Abstract
PURPOSE
To evaluate whether quantity of choroidal tissue directly adjacent to the optic nerve differs between eyes with and without glaucoma and whether beta-zone parapapillary atrophy influences this relationship.
DESIGN
Prospective cohort study.
METHODS
Subjects were enrolled in a longitudinal, observational study at our institution. We studied one eye of 63 primary open-angle glaucoma (POAG), 30 ocular hypertension (OH), and 48 control subjects. Using optical coherence tomography enhanced depth imaging, we acquired twelve radial scans centered on the optic nerve head with 15 degrees of separation between scans. After images were enhanced, segmented, and corrected for ocular magnification, juxtapapillary choroidal volumetric parameters were calculated using raw thickness measurements and standard interpolation techniques. Juxtapapillary choroidal volume was then compared by diagnosis and by beta-zone parapapillary atrophy status.
RESULTS
Total juxtapapillary choroidal volume was significantly reduced in POAG versus OH and control eyes (1.057 vs. 1.228 vs 1.255 μl, p=0.04) and it was reduced in eyes with versus without beta-zone parapapillary atrophy (1.076 μl, n=80 vs. 1.306 μl, n=61, p<0.001). Juxtapapillary choroidal volume did not differ between POAG, OH, and control eyes when beta-zone parapapillary atrophy was absent, but juxtapapillary choroidal volume was significantly reduced in POAG versus control eyes when beta-zone parapapillary atrophy was present (0.957 vs. 1.196 μl, p=0.02).. Furthermore, POAG eyes with beta-zone parapapillary atrophy had substantially lower juxtapapillary choroidal volume compared to POAG eyes without beta-zone parapapillary atrophy (0.957 vs. 1.356 μl, p<0.001).
CONCLUSIONS
The volume of choroid adjacent to the optic nerve was significantly reduced in POAG eyes when beta-zone parapapillary atrophy was present, suggesting that beta-zone parapapillary atrophy may be a biomarker for juxtapapillary choroidal atrophy and associated vascular compromise in POAG.
Introduction
A growing body of evidence suggests vascular perfusion abnormality is a frequent contributor to glaucoma pathophysiology. Specifically, several large epidemiologic studies have identified reduced ocular perfusion as a risk factor for glaucoma prevalence,1 incidence,2 and progression.3 Glaucoma is more common in patients with co-morbid systemic vascular disorders3–5 and several authors have shown, using a variety of techniques, that eyes with glaucoma commonly exhibit reduced blood flow compared to eyes without glaucoma.6,7 Despite these reports, the specific underlying anatomic and physiologic factors that explicitly contribute to or result in vascular compromise and ultimately optic nerve damage remain unclear.
The primary blood supply for the prelaminar and in some eyes the laminar portions of the optic nerve is principally provided by vessels that arise in the region of the choroid that lies immediately adjacent to the optic nerve,6 leading to hypotheses that disturbance of choroidal blood flow could contribute to glaucomatous optic neuropathy.6,8,9 The development of spectral domain optical coherence tomography (SD-OCT) with enhanced depth imaging has facilitated greater resolution of deeper structures of the posterior segment, 10,11 leading to many recent studies of choroidal thickness in various ocular disease processes.12–14 In studies utilizing SD-OCT to investigate choroidal thickness in glaucoma, results have been mixed with some reports finding no difference in choroidal thickness between glaucomatous and normal eyes15–21 and others reporting reduced choroidal thickness in glaucomatous eyes.22–26 It is important to note, however, that none of these investigations systematically measured choroidal thickness in the region directly adjacent to the optic nerve. Rather, choroidal thickness was generally quantified either subfoveally or beneath the circular circumpapillary ring that is typically used for retinal nerve fiber layer measurement. Considering that the region of the choroid that provides blood supply to the optic nerve lies directly adjacent to the optic nerve,6 it is plausible that investigation of this region might provide more specific and direct evidence regarding the relationship between choroidal thickness and glaucoma.
This study was therefore designed to use SD-OCT to image the region of the choroid that lies directly adjacent to the optic nerve, an area that might best be termed juxtapapillary.27 By measuring choroidal thickness in this region and then using linear interpolation techniques to develop total and sectoral volumetric parameters from these measures, we planned to compare juxtapapillary choroidal volume parameters in eyes with and without glaucoma. Additionally, because beta-zone parapapillary atrophy has been linked to both glaucoma28–31 and reduced choroidal volume,32,33 we planned to use SD-OCT to identify and quantify beta-zone parapapillary atrophy34–36 so that we could investigate inter-relationships between beta-zone parapapillary atrophy, juxtapapillary choroidal volume, and glaucoma.
Methods
This investigation was a prospective cohort study, using subjects enrolled in a longitudinal, observational glaucoma research study at the Albuquerque Veterans Affairs Medical Center. The study adhered to the tenets of the Declaration of Helsinki, conformed to HIPAA regulations, was approved by the University of New Mexico IRB, and all subjects completed informed consent prior to participation. Enrollment criteria were: age ≥ 40 years; open, normal angles in each eye on gonioscopic examination; no corneal pathology that could affect intraocular pressure (IOP) measurement; refractive error ≤ 5 diopters and astigmatism ≤ 3 diopters; no prior refractive, corneal, or incisional glaucoma surgery; no secondary glaucoma diagnoses; no significant retinal disorders; and no visual field loss due to non-glaucomatous pathology (including retinal, optic nerve or visual pathway disorders). Subjects with uncomplicated cataract surgery at least three months prior to SD-OCT choroidal imaging were eligible.
Study Protocol
All subjects had comprehensive examinations including Goldmann applanation tonometry, axial length (AL) and corneal curvature measurement (IOL Master, Carl Zeiss Meditec Inc., Dublin, CA), and central corneal thickness (CCT) measurement using ultrasound pachymetry. Slit lamp biomicroscopy, gonioscopy, standard automated perimetry (SAP), dilated fundus examination with indirect ophthalmoscopy, and SD-OCT imaging (Spectralis, Heidelberg Engineering, Heidelberg, Germany) were also performed. On the date that choroidal images were acquired for this investigation, we also measured systolic (SBP) and diastolic (DBP) blood pressure and gathered current data from the medical record for the following items: body mass index (BMI), cigarette smoking status, glycosylated hemoglobin (A1C), concurrent use of systemic hypertension medications, concurrent use of systemic lipid-lowering medications, and concurrent systemic health conditions that might impact optic nerve head perfusion (including diabetes mellitus, hypertension, hyperlipidemia, congestive heart failure, coronary artery disease, sleep apnea, chronic kidney disease, and asthma/chronic obstructive pulmonary disease). All SAP testing was performed with optimal near-point correction using the Humphrey Visual Field Analyzer II, 24-2 SITA-standard program (Carl Zeiss Meditec, Inc., Dublin, CA). Visual fields were required to meet reliability criteria (false positives and false negatives < 15%; fixation losses < 33% unless gaze-tracking demonstrated steady fixation in which case fixation was deemed acceptable).
