Abstract
Objective The nasoseptal flap (NSF) provides vascularized tissue for repair of skull base defects of various etiologies. However, the NSF repair after skull base resection for anterior cranial base malignancies may demonstrate radiologic findings confusing for recurrent or residual disease on postoperative surveillance imaging. The objective of the current study was to review neuroradiologic misinterpretations of NSF reconstruction following anterior cranial base malignancies.
Methods A multicenter review of patients reconstructed with the NSF after endoscopic resection of anterior cranial base malignancies from 2008 to 2013 was performed. Data were collected regarding etiology, surgical technique, locoregional control, and postoperative radiologic assessments. Only patients with at least one postoperative surveillance scan with inaccurate assessment of residual or recurrent malignancy were included in the study.
Results Over 5 years, 13 patients were identified who had erroneous reporting of malignancy due to NSF reconstruction. On average, two neuroradiologists interpreted the NSF as persistent or recurrent malignancy over this time period (range: 1–7). The key findings suspicious for recurrence were enhancement and soft tissue thickening of the NSF. These findings were present in at least one postoperative scan in all patients.
Conclusion Neuroradiologists and rhinologists performing surveillance on patients with a history of skull base malignancy with NSF reconstruction should maintain collaborative efforts to accurately interpret radiologic findings of the NSF during postoperative imaging.
Keywords: nasoseptal flap, endoscopic skull base surgery, cranial base, skull base tumor, endoscopic resection, endonasal skull base resection, skull base malignancy
Introduction
The nasoseptal flap (NSF) is frequently used to reconstruct skull base defects during endoscopic endonasal cranial base surgery.1 2 3 4 5 6 7 8 The flap has a robust vascular supply from the nasoseptal branch of the posterior septal artery, resulting in rapid healing, decreased crusting, and prevention of postoperative cerebrospinal fluid (CSF) leak. Additionally, the flap allows a wide arc of rotation, so the flap may be positioned from the frontal sinus to the clivus. In cases of malignant disease processes, postoperative surveillance via radiologic studies as well as direct clinical endoscopic visualization are the standards of care. The radiologic appearance of the NSF has limited description in the immediate postoperative setting following resection of skull base malignancies,9 10 and it has not been well described in the interval surveillance radiologic surveys. Although early enhancement on postcontrast T1 magnetic resonance imaging (MRI) sequences may indicate flap viability, the impact on long-term postoperative radiographic appearance may interfere with cancer surveillance. This study examined the neuroradiologic misinterpretations of NSF reconstruction in postresection surveillance imaging for tumor recurrence.
Methods
After obtaining approval from the institutional review board at the University of Alabama at Birmingham and the University of New South Wales, neuroradiology imaging reports were reviewed for all patients with anterior cranial base tumors treated with endonasal endoscopic skull base resection and NSF repair of the skull base defect by B.A.W. and R.J.H. from 2008 to 2013. The technique for harvesting the NSF was performed as previously described,11 with multilayered closure with underlay grafting (porcine small intestine submucosal graft commercially available as Biodesign, Cook Medical, West Lafayette, Indiana, United States, or Durepair, Medtronic, Jacksonville, Florida, United States) with and without overlay grafting and a vascularized pedicled NSF (Fig. 1).12 13 14 15 Clinical data collected included disease etiology, surgical technique, locoregional control, and postoperative radiologic assessments. Only patients with at least one postoperative surveillance scan with inaccurate assessment of residual or recurrent malignancy were included in the study. Only patients with preoperative and postoperative MRI scans interpreted by neuroradiologists were included for evaluation. Postoperative NSF appearance was at least similar to avid enhancement of the original tumors (Figs. 2 and 3).
Fig. 1.
