Abstract
Matrix metalloproteinases (MMPs) are important in injury and recovery in ischemic injury. They are proteolytic enzymes that degrade all components of the extracellular matrix (ECM). They are secreted in a latent form, protecting the cell from damage, but once activated induce injury prior to rapid inactivation by four tissue inhibitors to metalloproteinases (TIMPs). Normally the constitutive enzymes, MMP-2 and membrane type MMP (MMP-14), are activated in a spatially specific manner and act close to the site of activation, while the inducible enzymes, MMP-3 and MMP-9, become active through the action of free radicals and other enzymes during neuroinflammation. Because of the complex nature of the interactions with tissues during development, injury and repair, the MMPs have multiple roles, participating in the injury process in the early stages and contributing to recovery during the later stages. This dual role complicates the planning of treatment strategies.
Keywords: Matrix metalloproteinases, Blood-brain barrier, Hypoxia/ischemia, Recovery, Angiogenesis
Introduction
Matrix metalloproteinases (MMPs) are proteolytic enzymes that degrade all components of the extracellular matrix (ECM).(Vandooren et al., 2014) MMPs are a large family of enzymes that range from the smallest member of the family, matrilysin (MMP-7), to the large transmembrane MMPs, such as MMP-14. They have a standard configuration consisting of the catalytic zinc site, the propeptide portion, which forms a cysteine switch that maintains latency, and various other entities, such as the hemopexin site, the fibronectin binding site, and the transmembrane site. They are secreted in a latent form that protects the cell from damage, and once activated are generally rapidly inactivated by a series of mechanisms, mainly involving the four tissue inhibitors to metalloproteinases (TIMPs). They can be divided into constitutive enzymes, including MMP-2 and MMP-14, and inducible enzymes, MMP-3 and MMP-9. Normally the constitutive enzymes are activated in a spatially specific manner and act close to the site of activation. They maintain the integrity of the basement membranes, preventing overgrowth of the ECM. Inducible enzymes, on the other hand, are held in an inactive state until a neuroinflammatory process begins and they become active through the action of free radicals and other enzymes. Once the inducible MMPs are activated, they are not constrained to act close to the site of activation and lead to more extensive tissue damage. The timing of the activation cascades is critical in determining the role that the enzymes play during normal tissue maintenance, injury and recovery. Because of the complex nature of the interactions with tissues during development, injury and repair, the MMPs have multiple roles, participating in the injury process in the early stages and contributing to recovery during the later stages. This dual role makes planning of treatment strategies complicated. (Yang et al., 2011a)
The main constitutive enzymes in the brain are gelatinase A (MMP-2) and membrane type MMP (MMP-14).(Yong et al., 2001) These are present normally in brain cells, particularly in astrocytes where the foot processes are intimately connected to the endothelial cells (EC). Relatively high concentrations of MMP-2 are found in the cerebrospinal fluid (CSF). Activation of MMP-2 involves a trimolecular complex composed on MMP-2, TIMP-2, and MMP-14 (also MT1-MMP).(English et al., 2006) When the three molecules come together the MMP-14 activates the MMP-2, utilizing TIMP-2 as a bridging molecule. Because the MMP-14 is bound to the membrane, the activity of the MMP-2 is constrained to the region close to the activation site. This prevents the MMP-2 from doing extensive damage, but allows for the removal of excess ECM, maintaining the integrity of the basal lamina around the blood vessels.
The inducible MMPs primarily active in the brain are MMP-3 and MMP-9. Microglia, macrophages, and infiltrating neutrophils are the primary sources. Other MMPs involved in injury cascades include MMP-8 and MMP-13.(Cuadrado et al., 2009) Activator protein-1 (AP-1) and nuclear factor-kB (NF-kB) transcription sites are involved in the formation of MMP-3 and MMP-9. Cytokines activate the transcription sites leading to the formation of the latent forms of the enzymes. Once they are formed nitrosylation and other free radical actions lead to their activation. MMP-9 is activated by active MMP-3. Tumor necrosis factor-alpha (TNF-α) and interleukin-1beta (IL-β) are involved the transcription of MMPs. During inflammation there is a number of cytokines released and the exact combination and effect on the MMPs is dependent on the underlying disease process. Both MMPs and cytokines may be expressed in the blood during an inflammatory response to injury and infection. In bacterial meningitis, MMP-8 degrades the tight junction proteins (TJP), occludin, a component of the blood-brain barrier (BBB), and induces neuroinflammation.(Schubert-Unkmeir et al., 2010) MMP-8 is also critical in mediating microglial activation by modulating TNF-α activity, which suggests a proinflammatory role of microglial MMP-8.