For this investigation, we identified all subjects from our longitudinal study database who had undergone choroidal imaging and also met the diagnostic criteria for glaucoma (POAG), ocular hypertension (OH) or control. POAG eyes were characterized by clinical findings consistent with glaucomatous optic neuropathy (e.g. thinning, excavation, rim erosion or notch of the neuroretinal rim) in conjunction with glaucomatous visual field defects that were reproducible on at least three consecutive examinations. Minimum criteria for glaucomatous visual field defect included glaucoma hemifield test (GHT) result outside normal limits and/or the presence of at least three contiguous test points on the pattern deviation plot with P < 1% and at least one at P < 0.05%, not including points on the edge of the field. Intraocular pressure (IOP) was not used as a diagnostic criterion for POAG. OH subjects had IOP > 21 mm Hg on at least one occasion but did not have definitive glaucomatous optic neuropathy or visual field loss. Control subjects had normal optic nerves, visual fields and all IOP measurements < 22mm Hg.
Spectral-doman optical coherence tomography choroid data
In 2012, we began to acquire SD-OCT imaging of the optic nerve head and imaged all study subjects consecutively. With the enhanced depth imaging mode, we acquired twelve 20-degree radial scans centered on the optic nerve head with each scan separated by 15 degrees as shown in Figure 1. This pattern was selected in part so that we could obtain choroidal thickness measurements directly adjacent to the optic nerve.
Figure 1.
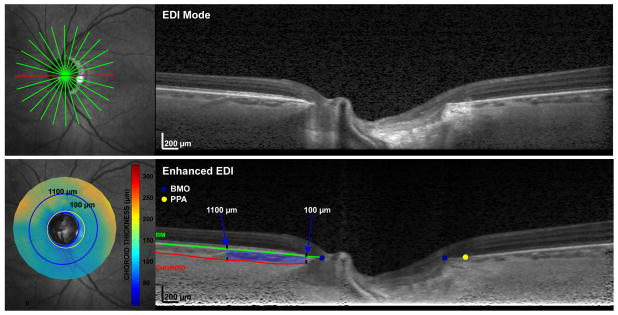
Optical coherence tomography enhanced depth imaging strategy for measuring juxtapapillary choroidal volume and beta-zone parapapillary atrophy in eyes with and without primary open-angle glaucoma
1 Top Left: Infrared-scanning laser ophthalmoscopy (IR-SLO) image with 12 superimposed radial scans centered on the optic nerve head
2 Top Right: Raw SD-OCT/enhanced depth image of one of the 12 radial b-scans
3 Bottom Left: Juxtapapillary choroidal thickness map; The inner blue ring corresponds to a location that is 100 μm distal to Bruch’s membrane opening; the outer blue ring corresponds to a location that is 1100 μm distal to Bruch’s membrane opening; the yellow ring corresponds to the outermost boundary of β-zone parapapillary atrophy
4 Bottom Right: Enhanced SD-OCT/enhanced depth image; blue dots correspond to Bruch’s membrane opening; yellow dot corresponds to boundary of beta-zone parapapillary atrophy (RPE no longer covers Bruch’s membrane); red line denotes choroid-sclera border and green line denotes Bruch’s membrane; the choroidal area located between 100-1100 μm and between the sclera and Bruch’s membrane was quantified on each side of the optic nerve head for each b-scan and then interpolated to create volumetric parameters for statistical analysis
We excluded scans if the image series was significantly de-centered, the b-scan was cut-off because the image was too low, if tissue resolution was poor, or if the choroid/sclera borders were not visible after image enhancement. Scan series were also excluded if choroidal segmentation could not be achieved in at least 75% of the scan length of any b-scan or if two adjacent scans within a regional sector could not be adequately segmented as this would be expected to degrade the validity of sectoral choroidal volume measurements. We also excluded eyes that exhibited parapapillary atrophy characterized on SD-OCT by bare sclera without overlying Bruch’s membrane (termed gamma-zone), since this form of atrophy is associated with myopia but not glaucoma.33,34 One eye was randomly chosen for analysis if both eyes met eligibility criteria.
After acquisition, raw (*.vol) data were exported and analyzed using custom Matlab algorithms, where the scans were adjusted for lateral magnification based on axial length and corneal curvature.37 This adjustment was deemed important to avoid the confounding effects of magnification error on choroidal thickness measurements. Scans were then enhanced using a previously published algorithm in an effort to optimize the contrast between the choroid and sclera as exhibited in Figure 1.38 To obtain choroidal thickness measurements for each of the b-scans, the instrument-derived segmentation of Bruch’s membrane was evaluated and manually corrected as necessary by a trained observer (NP), who also manually delineated the choroid-sclera border. After Bruch’s membrane opening (BMO) was marked, choroidal thickness was quantified in the region of the b-scan from 100 μm to 1100μm from the BMO. To reduce bias, images were randomly represented, and diagnostic data were unavailable during segmentation. Choroidal volumetric parameters were calculated using raw thickness measurements and standard interpolation techniques. Global and sectoral (superior, temporal, inferior, nasal) volumes were computed for use in statistical analyses.
With the aid of the corresponding infrared-scanning laser ophthalmoscope images, a trained observer also manually marked the beta-zone parapapillary atrophy boundary on each b-scan image where the retinal pigment epithelium (RPE) dissipated.36 The manually marked locations for the BMO and beta-zone parapapillary atrophy were then fit with a best-fit ellipse, and beta-zone parapapillary atrophy was quantified as the thickness and area differences between the BMO and beta-zone parapapillary atrophy boundary. All measures incorporated scaling using a three surface schematic eye, to minimize effects of ocular magnification. Eyes were classified as beta-zone parapapillary atrophy negative if RPE ended at the BMO in all b-scan images, and beta-zone parapapillary atrophy positive if RPE ended before the BMO in any of the b-scan images.
Statistical Analysis
Parametric and non-parametric tests were employed to compare parameters between diagnostic groups based on the normality of the parameter distributions. Specifically, Kruskal-Wallis tests were used to compare non-normally distributed variables between diagnostic groups, followed by Student-Newman-Keuls testing for post-hoc comparison between groups as needed. Univariate and multivariate regression analyses were used to investigate relationships between choroidal volume variables and various clinical parameters. Statistical significance was defined as p < 0.05, and all statistical analyses were performed in conjunction with a professional biostatistician (CQ) using SAS (SAS Institute Inc., Cary NC, Version 9.2) and MedCalc (MedCalc, Ostend, Belgium, Version 12.4.0.0).
