Abstract
Background
Simple and reliable ECG marker(s) for sudden cardiac arrest (SCA) could be very useful in assessing high-risk populations. Since ischemic repolarization abnormalities in the left ventricular (LV) apex are strongly correlated with discordant T waves in lead aVR, we sought to evaluate the clinical and prognostic significance of this feature in ischemic cardiomyopathy.
Methods
The PAREPET trial enrolled patients with ischemic cardiomyopathy eligible for a primary prevention implantable cardiac defibrillator (ICD). Those with persistent pacing or left bundle branch block were excluded. Amplitudes of T/aVR were automatically computed from median ECG beats at enrollment and endpoints were blindly adjudicated.
Results
The sample was mainly composed of older men (n=138, age 65±12, 91% male, EF 29±9%). At enrollment, amplitude of T/aVR significantly correlated with EF, indexed LV end-diastolic volume, B-type natriuretic peptide (BNP), regional scar volume, and PET-quantified denervated myocardium. After a median follow up of 4.2 years, there were 23 (17%) adjudicated SCA. In multivariate analysis, the presence of discordant T/aVR (>0mm, n=42, 30%) was a significant and independent predictor of SCA (hazard ratio 2.0 [95% CI 1.0–4.9]) and cardiac death (hazard ratio 1.9 [95% CI 1.0–3.7]).
Conclusions
In subjects with ischemic cardiomyopathy, discordant T waves in Lead aVR are associated with high-risk clinical parameters including lower ejection fraction, greater ventricular volume, higher BNP, and more denervated myocardium. Furthermore, discordant TaVR remained an independent predictor of SCA and cardiovascular mortality even after accounting for these prognostic factors.
Keywords: electrocardiogram, T wave, sudden cardiac arrest, lead aVR
INTRODUCTION
Despite medical advances over the past 30 years, sudden cardiac arrest (SCA) still accounts for nearly 50% of coronary artery disease deaths with nearly 450,000 deaths occurring annually in the United States alone. 1, 2 Reduced left ventricular (LV) ejection fraction (EF) remains the only clinically used tool to risk stratify candidates for the primary prevention of SCA, but this approach is neither sensitive nor specific.3 Twelve-lead electrocardiograms (ECGs) are routinely obtained in patients at high risk of SCA, and offer the potential to provide inexpensive marker(s) to assist in the prediction of SCA risk. As an initial test of this hypothesis we recently evaluated a wide array of classic ECG risk markers in patients with ischemic cardiomyopathy.4 However, with the exception of QTc prolongation, all previously identified ECG risk factors predicting increased cardiovascular mortality were not able to predict cause specific mortality from SCA in this population.
Previous reports suggest that T wave amplitude in lead aVR (T/aVR) is related to cardiac dysfunction and severity of coronary artery occlusion,5 which has been shown to predict cardiovascular (CV) mortality in various populations.6–9 Since wall tension is an important determinant of myocardial oxygen tension, and since most dramatic abnormalities of repolarization occur in the thinnest part of LV (apex),10 it follows that T/aVR can provide valuable information on LV wall tension and LV ischemia, and can hence improve SCA risk stratification in ischemic cardiomyopathy. In addition, give that regional sympathetic myocardial denervation is evident in ischemic cardiomyopathy,11 better understanding of the physiologic correlates with T/aVR can support its role in SCA-specific risk stratification in this unique patient population. Accordingly, we sought to evaluate the clinical correlates of T/aVR amplitude and discordance, as well as its potential to predict SCA in ischemic cardiomyopathy.