Neurovascular unit (NVU)
The neurovascular unit protects the brain microenvironment from substances circulating in the blood. The cells form a series of cellular layers beginning with ECs that form the major interface with the blood. Surrounding the ECs is a basal lamina composed of type IV collagen, laminin, heparin sulfate, fibronectin and other extracellular molecules. Contiguous with the basal lamina and embedded in it are the pericytes, which are the macrophage-like cells that play major roles both in injury and in angiogenesis. The final layer is formed by astrocytic end-feet that encircle the basal lamina.
TJP are located in the clefts that join the ECs together. Tethered to the endothelial membranes are the zona occludens that traverse the cell wall and enter the space between the edges of the cells. Within the clefts are the cells that constitute the tight junctions, namely, occluldin and claudins, which provide the main barrier between the blood and the interstitial fluid. The basal lamina most likely provides a molecular filter through its charge and intertwined proteins. The end-feet of the astrocytes take up molecules and water; they contain the water pores formed by aquaporin.(Higashida et al., 2011) When the cells swell, the astrocytic end-feet are the first to absorb the excess water and expand.(Kuchiwaki et al., 1990)
MMPs act at several sites in the neurovascular unit to regulate the permeability of the NVU. MMPs are extracellular-degrading enzymes, and a major site of their action is on the proteins in the basal lamina and the TJPs, but recent studies indicate they also act intracellularly.(Yang et al., 2007; Yang et al., 2010) Astrocytes normally secrete MMP-2 from the end-feet where they act on the contiguous structures. MMP-9 and MMP-3 are produced by the ECs and particularly by the microglia and by pericytes, which are a major source of MMP-3. Neutrophils are the source of MMP-8.(Owen et al., 2004)
In acute stroke, the first response seen in the MMPs occurs in MMP-2, which is probably due to the fact that it is present in a latent form normally and does not need to be produced. Activation of MMP-2 begins during hypoxia once MMP-14 is activated by furin, which is produced by hypoxia inducible factor-alpha (HIF-1α). Since MMP-2, MMP-14 and TIMP-2 are normally present, the activation of MMP-2 begins the disruption of the ECM proteins in the basal lamina and eventually attacks the TJPs. If the hypoxic stress does not continue, and there is no further MMP activation, there can be a restoration of the basal lamina integrity (FIGURE 1A).
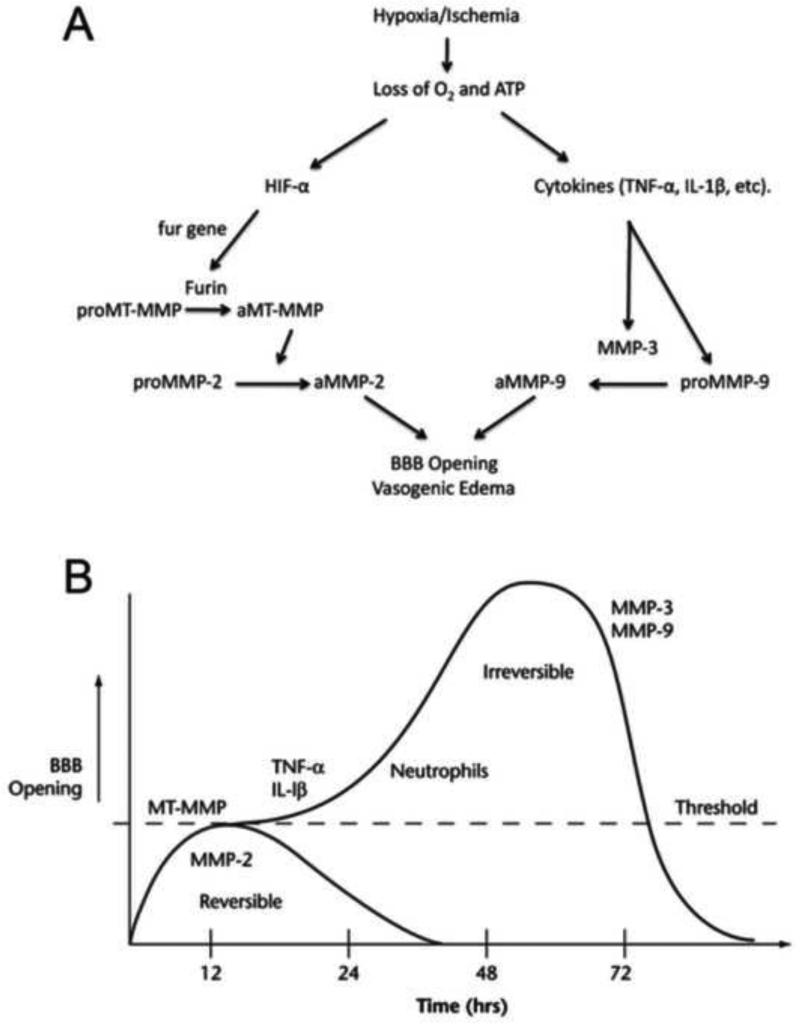
Figure 1.