Results
One hundred seventy-two subjects were consecutively imaged, and 141 (82%) had at least one eye that met all image quality criteria. Of those included, 63 (45%), 30 (21%) and 48 (34%) eyes were diagnosed POAG, OH, and control respectively. Of the 31 excluded subjects in which global or sectoral juxtapapillary choroidal volume could not be reliably measured, 14 were diagnosed POAG, 12 were diagnosed OH, and 5 were controls representing 18%, 29%, and 10% of each diagnostic group. Only one eye (diagnosed POAG) was excluded because its parapapillary atrophy was characterized by gamma zone.
Subject characteristics, stratified by diagnostic category, are listed in Table 1. Compared to the OH and control groups, the POAG group had higher age, lower systolic and diastolic blood pressure, thinner RNFL, and worse visual field sensitivity (lower mean defect and higher pattern standard deviation). Conversely, the OH group had higher IOP, systolic and diastolic blood pressure, and CCT compared to the control and POAG groups. The control group had significantly shorter AL than the other two groups, thicker CCT and lower IOP than the POAG group, and thicker RNFL than the OH group. Of note, IOP results were impacted by treatment as 86%, 23% and 0% of POAG, OH, and control subjects were using topical IOP-lowering agents. Beta-zone parapapillary atrophy was disproportionately present being significantly more common and extensive in POAG compared to the other two groups. See Table 1.
Table 1.
Juxtapapillary choroidal volume and beta-zone parapapillary atrophy in eyes with and without primary open-angle glaucoma: comparison of systemic and ocular parameters by diagnostic category
| POAG (n=63) | OH (n=30) | Control (n=48) | |||||
|---|---|---|---|---|---|---|---|
| Mean ±SD 95% CI |
Median (IQR) | Mean ±SD 95% CI |
Median (IQR) | Mean ±SD 95% CI |
Median (IQR) | p-value | |
| Age (yrs) | 67.2 ± 8.2 (54.1,82.9) | 66.0 (61.3,73.8) | 61.9 ± 7.1 (52.0,78.0) | 61.9 (57.0,66.0) | 62.8 ± 9.2 (45.2,86.0) | 62.0 (58.5,64.5) | 0.001 1 |
| BMI | 28.9 ± 5.4 (19.6,39.6) | -- | 29.8 ± 5.7 (19.9,40.7) | -- | 28.8 ± 4.7 (20.8,39.5) | -- | 0.69 |
| SBP (mm Hg) | 127.7 ± 13.5 (105.1,150.0) | -- | 135.3 ± 13.7 (106.6,171.0) | -- | 131.5 ± 12.9 (109.1,157.1) | -- | 0.04 2 |
| DBP (mm Hg) | 76.1 ± 6.9 (65.1,89.9) | -- | 80.6 ± 5.3 (70.0,90.0) | -- | 78.8 ± 6.7 (66.1,91.5) | -- | 0.006 3 |
| A1C (%) | 6.24 ± 0.87 (5.21, 8.10) | 5.90 (5.70,6.50) | 6.15 ± 0.93 (4.93, 8.18) | 5.90 (5.60,6.40) | 6.26 ± 0.97 (5.37, 9.22) | 6.00 (5.70,6.35) | 0.90 |
| AL (mm) | 24.41 ± 0.96 (22.10, 26.55) | 24.31 (23.76,24.92) | 24.31 ± 1.00 (22.65, 27.27) | 24.13 (23.80,24.80) | 23.77 ± 1.12 (21.31, 26.32) | 23.78 (23.22,24.27) | 0.004 4 |
| CCT (μm) | 543.7 ± 38.0 (480.2,638.2) | -- | 563.6 ± 35.9 (487.8,625.3) | 552.8 ± 32.1 (489.2,619.0) | 0.04 5 | ||
| GAT-IOP (mm Hg) | 15.5 ± 4.2 (7.0, 24.5) | 18.8 ± 4.4 (11.0, 30.8) | 14.5 ± 2.8 (8.0, 19.5) | <0.001 6 | |||
| MD (db) | −4.53 ± 4.07 (−15.50, 0.25) | −3.49 (−6.84, −1.73) | 0.22 ± 1.13 (−2.38, 2.51) | 0.37 (−0.61,0.95) | 0.09 ± 0.96 (−2.36, 1.71) | 0.12 (−0.54,0.71) | <0.001 3 |
| PSD (db) | 5.36 ± 3.61 (1.80, 13.99) | 3.77 (2.77,7.50) | 1.62 ± 0.41 (1.03, 2.70) | 1.50 (1.35,1.84) | 1.71 ± 0.46 (1.20, 3.03) | 1.58 (1.44,1.77) | <0.001 1 |
| RNFL (μm) | 66.0 ± 14.1 (39.1, 88.8) | 68.0 (57.0,74.8) | 88.9 ± 11.7 (66.0, 112.0) | 90.5 (80.0,95.0) | 97.7 ± 10.7 (75.7, 117.2) | 99.5 (91.5,104.5) | <0.001 7 |
| β-PPA presence (n, %) | 48/63 (76.2%) | 14/30 (46.7%) | 18/48 (37.5%) | <0.001 1 | |||
| β-PPA Area (mm) | 1.09 ± 0.59 (0.40, 2.72) | 0.96 (0.64,1.35) | 0.75 ± 0.47 (0.13,2,23) | 0.61 (0.41, 0.96) | 0.63 ± 0.43 (0.74, 1.91) | 0.53 (0.41, 0.73) | <0.001 1 |
POAG: primary open-angle glaucoma; OH: ocular hypertension; BMI: body mass index; SBP: systolic blood pressure; DBP diastolic blood pressure; A1C: glycosylated hemoglobin; AL: axial length; CCT: central corneal thickness; GAT-IOP: intraocular pressure by Goldmann applanation tonometry; MD: mean defect; PSD: pattern standard deviation; RNFL: global retinal nerve fiber layer thickness; β-PPA: beta-zone parapapillary atrophy Mean value ± standard deviation with 95% confidence intervals presented for normally distributed variables Median value with interquartile range (IQR) also presented for non-normally distributed variables
P-value derived from One-way analysis of variance in normally distributed variables and Kruskal-Wallis test in non-normally distributed variables; sub-group comparisons done using post-hoc pair-wise comparisons (Student-Newman-Keuls test)
Glaucoma>Control and Ocular Hypertension
Glaucoma<Ocular Hypertension
Glaucoma<Ocular Hypertension and Control
Control <Glaucoma and Ocular Hypertension
Ocular hypertension >Glaucoma
Ocular hypertension>Glaucoma and Control
Control >Ocular hypertension>Glaucoma
Descriptive data for total and sectoral juxtapapillary choroidal volume parameters, stratified by diagnosis, are shown in Table 2. The POAG group demonstrated significantly smaller total, inferior, and superior juxtapapillary choroidal volume compared to the OH and control groups. Juxtapapillary choroidal volume demonstrated wide variability (3 to 4 fold difference between high and low ends of the range) and significant overlap between diagnostic groups. Total juxtapapillary choroidal volume was weakly correlated with RNFL (p=0.02, r2=0.05) and mean defect (p=0.09, r2=0.03).
