Abstract
The central nervous system (CNS) has long been recognized as a site of ‘immune privilege’ because of the existence of the blood brain barrier (BBB) which presumably isolates CNS from the peripheral immunosurveillance. Different from the peripheral organs, CNS is unique in response to all forms of CNS injury and disease which is mainly mediated by resident microglia and astrocyte. There is increasing evidence that immune cells are not only involved in neuroinflammation process but also the maintenance of CNS homeostasis. T cells, an important immune cell population, are involved in the pathogenesis of some neurological diseases by inducing either innate or adaptive immune responses. Astrocytes, which are the most abundant cell type in the CNS, maintain the integrity of BBB and actively participate in the initiation and progression of neurological diseases. Surprisingly, how astrocytes and T cells interact and the consequences of their interaction are not clear. In this review we briefly summarized T cells diversity and astrocyte function. Then, we examined the evidence for the astrocytes and T cells interaction under physiological and pathological conditions including ischemic stroke, multiple sclerosis, viral infection, and Alzheimer’s disease.
Keywords: T cells, astrocyte, central nervous system, stroke, Alzheimer’s disease, multiple sclerosis
1. Introduction
The central nervous system (CNS) has long been recognized as a site of ‘immune privilege’ because of the existence of the blood brain barrier (BBB) which presumably isolates CNS from the peripheral immunosurveillance. Different from the peripheral organs, CNS is unique in response to all forms of CNS injury and disease which are mainly mediated by resident microglia and astrocyte (Ransohoff and Brown, 2012; Ransohoff and Engelhardt, 2012). This has led to the introduction of the term “neuroinflammation” to distinguish inflammation reaction in the CNS from inflammation in other tissues (Xanthos and Sandkuhler, 2014). There is increasing evidence that immune cells are involved in the maintenance of CNS homeostasis and neuroinflammation process. The interaction between the peripheral immune system and the CNS both in physiological and pathological condition is becoming a new research of interest. As an important cellular component of the immune system, T cells exert their functions in the immune surveillance, immune attack and immune tolerance. As the most abundant cells in the CNS, astrocytes support and maintain CNS integrity response to all form of CNS injury in term of reactive astrogliosis. In addition, astrocytes display an array of receptors involved in innate CNS immunity (Farina et al., 2007). How astrocytes and T cells interactand the consequences of their interaction are not clear. In this review we will briefly summarize T cells diversity and astrocyte function. Then, we will examine the evidence for the interaction between astrocytes and T cells under physiological and pathological conditions.
2. T cell heterogeneity
T cells are the cellular components of adaptive immunity in the body. Originated from hematopoietic stem cells and developing in the thymus, each T cell targets a unique antigen epitope with their specified antigen recognition receptors on their surface (Reiner, 2009). T cells can be grouped into various subsets based on their effector functions and molecular phenotype. Distinct T cell subsets promote different types of immune response.
2.1. αβ T cells
About 95% of T cells are αβ T cells, named after the T-cell receptor (TCR) consisting of an α and a β chain (Clambey et al., 2014). The thymus is the main producer of αβ T cells. αβ T cells exit from the thymus to enter the blood circulation and home to secondary lymphoid organ/tissues such as spleen, lymph nodes, tonsils, Peyer’s patches and mucosa associated lymphoid tissue (MALT) (Thompson, 2012). Based on the expression of surface CD4 and CD8 glycoprotein, αβ T cells can be further divided into CD4+ and CD8+ T cells.
2.1.1. CD4+ T cells
Also known as helper T cells, CD4+ T cells are crucial in achieving effective adaptive immune response to pathogens. Naive CD4+T cells are activated after interaction with antigen-MHC complex presented by antigen presenting cells (APCs) and differentiate into specific subtypes depending on the cytokine milieu of the microenvironment (Luckheeram et al., 2012). CD4+ T cells help B cells make antibody, enhance and maintain responses of CD8+ T cells, and regulate macrophage function. In addition, CD4+ T cells play critical role in immunologic memory (Zhu et al., 2010). CD4+ T cells can be activated in response to a particular cytokine milieu and may differentiate into one of several lineages, including Th1, Th2, Th17, Th9, Treg, and TFH, as defined by their pattern of cytokines production and function (Zhu et al., 2010). Table 1 summarizes the differentiation and functions of the major CD4+ T cell subtypes. CD4+ T cells and their subtypes are broadly involved in the neurological disorders mentioned in this review, especially in ischemic stroke and multiple sclerosis.
Table 1.
CD4+ T cell subtypes
| Subtype | Inducing cytokine | Master regulator | Effector Cytokine | Functions |
|---|---|---|---|---|
| Th1 | IL-12, IFN-γ | T-bet, STAT1 | IFN-γ, LTα/β | Anti-viral and anti-bacterial immunity |
| Th2 | IL-4, IL-2 | GATA-3, STAT6 | IL-4, IL-5, IL-9, IL-10, IL-13 | Extracellular parasites immunity |
| Th17 | TGF-β, IL-6, IL-21, IL-23 | RORγt, STAT3 | IL-17a, IL-21, IL-22 | Inflammation, autoimmunity |
| Th9 | TGF-β, IL-4 | IRF4* | IL-9 | Inflammation, autoimmunity, anti-tumor immunity |
| Treg | TGF-β | Foxp3 | IL-10, TGF-β, IL-35 | Anti-inflammation, Anti-autoimmunity |
| TFH | IL-6, IL-21 | STAT3* | IL-10, IL-21, IL-4 | Help B cell differentiation |
Important but might not be the master regulator.
2.1.2. CD8+ T cells
CD8+ T cells, also known as cytotoxic T cells (CTLs), are the major fighters against viral infections but also participate in defense against bacterial and protozoal infections (Zhang and Bevan, 2011). Resting naive CD8+ T cells react to pathogens by massive expansion and differentiation into cytotoxic effector cells that migrate to all regions of the body to clear the infection. Pro-inflammatory cytokines, such as IL-12, play a key role in terminal differentiation of CD8+ effector T cells (Grabie et al., 2003; Starbeck-Miller et al., 2014). CTLs are equipped with effector agents such as IFN-γ, TNF-α, perforin, granzyme B and Fas ligand to kill pathogen-infected or dysfunctional somatic cells. As active fighters against viral infection, CD8+ T cells are mainly involved in CNS viral infection.
2.2. γδ T cells
γδ T cells are a unique and conserved population of lymphocytes, representing a small fraction (1–5%) of the overall T cell population. Their TCR is composed of a γ and a δ chain, recognizing a broad set of antigens including both foreign pathogens and self-antigens (Chien et al., 2014). γδ T cells mature in the thymus. Unlike αβ T cells, they do not require further peripheral maturation or extensive clonal expansion to initiate terminal effector functions (Vantourout and Hayday, 2013). They can kill infected, activated or transformed cells, through pathways that involve the engagement of death-inducing receptors, such as FAS and TNF-related apoptosis-inducing ligand receptors (TRAILR), and the release of cytotoxic effector molecules, such as perforin and granzymes (Bonneville et al., 2010). Moreover, they contribute to pathogen clearance directly through the production of bacteriostatic or lytic molecules, such as granulysin and defensins, and indirectly through the induction of antibacterial functions of other immune effector cells and epithelial cells (Bonneville et al., 2010). γδ T cells can also produce immunomodulatory cytokines that are involved in protective immunity. They might be pathogenic in ischemic stroke and multiple sclerosis.
3. Astrocytes
Astrocytes constitute the most abundant cell typeand play diverse anatomical and functional roles in the CNS. The main task of astrocytes is to maintain the physiological homeostasis of CNS by providing a stable microenvironment and growth factors (Gimsa et al., 2013). Astrocytes uptake excess neurotransmitters and buffer the ionic content in the brain, so as to sense and regulate formation, stability, and efficacy of synapses. The processes of astrocytes form the glia limitans which is part of the BBB (Abbott et al., 2006). Recently, astrocytes have been shown to play a role in synaptic activity (Ota et al., 2013), regulating neuronal circuitry (Kim et al., 2014), modulating blood flow (Howarth, 2014), and even acting as a source of neural stem/progenitor cells (Chojnacki et al., 2009; Doetsch et al., 1999). Several cytokines and cell signaling pathways have been found to be essential for astrocyte development, such as PDGF, CNTF, EGF, BMP, BRAF/Mek/ERK signaling, sonic hedgehog signaling and Notch signaling, etc (Gallo and Deneen, 2014).
