Abstract
Lessons learned during a 6-year experience with more than 1200 patients undergoing targeted prostate biopsy via MRI/US fusion are reported: (1) The procedure is safe and efficient, requiring some 15–20 minutes in an office setting; (2) MRI is best performed by a radiologist with specialized training, employing a trans-abdominal multi-parametric approach and preferably a 3T magnet; (3) Grade of MRI suspicion is the most powerful predictor of biopsy results, e.g., Grade 5 usually represents cancer; (4) Some potentially-important cancers (15%–30%) are MRI-invisible; (5) Targeted biopsies provide >80% concordance with whole-organ pathology. Early enthusiasm notwithstanding, cost-effectiveness is yet to be resolved, and the technologies remain in evolution.
Keywords: targeted prostate biopsy, MRI-ultrasound fusion, multiparametric MRI, direct in-bore biopsy, cognitive fusion biopsy
Disruptive innovation, n.: One that helps to create a new market and value network, eventually … displacing an earlier technology.
— Clayton M. Christensen, Harvard Business Review1
Introduction
Targeted prostate biopsy, obtaining tissue samples from a defined region of interest within the organ, is a disruptive innovation. While still evolving, targeted biopsy often detects serious prostate cancer (CaP) missed by conventional biopsy and, when negative, provides a degree of reassurance not previously possible. Multi-parametric MRI (mpMRI) has become the preferred method for detecting cancer-suspicious regions in the prostate, and with a few caveats, is capable of identifying many serious cancers. MRI targeting is substantially more accurate than ultrasound guidance, the latter usually failing to reveal a valid target within the prostate. With clinical experience increasing rapidly, and as research intensifies into improving methodologies, a review of lessons learned midst the evolution of this important innovation is herein presented.
Conventional Prostate Biopsy
Biopsy guidance via trans-rectal ultrasound (TRUS), introduced by Stamey and colleagues in the 1980s2, became widely adopted; an estimated one million prostate biopsies are performed annually by this ‘systematic sampling’ technique in the United States3. Over the next several decades, the only major improvements made in TRUS-guided biopsy were the use of local anesthesia4 and the increase in number of biopsy cores obtained5. Despite increased sampling, the sensitivity of TRUS-guided biopsy remains low6; under-grading of cancer remains high7; and repeated biopsy sessions, driven by persistently elevated PSA levels, remain routine3. Prostate cancers of the 1980s were larger and easier to detect by systematic sampling than those diagnosed a few decades later8, as PSA screening became prevalent and sizeable lesions were removed from the pool. Moreover, TRUS-guided ‘systematic’ sampling is not always truly systematic, as operator bias using the freehand method often results in sub-optimal spacing within the desired schema9. Thus, the ‘blind’ TRUS-guided biopsy has important limitations.
Advent of MRI to Detect Prostate Cancer (CaP)
The goal of targeted prostate biopsy is three-fold: (1) to detect clinically significant disease, (2) to accurately categorize insignificant disease, and (3) to assess disease burden using the most efficient method possible. Detection of clinically important cancer via contemporary MRI is an important step toward achieving these goals. In his 2008 Whitmore address, Dr. Patrick Walsh said, “If you want to make a substantial contribution to medicine for this decade and maybe for the century, address yourself to the problem of imaging cancer within the prostate gland.10” Years before, in 1983, Hricak and associates published one of the first MR images of a prostate, which was obtained using a magnet of 0.3 Tesla field strength; she wrote that this new modality might in the future have an important role in evaluation of CaP11. MRI technology has advanced rapidly: as recently as 1999, the pioneer Jelle Barentsz wrote from the Netherlands that prostate MRI should be considered “… still at an exploratory phase.12” However, since that time, MR field strength has increased 10-fold; scan time has decreased; resolution has improved dramatically; and multi-parametric imaging has evolved. Landmarks along the way include attempts at standardization, the Prostate Imaging Reporting and Data System (PI-RADS) score13, and numerous reports validating MRI-guided diagnosis of CaP14. In expert hands, no other imaging modality compares with the sensitivity and specificity of mpMRI for visualization of cancer within the prostate. A patient example, demonstrating the value of mpMRI in the diagnosis of CaP, is shown in Figure 1.