Table 2.
Comparison of global and sectoral juxtapapillary choroidal volume parameters in eyes with and without primary open-angle glaucoma
| Glaucoma (n=63) | Ocular Hypertension (n=30) | Control (n=48) | ||
|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | P-value | |
| Total JCV | 1.057 (0.878, 1.263) | 1.228 (0.890, 1.524) | 1.255 (1.040, 1.381) | 0.04 1 |
| Inferior JCV | 0.220 (0.188, 0.288) | 0.300 (0.216, 0.364) | 0.287 (0.230, 0.362) | 0.001 2 |
| Nasal JCV | 0.285 (0.242, 0.353) | 0.342 (0.245, 0.383) | 0.337 (0.258, 0.392) | 0.12 |
| Superior JCV | 0.287 (0.233, 0.352) | 0.335 (0.249, 0.386) | 0.332 (0.286, 0.393) | 0.04 1 |
| Temporal JCV | 0.265 (0.208, 0.335) | 0.314 (0.238, 0.361) | 0.297 (0.242, 0.355) | 0.19 |
JCV: juxtapapillary choroidal volume; all CV units are in cubic microliters (μl); data presented as median and interquartile range (IQR)
Glaucoma < Control
Glaucoma < Ocular Hypertension and Control
p-value determined by Wilcoxon test for paired samples that are non-normally distributed; sub-group comparisons done using post-hoc pair-wise comparisons (Student-Newman-Keuls test)
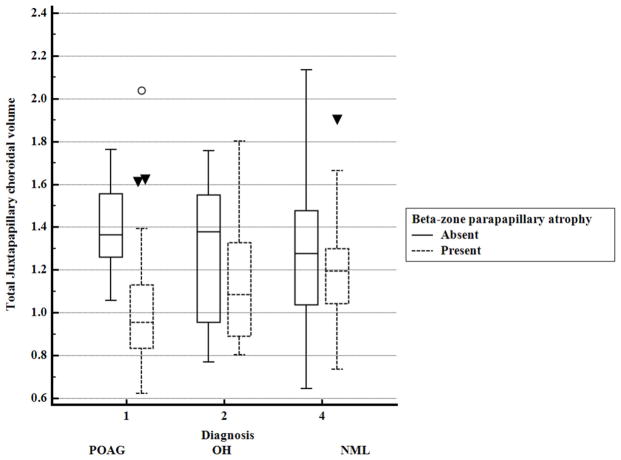
Eyes with beta-zone parapapillary atrophy (n=80) had significantly smaller (p<0.0001) total and sectoral juxtapapillary choroidal volume measures compared to eyes without beta-zone parapapillary atrophy (n=61). This relationship was primarily driven by POAG eyes as shown in Table 3. When eyes with beta-zone parapapillary atrophy were compared by diagnostic group, total, superior, and inferior juxtapapillary choroidal volume were reduced in POAG compared to control eyes and inferior juxtapapillary choroidal volume was also significantly lower in POAG versus OH eyes as shown in Table 4. Conversely, in eyes without beta-zone parapapillary atrophy, no differences in juxtapapillary choroidal volume were present between diagnostic groups as shown in Figure 2.
Table 3.
Global and sectoral juxtapapillary choroidal volume: comparisons between subjects with and without beta-zone parapapillary atrophy stratified by eyes with and without primary open-angle glaucoma
| POAG | OH | CONTROL | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β-PPA present (n=48) | β-PPA absent (n=15) | p-value | β-PPA present (n=14) | β-PPA absent (n=16) | p-value | β-PPA present (n=18) | β-PPA absent (n=30) | p-value | |
| Total JCV | 0.957 (0.835,1.131) | 1.365 (1.259,1.557) | <0.001 | 1.086 (0.890,1.327) | 1.378 (0.955,1.550) | 0.27 | 1.196 (1.043,1.299) | 1.277 (1.038,1.478) | 0.48 |
| Inferior JCV | 0.215 (0.179,0.249) | 0.300 (0.269,0.366) | <0.001 | 0.285 (0.216,0.329) | 0.313 (0.219,0.385) | 0.35 | 0.264 (0.226,0.337) | 0.320 (0.253,0.364) | 0.38 |
| Nasal JCV | 0.249 (0.230,0.306) | 0.366 (0.337,0.418) | <0.001 | 0.281 (0.231,0.362) | 0.373 (0.264,0.430) | 0.14 | 0.332 (0.225,0.368) | 0.337 (0.260,0.400) | 0.58 |
| Superior JCV | 0.260 (0.217.0.315) | 0.372 (0.354,0.421) | <0.001 | 0.284 (0.238,0.365) | 0.353 (0.278,0.420) | 0.33 | 0.324 (0.268,0.355) | 0.358 ((0.287,0.412) | 0.30 |
| Temporal JCV | 0.242 (0.204,0.291) | 0.389 (0.327,0.406) | <0.001 | 0.245 (0.219,0.356) | 0.325 (0.263,0.370) | 0.54 | 0.273 (0.243,0.318) | 0.308 (0.238,0.369) | 0.35 |
JCV: juxtapapillary choroidal volume; all JCV units are in cubic microliters (μl); data presented as median (IQR)
POAG: primary open-angle glaucoma; OH: ocular hypertension; β-PPA: beta-zone parapapillary atrophy
P-value derived from Kruskal-Wallis test
Table 4.
Global and sectoral juxtapapillary choroidal volume: comparisons between diagnostic groups in the presence or absence of beta-zone parapapillary atrophy
| β-PPA present | β-PPA absent | |||||||
|---|---|---|---|---|---|---|---|---|
| POAG (n=48) | OH (n=15) | CONTROL (n=18) | p-value | POAG (n=15) | OH (n=16) | CONTROL (n=30) | p-value | |
| Total JCV | 0.957 (0.835,1.131) | 1.086 (0.890,1.327) | 1.196 (1.043,1.299) | 0.031 | 1.365 (1.259,1.557) | 1.378 (0.955,1.550) | 1.277 (1.038,1.478) | 0.37 |
| Inferior JCV | 0.215 (0.179,0.249) | 0.285 (0.216,0.329) | 0.264 (0.226,0.337) | 0.0042 | 0.300 (0.269,0.366) | 0.313 (0.219,0.385) | 0.320 (0.253,0.364) | 0.97 |
| Nasal JCV | 0.249 (0.230,0.306) | 0.281 (0.231,0.362) | 0.332 (0.225,0.368) | 0.11 | 0.366 (0.337,0.418) | 0.373 (0.264,0.430) | 0.337 (0.260,0.400) | 0.43 |
| Superior JCV | 0.260 (0.217.0.315) | 0.284 (0.238,0.365) | 0.324 (0.268,0.355) | 0.021 | 0.372 (0.354,0.421) | 0.353 (0.278,0.420) | 0.358 ((0.287,0.412) | 0.46 |
| Temporal JCV | 0.242 (0.204,0.291) | 0.245 (0.219,0.356) | 0.273 (0.243,0.318) | 0.13 | 0.389 (0.327,0.406) | 0.325 (0.263,0.370) | 0.308 (0.238,0.369) | 0.14 |
JCV: juxtapapillary choroidal volume; all JCV units are in cubic microliters (μl); data presented as median (IQR)
β-PPA: beta-zone parapapillary atrophy; POAG: primary open-angle glaucoma; OH: ocular hypertension
P-value derived from Kruskal-Wallis test
POAG < CONTROL
POAG < OH and CONTROL
Figure 2.
