurosurgery 2000474843–848.; discussion 848–849 [DOI] [PubMed] [Google Scholar]
- 16.Yang S Y, Zhu T, Zhang J N, Sun Y S. Transsphenoidal microsurgical management of pituitary adenomas. Microsurgery. 1994;15(11):754–759. doi: 10.1002/micr.1920151103. [DOI] [PubMed] [Google Scholar]
- 17.Ciric I, Mikhael M, Stafford T, Lawson L, Garces R. Transsphenoidal microsurgery of pituitary macroadenomas with long-term follow-up results. J Neurosurg. 1983;59(3):395–401. doi: 10.3171/jns.1983.59.3.0395. [DOI] [PubMed] [Google Scholar]
- 18.Brada M Jankowska P Radiotherapy for pituitary adenomas Endocrinol Metab Clin North Am 2008371263–275., xi [DOI] [PubMed] [Google Scholar]
- 19.McCord M W, Buatti J M, Fennell E M. et al. Radiotherapy for pituitary adenoma: long-term outcome and sequelae. Int J Radiat Oncol Biol Phys. 1997;39(2):437–444. doi: 10.1016/s0360-3016(97)00335-0. [DOI] [PubMed] [Google Scholar]
- 20.Littley M D, Shalet S M, Beardwell C G, Ahmed S R, Applegate G, Sutton M L. Hypopituitarism following external radiotherapy for pituitary tumours in adults. Q J Med. 1989;70(262):145–160. [PubMed] [Google Scholar]
- 21.Minniti G, Traish D, Ashley S, Gonsalves A, Brada M. Risk of second brain tumor after conservative surgery and radiotherapy for pituitary adenoma: update after an additional 10 years. J Clin Endocrinol Metab. 2005;90(2):800–804. doi: 10.1210/jc.2004-1152. [DOI] [PubMed] [Google Scholar]
- 22.Pollock B E Carpenter P C Stereotactic radiosurgery as an alternative to fractionated radiotherapy for patients with recurrent or residual nonfunctioning pituitary adenomas Neurosurgery 20035351086–1091.; discussion 1091–1094 [DOI] [PubMed] [Google Scholar]
- 23.Mingione V, Yen C P, Vance M L. et al. Gamma surgery in the treatment of nonsecretory pituitary macroadenoma. J Neurosurg. 2006;104(6):876–883. doi: 10.3171/jns.2006.104.6.876. [DOI] [PubMed] [Google Scholar]
- 24.Lim Y L, Leem W, Kim T S, Rhee B A, Kim G K. Four years' experiences in the treatment of pituitary adenomas with gamma knife radiosurgery. Stereotact Funct Neurosurg. 1998;70 01:95–109. doi: 10.1159/000056412. [DOI] [PubMed] [Google Scholar]
- 25.Sheehan J P Kondziolka D Flickinger J Lunsford L D Radiosurgery for residual or recurrent nonfunctioning pituitary adenoma J Neurosurg 200297(5, Suppl):408–414. [DOI] [PubMed] [Google Scholar]
- 26.Leber K A, Berglöff J, Pendl G. Dose-response tolerance of the visual pathways and cranial nerves of the cavernous sinus to stereotactic radiosurgery. J Neurosurg. 1998;88(1):43–50. doi: 10.3171/jns.1998.88.1.0043. [DOI] [PubMed] [Google Scholar]
- 27.Ove R, Kelman S, Amin P P, Chin L S. Preservation of visual fields after peri-sellar gamma-knife radiosurgery. Int J Cancer. 2000;90(6):343–350. doi: 10.1002/1097-0215(20001220)90:6<343::aid-ijc6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 28.Duma C M Lunsford L D Kondziolka D Harsh G R IV Flickinger J C Stereotactic radiosurgery of cavernous sinus meningiomas as an addition or alternative to microsurgery Neurosurgery 1993325699–704.; discussion 704–705 [DOI] [PubMed] [Google Scholar]
- 29.El-Shehaby A M Reda W A Tawadros S R Abdel Karim K M Low-dose Gamma Knife surgery for nonfunctioning pituitary adenomas J Neurosurg 2012117(Suppl):84–88. [DOI] [PubMed] [Google Scholar]
- 30.Liscák R Vladyka V Marek J Simonová G Vymazal J Gamma knife radiosurgery for endocrine-inactive pituitary adenomas Acta Neurochir (Wien) 200714910999–1006.; discussion 1006 [DOI] [PubMed] [Google Scholar]
- 31.Iwai Y Yamanaka K Yoshioka K Radiosurgery for nonfunctioning pituitary adenomas Neurosurgery 2005564699–705.; discussion 699–705 [DOI] [PubMed] [Google Scholar]
- 32.Stafford S L, Pollock B E, Leavitt J A. et al. A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2003;55(5):1177–1181. doi: 10.1016/s0360-3016(02)04380-8. [DOI] [PubMed] [Google Scholar]
- 33.Leber K A, Berglöff J, Langmann G, Mokry M, Schröttner O, Pendl G. Radiation sensitivity of visual and oculomotor pathways. Stereotact Funct Neurosurg. 1995;64 01:233–238. doi: 10.1159/000098784. [DOI] [PubMed] [Google Scholar]
- 34.Kuo J S Chen J C Yu C et al. Gamma knife radiosurgery for benign cavernous sinus tumors: quantitative analysis of treatment outcomes Neurosurgery 20045461385–1393.; discussion 1393–1394 [DOI] [PubMed] [Google Scholar]
- 35.Abe T, Yamamoto M, Taniyama M, Tanioka D, Izumiyama H, Matsumoto K. Early palliation of oculomotor nerve palsy following gamma knife radiosurgery for pituitary adenoma. Eur Neurol. 2002;47(1):61–63. doi: 10.1159/000047951. [DOI] [PubMed] [Google Scholar]
- 36.Yoon S C, Suh T S, Jang H S. et al. Clinical results of 24 pituitary macroadenomas with linac-based stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 1998;41(4):849–853. doi: 10.1016/s0360-3016(98)00124-2. [DOI] [PubMed] [Google Scholar]
- 37.Vladyka V Liscák R Novotný J Jr Marek J Jezková J Radiation tolerance of functioning pituitary tissue in gamma knife surgery for pituitary adenomas Neurosurgery 2003522309–316.; discussion 316–317 [DOI] [PubMed] [Google Scholar]
- 38.Xu Z Lee Vance M Schlesinger D Sheehan J P Hypopituitarism after stereotactic radiosurgery for pituitary adenomas Neurosurgery 2013724630–637., 636–637 [DOI] [PubMed] [Google Scholar]