Astrocytes are the main innate immune neuroglia at the CNS (Farina et al., 2007; Ransohoff and Brown, 2012). Like T cells, astrocytes are heterogeneous. Classically, astrocytes are classified into different subtypes based on distinct morphological pattern, lineage and antigenic phenotype, anatomical locations, and transporter/receptor expression (Matyash and Kettenmann, 2010; Zhang and Barres, 2010). However, our understanding of astrocyte development and heterogeneity has lagged behind that of other cell brain cell types (Freeman, 2010), and a universal classification of astrocytes has yet been proposed. Under stress or pathological insults, astrocytes usually respond by astrocytic hypertrophy, increase in the number of astrocytes, and increased astrocytic processes (astrogliosis) (Sofroniew and Vinters, 2010). Meanwhile, reactive astrocytes produce/release diverse pro- or anti-inflammatory cytokines, chemokines and neurotrophins to cause tissue damage or repair (Choi et al., 2014; Fields, 2010; Shen et al., 2012; Yang et al., 2012b), depending on the temporal and spatial progression of astrocytic reaction, as well as the interaction between astrocytes with multiple cell populations such as neurons, microglia and even neural stem/progenitor cells.
4. Interaction between astrocytes and T cells under physiological condition
CNS is an immune-privileged organ, featured by the lack of lymphatic vessels, the lack of professional APCs, and insignificant adaptive immune response if foreign antigen is introduced into the CNS (Wilson et al., 2010). It was once considered that T cells cannot successfully penetrate the intact BBB to reach the CNS parenchyma in the steady state. However, it has been proposed that activated T cells readily cross the undamaged BBB to enter the CNS (Hickey et al., 1991). Recent research has demonstrated that a group of leukocytes including T cells are present in the normal human cerebrospinal fluid, probably being recruited across the choroid plexus (de Graaf et al., 2011; Kivisakk et al., 2002). These T cells could contribute to immune surveillance and respond to recurrent pathogen exposure in the CNS. It has been indicated that the existence of T cells in the parenchyma of normal mouse CNS contributes to immune tolerance and immune memory (Brabb et al., 2000). Our recent study demonstrated that Foxp3+ regulatory T cells (Tregs) are present in the normal rat brain cortex, subcortical region, hippocampus, and choroid plexus, constituting more than 15% of the cerebral CD4+ T-cell compartment (Xie et al., 2014).
There are two possible sites for recruitment of leukocyte into the brain. Choroid plexus lacks tight junction between vascular endothelial cells and the glia limitans (Ransohoff and Engelhardt, 2012). Leukocytes in capillaries can cross the choroidal endothelium into the choroidal stroma, then, penetrate the choroidal epithelium to enter the CSF. However, it has not been determined whether leukocytes can go further into the bran parenchyma. Another possible site for recruitment of leukocyte into CNS is the vascular network at the subventricular zone (SVZ). SVZ contains a rich plexus of blood vessels that snake along and within neuroblast chains. The vasculature has a leaky BBB, due to the lack of endothelial tight junctions, pericytes and astrocytic endfeet (Goldberg and Hirschi, 2009; Tavazoie et al., 2008). It has been indicated that blood-borne signals can enter the SVZ, although the direct evidence of T cell recruitment is still lacking. The exact location of T cell entry needs further investigations.
In vivo imaging of BBB showed that sheathing of subpial vessels by astrocyte processes was continuous along all capillaries, arterioles, and veins, comprising a highly interconnected pathway through which signals could feasibly be relayed over long distances via gap junctions (McCaslin et al., 2011). Once T cells have crossed the blood vasculature, the first cellular structure they encounter would be the endfeet or processes of astrocytes. However, there are not enough evidences demonstrating the direct interactions between astrocytes and T cells in vivo. Recent in vitro studies provided clues of the effect of astrocytes on T cells. Eléonore Beure et al found that culturing mouse CD4+ T-cells on mouse primary astrocytes without supplements of additional cytokines modified T-cell polarization to Th1 and Treg subtypes (Beurel et al., 2014). This modified T-cell polarization was diminished by inflammatory activation of astrocytes. Astrocytes-conditioned medium could not induce Th1 cell differentiation, suggesting that it is not an astrocyte-derived soluble factor that promotes Th1 cell production. Instead, it seems that CD4+ T cells stimulate astrocytes to release an unidentified factor that promotes Th1 differentiation. Interestingly, CD4+ T cells cultured on astrocytes showed a higher rate of cell division than undifferentiated CD4+ T cells, suggesting the factor(s) would be mitogenic. Our recent study showed that primary astrocytes are capable of maintaining Foxp3 expression of peripheral Tregs and support Treg survival through activation of IL-2-STAT5 signaling in vitro (Xie et al., 2014). In our study, astrocytes did not induce the generation of Tregs from non-Treg T cells, but rather act as a substitutive source of IL-2, which is usually supplied by activated T cells (Gasteiger and Kastenmuller, 2012). Besides IL-2, astrocytes might affect T cells via other mechanisms. For example, glutamate promotes Th1 cell production in the presence of anti-IL-4 and IL-12 (Beurel et al., 2014). Addition of glutamate on CD4+ T cells was sufficient to increase T-bet expression. It is noteworthy that an important function of astrocytes is to buffer glutamate. Thus, we may speculate that normal astrocytes would bias the CD4+ T cell polarization through regulating the extracellular glutamate level. Moreover, T cells may impact astrocytes through glutamate. Sanjay K. Garg and his colleagues found that cultured T cells caused glutamate accumulation, which was efficiently cleared when T cells were co-cultured with astrocytes (Garg et al., 2008). The T cell-derived glutamate elicited in turn, the release of neuroprotective thiols (cysteine, glutathione, and cysteinyl-glycine) and lactate from astrocytes, suggesting T cells endow astrocytes with a neuroprotective phenotype. In the above-mentioned studies, primary astrocytes were not stimulated with cytokines, Toll-like receptors or other astrocytic agonists. Therefore, these studies provide valuable clues on how astrocytes and T cells modulate each other in physiological condition. However, whether these interactions indeed exist in vivo is still unclear. Primary astrocyte culture might not precisely reflect the naive astrocytes in vivo, since the primary astrocytes are usually isolated from neonates, not from adults. Thus, further research using purified adult astrocytes will be necessary to confirm or amend the pattern of interaction between astrocytes and T cells.
5. Interaction between astrocytes and T cells under pathological conditions
The BBB is a multicellular vascular structure that separates the CNS from the peripheral blood circulation. Every constituent cell type makes an indispensable contribution to the BBB’s integrity. If one component of the BBB fails, subsequently the barrier breaks down, and neuroinflammation and neurodegeneration can occur (Obermeier et al., 2013). BBB breakdown can be the cause and/or the consequence of neurological disorders, facilitating the entry of peripheral cells and humoral components into the CNS. Both CD4+ and CD8+ T cells can disrupt the BBB (Johnson et al., 2014; Kebir et al., 2007; Smorodchenko et al., 2007; Suidan et al., 2006). Although there is little supporting evidence, it would be plausible to speculate that astrocytes could regulate T cell activity to maintain or impair the tight junction of the vascular endothelial cells. Since astrocytes are part of the BBB, the impact of T cells on astrocytes would also influence the permeability of the BBB. However, solid study is in demand to test the hypothesis and evaluate the consequence of the interaction. The neuroinflammation and/or neurodegeneration affect the biological features of both the innate astrocytes and infiltrating T cells. It has been indicated that astrocytes are important for modulation of immune cell responses in the CNS through multiple mechanisms. Furthermore, infiltrating immune cells including T cells may regulate astrocytic functions as well. The interaction between astrocytes and T cells might be either beneficial or detrimental, depending on the type of the disease, the cytokine microenvironment, and the responses of different cell classes.