METHODS
This analysis was performed from all eligible patients enrolled in the Prediction of Arrhythmic Events using Positron Emission Tomography (PAREPET) study. This NIH-funded, observational study recruited ambulatory patients with ischemic cardiomyopathy who were eligible for an implantable cardiac defibrillator (ICD) for the primary prevention of SCA. Thus, they all had coronary artery disease and pre-enrollment LVEF ≤ 35%. The data collection and analysis methods are reported elsewhere.11 In short, eligible subjects completed a 24-hour Holter ECG, echocardiogram, and positron emission tomography (PET) scans at start of study with follow-up at 3-month intervals. Volume of denervated myocardium was quantified using PET and 11C-meta-hydroxyephedrine scans. Other demographic and clinical parameters (e.g., B-type natriuretic peptide) were obtained from electronic medical records. The primary endpoint was SCA, which included arrhythmic death or ICD discharge for ventricular fibrillation or rapid ventricular tachycardia (>240 beats per minute).11 The secondary endpoint was total cardiac death as determined by a blinded adjudication committee of cardiologists after reviewing all available information. Of note, 78% of subjects had an ICD implanted prior to an event. The study was approved by appropriate institutional review boards and informed consent was obtained from all subjects.
ECG Processing & Analysis
High-fidelity, 24-hour, 12-lead ECG recordings were obtained in the Mason-Likar configuration using H12+ Electrocardiographs (V3.12, Mortara Instrument, Milwaukee, WI). Of note, initial 5-minute recordings were obtained while subjects were resting supine. Recorded ECG streams were then processed using H-Scribe 5.11 (frequency rate 0.05-300Hz, sampling rate 1,000 s/s) and 1-minute signal averaged ECG beats from initial recordings were computed using Super-ECG (Mortara Instrument). T/aVR amplitudes were automatically computed and three T/aVR permutations were evaluated by a reviewer blinded to all clinical data-: (1) T/aVR amplitude (mm); (2) QRS-corrected T/aVR amplitude (max T/aVR divided by max QRS/aVR); and (3) discordant T/aVR (>0 mm). There were five patients with upright dominant QRS/aVR; of which three had concordant upright T/aVR and two had discordant negative T/aVR. Only those with discordant T/aVR were classified as abnormal for the predictor of interest. Moreover, given that upright T/aVR is typically associated with (1) T wave inversion in lateral precordial leads (i.e., both V5 and V6)9; (2) more extensive disease with myocardial scarring involving the apex;5 and (3) spatial-temporal changes in ventricular repolarization;5 T wave inversion in V5-V6, Selvester scores,12 and spatial QRS-T angle13 were evaluated for all patients and used as control analyses. Since QTc prolongation (i.e., >450 milliseconds) was previously shown to predict SCA in this population,4 this variable was included as a control variable as well.. The final sample included 138 subjects after excluding those with persistent pacing (n=42), left bundle branch block (n=11), and missing high-resolution data (n=6). All patients had narrow (<110 ms) QRS complexes.
Statistical Analysis
Values are presented as mean ± SD or n (%). Groups were compared using chi-square for categorical variables and independent t-test for continuous variables. Correlations between T amplitude and clinical findings were evaluated using Pearson’s r coefficients. Time-to-event data was analyzed using proportional hazards Cox-regression and significant predictors were presented using Kaplan-Meier events probability curves with log rank test. Only predictors significant in the univariate model were entered simultaneously in a multivariate Cox-regression model with backward selection. T/aVR permutations (i.e., absolute and QRS-corrected T amplitudes, and discordant T/aVR >0 mm) were included in the multivariate model only one at a time. Significance was set at p<0.05 and all analyses were done using SPSS 22.0 for Windows.