A. Mechanism for MMP-mediated blood–brain barrier (BBB) disruption in hypoxia/ischemia. Induction of hypoxia inducible factor-1β by loss of oxygen and ATP leads to activation of MMP-2. The constitutive enzyme pro-membrane-type 1 metalloproteinase-MMP is activated by the convertase, Furin, and it activates proMMP-2. Secondary neuroinflammation with the formation of cytokines (TNF-α and IL-1β) and induction of MMP-9 and MMP-3 occurs. ProMMP-9 is activated by MMP-3 and free radicals. Active MMPs degrade the basal lamina and tight junctions of ECs, thereby opening the BBB. Opening of the BBB leads to vasogenic edema. B. Schematic drawing to show theoretical mechanisms leading to the initial reversible opening of the BBB and later, the more slowly irreversible opening. Reperfusion injury leads to a biphasic opening of the BBB. The early opening occurs several hours after the onset of reperfusion because of activation of the constitutive MMP-2. This initial opening is transient and reversible. At 24 to 72 hours later, the inflammatory response leads to the induction of MMP-3 and MMP-9, which induce more intense and irreversible damage to the blood vessel. Modified from (Yang and Rosenberg, 2011b)
In the reperfusion model of cerebral ischemia, a second phase of injury begins around 24 to 48 h after the ischemic insult.(Rosenberg et al., 1998) The delayed process results in the activation of more disruptive MMPs because they are not bound to the membranes, and move more freely through the extracellular space to attack molecules. Normally present in very low levels, there is induction of MMP-9, which is activated by MMP-3 and free radicals. By this later stage, inflammatory cells have been activated or recruited, including microglia, macrophages and neutrophils, which secrete additional MMPs, causing major damage to the cerebral vessels. The combination of hypoxia and ischemia causes cyotoxic edema as the cellular energy levels fall dramatically. In addition, there is extensive damage to the cerebral blood vessels with vasogenic edema (Figure 1B).