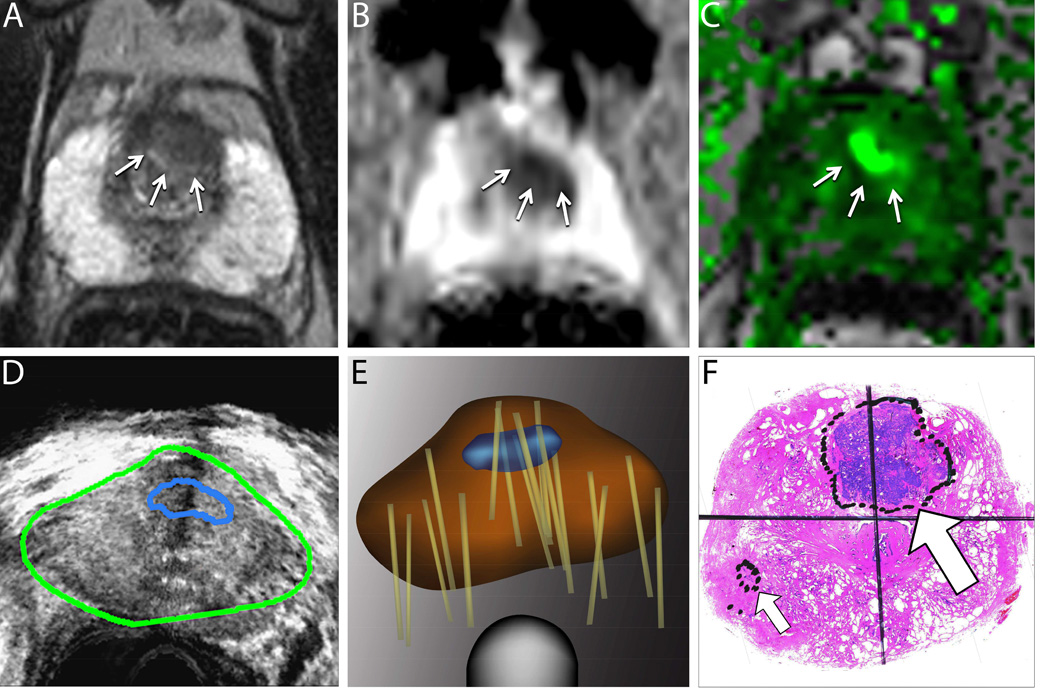
Figure 1. Value of Targeted Biopsy to Evaluate Men for Active Surveillance (Example Case).
A man aged 68 years was referred for active surveillance when a conventional biopsy revealed a small focus of low-grade prostate cancer. A confirmatory biopsy was later performed using magnetic resonance imaging/ultrasound (MRI/US) fusion to target a suspicious region. Top row: Multiparametric MRIs from a T2-weighted image (A), a diffusion-weighted image (B), and a dynamic contrast-enhanced image (C). The delineated region of interest (arrows) was assigned a grade 4 or 5 level of suspicion21. Bottom row: During fusion biopsy, the region of interest from an MRI is superimposed on a US image (D). Systematic and targeted biopsy cores (tan lines) were obtained and recorded on a digital reconstruction of the prostate using an MRI/US fusion device (Artemis; Eigen, Grass Valley, Calif) (E).
A targeted biopsy revealed potentially lethal, high-grade cancer, and radical prostatectomy was performed. A whole-mount prostate sample reveals high-grade cancer (large arrow) that was not detected on an earlier conventional biopsy (F). The small arrow indicates a focus of low-grade cancer that was identified on an earlier “blind” biopsy. Targeted biopsy provides more accurate characterization of whole-gland pathology than conventional (blind) biopsy and is increasingly used to evaluate men for active surveillance34. In the example case, a targeted biopsy revealed that the patient was a poor candidate for active surveillance. Reprinted with permission, American Cancer Society47.
Use of MRI to Guide Prostate Biopsy (Table 1)
Table 1.
Approaches to Targeted Prostate Biopsy
| Targeted Biopsy Method |
Illustration | Operational Technique |
Advantages & Disadvantages |
References |
|---|---|---|---|---|
| In-bore |  |
Radiologist uses MRI to aim directly in-bore at region of interest (ROI) obtained from previous MRI |
Most direct approach, BUT lengthy, resource- intensive, 2 MRIs required, mapping biopsies not taken |
Roethke et al.16 Hambrock et al.38 |
| Cognitive fusion |  |
Operator derives location of ROI from pre-biopsy MRI and aims at inferred ROI using US guidance |
Quick and least expensive, BUT operator-dependent, may miss small lesions, no tracking |
Wysock et al.25 Puech et al.26 Cool et al.27 |
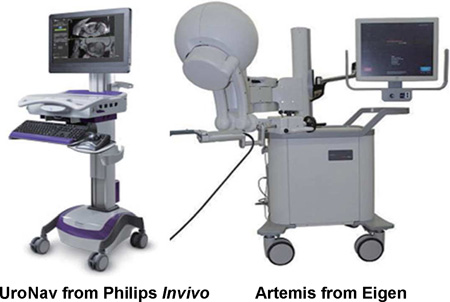
| Software-based MRI/US fusion |
 |
Operator uses a device to fuse stored MRI with real-time US and aims at ROI, delineated by radiologist, appearing on a 3D model |
Brings accuracy of MRI into a clinic setting, quick and efficient, BUT dependent on co- registration |
Natarajan et al.21 Siddiqui et al.23 Xu et al.24 Sonn et al.31 |
Direct In-Bore Biopsy
D’amico and associates were among the first to report use of MRI to guide prostate biopsy15. In an open-source MRI scanner with a field strength of 0.5 T, these authors successfully targeted MRI regions of interest via a trans-perineal approach in a single patient. Cancer was found in MRI targets, but not in random samples outside of targets, thus establishing the basis for clinical trials of MR-guided biopsy16–19. In 2009, the Philips/InVivo Corporation brought to market the DynaTrim Device, an MRI-compatible biopsy frame for performance of trans-rectal prostate biopsies within a closed MRI scanner. This device provided a standard approach to the procedure and helped to facilitate direct in-bore biopsy. Direct biopsy is performed by a radiologist and involves two separate trips to the MR scanner: first to obtain the diagnostic sequences (which must then be processed) and second to undergo the biopsy. In-bore biopsy involves aiming the needle at the perceived ROI, which is done outside the scanner, then confirming needle position by re-scanning. The procedure of moving the patient in and out of the scanner is repeated for each core obtained.