RESULTS
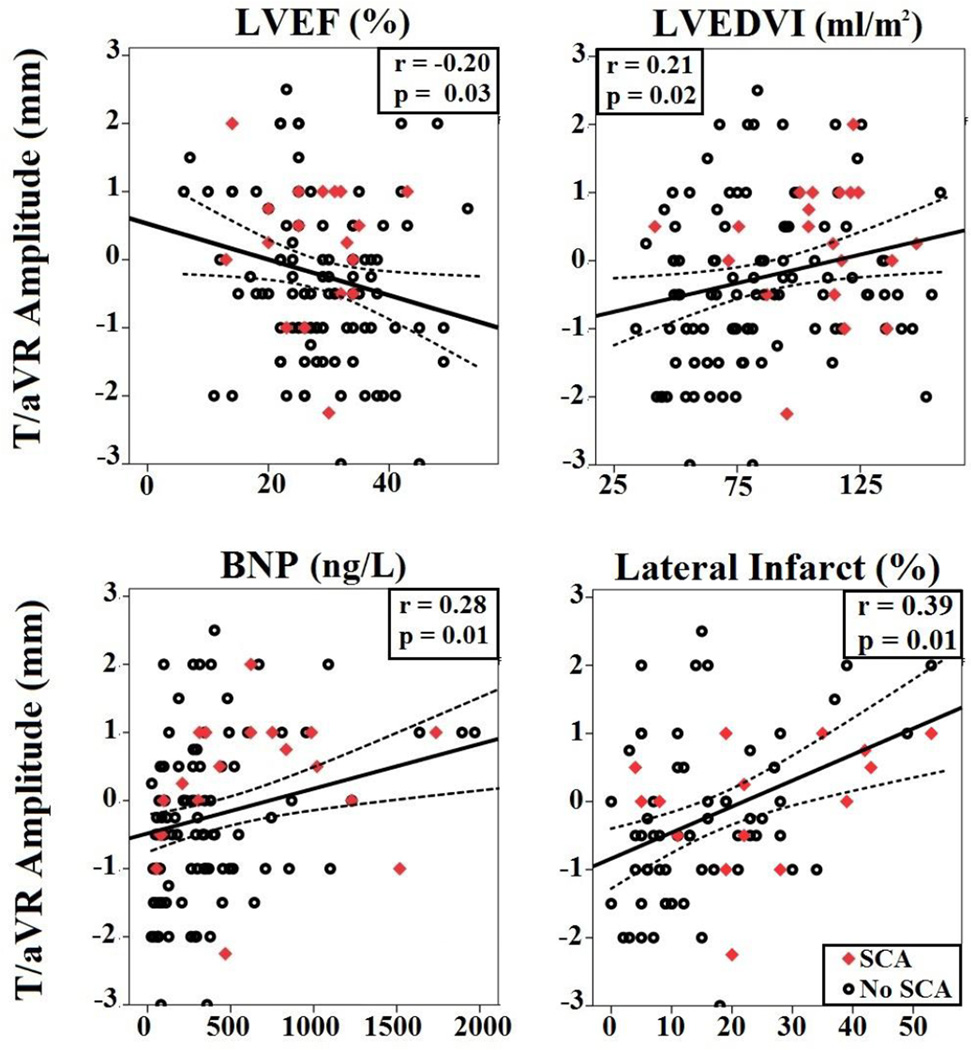
The sample was representative of those with ischemic cardiomyopathy receiving a primary prevention ICD (n=138, age 65±12, 91% male, LVEF 29±9%). Patients were optimally managed by β-blockers (96%) and angiotensin inhibition therapy (92%). Nearly one third of the sample (n=42, 30%) had discordant T/aVR pattern (Figure 1). There were no differences between those with and without discordant T/aVR with regard to age, sex, body mass index, New York Heart Association (NYHA) class, LVEF, or other comorbidities (i.e., diabetes) (Table 1). However, T/aVR amplitude was significantly correlated with LVEF (r=−0.20), indexed LV end-diastolic volume (LVEDVI, r=0.21), B-type natriuretic peptide (BNP, r=0.28) (Figure 2). Although total infarct size was not different between the groups (Table 1), those with discordant T/aVR had more infarction of the left circumflex coronary distribution (24±15% vs. 14±10%, p<0.01, Figure 2); but not left anterior descending 23±14% vs. 24±15%, p=0.68; or right coronary 13±17% vs. 16±12%, p=0.20. As expected, there was a significant correlation between T/aVR amplitude and QRS-T angle (r=0.44), T wave inversion in the lateral leads, and Selvester score (r=0.27). In addition, T/aVR amplitude also correlated with positron-emission tomography (PET)-quantified denervated myocardium (% LV, r=0.23).