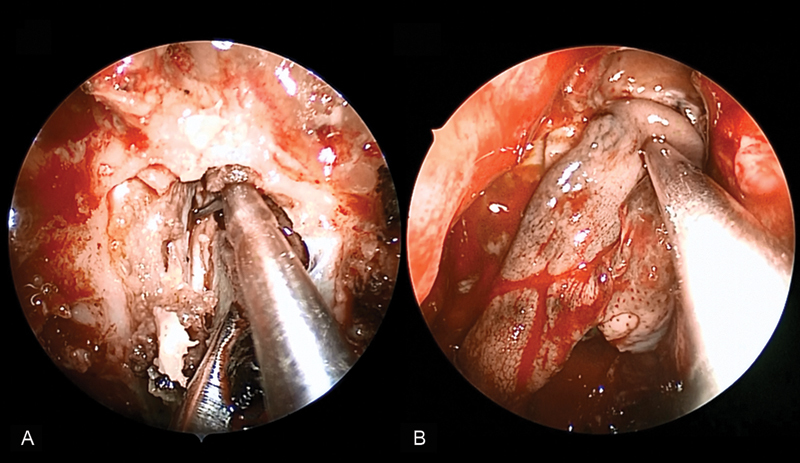
(A) Transnasal endoscopic resection of an adenocarcinoma of the anterior cranial base. (B) The nasoseptal flap is used to reconstruct the defect.
Fig. 2.
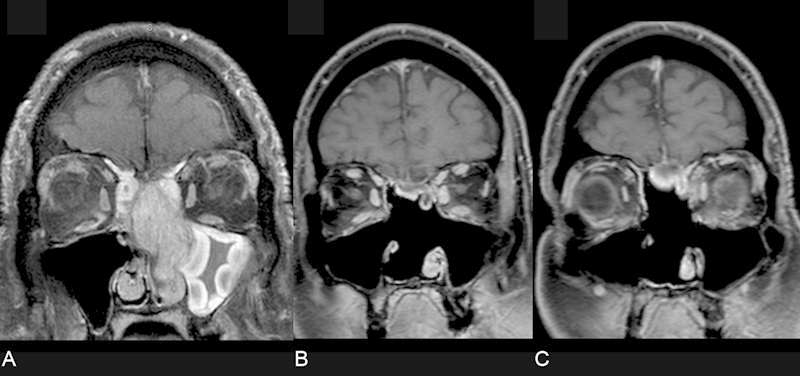
Coronal T1 magnetic resonance imaging (MRI) scan with contrast and fat suppression. (A) The tumor is pedicled on the skull base. (B, C) Postresection surveillance imaging reveals a nodular enhancing structure at the floor of the cranial vault.
Fig. 3.
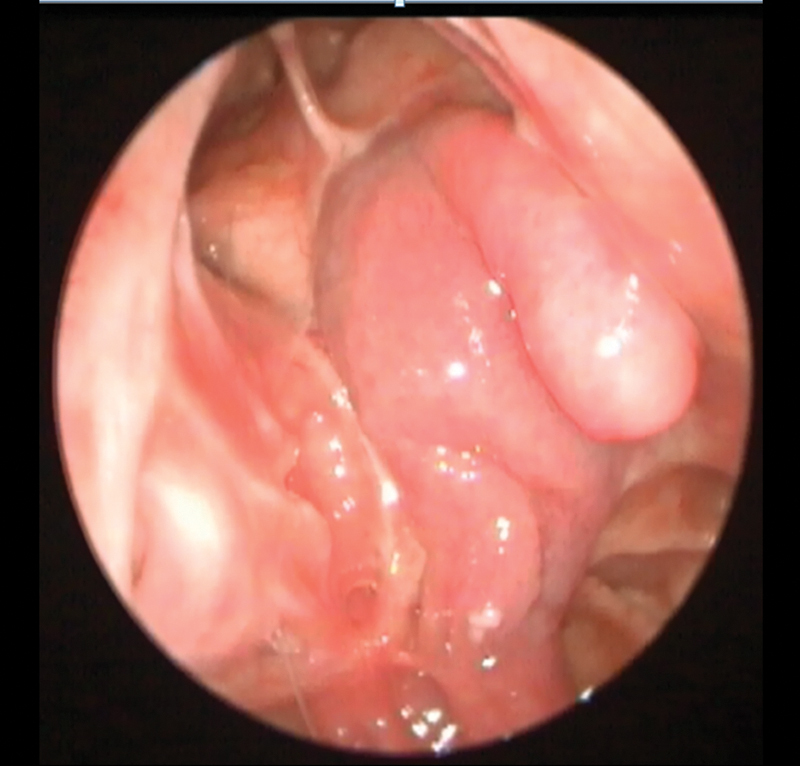
The nasoseptal flap covering the anterior cranial base to the inferior posterior table of the frontal sinus represents the nodular structure demonstrated in Fig. 2. Note the tip of the flap is draped inferiorly along the superior orbit providing the double nodule seen in the magnetic resonance imaging scan.