Software Fusion Biopsy
Coincident with development of in-bore biopsy methods, others were developing methods to target MRI-derived images out of bore, away from an MRI scanner, for use in a clinic setting. The process of MRI/US fusion combines the imaging advantage of MRI with the clinical efficiency of ultrasound. In the United States, six image-fusion devices for targeted prostate biopsy are now commercially available20. All employ co-registration of previously-acquired MR images with real-time ultrasound via proprietary software.
Targeted biopsy, aiming at a MRI-derived region of interest, is enabled by image guidance, which in this section refers to MRI/US fusion; when a target is present, as in some three-quarters of cases, samples are taken from the target. When a target is not present (and even when one is present), a template mapping is obtained, using a scalable grid built into the software of the device (Fig. 2). Further, using a fusion device, biopsy site locations are recorded via built-in software for tracking purposes21. Thus, the term ‘fusion biopsy’ incorporates, targeting, mapping, and tracking.
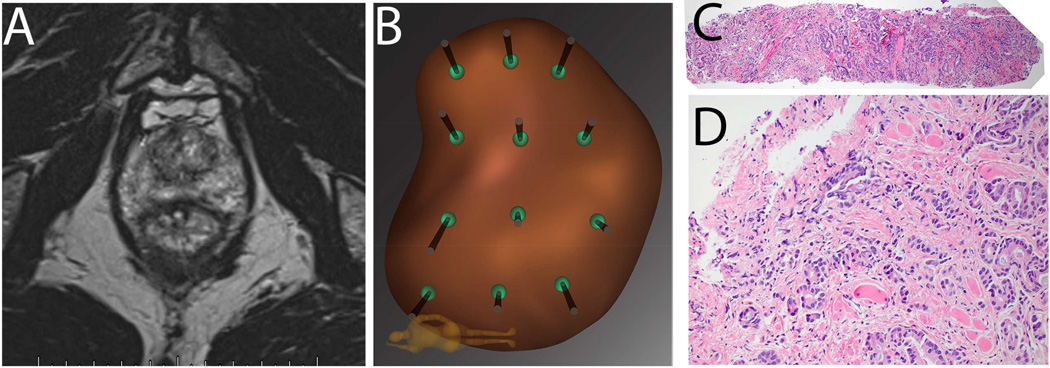
Figure 2. Example of Falsely Negative MRI in Patient P.M.
Patient is a 68 year old Caucasian male (PSA 3.8 ng/ml), who on a previous conventional biopsy was found to have a microfocus of Gleason 3+3=6 prostate cancer. He was considered for active surveillance, and mpMRI of prostate was obtained (A): prostate volume was found to be 35cc; no region of interest (ROI) was identified, even retrospectively. Mapping biopsy was performed by following the 12-point template of the Artemis device (B). A tissue core from the left lateral apex revealed 6 mm of Gleason 3+5=8 prostate cancer (C, 4×; D, 20×). Radical prostatectomy was performed, revealing a tumor on the left side of the prostate with diameters of 15mm × 12mm × 9 mm.
In our experience, the incidence of falsely negative MRI (i.e., Gleason Score ≥7 with no MRI evidence of tumor) is 15% when using biopsy evidence29 and approaches 30% when using whole mount prostatectomy evidence30. When biopsy is clinically indicated, a negative MRI should not preclude mapping biopsy.
Two image-fusion devices, most thoroughly evaluated in the U.S., are the UroNav (Philips/InVivo, FDA-approved in 2009) and the Artemis (Eigen, Grass Valley, CA; FDA-approved in 2008). Both systems are intended to assist trans-rectal ultrasound-guided biopsy with the patient positioned in the familiar lateral decubitus position. Trans-perineal biopsy systems also exist22, but experience with this approach is limited to date. The UroNav system was developed in the U.S. at the National Cancer Institute in collaboration between engineers from the Philips/Invivo Corp. and scientists at the NCI23,24. The UroNav system achieves image fusion via an electro-magnetic tracker positioned over the patient’s pelvis to determine spatial location of the biopsy cores. Probe manipulation is free-hand. The Artemis system originated in the labs of Dr. Aaron Fenster at the University of Western Ontario; the patents were licensed to the Eigen Corp, which developed it commercially; marketing is by Hitachi-Aloka. The Artemis system achieves image fusion and targeted biopsy via a series of encoders (angle-sensing devices) built into a robotic arm21. Probe manipulation with the Artemis device is along a fixed axis established at the beginning of the procedure. Both systems incorporate motion-compensation software. Other FDA-approved devices include the Urostation (Koelis, France), the Virtual Navigator (Esaote, France), the BiopSee (Pi Medical/MedCom, Germany), and the Hi RVS (Hitachi, Japan). The systems have been compared elsewhere20, and a detailed review of all forms of MRI-guided versus conventional biopsy was published recently by Moore and colleagues14.