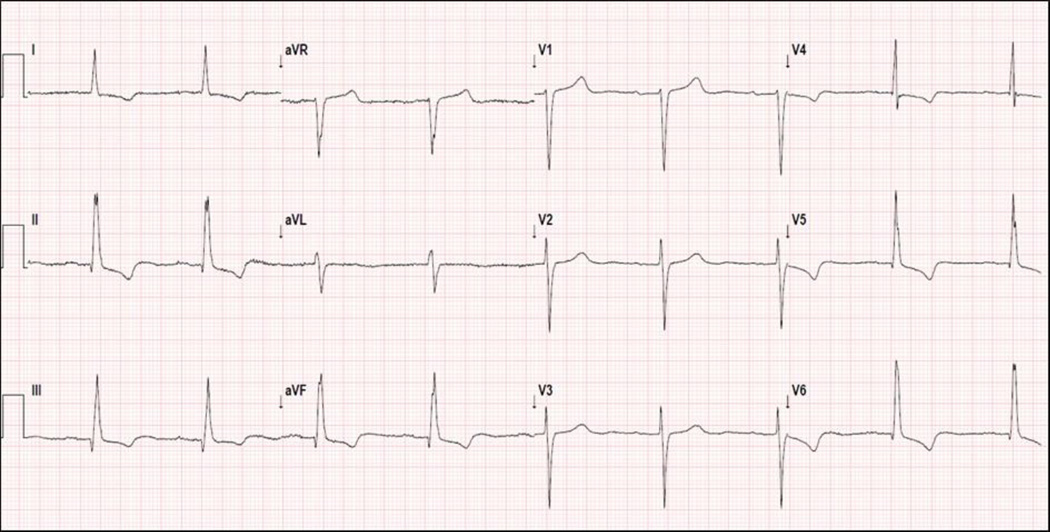
Figure 1. Representative 12-Lead ECG Showing Positive T waves in Lead aVR.
Resting 12-lead ECG of a 68 year-old male who experienced SCA after 14 months of follow-up. The ECG shows sinus bradycardia with T wave inversion in antero-lateral leads, and a positive and discordant T wave in lead aVR.
Table 1.
Demographic and Clinical Data at Enrollment
| Characteristics | All Patients (n=138) |
T/aVR Pattern |
p | |
|---|---|---|---|---|
| Concordant (n=96, 70%) |
Discordant (n=42, 30%) |
|||
| Age (years) | 66.5 ± 12.1 | 66.9 ± 12.2 | 65.4 ± 12.0 | 0.49 |
| Male Sex (%) | 126 (91%) | 87 (91%) | 39 (93%) | 0.67 |
| Race (% Black) | 22 (16%) | 13 (14%) | 9 (21%) | 0.24 |
| Body Mass Index | 28.4 ± 4.8 | 28.6 ± 5.0 | 27.8 ± 4.2 | 0.36 |
| NYHA class | 2.2 ± 0.8 | 2.2 ± 0.9 | 2.2 ± 0.8 | 0.88 |
| Diabetes Mellitus | 61 (44%) | 43 (45%) | 18 (43%) | 0.83 |
| BNP (ng/L) | 417 ± 489 | 296 ± 290 | 689 ± 698 | <0.01 |
| LV Ejection Fraction (%) | 28 ± 9 | 28 ± 9 | 28 ± 9 | 0.92 |
| LVEDVI (ml/m2) | 88 ± 30 | 85 ± 30 | 93 ± 29 | 0.03 |
| Infarct Size (%) | 19 ± 8 | 18 ± 7 | 19 ± 9 | 0.82 |
| Regional Infarct (% Territory) | ||||
| LAD | 24 ± 1517 ± | 24 ± 15 | 23 ± 14 | 0.68 |
| LCX | 13 | 14 ± 10 | 24 ± 15 | <0.01 |
| RCA | 16 ± 11 | 16 ± 12 | 13 ± 7 | 0.20 |
| Denervated Myocardium (% LV) | 0.26 ± 0.13 | 0.24 ± 0.13 | 0.29 ± 0.13 | 0.05 |
| QRS-T Angle | 122 ± 38 | 113 ± 40 | 145 ± 21 | <0.01 |
| Selvester Score | 6.8 ± 5.1 | 4.3 ± 0.7 | 5.3 ± 0.8 | 0.04 |
| QTc Prolongation (> 450 ms) | 48 (35%) | 29 (30%) | 19 (45%) | 0.09 |
| T wave Inversion (V5 and V6) | 40 (33%) | 20 (21%) | 20 (48%) | <0.01 |
| T/aVR Amplitude (mm) | −0.2 ± 1.2 | −0.9 ± 0.7 | 1.1 ± 0.7 | <0.01 |
| QRS Amplitude (mm) | −5.1 ± 3.1 | −4.7 ± 2.6 | −5.7 ± 3.7 | 0.14 |
| QRS-corrected T/aVR Amplitude | 0.07 ± 0.27 | 0.21 ± 0.22 | −0.19 ± 0.16 | <0.01 |
| Total Cardiac Death (%) | 41 (30%) | 21 (22%) | 20 (48%) | 0.01 |
| Sudden Cardiac Arrest (%) | 23 (17%) | 11 (11%) | 12 (29%) | 0.01 |
NYHA: New York Heart Association class, BNP: B-type natriuretic peptide; LVEDVI: indexed-left ventricular end diastolic volume, LAD: left anterior descending artery, LCX: left circumflex artery, RAC: right coronary artery
Figure 2. Physiologic Correlates of T/aVR Amplitude.