5.1. Ischemic stroke
Increasing evidence indicates that acute stroke is followed by a complex interplay between the CNS and the immune system (Chamorro et al., 2012; Iadecola and Anrather, 2011). It is well documented that different T cell types, including CD4+ non-Treg T cells (Gelderblom et al., 2009; Kleinschnitz et al., 2010), CD8+ T cells (Gelderblom et al., 2009; Kleinschnitz et al., 2010), Tregs (Kleinschnitz et al., 2013; Planas and Chamorro, 2009; Xu et al., 2013) and γδT cells (Gelderblom et al., 2014; Shichita et al., 2009) infiltrate into the brain parenchyma after ischemic stroke. There are increasing evidence that T cells, but not B cells, are detrimental during stroke (Hurn et al., 2007; Iadecola and Anrather, 2011; Kleinschnitz et al., 2010). However, the action of T cells in ischemic stroke may be subtype-specific as Treg cells have been indicated to be cerebroprotective in acute experimental stroke (Liesz et al., 2009). It has also been suggested that the effect of T cells in the acute phase of experimental cerebral ischemia was neither related to adaptive immunity nor thrombus formation (Kleinschnitz et al., 2010).
Astrocytes are active participants in initiation and maintenance of post-ischemic inflammation. Excitotoxicity and oxidative stress caused by the initial ischemic event activate astrocytes which react by secreting cytokines, chemokines, NO and matrix metalloproteases (Lakhan et al., 2009). Although in the lack of literature showing the direct interaction between astrocytes and T cells after ischemia, there are clues suggesting the potential effects of either cell type to another. For example, in the penumbra area, astrocytes produce lysophosphatidylcholine to up-regulate MCP-1 and CCL2 expression in microglia (Inose et al., 2014). CCL2 production by resident CNS cells is required for optimal accumulation of macrophages and dendritic cells, which both recruit and activate T cells (Dogan et al., 2008). CCL-2 is also involved in astrocyte-mediated extravasation of T cells in the brain (Carrillo-de Sauvage et al., 2012; Soria et al., 2014). In addition, ischemic stroke causes elevation of glutamate (Cui et al., 1999; Seki et al., 1999; Soria et al., 2014), which either impairs or enhances T cell activation depending on the glutamate receptor type on T cells (Pacheco et al., 2006; Pacheco et al., 2007). Glutamate-buffering astrocytes might regulate the glutamate amount to influence T cell activities, positively or negatively. Anton Kichev et al (Kichev et al., 2014) showed that astrocytes are the predominant source of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in a hypoxia-ischemia model. TRAIL can induce apoptosis of T cells (Jeremias et al., 1998), and this might be one of the mechanisms by which T cells are cleared in the late phase of ischemic stroke.
5.2. Multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE)
MS (and its animal model EAE) is a chronic, progressive inflammatory disorder of the brain and spinal cord. The disease is mediated by pathogenic T cell responses against myelin antigens, followed by a broader neurodegenerative process (Fletcher et al., 2010). The autoreactive T cells migrate across the BBB and mediate damage against the neurons and their myelin sheaths, in particular, but also their axons. Th1 cells (Hedegaard et al., 2008) were thought originally to be the main pathogenic T cells in MS. Recent discoveries suggest that Th17 cells (Conti et al., 2012; Grifka-Walk et al., 2013; Rothhammer et al., 2011), CD8+ T cells (Weiss et al., 2007) and γδ T cells (Sutton et al., 2009) are also pathogenic in the MS and EAE, while Th2 cells (Fernando et al., 2014) and Tregs (Kohm et al., 2002) are likely protective. The role of Th9 cells in MS and EAE is unclear, since Th9 cells reportedly exert both aggravating and suppressive roles on EAE (Elyaman et al., 2009; Jager et al., 2009; Li et al., 2010; Nowak et al., 2009).
Astrocytes may play a role in T cell recruitment through chemokine production. For example, astrocytes have been shown to produce CCL5, CCL2, CCL3, CCL12, CXCL1, CXCL2, CXCL8, CXCL10 during inflammation (Chastain et al., 2011; Choi et al., 2014; Dong and Benveniste, 2001). However, the exact role of astrocytes on T cell recruitment needs further investigation.
It has been indicated that astrocytes can positively or negatively regulate distinct T cell subtypes in MS and EAE. Because of the adaptive autoimmune feature of multiple sclerosis and EAE, the research has been focusing on the T cell priming function of astrocytes during the past decades. T cell priming demands antigen presentation to T cells by major histocompatibility complex class I (MHC-I) or major histocompatibility complex class II (MHC-II), ligation of co-stimulatory molecules, immune synapse formation and instructive cytokine expression. Astrocytes express MHC-I and MHC-II molecules in vitro (Cornet et al., 2000; Wong et al., 1984; Zeinstra et al., 2006) and up-regulate expression of the co-stimulatory molecules CD80 (B7-1) and CD86 (B7-2) upon treatment with IFN-γ (Cornet et al., 2000; Nikcevich et al., 1997). Although some studies did not find CD80 or CD86 expression on astrocytes in EAE (Aloisi et al., 1998; Cross and Ku, 2000), a more recent study found that astrocytes in chronic MS lesions do express CD80 and CD86 (Zeinstra et al., 2003). CD44 could be involved in the adhesive interactions between T cells and astrocytes (Haegel et al., 1993). Astrocyte also express other adhesion molecules such as intracellular adhesion molecule-1 (ICAM-1) (Lee et al., 1999; Shrikant et al., 1994) and vascular cell adhesion molecule-1 (VCAM-1) (Rosenman et al., 1995; Winkler and Beveniste, 1998), which might facilitate adhesion between T cells and astrocytes. Furthermore, supporting evidence indicates that astrocytes are capable of inducing Th1 differentiation and proliferation of naïve myelin-specific T cells (Carpentier et al., 2005; Constantinescu et al., 2005; Kort et al., 2006; Soos et al., 1999; Tan et al., 1998). However, compared with professional APCs such as dendritic cells and macrophages, the T cells priming effect of astrocytes are relatively weak. And the evidence confirming the formation of immune synapse between astrocytes and T cells in MS or EAE is still lacking. Thus, it is possible that astrocytes contribute to but is not the major player in the antigen presentation and co-stimulatory molecule recognition in MS and EAE. Interestingly, although most research indicated that astrocytes prime T cells for stimulation and polarization, which exacerbate the disease, one study stated that antigen presentation by IFN-γ-treated astrocytes primed rat T cells for apoptosis in a contact-dependent manner (Gold et al., 1996). Indeed, earlier and current studies indicated that astrocytes may induce T cell apoptosis through different mechanisms. It has been revealed that TNF-treated astrocytes up-regulates Galectin-9 to promote encephalitogenic T-cell apoptosis (Steelman et al., 2013). Xu Wang et al (Wang et al., 2013) used GFAP-Cre FasLfl/fl mice in EAE model and concluded that astrocytes induced apoptosis of Fas+ activated CD4+ T cells and to increase numbers of Foxp3+ Treg cells beyond the time point of maximal clinical disease. The FasL expression on astrocytes and its role in induction of T cell apoptosis have also been documented in other literatures. FasL and Fas are expressed constitutively in human astrocytes and the expression increases with IL-1, IL-6, TNF-α, or IFN-γ (Choi et al., 1999). Ingo Bechmann et al (Bechmann et al., 2002) revealed that astrocytes co-localized with apoptotic lymphocytes in vivo and induce apoptosis of transformed T cells in vitro. T cell apoptosis measured by Annexin V binding and DNA fragmentation was significantly lower using CD95 ligand-deficient astrocytes compared to non-deficient controls. Moreover, neutralizing anti-CD95 ligand antibody reduced astrocyte-induced T cell apoptosis. And this finding is supported by an independent study showing the similar results (Kohji and Matsumoto, 2000).