Cognitive-fusion Biopsy
A third method employs operator awareness of the MRI-derived target, then visual translation of that impression into ultrasound-guided needle placement. Cognitive registration uses a standard TRUS probe for biopsy needle guidance and requires the operator to infer the location of the MRI-suspicious lesion within the TRUS images. The cognitive fusion method requires no fusion device, is relatively quick, and has an obvious cost-advantage. However, biopsy-site tracking is not possible, and small lesions may be missed by the cognitive method25–27.
Targeted Biopsy as a Urological Tool
The Artemis system (Eigen/Hitachi) received FDA approval in May 2008, and it was first exhibited at the annual meeting of the American Urological Association later that month in Orlando, FL. The Department of Urology at UCLA obtained the device in March 2009. A multi-disciplinary group was formed to study the clinical utility of the device. Core members of the group include a radiologist with advanced MRI experience (DM), a urologist (LM), a uropathologist (JH), a biomedical engineer (SN), and a trained device operator (MM). Close collaboration among these principles, who have continued working together since the inception, has led to numerous publications, presentations, and refinements.
After several months of pre-clinical testing, tracking biopsy was started in September, 2009 and MRI/US fusion biopsy was started in March, 2010. Since its arrival, and through several upgrades, the device has been used more than 1600 times in more than 1200 men to obtain nearly 25,000 cores of prostate tissue. All data were gathered prospectively using an IRB-approved protocol (Ref Natarajan 2011). This extensive multi-disciplinary experience, throughout a continuing evolution of targeted biopsy technology, has led to many lessons learned.* The following five are standouts:
Lesson 1: Targeted biopsy is safe and efficient
Targeting and mapping (systematic sampling directed by a built-in grid) can be performed in an office setting under local anesthesia in 15–20 minutes with no more morbidity than conventional biopsy. Beginners should reach this level of efficiency after completing 30 or fewer cases. Sedation of the patient is not necessary. Complications after targeted biopsy at our institution are no more common than with conventional biopsy. Post-biopsy hospitalization for sepsis has become an extremely rare event, seen only twice during the last 500 targeted biopsy cases, after antibiotic prophylaxis was changed to include a single injection of ceftriaxone in addition to a 5-day regimen of a quinolone.
Lesson 2: High-quality MRI is critical to the success of targeted biopsy
Three components of this lesson require attention. First, the equipment used to obtain the images should be up to date, including both the hardware and the software used to delineate the targets. 3T magnet strength is rapidly becoming state-of-the art, but satisfactory results can also be obtained with a 1.5T magnet with an endorectal coil. An endorectal coil is generally not necessary for diagnostic purposes, but may add definition of the capsule in staging evaluations. Multi-parametric imaging (T2, DWI, and DCE) should be obtainable in about 30 minutes of in-bore time; complete processing requires at least a few hours after the images are obtained. Second, the radiologist should be an experienced reader of prostate MRI studies. A proctored experience with about 50 prostate cases should enable a radiologist to make satisfactory interpretation of the images. Radiological training for interpretation of prostate MRI has yet to be standardized, but an online certification is available through the American Roentgen Ray Society (http://www.arrs.org/prostatemri/). Third, every attempt should be made to standardize how the images are obtained, how they are interpreted, and how they are reported. MRI regions of interest are graded according to level of cancer suspicion. At present, most support exists for adoption of the PI-RADS grading system13, but other grading systems have also proved effective28. Best results will likely come from high-volume centers.
Lesson 3: Degree of suspicion on MRI is single most important determinant of biopsy findings
In comparison with other factors (e.g., PSA, age, race, palpation), the grade of suspicion of cancer derived from the MRI most reliably predicts biopsy results. A grading system to quantify degree of suspicion was developed and proposed by the UCLA group even before the PI-RADS system was described21. Other grading systems have also been described28. Regardless of which system is used, in-house standardization is all-important. At our institution, among the first 1000 men studied, when the radiologist assigns a Grade 5 to an MRI target, i.e., the most suspicious grade, either by PI-RADS or the UCLA system, the chances of a targeted biopsy showing cancer approaches 85%, and of the cancers found in such a lesion, more than 80% are high-grade (GS≥7)29. Conversely, when MRI shows only minimal or no abnormalities, the chances of a serious cancer found on mapping biopsy fall to approximately 15%. Among the clinical variables studied in the 1000-man report cited above, PSA density (PSAD) was highly associated with prostate cancer (odds ratio of 7.2)29.
Lesson 4: Both targeted and mapping biopsy should routinely be performed
The MRI-invisible cancer is not a rarity, i.e., even sophisticated mpMRI does not detect all serious prostate cancers. In recent study of 122 men undergoing radical prostatectomy, pre-operative MRI failed to detect GS>7 tumors and tumors >1 cm diameter in 28% of cases each30. In Figure 2 is shown an example of how prostate cancer may elude MRI detection. In the example case, a man with potentially lethal CaP (Gleason Score 8) had a mpMRI that in very experienced hands, using state-of-the-art equipment, was unrevealing. Clearly, targeting of suspicious MRI regions results in the detection of more serious cancers and fewer insignificant cancers than conventional biopsy31, but to ensure maximal sensitivity, both targeted and mapping biopsy should be employed. If biopsy is indicated on clinical grounds, a negative MRI should not preclude it. Why some potentially serious cancers escape MRI detection is not known, but this observation has been repeatedly made by our group and by others32.