Comparison of juxtapapillary choroidal volume by presence or absence of beta-zone parapapillary atrophy in eyes with and without primary open-angle glaucoma
POAG: Primary open-angle glaucoma; OH: ocular hypertension
Univariate regression analysis showed that total juxtapapillary choroidal volume was directly associated with POAG diagnosis, body-mass index, systolic blood pressure, and RNFL while being inversely associated with age, presence, and extent of beta-zone parapapillary atrophy. In multivariate analysis, presence and extent of beta-zone parapapillary atrophy along with body-mass index were the only factors independently associated with total juxtapapillary choroidal volume as shown in Table 5. Results from regression analyses using sectoral parameters were very similar to results for total juxtapapillary choroidal volume. In multivariate analyses using presence and extent of beta-zone parapapillary atrophy as dependent variables, POAG diagnosis and decreasing juxtapapillary choroidal volume were independently related to both variables while extent of beta-zone parapapillary atrophy was also independently related to age and axial length.
Table 5.
Linear Regression Analysis of factors associated with total juxtapapillary choroidal volume in eyes with and without primary open-angle glaucoma
| UNIVARIATE ANALYSIS | MULTIVARIATE ANALYSIS | |||
|---|---|---|---|---|
| Factor | β-coefficient (SE) | p-value | β-coefficient (SE) | p-value |
| CLINICAL DIAGNOSIS | −0.21 (0.00539) | 0.026 | ||
| AGE | −0.31 (0.00305) | <0.001 | ||
| BMI | 0.19 (0.00520) | 0.016 | 0.21 (0.00471) | 0.006 |
| SBP | 0.16 (0.00201) | 0.049 | ||
| DBP | 0.08 (0.00406) | 0.083 | ||
| HbA1C | 0.02 (0.03039) | 0.833 | ||
| TIME OF SCAN | −0.16 (0.00010) | 0.056 | ||
| ACTIVE CIGARETTE SMOKER | 0.12 (0.07517) | 0.144 | ||
| CCT | −0.07 (0.00076) | 0.392 | ||
| GAT-IOP | 0.10 (0.00647) | 0.237 | ||
| MD | 0.16 (0.00755) | 0.070 | ||
| RNFL | 0.19 (0.00142) | 0.025 | ||
| β-PPA PRESENCE | −0.35 (0.05183) | <0.001 | −0.21 (0.05978) | 0.021 |
| β-PPA AREA | −0.44 (0.04475) | <0.001 | −0.31 (0.05401) | 0.001 |
POAG: primary open-angle glaucoma; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; A1C: glycosylated hemoglobin; SD-OCT: spectral domain optical coherence tomography; CCT: central corneal thickness; GAT-IOP: Intraocular pressure (IOP) by Goldmann applanation tonometry; MD: mean defect; RNFL: average retinal nerve fiber layer thickness; β-PPA: beta-zone parapapillary atrophy
Discussion
We utilized SD-OCT enhanced depth imaging to simultaneously measure both the volume of choroid and the area of beta-zone parapapillary atrophy directly surrounding the optic nerve in eyes with and without glaucoma. While we found that juxtapapillary choroidal volume was reduced in eyes with POAG compared to control and OH eyes, this result was dependent on the coexistence of beta-zone parapapillary atrophy. Specifically, juxtapapillary choroidal volume was not different between POAG, OH, and control eyes when beta-zone parapapillary atrophy was absent, but when beta-zone parapapillary atrophy was present, POAG eyes had significantly reduced juxtapapillary choroidal volume compared to the control group. Furthermore, POAG eyes with beta-zone parapapillary atrophy had 30% lower juxtapapillary choroidal volume compared to POAG eyes without beta-zone parapapillary atrophy. Finally, multivariate regression analyses identified both presence and extent of beta-zone parapapillary atrophy as being independently and inversely related to juxtapapillary choroidal volume. Taken together, these findings suggest a substantial inter-dependence between juxtapapillary choroidal volume, beta-zone parapapillary atrophy, and glaucoma in which vascular insufficiency appears to be the common link.
Given that vascular support for the prelaminar optic nerve originates in the portion of the choroid immediately adjacent to the optic nerve,6,7 that beta-zone parapapillary atrophy has been linked to vascular insufficiency,9 and that numerous prior studies point to vascular compromise as a contributor to glaucoma pathophysiology,1–9 we designed this study to specifically investigate the anatomy of the juxtapapillary region. While several clinico-pathologic studies of this region have been reported, we are aware of only one published study that used SD-OCT to investigate juxtapapillary choroid anatomy. In that study, Sigler et al22 obtained five horizontal SD-OCT raster scans through the optic nerve and then measured choroidal thickness in the nasal and temporal sectors. Consistent with the results in this study, the authors reported that juxtapapillary choroidal thickness was significantly reduced in glaucomatous versus control eyes. Results from clinico-pathologic studies have also shown that juxtapapillary choroidal thickness is reduced in eyes with glaucoma39 and in eyes with beta-zone parapapillary atrophy.31,32 Interestingly, thinning or loss of the choriocapillaris was identified as the primary reason for reduced choroidal thickness in these studies, suggesting compromised perfusion in these eyes. These results are also consistent with reports of deficient peripapillary choroidal filling in glaucomatous eyes during fluorescein angiography.40,41 Yet, while reduction of choroidal thickness appears to be a relatively consistent finding in studies of the juxtapapillary region, results from studies in which choroidal measurements were obtained from regions other than the juxtapapillary region have been mixed. Specifically, some studies of peripapillary and macular choroidal thickness report thinner choroid in glaucomatous versus non-glaucomatous eyes,22–26 while other studies fail to confirm this relationship.15–21 This collection of results suggests that measurement location may be important when evaluating the relationship between the choroid and glaucoma.