Besides direct priming of T cells, astrocytes may also regulate T cell activation and differentiation by providing instructive cytokines or unidentified factors. During inflammation, astrocytes are producers of a variety of cytokines including IL-1, IL-6, TNF-α, IL-10, and TGF-β (Dong and Benveniste, 2001; Falsig et al., 2006). TGF-β and IL-6 are related to Th17 and Treg cell generation in the periphery. However, whether astrocytes-derived TGF-β and IL-6 affect Th17 or Treg cells needs further investigation. Cris S. Constantinescu et al (Constantinescu et al., 2005) showed that IFN-γ-stimulated astrocytes and microglia produce biologically active IL-12p70. In addition, astrocytes expressed IL-12p35 mRNA constitutively, and IL-23 p19 after stimulation. Thus, under inflammatory conditions, astrocytes express all subunits of IL-12/IL-23. Astrocytes’ ability to present antigen to encephalitogenic T cells can be blocked by neutralizing anti-IL-12/IL-23p40 antibodies. This study poses the possibility that astrocytes not only induce Th1 polarization, but also favor the generation of Th17 cells and IL-17-producing γδ T cells, since IL-23 is essential for expansion of Th17 cells and IL-17-producing γδ T cells. Indeed, Djordje Miljkovic et al (Miljkovic et al., 2007) reported that astrocytes up-regulated IL-17 and IFN-γ gene expression and protein synthesis in T cells, which coincided with astrocytes’ ability to express IL-23 subunit p19 and common IL-12/IL-23 subunit p40. Philippe Saikali et al (Saikali et al., 2010) found that human astrocytes in primary cultures increased surface IL-15 levels upon activation with combinations of IFN-γ plus TNF or IFN-γ plus IL-1β. The astrocyte-derived IL-15 promoted lytic enzyme content, NKG2D expression, and Ag-specific cytotoxicity of myelin-specific autoreactive CD8+ T cells. Meanwhile, other studies suggest astrocytes can suppress autoreactive T cell response. An early study found that astrocytes derived from human embryonic brain were able to suppress PBMC-dependent proliferation and IFN-γ production of antigen-specific CD4+ T cell lines (Meinl et al., 1994). Prostaglandins were partially involved in the suppressive effect, while IL-4, IL-10 and TGF-β2 were not. The inhibitory effect was observed in the presence of both HLA matched and mismatched astrocytes and was mediated by astrocyte-derived soluble factor(s) rather than by direct cellular contact. Vladimir Trajkovic et al (Trajkovic et al., 2004) reported that astrocytes mitigate CNS autoimmunity by inducing suppressive activity of both CD4+ and CD8+ T cell compartment. Interestingly, Heat-sensitive soluble T-cell factors, not including transforming growth TGF-β or IL-10, were solely responsible for the observed suppression. Hui-Rong Jiang and colleagues (Jiang et al., 2012) reported that IL-33, a member of the IL-1 family, is expressed in astrocytes and neurons in EAE. IL-33-treated mice attenuated EAE, having reduced levels of IL-17 and IFN-γ but produced increased amounts of IL-5 and IL-13. Moreover, Lymph node and splenic macrophages of IL-33-treated mice showed polarization toward an alternatively activated macrophage, which are anti-inflammatory. JF Yang et al (Yang et al., 2012a) found that in vitro astrocytes inhibited the proliferation and IFN-γ, IL-4, IL-17 and TGF-β secretion of MOG35–55-specific lymphocytes, an effect that could be ameliorated by IL-27 neutralization. However, when astrocytes were pretreated with IFN-γ, they could promote the proliferation and secretion levels of MOG35–55-specific lymphocytes. These studies pose the possibility that resting and activated astrocytes possess distinct, even opposite regulatory functions towards T cells.
Although most studies focused on astrocyte-induced changes in T cell behavior, the interaction between astrocytes and T cells in MS and EAE is likely bidirectional. T cells also induce biological changes in astrocytes, either attenuate or exacerbate the disease. IFN-γ, which is produced by Th1 cells, has been repeatedly proved to be a potent stimuli for astrocytes (Lee et al., 2013; Nikcevich et al., 1997; Vardjan et al., 2012; Yong et al., 1991). Yan Zhou et al (Zhou et al., 2011) reported that the IL-9 receptor complex is constitutively expressed in astrocytes. T-cell-derived IL-9 induces astrocytes to produce CCL20 to induce Th17 cell migration in vitro. Treating with anti–IL-9 neutralizing antibody attenuates experimental autoimmune encephalomyelitis, decreases the number of infiltrating Th17 cells, and reduces CCL20 expression in astrocytes. Zizeng Kang and colleagues (Kang et al., 2010) found that Th17 induced IL-17 signaling in astrocytes through Act1, which ameliorates EAE. IL-17- and IL-17+TNF-induced inflammatory gene expression was reduced in Act1-deficient astrocytes. In addition, other effects of IL-17 on astrocytes have been discovered, including stimulating inducible nitric oxide synthase activation (Trajkovic et al., 2001), inducing MIP-1α expression (Yi et al., 2014), enhancing IL-6 signaling Cascade (Ma et al., 2010).
5.3. Viral infection
Studies on viral infection in the CNS suggest that astrocytes are able to present viral antigens to virus-specific T cells, thus helping clearance of virus. The information about the immune synapse formation between astrocytes and T cells is generally derived from studies on virus-infected astrocytes. Carlos Barcia et al (Barcia et al., 2006) observed in vivo formation of the supramolecular activation clusters between effector CD8+ T cells and adenovirus-infected astrocytes precedes and mediates clearance of virally infected astrocytes. In this study, each type of T cell makes a distinctive contribution to virus clearance, being shown by the fact that CD4+ T cells remained in the perivascular compartment, whereas CD8+ T cells were in the area where infected astrocytes were located. Astrocytes displayed MHC-I on the plasma membrane, thus constituting a potential target for activated CD8+ T cells. CD8+ T cells established frequent close anatomical contacts with infected brain cells. Furthermore, brain-infiltrating CD8+ T cells increased tyrosine kinase cascade phosphorylation induced by TCR signaling, indicating that the CD8+ T cells were activated through interaction with antigenic peptides on MHC-I expressed on astrocytes. Interestingly, a later study demonstrated that this synapse formation also induced polarization of brain astrocytes in vivo and in vitro (Barcia et al., 2008). Rather than causing astrocyte hypertrophy, antiviral T cells cause a major structural reorganization of target virally infected astrocytes. Thus, the immune synapse might trigger and facilitate bidirectional signaling in participating cells, like APCs and T cells in peripheral immune priming.
Other studies also showed the interplay between T cells and astrocytes during viral infection. Astrocytes infected by T-cell lymphotropic virus type 1 serve as immunological targets for HTLV-1-specific cytotoxic T cells, resulting in parenchymal damage (Mendez et al., 1997). It has been demonstrated that T-cell lymphotropic virus type 1-infected T cells decreased uptake of extracellular glutamate by astrocytes through reducing expression of the glial transporters GLAST and GLT-1 (Szymocha et al., 2000). In a Theiler’s murine encephalomyelitis virus (TMEV) infection model, IFN-γ-pretreated astrocytes were able to process and present all the predominant T cell epitopes of TMEV to virus-specific T cells. These T cells mediate lysis of the astrocytes in vitro in a Fas-dependent mechanism (Palma et al., 1999). Thus, astrocytes and T cells may collaborate to clear virus-infected target cells in the CNS. However, recent studies on human immunodeficiency virus (HIV) indicated that HIV could infect astrocytes, and astrocytes released infectious virus that could be transmitted to CD4+ T cells, thus spreading the virus and exacerbate the infection (Clarke et al., 2006; Gray et al., 2014). Taken together, the relationship between T cells and astrocytes during virus infection may be complicated, depending on the viral types and the microenvironmental changes.