Lesson 5: MRI-guided biopsy provides a more accurate reflection of whole-organ pathology than conventional biopsy
When radical prostatectomy is performed after conventional biopsy, the final pathology is often different from the biopsy-derived information. In particular, under-grading has been reported to occur in 30–50% of cases so studied6,7. When whole-organ histo-pathology is correlated with targeted biopsy findings, the prediction of final pathology is more accurate than when blind biopsy specimens are employed33. In a recent study of 54 men undergoing fusion biopsy followed by radical prostatectomy, the highest Gleason pattern at prostatectomy was detected by systematic mapping biopsy in 54%, targeted biopsy in 54% and a combination in 81% of cases33.
Further, for men with low-grade, low stage lesions believed to be candidates for active surveillance on the basis of conventional biopsy, many will be re-classified when subjected to confirmatory biopsy using MRI guidance34. Confirmation of low-risk pathology by MRI-guided biopsy appears increasingly important prior to enrollment in active surveillance programs. The role of serial MRI to evaluate disease progression in men during active surveillance is currently under study. Information derived from MRI-guided biopsy may also have important utility for management of radiation therapy35. Thus, MRI/US fusion biopsy leads to improved whole-organ characterization and better-informed treatment decisions.
Targeting Approaches: Urology vs Radiology
With the approval of the DynaTrim device in 2010 (Table 1), radiologists gained the ability to perform MRI-targeted prostate biopsy via a direct in-bore approach. Several reports attest to the potential value of the method of the radiologist36–39. However, compared to the MRI/US fusion method, which is increasingly performed by urologists in the clinic setting, the in-bore method is expensive, time-consuming, labor-intensive, and most importantly, because of time constraints does not usually include mapping. Either method is capable of obtaining tissue samples from a MRI target, but obtaining cores only from a MRI target results in missing a sizable number of high-grade cancers, which may be MRI-invisible19,31,33. Further, radiological training is deficient in experience with recognition and treatment of post-biopsy complications. The combination of lower cost, wider availability, greater efficiency, and routine inclusion of mapping biopsies give MRI/US fusion biopsy a substantial advantage over in-bore biopsy. Where MRI/US fusion technology is not available, the in-bore approach would appear to compare favorably with US guidance alone.
Cost-effectiveness
Economic aspects of targeted prostate biopsy have been little studied to date40,41. Issues include the increased cost of pre-biopsy MRI and start-up costs of an image-fusion device versus the reduced number of biopsy sessions required to obtain a correct diagnosis and increased avoidance of inappropriate surgery or radiation therapy. One authority, representing the very cost-conscious British healthcare system, has stated that prostate MRI will not “break the bank…and when looked at from a cost-of-care perspective it not only generates better health outcomes, but it does so at less cost.42” When patients are given a choice between blind and targeted biopsy, their preference is clear, not unlike their choice between laparoscopic and open prostatectomy. How this preference (and the supportive data) will affect decisions of 3rd party payers is not yet clear.
The Future
Like any valuable new technology, targeted prostate biopsy is evolving rapidly, and current methods are certain to improve in the near future. Refinements in MRI are on the near horizon, and radiologists trained to perform and interpret prostate MRI will increase in number. Under study is the possibility of omitting dynamic contrast enhancement (DCE) from mpMRI, leaving a ‘bi-parametric’ test which would be quicker and cheaper than the 3-part mpMRI currently employed. Compared to the other 2 parameters (T2 and diffusion), DCE adds the least in terms of target definition. In one recent study, omitting DCE was found to detract little from the diagnostic value of standard mpMRI43, raising the potential for a rapid, image-based screening test.
The evolution of the most powerful parametric pulse sequence, diffusion-weighted imaging, by using complex image processing techniques on diffusion tensor imaging, can produce improved maps such as restriction spectrum imaging (RSI). RSI improves upon DWI by discriminating between hindered and restricted diffusion and is therefore more sensitive to changes in cell size and shape, and incorporates geometric distortion correction to improve co-registration44. Moreover, pulse sequences such as RSI may allow standardization of diffusion imaging across scanners, which is currently lacking. When RSI was applied to diagnosis of brain tumors, the tumor signal was enhanced by 10-fold over conventional diffusion imaging45. Preliminary studies indicate that RSI may also be of great value in prostate cancer44.
Industry-driven improvements in image-fusion hardware and software are expected soon, because of the large market potential for improving prostate biopsy. Image-fusion devices will become smaller and easier to use than at present. Co-registration accuracy will increase, and graphical user interfaces will become more intuitive. Already, targeted prostate biopsy has a serious online visibility, and various support groups are making it a priority item. Attempts to image CaP via modalities simpler than MRI (e.g., elastography, contrast-enhanced ultrasound) will continue. Trans-perineal biopsy systems employing MRI/US fusion guidance are under development. Pilot research into focal therapy, using technologies derived from image-guided biopsy, has already begun46. A comparison of the present time in targeted prostate biopsy with the mid-1970s in personal computers seems entirely appropriate!