Role of MMPs in acute damage in ischemic stroke
In the first phases after stroke, the dysregulation of MMPs has been linked to increased acute neurovascular disruption and cerebral injury. In particular, MMP-mediated alterations lead to BBB leakage, cerebral edema, hemorrhage, leukocyte infiltration and progressive inflammatory reactions underlying brain tissue loss. (Lee et al., 2004; Lenglet et al., 2013; Rosenberg and Yang, 2007; Si-Tayeb et al., 2006; Wasserman and Schlichter, 2007; Yang et al., 2010) Cerebrovascular endothelial tight junctions restrict molecules from moving between blood and brain. TJPs join together with ECs to form the first barrier of BBB between the blood and the brain. In rodents and adult human brains, ZO-1, claudin-1, claudin-5, and occludin have been found to be present in brain endothelial tight junctions forming the BBB.(Yang et al., 2007) Proteins of tight junction change expression, subcellular location, posttranslational modification, and protein–protein interactions under both physiological and pathophysiological conditions. Occludin, claudin-5, and ZO-1, which are the main structural barrier proteins, are considered sensitive indicators of normal and disturbed functional states of the BBB. In a reperfusion model, elevation in MMP-2 in the early stages of the injury has been observed in rodents and nonhuman primates.(Chang et al., 2003; Yang and Rosenberg, 2011a) Claudin-5 and occludin are degraded by MMP-2, but remain within the vessels after 3 h of reperfusion. Treatment with MMP inhibitors or MMP neutralizing antibodies decreases infarct size and prevents BBB breakdown after focal ischemic stroke (Rosenberg et al., 1998). Treated with the MMP inhibitor, BB1101, after the onset of reperfusion, significantly attenuates degradation of claudin-5 and occludin, blocking the early BBB opening. However, by 24 h the TJPs are no longer seen in the vessels.(Yang et al., 2007) (Rosenberg et al., 1998) During this phase there is marked increase in MMP-9, which leads to more extensive damage to TJPs, especially ZO-1 in the blood vessels. (Bauer et al., 2010; Cui et al., 2012)
BBB disruption occurs early enough to be within the thrombolytic time window. (Jin et al., 2012) Animal and human studies found that thrombolysis-associated cerebral hemorrhage occurred in the brain regions where the BBB was compromised. (Bang et al., 2007; Hjort et al., 2008; Hom et al., 2011; Kassner et al., 2009; Sun et al., 2010; Wu et al., 2009) These studies suggest that loss of BBB integrity during cerebral ischemia may predispose ischemic brain tissue to bleeding during reperfusion with thrombolysis. In middle cerebral artery occlusion (MCAO) model, dextran leaks in the ischemic ventromedial striatum and the preoptic area, where high (2000 kDa) and low (70 kDa) molecular weight dextrans displayed almost identical leakage patterns. Increased gelatinolytic activity co-localized with dextran leakage, and MMP-2 was found to be the major enzymatic source on gelatin zymograms. Pretreatment with MMP inhibitor GM6001 significantly reduced dextran leakage induced by 2 and 3 h MCAO.(Jin et al., 2012) Further studies demonstrated that hypoxia-induced secretion of MMP-2 in ECs mediated occludin degradation, and caveolin-1-mediated claudin-5 redistribution.(Liu et al., 2012) In reperfusion model of rat, treatment with normobaric hyperoxia (NBO) initiated 5 min after MCAO onset for one h, decreases degradation of TJPs-mediated by MMP-2 and -9 and attenuates disruption of the BBB in reperfusion injury of 48 h.(Jin et al., 2013; Liu et al., 2009) When hyperoxia is used with tPA, there is reduction in hemorrhagic complications from tPA, probably because of the protection of the BBB. Rats treated with combined normobaric hyperoxia and tPA showed significantly reduced tPA-associated mortality, brain edema, hemorrhage, and MMP-9 induction compared with tPA alone.(Liu et al., 2009)
Neuronal apoptosis following ischemic injury has been proposed as a mechanism of cell death.(Gu et al., 2002) Treatment with inhibitors of MMPs blocks cell death in transient focal ischemia. Several studies implicated involvement of MMP activation in oxidative stress and cell apoptosis in cerebral ischemic brains.(Gasche et al., 2001; Kelly et al., 2008; Wakisaka et al., 2010) MMP-2 activity has been considered as an early and key event in oxidative stress-related injury to the heart after ischemia-reperfusion.(Ali and Schulz, 2009) It has been reported that oxidative stress can promote the increase of MMP-2 and -9 activities.(Haorah et al., 2007; Pustovrh et al., 2005) In addition to triggering DNA damage, oxidative stress activates MMP-2 without proteolytic removal of the propeptide, resulting in an active, full-length MMP-2.(Ali and Schulz, 2009; Sariahmetoglu et al., 2007)