Best-fit linear regression line and its 95% CI of the mean between T/aVR amplitude (mm) and left ventricular ejection fraction (LVEF [%], upper left graphs), left ventricular end-diastolic volume index (LVEDVI, upper right graphs), B-type natriuretic peptide (BNP, left lower graphs), and regional scar volume of left circumflex artery (right lower graphs).
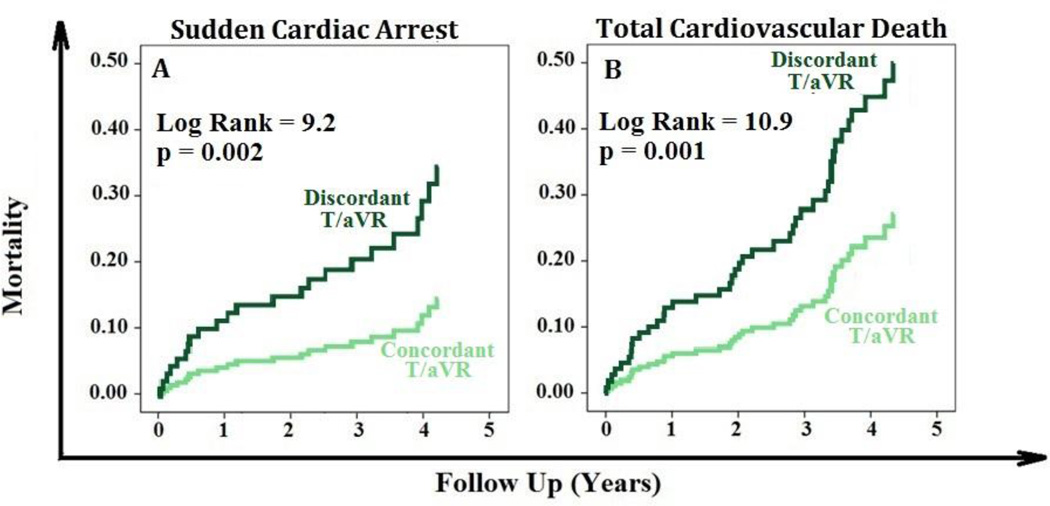
After a median follow up of 4.2 years, 23 patients (17%) experienced SCA. No demographic or clinical differences (e.g., age) were observed between those who experienced arrhythmic death (n=15) versus those displaying ICD discharges (n=8). In univariate analysis, 29% of those with discordantT/aVR (12 out of 42) died from SCA, compared to only 11% of those with concordant T/aVR (11 out of 96, table 1). LVEDVI, BNP, and QTc prolongation were univariately predictive of SCA, but not lateral precordial T wave inversion or QRS-T angle (Table 2). In multivariate analysis, discordant T/aVR was an independent predictor of SCA (Hazard Ratio 2.0 [95% CI 1.0–4.9], Figure 3A). The area under the curve (AUC) for SCA classification performance using T/aVR amplitude (mm) was 0.67 (95% CI 0.56–0.79). The sensitivity and specificity to predict SCA cases using discordant T/aVR (>0 mm) were 57% and 75%, respectively.