ACKNOWLEDGEMENTS
The work was supported by Award Number R01CA158627 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. Additional support was provided by the Beckman Coulter Foundation, the Jean Perkins Foundation and the Steven C. Gordon Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
While the UCLA experience has been with the Artemis device, the reported experience targeting with the UroNav device at the National Cancer Institute is qualitatively similar.
Presented in part at Annual Meetings of the American Urological Association, 2014 and 2015).
REFERENCES
- 1.Bower JL, Christensen CM. Disruptive Technologies - Catching the Wave. Harvard Bus Rev. 1995;73:43–53. PMID:ISI:A1995PZ57400009. [Google Scholar]
- 2.Hodge KK, McNeal JE, Terris MK, Stamey TA. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. The Journal of urology. 1989;142:71–74. doi: 10.1016/s0022-5347(17)38664-0. discussion 4–5 PMID:2659827. [DOI] [PubMed] [Google Scholar]
- 3.Welch HG, Fisher ES, Gottlieb DJ, Barry MJ. Detection of prostate cancer via biopsy in the Medicare-SEER population during the PSA era. Journal of the National Cancer Institute. 2007;99:1395–1400. doi: 10.1093/jnci/djm119. PMID:17848671. [DOI] [PubMed] [Google Scholar]
- 4.Autorino R, De Sio M, Di Lorenzo G, et al. How to decrease pain during transrectal ultrasound guided prostate biopsy: a look at the literature. The Journal of urology. 2005;174:2091–2097. doi: 10.1097/01.ju.0000181212.51025.06. PMID:16280735. [DOI] [PubMed] [Google Scholar]
- 5.Eichler K, Hempel S, Wilby J, Myers L, Bachmann LM, Kleijnen J. Diagnostic value of systematic biopsy methods in the investigation of prostate cancer: a systematic review. The Journal of urology. 2006;175:1605–1612. doi: 10.1016/S0022-5347(05)00957-2. PMID:16600713. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro RH, Johnstone PA. Risk of Gleason grade inaccuracies in prostate cancer patients eligible for active surveillance. Urology. 2012;80:661–666. doi: 10.1016/j.urology.2012.06.022. PMID:22925240. [DOI] [PubMed] [Google Scholar]
- 7.King CR, McNeal JE, Gill H, Presti JC., Jr Extended prostate biopsy scheme improves reliability of Gleason grading: implications for radiotherapy patients. International journal of radiation oncology, biology, physics. 2004;59:386–391. doi: 10.1016/j.ijrobp.2003.10.014. PMID:15145152. [DOI] [PubMed] [Google Scholar]
- 8.Stamey TA, Caldwell M, McNeal JE, Nolley R, Hemenez M, Downs J. The prostate specific antigen era in the United States is over for prostate cancer: what happened in the last 20 years? The Journal of urology. 2004;172:1297–1301. doi: 10.1097/01.ju.0000139993.51181.5d. PMID:15371827. [DOI] [PubMed] [Google Scholar]
- 9.Han M, Chang D, Kim C, et al. Geometric evaluation of systematic transrectal ultrasound guided prostate biopsy. The Journal of urology. 2012;188:2404–2409. doi: 10.1016/j.juro.2012.07.107. PMID:23088974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walsh P. Annual Meeting of the American Urological Association. Orlando, Fl: 2008. Whitmore address. [Google Scholar]
- 11.Hricak H, Williams RD, Spring DB, et al. Anatomy and pathology of the male pelvis by magnetic resonance imaging. AJR American journal of roentgenology. 1983;141:1101–1110. doi: 10.2214/ajr.141.6.1101. PMID:6196961. [DOI] [PubMed] [Google Scholar]
- 12.Barentsz JO, Engelbrecht MR, Witjes JA, de la Rosette JJ, van der Graaf M. MR imaging of the male pelvis. European radiology. 1999;9:1722–1736. doi: 10.1007/s003300050916. PMID:10602944. [DOI] [PubMed] [Google Scholar]
- 13.Hamoen EH, de Rooij M, Witjes JA, Barentsz JO, Rovers MM. Use of the Prostate Imaging Reporting and Data System (PI-RADS) for Prostate Cancer Detection with Multiparametric Magnetic Resonance Imaging: A Diagnostic Meta-analysis. European urology. 2014 doi: 10.1016/j.eururo.2014.10.033. PMID:25466942. [DOI] [PubMed] [Google Scholar]
- 14.Moore CM, Robertson NL, Arsanious N, et al. Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. European urology. 2013;63:125–140. doi: 10.1016/j.eururo.2012.06.004. PMID:22743165. [DOI] [PubMed] [Google Scholar]
- 15.D'Amico AV, Tempany CM, Cormack R, et al. Transperineal magnetic resonance image guided prostate biopsy. The Journal of urology. 2000;164:385–387. PMID:10893591. [PubMed] [Google Scholar]
- 16.Roethke M, Anastasiadis AG, Lichy M, et al. MRI-guided prostate biopsy detects clinically significant cancer: analysis of a cohort of 100 patients after previous negative TRUS biopsy. World J Urol. 2012;30:213–218. doi: 10.1007/s00345-011-0675-2. PMID:21512807. [DOI] [PubMed] [Google Scholar]