Results
Over the study period, 32 patients met the criteria for assessment. Thirteen individuals (average age: 59; range: 43–82) had erroneous reporting of recurrent or residual tumor on postoperative imaging following NSF reconstruction. Patient diagnoses, number of postoperative scans, radiation treatment, number of neuroradiologists indicating NSF as a possibly recurrent disease, and clinical follow-up are described in Table 1.
Table 1. Tumor characteristics.
| Histology | No. of MRIs | XRT | No. of NRs | Follow-up, mo |
|---|---|---|---|---|
| Adenocarcinoma | 5 | Y | 4 | 48 |
| Adenocarcinoma | 1 | Y | 1 | 16 |
| Adenoid cystic carcinoma | 5 | N | 2 | 57 |
| Esthesioneuroblastoma | 2 | N | 1 | 18 |
| Squamous cell carcinoma | 2 | N | 1 | 24 |
| Esthesioneuroblastoma | 15 | Y | 7 | 62 |
| Esthesioneuroblastoma | 11 | Y | 1 | 62 |
| Melanoma | 1 | Y | 1 | 24 |
| Squamous cell carcinoma | 9 | Y | 4 | 33 |
| Adenoid cystic carcinoma | 7 | Y | 2 | 31 |
| Esthesioneuroblastoma | 6 | Y | 1 | 55 |
| Esthesioneuroblastoma | 5 | Y | 1 | 36 |
| Squamous cell carcinoma | 5 | Y | 1 | 38 |
Abbreviations: MRI, magnetic resonance imaging; N, no; NR, number of neuroradiologists; XRT, radiation therapy; Y, yes.
Patients had an average clinical follow-up of 38.7 months (range: 16–62 months). Most of the patients (70%) underwent postoperative radiation therapy and demonstrated stable thickness and appearance of the flap over time (Fig. 4). Additionally, one patient with a diagnosis of adenoid cystic carcinoma underwent postoperative proton beam therapy. On average, three neuroradiologists per patient interpreted the NSF on postoperative surveillance imaging as concerning for residual tumor or recurrent disease (range: 1–7). Key findings suspicious for residual/recurrence were enhancement and soft tissue thickening of the NSF. For example, terminology frequently noted in these radiologic reports included “findings of enhancement and prominent soft tissue in the surgical bed concerning for residual tumor or recurrent disease,” which upon review by the otolaryngologist represented the NSF. Other select interpretations are listed in Table 2. All 13 patients had imaging interpretation by a neuroradiologist including this terminology on at least one postoperative MRI study. All studies interpreted with concern for recurrent tumor were reevaluated by neuroradiology after discussion with a rhinologist or neurosurgeon with communication of the expected appearance of NSF. All patients had complete locoregional control based on endoscopic surveillance, computed tomography/positron emission tomography (CT/PET) scans, stable MRI changes, and further explanation of MRI findings with the neuroradiologist. One patient with an esthesioneuroblastoma developed middle cranial fossa dural-based metastases separate from the resection site treated with open resection and Gamma Knife therapy, and another patient with melanoma developed pulmonary, hepatic, and osseous metastases 2 years after surgery.
Fig. 4.
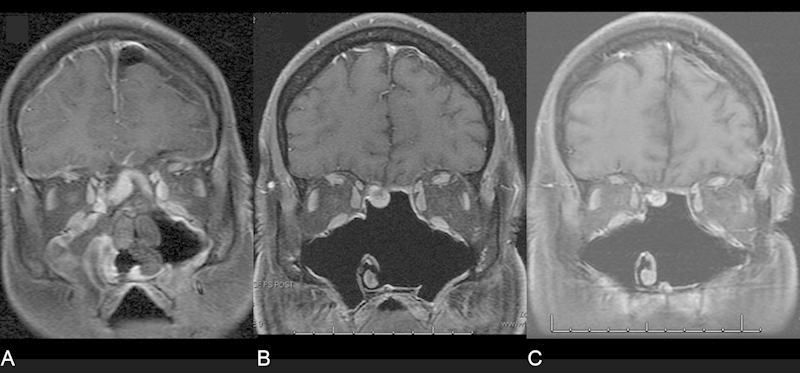
Magnetic resonance imaging scans (A) immediately postoperatively, (B) 3 months, and (C) 2 years after resection of an esthesioneuroblastoma. Note the stable enhancement over time despite radiotherapy.