Table 2.
Univariate and Multivariate Predictors of Sudden Cardiac Arrest and Total Cardiac Death
| Characteristic | SCA (n = 23, 17%) | Total Cardiac Death (n = 41, 30%) | ||
|---|---|---|---|---|
| Univariate HR (95% CI) |
Multivariate HR (95% CI) |
Univariate HR (95% CI) |
Multivariate HR (95% CI) |
|
| Age (per 1 year increment) | NS | - | NS | - |
| Race (Black vs White) | NS | - | NS | - |
| Body Mass Index | NS | - | NS | - |
| NYHA class | NS | - | NS | - |
| Diabetes Mellitus (yes vs no) | NS | - | NS | - |
| BNP (per 500 units increment) | 1.64 (1.23–2.72) | 1.43 (1.08–2.20) | 1.64 (1.23–2.71) | 1.52 (1.20–2.02) |
| LVEF (per 1% decline) | NS | - | NS | - |
| LVEDVI (per 10 units increment) | 1.33 (1.15–1.60) | 1.31 (1.13–1.60) | 1.29 (1.16–1.45) | 1.28 (1.15–1.44) |
| Infarct Size | NS | - | NS | - |
| T wave Inversions in Leads V5 and V6 | NS | - | NS | - |
| Selvester Score | NS | - | NS | - |
| QRS-T Angle | NS | - | NS | - |
| QTc Prolongation (> 450 ms) | 3.33 (1.26–8.76) | NS | NS | - |
| T/aVR Amplitude (per 1 mm increment) | 1.34 (1.02–1.81) | NS | 1.31 (1.04–1.65) | NS |
| QRS-Corrected T/aVR Amplitude | NS | NS | 0.28 (0.08–0.96) | NS |
| Discordant T/aVR (>0 mm) | 3.33 (1.46–7.61) | 2.02 (1.04–4.92) | 2.70 (1.46–4.98) | 1.98 (1.03–3.67) |
Bold denotes statistical significance.
Figure 3. Discordant T/aVR Predicts Sudden Cardiac Arrest and Total cardiac Mortality.
Kaplan-Meier events probability curves showing that discordant T/aVR (>0 mm) predicts sudden cardiac arrest (A, left) and total cardiovascular death (B, right). p values are based on log rank test between curves.
In addition to those with SCA, 18 patients (13%) died of heart failure progression (total cardiac death=41, 30%). Nearly half of those with discordant T/aVR (20 out of 42) experienced cardiac death, compared to only 12% of those with concordant T/aVR (21 out of 96; Table 1). Absolute and QRS-corrected T/aVR amplitude were univariate predictors of cardiac death, as were LVEDVI and BNP (Table 2). Lateral precordial T wave inversion, and QRS-T angle were not predictive of total cardiac death in this cohort. In multivariate analysis, discordant T/aVR remained an independent predictor of cardiac death (Hazard Ratio 1.9 [95% CI 1.0–3.7], Figure 3B). The sensitivity and specificity of discordant T/aVR to predict cardiac death were 51% and 78%, respectively.
DISCUSSION
In this secondary analysis of the PAREPET study, we report that the pattern of discordant T/aVR significantly correlated with other clinical parameters associated with a poor prognosis and increased risk for SCA. These include more severely reduced cardiac function (lower LVEF), indices consistent with volume overload and LV wall stress (greater LVEDVI and higher BNP), and greater cardiac autonomic dysfunction (larger volumes of denervated myocardium). In addition, discordant T/aVR independently predicted both total cardiac death, and more importantly, cause-specific mortality from SCA. This is interesting given the fact that traditional demographic and clinical parameters (including LVEF) could not differentiate those at risk from SCA from survivors. Our findings, are similar to previous reports6–9 showing that +T/aVR could predict total CV death on long term-follow up in lower risk populations. However, this is the first study to show that a discordant T/aVR pattern is specifically linked to the risk of SCA, in a high-risk population.