Juxtapapillary choroidal volume was independently related to both presence and extent of beta-zone parapapillary atrophy in this study. Expressly, eyes with beta-zone parapapillary atrophy had reduced juxtapapillary choroidal volume compared to eyes without beta-zone parapapillary atrophy, and increasing size of beta-zone parapapillary atrophy was associated with decreasing juxtapapillary choroidal volume. These findings, which were present in the full sample as well as within each diagnostic group, suggest that diminution of underlying choroidal tissue is a common feature of eyes with beta-zone parapapillary atrophy. The degree to which juxtapapillary choroidal volume is reduced in eyes with beta-zone parapapillary atrophy, however, appears to differ by diagnostic group, being most pronounced in POAG eyes. This finding suggests that POAG eyes with beta-zone parapapillary atrophy either have developmentally reduced juxtapapillary choroidal volume or a more advanced form of choroidal vascular atrophy compared to non-glaucomatous eyes with beta-zone parapapillary atrophy. In either case, it is plausible that reduced juxtapapillary choroidal volume might contribute to vascular insufficiency of the optic nerve head in POAG eyes. Alternatively, reduced juxtapapillary choroidal volume in glaucoma may be a result of the glaucomatous disease process, rather than a cause.
Results from a recent study by Roberts et al24 may also support a relationship between beta-zone parapapillary atrophy and choroidal thickness. In that study, choroidal thickness was measured under the standard circumpapillary RNFL thickness ring in glaucomatous eyes that were stratified according to optic disc phenotype and in normal eyes. While their results showed that choroidal thickness was modestly but significantly thinner in glaucomatous versus non-glaucomatous eyes, more interesting and possibly analogous to our results was that this finding was driven by the glaucomatous eyes in their study that were characterized by the senile sclerotic optic disc phenotype. Specifically, they reported that eyes with senile sclerotic discs had a 25 to 30% reduction in choroidal thickness compared to normal eyes. This finding is very similar to our finding that POAG eyes with beta-zone parapapillary atrophy had approximately 25% less juxtapapillary choroidal volume compared to our control eyes. Thus, considering that beta-zone parapapillary atrophy is a prominent feature of the senile sclerotic disc phenotype42 while being less commonly associated with normal eyes and other glaucoma disc phenotypes, the thinner choroid found in eyes with senile sclerotic discs appears to be consistent with the relationship between juxtapapillary choroidal volume, beta-zone parapapillary atrophy, and glaucoma that we found in this investigation. Moreover, these results contribute to the premise that beta-zone parapapillary atrophy may be a biomarker for juxtapapillary choroidal atrophy and associated vascular compromise in glaucomatous disease.
In multivariate analyses, extent and presence of beta-zone parapapillary atrophy were independently associated with juxtapapillary choroidal volume, diagnosis, age, and AL. POAG eyes in this study had a higher prevalence and magnitude of beta-zone parapapillary atrophy compared to the other diagnostic groups, a finding that is consistent with prior reports.27 Additionally, although we excluded eyes with high myopia and eyes with gamma-zone parapapillary atrophy,34 we found that longer AL was correlated with beta-zone parapapillary atrophy magnitude. This finding suggests that AL may also contribute to beta-zone parapapillary atrophy pathophysiology, even at lower magnitudes. Additionally, we found that beta-zone parapapillary atrophy magnitude increased with higher age, both in the full sample and within each diagnostic group. This finding agrees with studies identifying an age-related enlargement of beta-zone parapapillary atrophy that occurs in eyes with and without glaucoma.28 While the reason for age-related enlargement of beta-zone parapapillary atrophy is unclear, one study suggested that excessive catecholamine leakage from juxtapapillary choroidal vessels may result in development and enlargement of beta-zone parapapillary atrophy. Specifically, the authors suggest that excessive catecholamine leakage may lead to severe vasoconstriction of the regional peripapillary vasculature, with resultant atrophy that appears clinically as beta-zone parapapillary atrophy.43 A separate study showed that when catecholamine load is particularly extreme, an optic neuropathy that is morphologically similar to glaucoma can even result.44 Several studies have also reported relatively elevated levels of endothelin-1, a potent vascoconstrictor derived from vascular endothelial cells, in eyes with glaucomatous optic neuropathy.45 Considered together, these findings suggest that beta-zone parapapillary atrophy development and enlargement are influenced by several factors including genetic predisposition, aging, AL, vascular mediator effects, and POAG.46 The specific manner in which these factors interact is likely important in explaining the wide clinical variation associated with beta-zone parapapillary atrophy.
In an effort to identify specific systemic health factors that might be related to juxtapapillary choroidal volume, beta-zone parapapillary atrophy, and glaucoma, we gathered data for blood pressure, glycosylated hemoglobin (A1C), concurrent systemic vascular disease conditions, and systemic medication use. We did not, however, find any significant relationships between juxtapapillary choroidal volume, beta-zone parapapillary atrophy, glaucoma and any systemic disease parameter nor did we find clinically significant differences for these parameters between diagnostic groups. Mean A1C was relatively elevated across our diagnostic groups but because prior reports suggest that diabetic eyes without retinopathy have choroidal thickness that is similar to non-diabetic eyes,47,48 and we excluded eyes with diabetic retinopathy, it seems unlikely that A1C impacted our results. Because vascular health factors are dynamic characteristics, it may be that using static vascular disease markers to identify dynamic vascular risk factors is insufficient. Longitudinal study designs that better reflect dynamic data might improve efforts to identify underlyling systemic vascular health relationships between juxtapapillary choroidal volume, beta-zone parapapillary atrophy, and glaucoma.49
Strengths of this study include its use of an enhanced depth SD-OCT imaging technique to optimize measurement of the choroid directly adjacent to the optic nerve, as this is the region of the choroid most likely to be affected in glaucoma. Furthermore, choroidal thickness measurements were corrected for magnification error related to both corneal curvature and axial length, thus permitting more direct comparisons between eyes. The study also employed an image enhancement technique that improved visualization of the sclera-choroid junction which served to optimize accuracy of the choroidal thickness measurements. We also masked the investigator who provided SD-OCT image segmentation to prevent bias, and we included data previously reported to be correlated to choroidal thickness (e.g. age, body-mass index, time of measurement, smoking status) so that we could account for confounding effects of these factors in statistical analyses.
The primary limitations of this study are its cross-sectional design and its use of a convenience sample which was recruited from our hospital outpatient clinic and therefore may not be representative of the population at large. We also did not account for the magnification effects of the intrinsic focusing mechanism of the Spectralis® which may have affected our efforts to control for magnification error. Because we excluded subjects with high refractive error, our results may not apply to subjects with high myopia or hyperopia. We were also unable to adequately measure choroidal volume in 29% of our OH eyes due to difficulties in detecting the sclera-choroid boundary. Because this difficulty primarily occurred in eyes with thicker choroids, it is likely that the mean juxtapapillary choroidal volume values for the OH group would have been somewhat higher had we been able to measure the choroid in these eyes. This would have then further enlarged the juxtapapillary choroidal volume difference between the OH and POAG groups. Additional study using technology that permits improved visualization of deeper choroidal structures is needed to better address this issue. Finally, we did not evaluate the spatial correspondence between beta-zone parapapillary atrophy and juxtapapillary choroidal volume. Future studies are needed to address relationships between glaucomatous optic neuropathy and these parameters.