Although best known for their roles in the proteolysis of extracellular protein targets, recent studies have revealed that MMPs are also localized to various intracellular sites, including the nucleus.(Ali and Schulz, 2009; Amantea et al., 2008; Kwan et al., 2004) and that non-extracellular-matrix molecules are also substrates.(Somerville et al., 2003) Proteolysis of numerous nuclear proteins has been implicated in DNA fragmentation and apoptosis. Oxidative DNA damage in injured neurons is a prominent event which occurs during the early stages after cerebral ischemia.(Alexander et al., 1998; Shi and Liu, 2007) We have observed active gelatinases (MMP-2/-9) in ischemic neuronal nuclei at 3 h reperfusion, suggesting a role for MMPs in nuclear proteolysis.(Yang et al., 2010) The intranuclear MMP activity, including MMP-2, -3, -9 and -13, have been observed in ischemic rat and human brains, which can become activated as early as 15-30 min after the onset of reperfusion in MCAO rat brains.(Amantea et al., 2008; Cuadrado et al., 2009; Gasche et al., 2001; Si-Tayeb et al., 2006) Our studies further demonstrate that the activated intranuclear MMP-2/-9 in ischemic neurons can degrade nuclear proteins poly-ADP-ribose polymerase-1 (PARP-1) and X-ray cross-complementary factor 1 (XRCC1) and concomitantly increase oxidative DNA damage levels 3 h of reperfusion after MCAO.(Yang et al., 2010) PARP-1 mediates cytotoxic as well as protective functions when involved in DNA repair, and both of these mechanisms are amenable to therapeutic targeting in models of ischemia. XRCC1 plays a central role in DNA base excision repair (BER) pathway where it serves as a scaffolding protein interacting with PARP-1 and other BER enzymes. We showed that treatment with a MMP inhibitor significantly reduced the degradation of PARP-1 and XRCC1, attenuated accumulation of oxidative DNA damage, and neuronal apoptotic death in ischemic rat brain tissue and in primary neurons subjected to oxygen-glucose deprivation (OGD).(Hill et al., 2012)
Neuroinflammation contributes to the pathophysiology cerebral ischemia. At an early stage of stroke, pro-inflammatory cytokines, such as tumor necrosis factor TNF-α, interleukin IL-1β, IL-6 and IL-18 are released by activated cells including neurons, astrocytes, microglia and ECs. Acute elevation of IL-1β, and consequent activation of the IL-1 receptor 1, are harmful to the injured cerebral tissue during ischemic stroke. Recent study demonstrate a significant increase of cortical IL-1β as early as 1 h after the beginning of reperfusion, being the cytokine mainly expressed in cortical neurons and, to a lesser extent, in pericallosal astroglial cells. Importantly, this early increased IL-1β, more specifically in neuronal nuclei, colocalizes with elevated intracellular gelatinolytic activity, mainly MMP2, indicating MMP-2 may contribute to IL-1b production early after the beginning of reperfusion.(Amantea et al., 2014) Using a TIMP-3 knockout (KO) mouse model of MCAO/R, we demonstrated that TIMP-3 contributes to oligodendrocyte (OL) death in brain white matter via TNF-α-mediated neuroinflammation. We detected an increased MMP-3 and -9 in astrocytes in white matter at 72 h after stroke.(Yang et al., 2011c) MMP-3 can proteolytically activate MMP-9.(Candelario-Jalil et al., 2009; Yang et al., 2011b) Therefore, increased MMP-3 and -9 in astrocytes in white matter may play a role in BBB damage with facilitation of infiltration of leukocytes including macrophages in WT mice. MMP-3 is associated with the neuroinflammatory response and was shown to activate microglia and increase the neuroinflammatory response with expression of cytokines such as TNF-β.(Kim et al., 2005)
Role of MMPs in brain edema and hemorrhage after stroke
Hemorrhage is a major complication of treatment of acute stroke with tissue plasminogen activator (tPA) because of hemorrhagic transformation and intracerebral hemorrhage. Cardioembolic strokes result in hemorrhagic transformation in approximately 60% of MRIs.(Hornig et al., 1993) Intracerebral hemorrhage is less frequent, but more life threatening due to high mortality associated with the mass effect. MMPs are implicated in both hemorrhagic transformation and intracerebral hemorrhage. Measurements of MMP-9 in the plasma correlate with the severity of the hemorrhagic changes in the brain.(Montaner et al., 2001)