The Clinical and Prognostic Significance of Discordant T/aVR
Indices of dispersion in ventricular repolarization have been repeatedly associated with the development of arrhythmias and SCA.14 However, an exact mechanism to link the direction of repolarization in the LV (i.e., discordant T/aVR) to SCA is challenging due to the numerous physiological and psychological factors that can account for non-specific repolarization changes in this patient population. These disparate factors likely explain the high prevalence of discordant T/aVR in ischemic cardiomyopathy (30% vs 7% in clinical populations8 and 3% in the general population9), as well as the moderate sensitivity and specificity of this pattern to predict SCA in this high-risk population. More importantly, the observed modest correlations of discordant T/aVR with LV dysfunction, volume overload and LV wall stress, and sympathetic denervation provide new insights in understanding the complex physiological mechanisms involved in SCA risk stratification using this simple risk marker.15, 16 In a recent analysis of 122 patients with anterior wall old myocardial infarction, Shinozaki and colleagues5 similarly noticed that discordant T/aVR is related to more severely reduced cardiac function, but they also found that this pattern is related to more severe anterior wall disease with the LAD wrapping the apex. On the contrary, more than 80% of our sample had multi-vessels disease, and we found that discordant T/aVR was related to more extensive left circumflex territory infarction. Furthermore, T wave inversions in the lateral precordial leads and QRS-T angle did not predict risk from SCA in this patient population. These observations suggest that the predictive accuracy of discordant T/aVR in ischemic cardiomyopathy is more direction specific than simply discordance of the T wave relative to the QRS complex (in which case the global QRS-T angle should have been most predictive). This observation supports the notion that T/aVR can provide valuable information on wall tension and ischemia in the LV apex given that most dramatic abnormalities of repolarization occur in the thinnest part of LV, the apex… Further research will be necessary to determine the complex interrelationships between neural control mechanisms, myocardial denervation,11 discordant T/aVR and other factors in the mechanism(s) of SCA in ischemic cardiomyopathy.
Limitations
This was a secondary analysis of the PAREPET study, which was exclusively conducted in subjects with ischemic cardiomyopathy. Thus, the relevance of these results to patients with non-ischemic cardiomyopathy is unknown. Furthermore, persistent pacing or left bundle branch block, in which the discordant T waves are almost invariably present, necessitated the exclusion of almost 30% of our subjects. Finally, our findings indicate that discordant T/aVR is only a fair SCA event-classifier (AUC<70%). Thus, discordant T/aVR may be useful in a multi-parameter risk prediction model for SCA, but will not be useful as a stand-alone marker.
CONCLUSIONS
Discordant T waves in lead aVR can be easily visualized on the 12-lead ECG without the need for advanced computerized algorithms, and in patients with ischemic cardiomyopathy who are eligible for a primary prevention ICD, this simple marker independently predicts risk for SCA.
Highlights.
-
-
Simple and reliable ECG markers for sudden cardiac arrest are needed.
-
-
Discordant T waves in lead aVR are associated with lower ejection fraction, greater ventricular volume, higher BNP, and more denervated myocardium.