In summary, this study found the volume of choroid surrounding the optic nerve was reduced in POAG eyes compared to OH and control eyes, but only when beta-zone parapapillary atrophy was present. This suggests that glaucoma pathophysiology may differ in eyes with, versus without, beta-zone parapapillary atrophy and that beta-zone parapapillary atrophy may be a biomarker for juxtapapillary choroidal atrophy and associated vascular compromise in glaucomatous disease.
Acknowledgments
Funding Support: NIH K23 EY021761 (Nimesh Patel); The sponsor or funding organization had no role in the design or conduct of this research.
Biography

Michael Sullivan-Mee, OD, FAAO, Diplomate (AAO Glaucoma)
Dr. Michael Sullivan-Mee is the Chief of Optometry and Director of Optometric Education at the Albuquerque Veterans Affairs Medical Center. He is actively involved with clinical research in glaucoma with particular interests in anterior segment biomechanics and posterior segment imaging.
Footnotes
Financial Disclosures: None of the authors have any financial disclosures.
Author Contributions: Design of the study (MSM,NBP,DP); conduct of the study (MSM,NBP, DP); collection of data (MSM, DP); analysis and interpretation of study data (MSM, NBP, DP, CQ); manuscript preparation (MSM,NBP, DP); review, enhanced depth imaging, and approval of manuscript (MSM,NBP,DP,CQ).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tielsch JM, Katz J, Sommer A, Quigley HA, Javitt JC. Hypertension, perfusion pressure, and primary open-angle glaucoma. A population-based assessment. Baltimore Eye Survey Arch Ophthalmol. 1995;113(2):216–21. doi: 10.1001/archopht.1995.01100020100038. [DOI] [PubMed] [Google Scholar]
- 2.Leske MC, Wu SY, Hennis A, Honkanen R, Nemesure B BES-Study group. Risk Factors for Incident Open-Angle Glaucoma: The Barbados Eye Studies. Ophthalmology. 2008;115(1):85–93. doi: 10.1016/j.ophtha.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114(11):1965–72. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Drance S, Anderson DR, Schulzer M Collaborative Normal-Tension Glaucoma Study Group. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol. 2001;131(6):699–708. doi: 10.1016/s0002-9394(01)00964-3. [DOI] [PubMed] [Google Scholar]
- 5.Hayreh SS, Zimmerman MB, Podhajsky P, Alward WL. Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. Am J Ophthalmol. 1994;117(5):603–24. doi: 10.1016/s0002-9394(14)70067-4. [DOI] [PubMed] [Google Scholar]
- 6.Hayreh SS. Blood supply of the optic nerve head and its role in optic atrophy, glaucoma, and oedema of the optic disc. Br J Ophthalmol. 1969;53(11):721–48. doi: 10.1136/bjo.53.11.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flammer J, Orgül S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21(4):359–93. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 8.Kur J, Newman EA, Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retin Eye Res. 2012;31(5):377–406. doi: 10.1016/j.preteyeres.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ulrich A, Ulrich C, Barth T, Ulrich WD. Detection of disturbed autoregulation of the peripapillary choroid in primary open angle glaucoma. Ophthalmic Surg Lasers. 1996;27(9):746–57. [PubMed] [Google Scholar]
- 10.Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147(5):811–5. doi: 10.1016/j.ajo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Spaide FR, Koizumi H, Pozonni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146(4):496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 12.Hua R, Liu L, Wang X, Chen L. Imaging evidence of diabetic choroidopathy in vivo: angiographic pathoanatomy and choroidal-enhanced depth imaging. PLoS One. 2013;8:e83494. doi: 10.1371/journal.pone.0083494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Querques G, Lattanzio R, Querques L, et al. Enhanced depth imaging optical coherence tomography in type 2 diabetes. Invest Ophthalmol Vis Sci. 2012;53(10):6017–24. doi: 10.1167/iovs.12-9692. [DOI] [PubMed] [Google Scholar]
- 14.Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29(10):1469–73. doi: 10.1097/IAE.0b013e3181be0a83. [DOI] [PubMed] [Google Scholar]
- 15.Maul EA, Friedman DS, Chang DS, et al. Choroidal thickness measured by spectral domain optical coherence tomography: factors affecting thickness in glaucoma patients. Ophthalmology. 2011;118(8):1571–9. doi: 10.1016/j.ophtha.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehrlich JR, Peterson J, Parlitsis G, Kay KY, Kiss S, Radcliffe NM. Peripapillary choroidal thickness in glaucoma measured with optical coherence tomography. Exp Eye Res. 2011;92(3):89–94. doi: 10.1016/j.exer.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Mwanza JC, Sayyad FE, Budenz DL. Choroidal thickness in unilateral advanced glaucoma. Invest Opthalmol Vis Sci. 2012;53(10):6695–701. doi: 10.1167/iovs.12-10388. [DOI] [PubMed] [Google Scholar]
- 18.Mwanza JC, Hochberg JT, Banitt MR, Feuer WJ, Budenz DL. Lack of association between glaucoma and macular choroidal thickness measured with enhanced depth-imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(6):3430–5. doi: 10.1167/iovs.10-6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lue L, Bian A, Zhou Q, Mao J. Peripapillary choroidal thickness in both eyes of glaucoma patients with unilateral visual field loss. Am J Ophthalmol. 2013;156(6):1277–84. doi: 10.1016/j.ajo.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Zhang X. Choroidal thickness and primary open-angle glaucoma: a cross-sectional study and meta-analysis. Invest Ophthalmol Vis Sci. 2014;55(9):6007–14. doi: 10.1167/iovs.14-14996. [DOI] [PubMed] [Google Scholar]