5.4. Alzheimer’s disease
Alzheimer’s disease (AD) is the most common dementing illness and is pathologically characterized by deposition of the 40–42 amino acid peptide, amyloid-β (Aβ), as senile plaques. A number of reports suggest that some T cells are activated in AD patients, and that these cells exist both in the periphery and as infiltrates in the brain (Buckwalter et al., 2006; Monsonego et al., 2003; Monsonego et al., 2013; Trieb et al., 1996; Weiner and Frenkel, 2006). Immunization with Aβ in a mouse model of AD results in the accumulation of T cells at Aβ plaques in the brain (Fisher et al., 2010). These accumulated Aβ-specific T cells, having a phenotype of Th1 cells secreting primarily IFN-γ, induced almost complete clearance of Aβ. However, another report suggested that the phenotype of T cells in the AD brain are activated but are not fully differentiated (Togo et al., 2002). The characteristics of Aβ-specific T cells have yet to be thoroughly elucidated. It is proposed that Aβ may be presented to T cells via co-localized APCs including microglia and recruited blood APCs (Monsonego et al., 2013). However, whether astrocytes can uptake and present Aβ peptide remains unknown. Astrogliosis is observed in brains of both AD patients and animal models (Verkhratsky et al., 2010). Astrocytes are known to be important for Aβ clearance and degradation, for providing trophic support to neurons, and for forming a protective barrier between Aβ deposits and neurons (Rubio-Perez and Morillas-Ruiz, 2012). Studies have observed astrocyte-mediated inflammation in AD, including increased IL-1 family members, TRAIL and IL-6 (Li et al., 2011; Rubio-Perez and Morillas-Ruiz, 2012). Rekha Bhat et al (Bhat et al., 2012) found that Aβ1–42 peptide leads to astrocyte senescence and elevated production of multiple inflammatory cytokines including IL-6, CCL5, IL-8, and ICAM-1. However, whether these astrocyte-derived cytokines influence Aβ-specific T cells is unknown due to the lack of study on the interaction between astrocytes and Aβ-specific T cells. Keith L. Mc Quillan et al (McQuillan et al., 2010) reported that cultured mixed glia (contains 80% astrocytes) acted as effective APCs for Aβ-specific Th1 and Th17 cells. Addition of Aβ-specific Th2 cells suppressed the Aβ-induced IFN-γ production by Th1 cells and IL-17 production by Th17 cells with glia as the APC. Aβ-specific Th1 or Th17 cells remarkably enhanced expression of MHC-II and co-stimulatory molecules on the microglia, while they only modestly enhanced MHC-II and CD86 expression on astrocytes. This study suggests there might be some interactions between astrocytes and Ab-specific T cells and future in vivo studies are warrant.
6. Conclusion and Perspective
As the most abundant cell type in the CNS, astrocytes play active roles in maintaining CNS homeostasis under steady state and repair tissue damage under neuropathological status. Under normal condition, the interaction between astrocytes and peripheral immune cells is blocked by the BBB. Stress and damage in the CNS compromise BBB integrity, permitting the access of blood immune cells to astrocytes and other CNS cellular components. The reactive astrocytes could mediate biological alterations of T cells through distinct mechanisms, depending on the types of diseases, the phases of diseases, and the intrinsic astrocytic activities. Astrocytes act as a source of cell surface receptor/ligands, cytokines and unidentified soluble factors to modulate both innate immune cells and adaptive immune cells in the neuropathy, and for exchange, immune cells also regulate astrocytic activities. Although current studies on the interactions of astrocytes and T cells are mainly derived from autoimmune disorders, it has been clear that T cells also play roles in other neurodegenerative diseases including AD, PD, and ischemic stroke. Surprisingly, the interaction of T cells and astrocytes has been rarely studied. Future research is warranted on the mutual regulation of T cells and astrocytes which will boost our understanding of CNS homeostasis, and provide new insights to the prevention and therapeutic interventions for distinct neurological disorders.
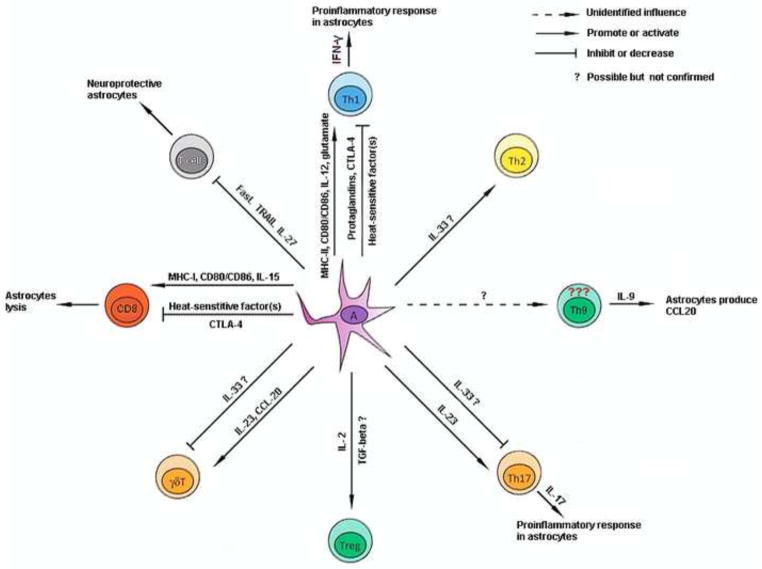
Figure 1. The summary of interaction between astrocytes and T cells.
This diagram displays the potential molecules responsible for the interaction of astrocyte and T cells, disregarding the activation status of astrocytes and the concrete neurological disorders.
Astrocytes are crucial for the homeostasis of the central nervous system.
T cells are involved in pathogenesis of several neurological disorders.
T cell-astrocyte interaction exists in some neurological disorders.
The interaction influences progression of several neurological disorders.
The interaction involves cell-cell contact, cytokines, and neurotransmitters.
Acknowledgments
Research support: This work was supported by National Institutes of Health grants NS054651, NS088596, NS087209.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Aloisi F, et al. Microglia are more efficient than astrocytes in antigen processing and in Th1 but not Th2 cell activation. J Immunol. 1998;160:4671–80. [PubMed] [Google Scholar]
- Barcia C, et al. In vivo mature immunological synapses forming SMACs mediate clearance of virally infected astrocytes from the brain. J Exp Med. 2006;203:2095–107. doi: 10.1084/jem.20060420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcia C, et al. T cells’ immunological synapses induce polarization of brain astrocytes in vivo and in vitro: a novel astrocyte response mechanism to cellular injury. PLoS One. 2008;3:e2977. doi: 10.1371/journal.pone.0002977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechmann I, et al. Astrocyte-induced T cell elimination is CD95 ligand dependent. J Neuroimmunol. 2002;132:60–5. doi: 10.1016/s0165-5728(02)00311-9. [DOI] [PubMed] [Google Scholar]
- Beurel E, et al. Astrocytes modulate the polarization of CD4+ T cells to Th1 cells. PLoS One. 2014;9:e86257. doi: 10.1371/journal.pone.0086257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R, et al. Astrocyte senescence as a component of Alzheimer’s disease. PLoS One. 2012;7:e45069. doi: 10.1371/journal.pone.0045069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneville M, O’Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10:467–78. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- Brabb T, et al. In situ tolerance within the central nervous system as a mechanism for preventing autoimmunity. J Exp Med. 2000;192:871–80. doi: 10.1084/jem.192.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter MS, et al. Increased T cell recruitment to the CNS after amyloid beta 1–42 immunization in Alzheimer’s mice overproducing transforming growth factor-beta 1. J Neurosci. 2006;26:11437–41. doi: 10.1523/JNEUROSCI.2436-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier PA, et al. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia. 2005;49:360–74. doi: 10.1002/glia.20117. [DOI] [PubMed] [Google Scholar]
- Carrillo-de Sauvage MA, et al. CCL2-expressing astrocytes mediate the extravasation of T lymphocytes in the brain. Evidence from patients with glioma and experimental models in vivo. PLoS One. 2012;7:e30762. doi: 10.1371/journal.pone.0030762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro A, et al. The immunology of acute stroke. Nat Rev Neurol. 2012;8:401–10. doi: 10.1038/nrneurol.2012.98. [DOI] [PubMed] [Google Scholar]
- Chastain EM, et al. The role of antigen presenting cells in multiple sclerosis. Biochim Biophys Acta. 2011;1812:265–74. doi: 10.1016/j.bbadis.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien YH, Meyer C, Bonneville M. gammadelta T cells: first line of defense and beyond. Annu Rev Immunol. 2014;32:121–55. doi: 10.1146/annurev-immunol-032713-120216. [DOI] [PubMed] [Google Scholar]
- Choi C, et al. Fas ligand and Fas are expressed constitutively in human astrocytes and the expression increases with IL-1, IL-6, TNF-alpha, or IFN-gamma. J Immunol. 1999;162:1889–95. [PubMed] [Google Scholar]