- 17.Beyersdorff D, Winkel A, Hamm B, Lenk S, Loening SA, Taupitz M. MR imaging-guided prostate biopsy with a closed MR unit at 1.5 T: initial results. Radiology. 2005;234:576–581. doi: 10.1148/radiol.2342031887. PMID:15616117. [DOI] [PubMed] [Google Scholar]
- 18.Engelhard K, Hollenbach HP, Kiefer B, Winkel A, Goeb K, Engehausen D. Prostate biopsy in the supine position in a standard 1.5-T scanner under real time MR-imaging control using a MR-compatible endorectal biopsy device. European radiology. 2006;16:1237–1243. doi: 10.1007/s00330-005-0100-6. PMID:16447048. [DOI] [PubMed] [Google Scholar]
- 19.Zangos S, Melzer A, Eichler K, et al. MR-compatible assistance system for biopsy in a high-field-strength system: initial results in patients with suspicious prostate lesions. Radiology. 2011;259:903–910. doi: 10.1148/radiol.11101559. PMID:21364080. [DOI] [PubMed] [Google Scholar]
- 20.Logan JK, Rais-Bahrami S, Turkbey B, et al. Current status of magnetic resonance imaging (MRI) and ultrasonography fusion software platforms for guidance of prostate biopsies. BJU international. 2014;114:641–652. doi: 10.1111/bju.12593. PMID:24298917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Natarajan S, Marks LS, Margolis DJ, et al. Clinical application of a 3D ultrasound-guided prostate biopsy system. Urologic oncology. 2011;29:334–342. doi: 10.1016/j.urolonc.2011.02.014. PMID:21555104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hadaschik BA, Kuru TH, Tulea C, et al. A novel stereotactic prostate biopsy system integrating pre-interventional magnetic resonance imaging and live ultrasound fusion. The Journal of urology. 2011;186:2214–2220. doi: 10.1016/j.juro.2011.07.102. PMID:22014798. [DOI] [PubMed] [Google Scholar]
- 23.Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. Jama. 2015;313:390–397. doi: 10.1001/jama.2014.17942. PMID:25626035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu S, Kruecker J, Turkbey B, et al. Real-time MRI-TRUS fusion for guidance of targeted prostate biopsies. Comput Aided Surg. 2008;13:255–264. doi: 10.1080/10929080802364645. PMID;18821344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wysock JS, Rosenkrantz AB, Huang WC, et al. A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR-targeted prostate biopsy: the PROFUS trial. European urology. 2014;66:343–351. doi: 10.1016/j.eururo.2013.10.048. PMID:24262102. [DOI] [PubMed] [Google Scholar]
- 26.Puech P, Rouviere O, Renard-Penna R, et al. Prostate cancer diagnosis: multiparametric MR-targeted biopsy with cognitive and transrectal US-MR fusion guidance versus systematic biopsy--prospective multicenter study. Radiology. 2013;268:461–469. doi: 10.1148/radiol.13121501. PMID:23579051. [DOI] [PubMed] [Google Scholar]
- 27.Cool DW, Zhang X, Romagnoli C, Izawa JI, Romano WM, Fenster A. Evaluation of MRI-TRUS fusion versus cognitive registration accuracy for MRI-targeted, TRUS-guided prostate biopsy. AJR American journal of roentgenology. 2015;204:83–91. doi: 10.2214/AJR.14.12681. PMID:25539241. [DOI] [PubMed] [Google Scholar]
- 28.Dickinson L, Ahmed HU, Allen C, et al. Scoring systems used for the interpretation and reporting of multiparametric MRI for prostate cancer detection, localization, and characterization: could standardization lead to improved utilization of imaging within the diagnostic pathway? Journal of magnetic resonance imaging : JMRI. 2013;37:48–58. doi: 10.1002/jmri.23689. PMID:22566285. [DOI] [PubMed] [Google Scholar]
- 29.Filson CP, Margolis D, Huang J, et al. MR-US fusion biopsy: importance of both systematic and targeted sampling to diagnose prostate cancer. New Orleans, LA: American Urological Association; 2015. [Google Scholar]
- 30.Le JD, Tan N, Shkolyar E, et al. Multifocality and Prostate Cancer Detection by Multiparametric Magnetic Resonance Imaging: Correlation with Whole-mount Histopathology. European urology. 2015;67:569–576. doi: 10.1016/j.eururo.2014.08.079. PMID:25257029. [DOI] [PubMed] [Google Scholar]
- 31.Sonn GA, Natarajan S, Margolis DJ, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. The Journal of urology. 2013;189:86–91. doi: 10.1016/j.juro.2012.08.095. PMID:23158413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenkrantz AB, Verma S, Turkbey B. Prostate cancer: top places where tumors hide on multiparametric MRI. AJR American journal of roentgenology. 2015;204:W449–W456. doi: 10.2214/AJR.14.13280. PMID:25794094. [DOI] [PubMed] [Google Scholar]