- 21.Zhang C, Tatham AJ, Medeiros FA, Zangwill LM, Yang Z, Weinreb RN. Assessment of choroidal thickness in healthy and glaucomatous eyes using swept source optical coherence tomography. PLoS ONE. 2014;9(10):e109683. doi: 10.1371/journal.pone.0109683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sigler EJ, Mascarenhas KG, Tsai JC, Loewen NA. Clinicopathologic correlation of disc and peripapillary region using SD-OCT. Optom Vis Sci. 2013;90(1):84–93. doi: 10.1097/OPX.0b013e318278fc15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirooka K, Tenkumo K, Fujiwara A, Baba T, Sato S, Shiraga F. Evaluation of peripapillary choroidal thickness in patients with normal-tension glaucoma. BMC Ophthalmol. 2012;28:12–29. doi: 10.1186/1471-2415-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts KF, Artes PH, O’Leary N, et al. Peripapillary choroidal thickness in healthy controls and patients with focal, diffuse, and sclerotic glaucomatous optic disc damage. Am J Ophthalmol. 2012;130(8):980–6. doi: 10.1001/archophthalmol.2012.371. [DOI] [PubMed] [Google Scholar]
- 25.Ho J, Branchini L, Regatieri C, et al. Analysis of normal peripapillary choroidal thickness via spectral domain optical coherence tomography. Ophthalmology. 2011;118(10):2001–7. doi: 10.1016/j.ophtha.2011.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park HL, Lee N, Shin H, Park CK. Analysis of macular and peripapillary choroidal thickness in glaucoma patients by enhanced depth imaging optical coherence tomography. J Glaucoma. 2014;23(4):25–231. doi: 10.1097/IJG.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 27.Gass JD, Braunstein R. Sessile and exophytic capillary angiomas of the juxtapapillary retina and optic nerve head. Arch Ophthalmol. 1980;98(10):1790–7. doi: 10.1001/archopht.1980.01020040642011. [DOI] [PubMed] [Google Scholar]
- 28.Jonas JB, Nguyen XN, Gusek GC, Naumann GO. Parapapillary chorioretinal atrophy in normal and glaucoma eyes. Invest Ophthalmol Vis Sci. 1989;30(5):908–18. [PubMed] [Google Scholar]
- 29.Rockwood EJ, Anderson DR. Acquired peripapillary changes and progression in glaucoma. Graefes Arch Clin Exp Ophthalmol. 1988;226(6):510–5. doi: 10.1007/BF02169197. [DOI] [PubMed] [Google Scholar]
- 30.Uchida H, Ugurle S, Caprioli J. Increasing peripapillary atrophy is associated with progressive glaucoma. Ophthalmology. 1998;105(8):1541–5. doi: 10.1016/S0161-6420(98)98044-7. [DOI] [PubMed] [Google Scholar]
- 31.Emdadi A, Kono Y, Sample PA, Maskaleris G, Weinreb RN. Parapapillary atrophy in patients with focal visual field loss. Am J Ophthalmol. 1999;128(5):595–600. doi: 10.1016/s0002-9394(99)00189-0. [DOI] [PubMed] [Google Scholar]
- 32.Curcio CA, Saunders PL, Younger PW, Malek G. Peripapillary chorioretinal atrophy. Ophthalmology. 2000;107(2):334–343. doi: 10.1016/s0161-6420(99)00037-8. [DOI] [PubMed] [Google Scholar]
- 33.Korte GE, Reppucci V, Henkind P. RPE destruction causes chorioretinal atrophy. Invest Ophthalmol Vis Sci. 1984;25(10):1135–45. [PubMed] [Google Scholar]
- 34.Dai Y, Jonas JB, Huang H, Wang M, Sun X. Microstructure of parapapillary atrophy: Beta zone and gamma zone. Invest Ophthalmol Vis Sci. 2013;54(3):2013–8. doi: 10.1167/iovs.12-11255. [DOI] [PubMed] [Google Scholar]
- 35.Kim M, Kim T, Weinreb RN, Lee EJ. Differentiation of parapapillary atrophy using spectral-domain optical coherence tomography. Ophthalmology. 2013;120(9):1790–7. doi: 10.1016/j.ophtha.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Lee KYC, Tomidokoro A, Sakata R, et al. Cross-sectional anatomic configurations of peripapillary atrophy evaluated with spectral domain-optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51(2):666–71. doi: 10.1167/iovs.09-3663. [DOI] [PubMed] [Google Scholar]
- 37.Patel NB, Garcia B, Harwerth RS. Influence of anterior segment power on the scan path and RNFL thickness using SD-OCT. Invest Ophthalmol Vis Sci. 2012;53(9):5788–98. doi: 10.1167/iovs.12-9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Girard MJA, Strouthidis NG, Ethier CR, Mari JM. Shadow removal and contrast enhancement in optical coherence tomography images of the human optic nerve head. Invest Ophthalmol Vis Sci. 2011;52(10):7738–48. doi: 10.1167/iovs.10-6925. [DOI] [PubMed] [Google Scholar]
- 39.Yin ZQ, Vaegan, Millar TH, Beaumont P, Sarks S. Widespread choroidal insufficiency in primary open-angle glaucoma. J Glaucoma. 1997;6(1):23–32. [PubMed] [Google Scholar]
- 40.Hayreh SS, Revie IH, Edwards J. Vasogenic origin of visual field defects and optic nerve changes in glaucoma. Br J Ophthalmol. 1970;54(7):461–72. doi: 10.1136/bjo.54.7.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laatikainen L. Fluorescein angiographic studies of the peripapillary and perilimbal regions in simple, capsular, and low-tension glaucoma. Acta Ophthalmol Suppl. 1971;111:3–83. [PubMed] [Google Scholar]
- 42.Geijssen HC, Greve EL. The spectrum of primary open angle glaucoma. I:Senile sclerotic glaucoma versus high tension glaucoma. Ophthalmic Surg. 1987;18(3):207–13. [PubMed] [Google Scholar]
- 43.Cohen AI. Is there a potential defect in the blood-retinal barrier at the choroidal level of the optic nerve canal ? Invest Ophthalmol. 1973;12(7):513–9. [PubMed] [Google Scholar]
- 44.Cioffi GA, Orgul S, Onda E, Bacon DR, Van Buskirk EM. An in vivo model of chronic optic nerve ischemia: the dose-dependent effects of endothelin-1 on the optic nerve microvasculature. Curr Eye Res. 1995;14(12):1147–53. doi: 10.3109/02713689508995821. [DOI] [PubMed] [Google Scholar]
- 45.Venkataraman ST, Flanagan JG, Hudson C. Vascular reactivity of optic nerve head and retinal blood vessels in glaucoma—a review. Microcirculation. 2010;17(7):568–81. doi: 10.1111/j.1549-8719.2010.00045.x. [DOI] [PubMed] [Google Scholar]
- 46.Healey PR, Mitchell P, Gilbert CE, et al. The inheritance of peripapillary atrophy. Invest Ophthalmol Vis Sci. 2007;48(6):2529–34. doi: 10.1167/iovs.06-0714. [DOI] [PubMed] [Google Scholar]
- 47.Vujosevic S, Martini F, Cavarzeran F, Pilotto E, Midena E. Macular and peripapillary choroidal thickness in diabetic patients. Retina. 2012;32:1781–90. doi: 10.1097/IAE.0b013e31825db73d. [DOI] [PubMed] [Google Scholar]
- 48.Lee HK, Lim JW, Shin MC. Comparison of choroidal thickness in patients with diabetes by spectral-domain optical coherence tomography. Korean J Ophthalmol. 2013;27(6):433–9. doi: 10.3341/kjo.2013.27.6.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goharian I, Sehi M. Is there any role for the choroid in glaucoma? J Glaucoma. 2014 Aug 25; doi: 10.1097/IJG0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]