- Choi SS, et al. Human astrocytes: secretome profiles of cytokines and chemokines. PLoS One. 2014;9:e92325. doi: 10.1371/journal.pone.0092325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chojnacki AK, Mak GK, Weiss S. Identity crisis for adult periventricular neural stem cells: subventricular zone astrocytes, ependymal cells or both? Nat Rev Neurosci. 2009;10:153–63. doi: 10.1038/nrn2571. [DOI] [PubMed] [Google Scholar]
- Clambey ET, et al. Molecules in medicine mini review: the alphabeta T cell receptor. J Mol Med (Berl) 2014;92:735–41. doi: 10.1007/s00109-014-1145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JN, et al. Novel pathway of human immunodeficiency virus type 1 uptake and release in astrocytes. Virology. 2006;348:141–55. doi: 10.1016/j.virol.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Constantinescu CS, et al. Astrocytes as antigen-presenting cells: expression of IL-12/IL-23. J Neurochem. 2005;95:331–40. doi: 10.1111/j.1471-4159.2005.03368.x. [DOI] [PubMed] [Google Scholar]
- Conti L, et al. Th17 cells in multiple sclerosis express higher levels of JAK2, which increases their surface expression of IFN-gammaR2. J Immunol. 2012;188:1011–8. doi: 10.4049/jimmunol.1004013. [DOI] [PubMed] [Google Scholar]
- Cornet A, et al. Role of astrocytes in antigen presentation and naive T-cell activation. J Neuroimmunol. 2000;106:69–77. doi: 10.1016/s0165-5728(99)00215-5. [DOI] [PubMed] [Google Scholar]
- Cross AH, Ku G. Astrocytes and central nervous system endothelial cells do not express B7-1 (CD80) or B7-2 (CD86) immunoreactivity during experimental autoimmune encephalomyelitis. J Neuroimmunol. 2000;110:76–82. doi: 10.1016/s0165-5728(00)00327-1. [DOI] [PubMed] [Google Scholar]
- Cui Y, et al. Ischemia-induced glutamate release in the dentate gyrus. A microdialysis study in the gerbil. Neurosci Lett. 1999;271:191–4. doi: 10.1016/s0304-3940(99)00556-x. [DOI] [PubMed] [Google Scholar]
- de Graaf MT, et al. Central memory CD4+ T cells dominate the normal cerebrospinal fluid. Cytometry B Clin Cytom. 2011;80:43–50. doi: 10.1002/cyto.b.20542. [DOI] [PubMed] [Google Scholar]
- Doetsch F, et al. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–16. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Dogan RN, Elhofy A, Karpus WJ. Production of CCL2 by central nervous system cells regulates development of murine experimental autoimmune encephalomyelitis through the recruitment of TNF- and iNOS-expressing macrophages and myeloid dendritic cells. J Immunol. 2008;180:7376–84. doi: 10.4049/jimmunol.180.11.7376. [DOI] [PubMed] [Google Scholar]
- Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–90. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- Elyaman W, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:12885–90. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsig J, et al. The inflammatory transcriptome of reactive murine astrocytes and implications for their innate immune function. J Neurochem. 2006;96:893–907. doi: 10.1111/j.1471-4159.2005.03622.x. [DOI] [PubMed] [Google Scholar]
- Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–45. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Fernando V, et al. Regulation of an autoimmune model for multiple sclerosis in Th2-biased GATA3 transgenic mice. Int J Mol Sci. 2014;15:1700–18. doi: 10.3390/ijms15021700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. Release of neurotransmitters from glia. Neuron Glia Biol. 2010;6:137–9. doi: 10.1017/S1740925X11000020. [DOI] [PubMed] [Google Scholar]
- Fisher Y, et al. T cells specifically targeted to amyloid plaques enhance plaque clearance in a mouse model of Alzheimer’s disease. PLoS One. 2010;5:e10830. doi: 10.1371/journal.pone.0010830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JM, et al. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2010;162:1–11. doi: 10.1111/j.1365-2249.2010.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MR. Specification and morphogenesis of astrocytes. Science. 2010;330:774–8. doi: 10.1126/science.1190928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Deneen B. Glial development: the crossroads of regeneration and repair in the CNS. Neuron. 2014;83:283–308. doi: 10.1016/j.neuron.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg SK, Banerjee R, Kipnis J. Neuroprotective immunity: T cell-derived glutamate endows astrocytes with a neuroprotective phenotype. J Immunol. 2008;180:3866–73. doi: 10.4049/jimmunol.180.6.3866. [DOI] [PubMed] [Google Scholar]
- Gasteiger G, Kastenmuller W. Foxp3+ Regulatory T-cells and IL-2: The Moirai of T-cell Fates? Front Immunol. 2012;3:179. doi: 10.3389/fimmu.2012.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom M, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–57. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- Gelderblom M, Arunachalam P, Magnus T. gammadelta T cells as early sensors of tissue damage and mediators of secondary neurodegeneration. Front Cell Neurosci. 2014;8:368. doi: 10.3389/fncel.2014.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimsa U, Mitchison NA, Brunner-Weinzierl MC. Immune privilege as an intrinsic CNS property: astrocytes protect the CNS against T-cell-mediated neuroinflammation. Mediators Inflamm. 2013;2013:320519. doi: 10.1155/2013/320519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold R, et al. Antigen presentation by astrocytes primes rat T lymphocytes for apoptotic cell death. A model for T-cell apoptosis in vivo. Brain. 1996;119(Pt 2):651–9. doi: 10.1093/brain/119.2.651. [DOI] [PubMed] [Google Scholar]
- Goldberg JS, Hirschi KK. Diverse roles of the vasculature within the neural stem cell niche. Regen Med. 2009;4:879–97. doi: 10.2217/rme.09.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabie N, et al. IL-12 is required for differentiation of pathogenic CD8+ T cell effectors that cause myocarditis. J Clin Invest. 2003;111:671–80. doi: 10.1172/JCI16867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LR, et al. HIV-1 entry and trans-infection of astrocytes involves CD81 vesicles. PLoS One. 2014;9:e90620. doi: 10.1371/journal.pone.0090620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifka-Walk HM, Lalor SJ, Segal BM. Highly polarized Th17 cells induce EAE via a T-bet independent mechanism. Eur J Immunol. 2013;43:2824–31. doi: 10.1002/eji.201343723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegel H, et al. Activated mouse astrocytes and T cells express similar CD44 variants. Role of CD44 in astrocyte/T cell binding. J Cell Biol. 1993;122:1067–77. doi: 10.1083/jcb.122.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard CJ, et al. T helper cell type 1 (Th1), Th2 and Th17 responses to myelin basic protein and disease activity in multiple sclerosis. Immunology. 2008;125:161–9. doi: 10.1111/j.1365-2567.2008.02837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991;28:254–60. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- Howarth C. The contribution of astrocytes to the regulation of cerebral blood flow. Front Neurosci. 2014;8:103. doi: 10.3389/fnins.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurn PD, et al. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab. 2007;27:1798–805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inose Y, et al. Activated microglia in ischemic stroke penumbra upregulate MCP-1 and CCR2 expression in response to lysophosphatidylcholine derived from adjacent neurons and astrocytes. Neuropathology. 2014 doi: 10.1111/neup.12182. [DOI] [PubMed] [Google Scholar]
- Jager A, et al. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009;183:7169–77. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeremias I, et al. TRAIL/Apo-2-ligand-induced apoptosis in human T cells. Eur J Immunol. 1998;28:143–52. doi: 10.1002/(SICI)1521-4141(199801)28:01<143::AID-IMMU143>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Jiang HR, et al. IL-33 attenuates EAE by suppressing IL-17 and IFN-gamma production and inducing alternatively activated macrophages. Eur J Immunol. 2012;42:1804–14. doi: 10.1002/eji.201141947. [DOI] [PubMed] [Google Scholar]