Table 2. Selected interpretations.
| “Findings are worrisome for residual/recurrent tumor along the midline ethmoid roof, possibly involving the overlying dura. The tissue could conceivably reflect surgically-placed soft tissue to close the cranial defect. However, based on MR imaging there is no way to exclude the possibility that this could represent residual/recurrent tumor.” |
| “There is soft tissue mass in the ethmoid region, 1.0 CC × 2.6 AP × 1.9 cm, immediately beneath the cribriform plate, with homogeneous enhancement suggesting possible tumor.” |
| “There is diffuse mucoperiosteal thickening with a nodular configuration just immediately inferior to the cribriform plate where it measures 1.3 × 0.7 cm. This nodular area is T2 hypointense and enhances with gadolinium.” |
| “On postcontrast images the midline soft tissue along the ethmoid roof enhances fairly prominently. This is worrisome for residual tumor.” |
Discussion
Endoscopic cranial base resection has become a widespread technique for select anterior cranial base malignancies due, in part, to the utility of the NSF. When the septum is not perforated or involved with malignancy, the NSF has become the standard first-choice reconstructive technique for many endoscopic skull base surgeons because it has been widely reported to decrease postoperative CSF leak.10 16 17 18 19 A robust vascular supply promoting mucosal ingrowth has been suggested as the reason for prevention of CSF leak rate. Limited reviews of the radiologic appearance of the NSF in skull base repair have been reported, focusing on the tissue enhancement as a correlation of viability and the risk of CSF leak.9 10 However, the current study aimed to recognize patient surveillance scenarios where neoplastic disease recurrence or persistence was reported erroneously in the setting of NSF reconstruction.
All patients included in this study had at least one interpretation noting concern for enhancing soft tissue or increased soft tissue within the surgical bed suggestive of residual or recurrent neoplasm. For individuals with several interval studies, the radiologists identified the stability of the soft tissue with or without enhancement and more readily reported this as benign or recognized it as a vascularized soft tissue repair. Notably, all patients have had complete locoregional control according to endoscopic follow up, CT/PET scans, and stable changes on surveillance MRI. This illustrates the importance of clinical examination with endoscopy and review of imaging by the rhinologist and neurosurgeon followed by open communication with neuroradiology colleagues.
Conclusion
Postoperative imaging modalities in combination with clinical assessment are mainstays of monitoring for residual or recurrent sinonasal neoplasms. Due to the variations in anatomy following endonasal endoscopic skull base resections, communication and education between the neuroradiologists, rhinologists, and neurosurgeons are critical to evaluate patients accurately for recurrent neoplasia.
Conflict of Interest Bradford A. Woodworth is a consultant for ArthroCare ENT, Gyrus ENT, and Cook Medical.
Notes
Presented at the North American Skull Base Society meeting, San Diego, CA, February 15, 2014.