- 33.Le JD, Stephenson S, Brugger M, et al. Magnetic resonance imaging-ultrasound fusion biopsy for prediction of final prostate pathology. The Journal of urology. 2014;192:1367–1373. doi: 10.1016/j.juro.2014.04.094. PMID:24793118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu JC, Chang E, Natarajan S, et al. Targeted prostate biopsy in select men for active surveillance: do the Epstein criteria still apply? The Journal of urology. 2014;192:385–390. doi: 10.1016/j.juro.2014.02.005. PMID:24512956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamrava M, Hegde JV, Abgaryan N, et al. Does the Addition of Targeted Prostate Biopsies to Standard Systemic Biopsies Impact Treatment Management for Radiation Oncologists? BJU international. 2015 doi: 10.1111/bju.13082. PMID:25684394. [DOI] [PubMed] [Google Scholar]
- 36.Quentin M, Blondin D, Klasen J, et al. Evaluation of a structured report of functional prostate magnetic resonance imaging in patients with suspicion for prostate cancer or under active surveillance. Urologia internationalis. 2012;89:25–29. doi: 10.1159/000338808. PMID:22677880. [DOI] [PubMed] [Google Scholar]
- 37.Puech P, Potiron E, Lemaitre L, et al. Dynamic contrast-enhanced-magnetic resonance imaging evaluation of intraprostatic prostate cancer: correlation with radical prostatectomy specimens. Urology. 2009;74:1094–1099. doi: 10.1016/j.urology.2009.04.102. PMID:19773038. [DOI] [PubMed] [Google Scholar]
- 38.Hambrock T, Hoeks C, Hulsbergen-van de Kaa C, et al. Prospective assessment of prostate cancer aggressiveness using 3-T diffusion-weighted magnetic resonance imaging-guided biopsies versus a systematic 10-core transrectal ultrasound prostate biopsy cohort. European urology. 2012;61:177–184. doi: 10.1016/j.eururo.2011.08.042. PMID:21924545. [DOI] [PubMed] [Google Scholar]
- 39.Labanaris AP, Zugor V, Smiszek R, Nutzel R, Kuhn R, Engelhard K. Guided e-MRI prostate biopsy can solve the discordance between Gleason score biopsy and radical prostatectomy pathology. Magnetic resonance imaging. 2010;28:943–946. doi: 10.1016/j.mri.2010.03.041. PMID:20418041. [DOI] [PubMed] [Google Scholar]
- 40.de Rooij M, Crienen S, Witjes JA, Barentsz JO, Rovers MM, Grutters JP. Cost-effectiveness of magnetic resonance (MR) imaging and MR-guided targeted biopsy versus systematic transrectal ultrasound-guided biopsy in diagnosing prostate cancer: a modelling study from a health care perspective. European urology. 2014;66:430–436. doi: 10.1016/j.eururo.2013.12.012. PMID:24377803. [DOI] [PubMed] [Google Scholar]
- 41.Lotan Y, Haddad AQ, Costa DN, Pedrosa I, Rofsky NM, Roehrborn CG. Decision analysis model comparing cost of multiparametric magnetic resonance imaging vs. repeat biopsy for detection of prostate cancer in men with prior negative findings on biopsy. Urologic oncology. 2015 doi: 10.1016/j.urolonc.2015.03.007. PMID:25858102. [DOI] [PubMed] [Google Scholar]
- 42.Emberton M. Is prostate magnetic resonance imaging going to break the bank? European urology. 2014;66:437–438. doi: 10.1016/j.eururo.2014.02.043. PMID:24613683. [DOI] [PubMed] [Google Scholar]
- 43.Rais-Bahrami S, Siddiqui MM, Vourganti S, et al. Diagnostic value of biparametric magnetic resonance imaging (MRI) as an adjunct to prostate-specific antigen (PSA)-based detection of prostate cancer in men without prior biopsies. BJU international. 2015;115:381–388. doi: 10.1111/bju.12639. PMID:24447678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rakow-Penner RA, White NS, Parsons JK, et al. Novel technique for characterizing prostate cancer utilizing MRI restriction spectrum imaging: proof of principle and initial clinical experience with extraprostatic extension. Prostate cancer and prostatic diseases. 2015;18:81–85. doi: 10.1038/pcan.2014.50. PMID:25559097. [DOI] [PubMed] [Google Scholar]
- 45.White NS, McDonald CR, Farid N, Kuperman JM, Kesari S, Dale AM. Improved conspicuity and delineation of high-grade primary and metastatic brain tumors using "restriction spectrum imaging": quantitative comparison with high B-value DWI and ADC. AJNR Am J Neuroradiol. 2013;34:958–964. S1. doi: 10.3174/ajnr.A3327. PMID:23139079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oto A, Sethi I, Karczmar G, et al. MR imaging-guided focal laser ablation for prostate cancer: phase I trial. Radiology. 2013;267:932–940. doi: 10.1148/radiol.13121652. PMID:23440319. [DOI] [PubMed] [Google Scholar]
- 47.Filson CP, Marks LS, Litwin MS. Expectant management for men with early stage prostate cancer. CA: a cancer journal for clinicians. 2015 doi: 10.3322/caac.21278. PMID:25958817. [DOI] [PubMed] [Google Scholar]