- Johnson HL, et al. Perforin competent CD8 T cells are sufficient to cause immune-mediated blood-brain barrier disruption. PLoS One. 2014;9:e111401. doi: 10.1371/journal.pone.0111401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Z, et al. Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity. 2010;32:414–25. doi: 10.1016/j.immuni.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebir H, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–5. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kichev A, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) signaling and cell death in the immature central nervous system after hypoxia-ischemia and inflammation. J Biol Chem. 2014;289:9430–9. doi: 10.1074/jbc.M113.512350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JG, et al. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat Neurosci. 2014;17:908–10. doi: 10.1038/nn.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivisakk P, et al. T-cells in the cerebrospinal fluid express a similar repertoire of inflammatory chemokine receptors in the absence or presence of CNS inflammation: implications for CNS trafficking. Clin Exp Immunol. 2002;129:510–8. doi: 10.1046/j.1365-2249.2002.01947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschnitz C, et al. Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood. 2010;115:3835–42. doi: 10.1182/blood-2009-10-249078. [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, et al. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood. 2013;121:679–91. doi: 10.1182/blood-2012-04-426734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohji T, Matsumoto Y. Coexpression of Fas/FasL and Bax on brain and infiltrating T cells in the central nervous system is closely associated with apoptotic cell death during autoimmune encephalomyelitis. J Neuroimmunol. 2000;106:165–71. doi: 10.1016/s0165-5728(00)00238-1. [DOI] [PubMed] [Google Scholar]
- Kohm AP, et al. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–6. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- Kort JJ, et al. Efficient presentation of myelin oligodendrocyte glycoprotein peptides but not protein by astrocytes from HLA-DR2 and HLA-DR4 transgenic mice. J Neuroimmunol. 2006;173:23–34. doi: 10.1016/j.jneuroim.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, McGeer E, McGeer PL. Neurotoxins released from interferon-gamma-stimulated human astrocytes. Neuroscience. 2013;229:164–75. doi: 10.1016/j.neuroscience.2012.10.033. [DOI] [PubMed] [Google Scholar]
- Lee SJ, et al. Transcriptional regulation of the intercellular adhesion molecule-1 gene by proinflammatory cytokines in human astrocytes. Glia. 1999;25:21–32. doi: 10.1002/(sici)1098-1136(19990101)25:1<21::aid-glia3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Li C, et al. Astrocytes: implications for neuroinflammatory pathogenesis of Alzheimer’s disease. Curr Alzheimer Res. 2011;8:67–80. doi: 10.2174/156720511794604543. [DOI] [PubMed] [Google Scholar]
- Li H, et al. Neutralization of IL-9 ameliorates experimental autoimmune encephalomyelitis by decreasing the effector T cell population. J Immunol. 2010;185:4095–100. doi: 10.4049/jimmunol.1000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesz A, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–9. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- Luckheeram RV, et al. CD4(+)T cells: differentiation and functions. Clin Dev Immunol. 2012;2012:925135. doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, et al. IL-17 enhancement of the IL-6 signaling cascade in astrocytes. J Immunol. 2010;184:4898–906. doi: 10.4049/jimmunol.1000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyash V, Kettenmann H. Heterogeneity in astrocyte morphology and physiology. Brain Res Rev. 2010;63:2–10. doi: 10.1016/j.brainresrev.2009.12.001. [DOI] [PubMed] [Google Scholar]
- McCaslin AF, et al. In vivo 3D morphology of astrocyte-vasculature interactions in the somatosensory cortex: implications for neurovascular coupling. J Cereb Blood Flow Metab. 2011;31:795–806. doi: 10.1038/jcbfm.2010.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillan K, Lynch MA, Mills KH. Activation of mixed glia by Abeta-specific Th1 and Th17 cells and its regulation by Th2 cells. Brain Behav Immun. 2010;24:598–607. doi: 10.1016/j.bbi.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Meinl E, et al. Multiple sclerosis. Immunomodulatory effects of human astrocytes on T cells. Brain. 1994;117(Pt 6):1323–32. doi: 10.1093/brain/117.6.1323. [DOI] [PubMed] [Google Scholar]
- Mendez E, et al. Astrocyte-specific expression of human T-cell lymphotropic virus type 1 (HTLV-1) Tax: induction of tumor necrosis factor alpha and susceptibility to lysis by CD8+ HTLV-1-specific cytotoxic T cells. J Virol. 1997;71:9143–9. doi: 10.1128/jvi.71.12.9143-9149.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miljkovic D, et al. Astrocytes stimulate interleukin-17 and interferon-gamma production in vitro. J Neurosci Res. 2007;85:3598–606. doi: 10.1002/jnr.21453. [DOI] [PubMed] [Google Scholar]
- Monsonego A, et al. Increased T cell reactivity to amyloid beta protein in older humans and patients with Alzheimer disease. J Clin Invest. 2003;112:415–22. doi: 10.1172/JCI18104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsonego A, Nemirovsky A, Harpaz I. CD4 T cells in immunity and immunotherapy of Alzheimer’s disease. Immunology. 2013;139:438–46. doi: 10.1111/imm.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikcevich KM, et al. IFN-gamma-activated primary murine astrocytes express B7 costimulatory molecules and prime naive antigen-specific T cells. J Immunol. 1997;158:614–21. [PubMed] [Google Scholar]
- Nowak EC, et al. IL-9 as a mediator of Th17-driven inflammatory disease. J Exp Med. 2009;206:1653–60. doi: 10.1084/jem.20090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19:1584–96. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota Y, Zanetti AT, Hallock RM. The role of astrocytes in the regulation of synaptic plasticity and memory formation. Neural Plast. 2013;2013:185463. doi: 10.1155/2013/185463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco R, et al. Glutamate released by dendritic cells as a novel modulator of T cell activation. J Immunol. 2006;177:6695–704. doi: 10.4049/jimmunol.177.10.6695. [DOI] [PubMed] [Google Scholar]
- Pacheco R, et al. Role of glutamate on T-cell mediated immunity. J Neuroimmunol. 2007;185:9–19. doi: 10.1016/j.jneuroim.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Palma JP, et al. Potential role of CD4+ T cell-mediated apoptosis of activated astrocytes in Theiler’s virus-induced demyelination. J Immunol. 1999;162:6543–51. [PubMed] [Google Scholar]
- Planas AM, Chamorro A. Regulatory T cells protect the brain after stroke. Nat Med. 2009;15:138–9. doi: 10.1038/nm0209-138. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122:1164–71. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. 2012;12:623–35. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- Reiner SL. Decision making during the conception and career of CD4+ T cells. Nat Rev Immunol. 2009;9:81–2. doi: 10.1038/nri2490. [DOI] [PubMed] [Google Scholar]
- Rosenman SJ, et al. Cytokine-induced expression of vascular cell adhesion molecule-1 (VCAM-1) by astrocytes and astrocytoma cell lines. J Immunol. 1995;154:1888–99. [PubMed] [Google Scholar]
- Rothhammer V, et al. Th17 lymphocytes traffic to the central nervous system independently of alpha4 integrin expression during EAE. J Exp Med. 2011;208:2465–76. doi: 10.1084/jem.20110434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Perez JM, Morillas-Ruiz JM. A review: inflammatory process in Alzheimer’s disease, role of cytokines. Scientific World Journal. 2012;2012:756357. doi: 10.1100/2012/756357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikali P, et al. Contribution of astrocyte-derived IL-15 to CD8 T cell effector functions in multiple sclerosis. J Immunol. 2010;185:5693–703. doi: 10.4049/jimmunol.1002188. [DOI] [PubMed] [Google Scholar]
- Seki Y, et al. Inhibition of ischemia-induced glutamate release in rat striatum by dihydrokinate and an anion channel blocker. Stroke. 1999;30:433–40. doi: 10.1161/01.str.30.2.433. [DOI] [PubMed] [Google Scholar]