References
- 1.Chaaban M R, Woodworth B A, Vattoth S, Tubbs R S, Owen Riley K. Surgical approaches to central skull base and postsurgical imaging. Semin Ultrasound CT MR. 2013;34(5):476–489. doi: 10.1053/j.sult.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Chaaban M R, Chaudhry A, Riley K O, Woodworth B A. Simultaneous pericranial and nasoseptal flap reconstruction of anterior skull base defects following endoscopic-assisted craniofacial resection. Laryngoscope. 2013;123(10):2383–2386. doi: 10.1002/lary.24071. [DOI] [PubMed] [Google Scholar]
- 3.Jones V, Virgin F, Riley K, Woodworth B A. Changing paradigms in frontal sinus cerebrospinal fluid leak repair. Int Forum Allergy Rhinol. 2012;2(3):227–232. doi: 10.1002/alr.21019. [DOI] [PubMed] [Google Scholar]
- 4.Chaaban M R, Conger B, Riley K O, Woodworth B A. Transnasal endoscopic repair of posterior table fractures. Otolaryngol Head Neck Surg. 2012;147(6):1142–1147. doi: 10.1177/0194599812462547. [DOI] [PubMed] [Google Scholar]
- 5.Blount A, Riley K, Cure J, Woodworth B A. Cerebrospinal fluid volume replacement following large endoscopic anterior cranial base resection. Int Forum Allergy Rhinol. 2012;2(3):217–221. doi: 10.1002/alr.21025. [DOI] [PubMed] [Google Scholar]
- 6.Virgin F, Barañano C F, Riley K, Woodworth B A. Frontal sinus skull base defect repair using the pedicled nasoseptal flap. Otolaryngol Head Neck Surg. 2011;145(2):338–340. doi: 10.1177/0194599811404527. [DOI] [PubMed] [Google Scholar]
- 7.Schuster D, Riley K O, Cure J K, Woodworth B A. Endoscopic resection of intracranial dermoid cysts. J Laryngol Otol. 2011;125(4):423–427. doi: 10.1017/S0022215110002823. [DOI] [PubMed] [Google Scholar]
- 8.Virgin F W Bleier B S Woodworth B A Evolving materials and techniques for endoscopic sinus surgery Otolaryngol Clin North Am 2010433653–672, xi. xi. [DOI] [PubMed] [Google Scholar]
- 9.Learned K O, Adappa N D, Loevner L A, Palmer J N, Newman J G, Lee J Y. MR imaging evaluation of endoscopic cranial base reconstruction with pedicled nasoseptal flap following endoscopic endonasal skull base surgery. Eur J Radiol. 2013;82(3):544–551. doi: 10.1016/j.ejrad.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 10.Adappa N D, Learned K O, Palmer J N, Newman J G, Lee J Y. Radiographic enhancement of the nasoseptal flap does not predict postoperative cerebrospinal fluid leaks in endoscopic skull base reconstruction. Laryngoscope. 2012;122(6):1226–1234. doi: 10.1002/lary.23351. [DOI] [PubMed] [Google Scholar]
- 11.Hadad G, Bassagasteguy L, Carrau R L. et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116(10):1882–1886. doi: 10.1097/01.mlg.0000234933.37779.e4. [DOI] [PubMed] [Google Scholar]
- 12.Phillips J, Riley K O, Woodworth B A. Porcine small intestine submucosal grafts for post-tumor resection orbital reconstruction. Laryngoscope. 2014;124(6):E219–E223. doi: 10.1002/lary.24515. [DOI] [PubMed] [Google Scholar]
- 13.Illing E, Chaaban M R, Riley K O, Woodworth B A. Porcine small intestine submucosal graft for endoscopic skull base reconstruction. Int Forum Allergy Rhinol. 2013;3(11):928–932. doi: 10.1002/alr.21206. [DOI] [PubMed] [Google Scholar]
- 14.Chaaban M R, Woodworth B A. Complications of skull base reconstruction. Adv Otorhinolaryngol. 2013;74:148–162. doi: 10.1159/000342291. [DOI] [PubMed] [Google Scholar]
- 15.Chaaban M R, Illing E, Riley K O, Woodworth B A. Spontaneous cerebrospinal fluid leak repair: a five-year prospective evaluation. Laryngoscope. 2014;124(1):70–75. doi: 10.1002/lary.24160. [DOI] [PubMed] [Google Scholar]
- 16.Patel M R, Stadler M E, Snyderman C H. et al. How to choose? Endoscopic skull base reconstructive options and limitations. Skull Base. 2010;20(6):397–404. doi: 10.1055/s-0030-1253573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cappabianca P, Cavallo L M, Esposito F, De Divitiis O, Messina A, De Divitiis E. Extended endoscopic endonasal approach to the midline skull base: the evolving role of transsphenoidal surgery. Adv Tech Stand Neurosurg. 2008;33:151–199. doi: 10.1007/978-3-211-72283-1_4. [DOI] [PubMed] [Google Scholar]
- 18.Kassam A B Thomas A Carrau R L et al. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap Neurosurgery 200863101ONS44–ONS52.; discussion ONS52–ONS53 [DOI] [PubMed] [Google Scholar]
- 19.Harvey R J, Parmar P, Sacks R, Zanation A M. Endoscopic skull base reconstruction of large dural defects: a systematic review of published evidence. Laryngoscope. 2012;122(2):452–459. doi: 10.1002/lary.22475. [DOI] [PubMed] [Google Scholar]