-
-
Discordant T in lead aVR is an independent predictor of sudden death in ischemic cardiomyopathy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Deo R, Albert CM. Epidemiology and Genetics of Sudden Cardiac Death. Circulation. 2012;125:620–637. doi: 10.1161/CIRCULATIONAHA.111.023838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart Disease and Stroke Statistics—2014 Update: A Report From the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buxton AE, Lee KL, Hafley GE, Pires LA, Fisher JD, Gold MR, Josephson ME, Lehmann MH, Prystowsky EN. Limitations of Ejection Fraction for Prediction of Sudden Death Risk in Patients With Coronary Artery Disease: Lessons From the MUSTT Study. Journal of the American College of Cardiology. 2007;50:1150–1157. doi: 10.1016/j.jacc.2007.04.095. [DOI] [PubMed] [Google Scholar]
- 4.Al-Zaiti SS, Fallavollita JA, Canty JM, Jr, Carey MG. Electrocardiographic predictors of sudden and non-sudden cardiac death in patients with ischemic cardiomyopathy. Heart & Lung: The Journal of Acute and Critical Care. 2014;43:527–533. doi: 10.1016/j.hrtlng.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shinozaki K, Tamura A, Kadota J. Associations of positive T wave in lead aVR with hemodynamic, coronary, and left ventricular angiographic findings in anterior wall old myocardial infarction. Journal of cardiology. 2011;57:160–164. doi: 10.1016/j.jjcc.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Badheka AO, Patel NJ, Grover PM, Shah N, Singh V, Deshmukh A, Mehta K, Chothani A, Hoosien M, Rathod A, Savani GT, Marzouka GR, Gupta S, Mitrani RD, Moscucci M, Cohen MG. ST-T Wave Abnormality in Lead aVR and Reclassification of Cardiovascular Risk (from the National Health and Nutrition Examination Survey-III) American Journal of Cardiology. 2013;112:805–810. doi: 10.1016/j.amjcard.2013.04.058. [DOI] [PubMed] [Google Scholar]
- 7.Torigoe K, Tamura A, Kawano Y, Shinozaki K, Kotoku M, Kadota J. Upright T waves in lead aVR are associated with cardiac death or hospitalization for heart failure in patients with a prior myocardial infarction. Heart and vessels. 2012;27:548–552. doi: 10.1007/s00380-011-0193-6. [DOI] [PubMed] [Google Scholar]
- 8.Tan SY, Engel G, Myers J, Sandri M, Froelicher VF. The Prognostic Value of T Wave Amplitude in Lead aVR in Males. Annals of Noninvasive Electrocardiology. 2008;13:113–119. doi: 10.1111/j.1542-474X.2008.00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anttila I, Nikus K, Nieminen T, Jula A, Salomaa V, Reunanen A, Nieminen MS, Lehtimäki T, Virtanen V, Kähönen M. Relation of Positive T Wave in Lead aVR to Risk of Cardiovascular Mortality. The American Journal of Cardiology. 2011;108:1735–1740. doi: 10.1016/j.amjcard.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 10.Warner RA, Hill NE, Mookherjee S, Smulyan H. Diagnostic significance for coronary artery disease of abnormal Q waves in the "lateral" electrocardiographic leads. Am J Cardiol. 1986;58:431–435. doi: 10.1016/0002-9149(86)90010-x. [DOI] [PubMed] [Google Scholar]
- 11.Fallavollita JA, Heavey BM, Luisi AJ, Jr, Michalek SM, Baldwa S, Mashtare TL, Hutson AD, deKemp RA, Haka MS, Sajjad M, Cimato TR, Curtis AB, Cain ME, Canty JM., Jr Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. J Am Coll Cardiol. 2014;63:141–149. doi: 10.1016/j.jacc.2013.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selvester R, Wagner G, Hindman N. The Selvester QRS scoring system for estimating myocardial infarct size. The development and application of the system. Archives of Internal Medicine. 1985;145:1877–1881. [PubMed] [Google Scholar]
- 13.Rautaharju PM, Prineas RJ, Zhang Z-M. A simple procedure for estimation of the spatial QRS/T angle from the standard 12-lead electrocardiogram. Journal of Electrocardiology. 2007;40:300–304. doi: 10.1016/j.jelectrocard.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Al-Zaiti SS, Fallavollita JA, Wu YB, Tomita MR, Carey MG. Electrocardiogram-Based Predictors of Clinical Outcomes: A Meta-Analysis of the Prognostic Value of ventricular repolarization. Heart & Lung. 2014;43:516–526. doi: 10.1016/j.hrtlng.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Verma VK, Alkeylani A. Can an upright t-wave in lead avr be a clinical marker for underlying myocardial disease? Chest. 2003;124:155S-a–155S. [Google Scholar]
- 16.Berger R, Huelsman M, Strecker K, Bojic A, Moser P, Stanek B, Pacher R. B-Type Natriuretic Peptide Predicts Sudden Death in Patients With Chronic Heart Failure. Circulation. 2002;105:2392–2397. doi: 10.1161/01.cir.0000016642.15031.34. [DOI] [PubMed] [Google Scholar]