- Shen Y, et al. Upregulation of mesencephalic astrocyte-derived neurotrophic factor in glial cells is associated with ischemia-induced glial activation. J Neuroinflammation. 2012;9:254. doi: 10.1186/1742-2094-9-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichita T, et al. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15:946–50. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- Shrikant P, et al. Regulation of intercellular adhesion molecule-1 gene expression by tumor necrosis factor-alpha, interleukin-1 beta, and interferon-gamma in astrocytes. J Neuroimmunol. 1994;51:209–20. doi: 10.1016/0165-5728(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Smorodchenko A, et al. CNS-irrelevant T-cells enter the brain, cause blood-brain barrier disruption but no glial pathology. Eur J Neurosci. 2007;26:1387–98. doi: 10.1111/j.1460-9568.2007.05792.x. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soos JM, et al. Differential expression of B7 co-stimulatory molecules by astrocytes correlates with T cell activation and cytokine production. Int Immunol. 1999;11:1169–79. doi: 10.1093/intimm/11.7.1169. [DOI] [PubMed] [Google Scholar]
- Soria FN, et al. Extrasynaptic glutamate release through cystine/glutamate antiporter contributes to ischemic damage. J Clin Invest. 2014;124:3645–55. doi: 10.1172/JCI71886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starbeck-Miller GR, Xue HH, Harty JT. IL-12 and type I interferon prolong the division of activated CD8 T cells by maintaining high-affinity IL-2 signaling in vivo. J Exp Med. 2014;211:105–20. doi: 10.1084/jem.20130901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steelman AJ, et al. Galectin-9 protein is up-regulated in astrocytes by tumor necrosis factor and promotes encephalitogenic T-cell apoptosis. J Biol Chem. 2013;288:23776–87. doi: 10.1074/jbc.M113.451658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suidan GL, Pirko I, Johnson AJ. A potential role for CD8+ T-cells as regulators of CNS vascular permeability. Neurol Res. 2006;28:250–5. doi: 10.1179/016164106X98116. [DOI] [PubMed] [Google Scholar]
- Sutton CE, et al. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–41. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Szymocha R, et al. Human T-cell lymphotropic virus type 1-infected T lymphocytes impair catabolism and uptake of glutamate by astrocytes via Tax-1 and tumor necrosis factor alpha. J Virol. 2000;74:6433–41. doi: 10.1128/jvi.74.14.6433-6441.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, et al. Presentation of proteolipid protein epitopes and B7-1-dependent activation of encephalitogenic T cells by IFN-gamma-activated SJL/J astrocytes. J Immunol. 1998;160:4271–9. [PubMed] [Google Scholar]
- Tavazoie M, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–88. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson EC. Focus issue: Structure and function of lymphoid tissues. Trends Immunol. 2012;33:255. doi: 10.1016/j.it.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Togo T, et al. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J Neuroimmunol. 2002;124:83–92. doi: 10.1016/s0165-5728(01)00496-9. [DOI] [PubMed] [Google Scholar]
- Trajkovic V, et al. Interleukin-17 stimulates inducible nitric oxide synthase activation in rodent astrocytes. J Neuroimmunol. 2001;119:183–91. doi: 10.1016/s0165-5728(01)00391-5. [DOI] [PubMed] [Google Scholar]
- Trajkovic V, et al. Astrocyte-induced regulatory T cells mitigate CNS autoimmunity. Glia. 2004;47:168–79. doi: 10.1002/glia.20046. [DOI] [PubMed] [Google Scholar]
- Trieb K, et al. APP peptides stimulate lymphocyte proliferation in normals, but not in patients with Alzheimer’s disease. Neurobiol Aging. 1996;17:541–7. doi: 10.1016/0197-4580(96)00068-1. [DOI] [PubMed] [Google Scholar]
- Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardjan N, et al. IFN-gamma-induced increase in the mobility of MHC class II compartments in astrocytes depends on intermediate filaments. J Neuroinflammation. 2012;9:144. doi: 10.1186/1742-2094-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, et al. Astrocytes in Alzheimer’s disease. Neurotherapeutics. 2010;7:399–412. doi: 10.1016/j.nurt.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. Astrocytic Fas ligand expression is required to induce T-cell apoptosis and recovery from experimental autoimmune encephalomyelitis. Eur J Immunol. 2013;43:115–24. doi: 10.1002/eji.201242679. [DOI] [PubMed] [Google Scholar]
- Weiner HL, Frenkel D. Immunology and immunotherapy of Alzheimer’s disease. Nat Rev Immunol. 2006;6:404–16. doi: 10.1038/nri1843. [DOI] [PubMed] [Google Scholar]
- Weiss HA, Millward JM, Owens T. CD8+ T cells in inflammatory demyelinating disease. J Neuroimmunol. 2007;191:79–85. doi: 10.1016/j.jneuroim.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Wilson EH, Weninger W, Hunter CA. Trafficking of immune cells in the central nervous system. J Clin Invest. 2010;120:1368–79. doi: 10.1172/JCI41911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler MK, Beveniste EN. Transforming growth factor-beta inhibition of cytokine-induced vascular cell adhesion molecule-1 expression in human astrocytes. Glia. 1998;22:171–9. doi: 10.1002/(sici)1098-1136(199802)22:2<171::aid-glia8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Wong GH, et al. Inducible expression of H-2 and Ia antigens on brain cells. Nature. 1984;310:688–91. doi: 10.1038/310688a0. [DOI] [PubMed] [Google Scholar]
- Xanthos DN, Sandkuhler J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci. 2014;15:43–53. doi: 10.1038/nrn3617. [DOI] [PubMed] [Google Scholar]
- Xie L, et al. Cerebral regulatory T cells restrain microglia/macrophage-mediated inflammatory responses via IL-10. Eur J Immunol. 2014 doi: 10.1002/eji.201444823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Li M, Jiang Y. The paradox role of regulatory T cells in ischemic stroke. Scientific World Journal. 2013;2013:174373. doi: 10.1155/2013/174373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JF, et al. Characterization of the interaction between astrocytes and encephalitogenic lymphocytes during the development of experimental autoimmune encephalitomyelitis (EAE) in mice. Clin Exp Immunol. 2012a;170:254–65. doi: 10.1111/j.1365-2249.2012.04661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, et al. Excessive astrocyte-derived neurotrophin-3 contributes to the abnormal neuronal dendritic development in a mouse model of fragile X syndrome. PLoS Genet. 2012b;8:e1003172. doi: 10.1371/journal.pgen.1003172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H, et al. IL-17A induces MIP-1alpha expression in primary astrocytes via Src/MAPK/PI3K/NF-kB pathways: implications for multiple sclerosis. J Neuroimmune Pharmacol. 2014;9:629–41. doi: 10.1007/s11481-014-9553-1. [DOI] [PubMed] [Google Scholar]
- Yong VW, et al. Gamma-interferon promotes proliferation of adult human astrocytes in vitro and reactive gliosis in the adult mouse brain in vivo. Proc Natl Acad Sci U S A. 1991;88:7016–20. doi: 10.1073/pnas.88.16.7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeinstra E, Wilczak N, De Keyser J. Reactive astrocytes in chronic active lesions of multiple sclerosis express co-stimulatory molecules B7-1 and B7-2. J Neuroimmunol. 2003;135:166–71. doi: 10.1016/s0165-5728(02)00462-9. [DOI] [PubMed] [Google Scholar]
- Zeinstra E, et al. Simvastatin inhibits interferon-gamma-induced MHC class II up-regulation in cultured astrocytes. J Neuroinflammation. 2006;3:16. doi: 10.1186/1742-2094-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bevan MJ. CD8(+) T cells: foot soldiers of the immune system. Immunity. 2011;35:161–8. doi: 10.1016/j.immuni.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr Opin Neurobiol. 2010;20:588–94. doi: 10.1016/j.conb.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Zhou Y, et al. IL-9 promotes Th17 cell migration into the central nervous system via CC chemokine ligand-20 produced by astrocytes. J Immunol. 2011;186:4415–21. doi: 10.4049/jimmunol.1003307. [DOI] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–89. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]