Hemorrhagic transformation can be modeled by the injection of bacterial collagenase into brains of animals; the bacterial collagenase causes multifocal sites of hemorrhage, which coalesce into a mass lesion.(Rosenberg et al., 1990) De novo production of MMPs occurs aggravating the bleeding.(Rosenberg et al., 1994) Treatment of the animal models with inhibitors of MMPs reduces the edema and tissue injury.(Power et al., 2003; Rosenberg et al., 1994; Rosenberg and Navratil, 1997; Wang and Tsirka, 2005) In the bacterial collagenase model there is an increase in RNA of several MMPs, including MMP-2, -3, -7, and -9 relative to sham-injected (control) animals. MMP-12 (macrophage metalloelastase) was the most highly induced MMP, and immunohistochemistry showed MMP-12 localized in activated monocytoid cells.(Power et al., 2003) MMPs are induced by cytokines, cyclooxigenases (COXs) and oxidative stress. These inflammatory factors induce expression and activation of MMPs, creating a cycle of injury. Nitric oxide is major factor in the activation of the MMPs.(Gu et al., 2002) (Jian and Rosenberg, 2005)
MMPs in white matter damage associated with neuroinflammation
White matter injury is a major complication of acute and chronic stroke. In acute stroke, there is necrosis and apoptosis of the cells in the white matter that results in rapid death and leaves cyst-like lesions.(Pantoni et al., 1996) In the chronic state, there is hypoperfusion of the white matter due to cerebral blood vessel narrowing from hypertension, diabetes and other vascular risk factors.(Brun, 1994) Since the deep white matter is a watershed region, it is vulnerable to changes in the tissue oxygen levels, and hypoxic hypoperfusion leads to incomplete infarction. MMPs play a primary role in the acute injury, which results in widespread activation of a number of MMPs. On the other hand, in chronic injury there is secondary damage to the white matter related to the MMPs that are expressed in the blood vessels, resulting in disruption to the BBB with production of vasogenic edema that releases toxic blood products that spread through the white matter.(Rosenberg and Yang, 2007) In addition to the damage from the edema, there is direct attack of the MMPs on the myelinated fibers. This combination of leakage of the BBB and breakdown of myelin results in the chronic changes, which are commonly seen in the white matter of elderly patients, leading in some to vascular cognitive impairment.
Several animal models mimic these changes in the white matter. Bilateral occlusion of the carotid arteries in the normotensive rat causes hypoxic hypoperfusion that primarily damages the white matter.(Kitamura et al., 2012) MMPs are induced by the hypoxic state and contribute to the white matter damage. Knockout mice lacking MMP-2 are protected from white matter injury, and agents that block the MMPs can reduce the white matter damage.(Nakaji et al., 2006) Spontaneously hypertensive stroke prone rats have white matter injury, making them another model that more closely mimics the clinical condition. Manipulation of the diet by adding salt or reducing protein can accelerate the injury of the white matter.(Sironi et al., 2004) Both the bilateral occlusion of the carotid in the normotensive rat and dietary manipulation in the hypertensive rat offer excellent models to test therapeutic agents. Blocking the action of the MMPs effectively reduces the injury to the white matter as does other agents, such as free radical inhibitors and anti-inflammatory agents.
MMP Inhibitors in Stroke Treatment
Use of the MMP inhibitors in animal studies has shown promise. A selective MMP-9 inhibitor, SB-3CT, was shown to reduce injury in acute stroke.(Gu et al., 2005) Minocycline is a tetracycline derivative that has anti-inflammatory actions. It reduces microglial activation, decreases caspases, and blocks the action of MMPs. It is an effective drug to reduce injury in acute stroke, and when given with tPA is reduces the bleeding that can occur.(Fagan et al., 2010; Xu et al., 2004; Yrjanheikki et al., 1999) Because of the ability to use the MMP inhibitors for a shorter time in the treatment of acute stroke, they are potential treatments that may overcome the problems encountered with long-term treatment in cancer.(Roycik et al., 2013) Although the MMP inhibitors show benefit during the acute phase of the injury, they slow recovery probably by interfering with angiogenesis. This suggests that the timing of the use of the MMP inhibitors in stroke will be critical and that short-term use may be all that is possible. Treatment with MMP inhibitors at 7 days after stroke in rats suppressed neurovascular remodeling, increased ischemic brain injury and impaired functional recovery at 14 days.(Zhao et al., 2006a)
MMPs in brain repair during recovery from stroke
MMPs produce detrimental effects during the early ischemic phase, but are beneficial during the recovery phase, particularly during angiogenesis and reestablishment of cerebral blood flow.(Lenglet et al., 2013) Three processes are implicated in neurorepair: angiogenesis, neurogenesis and synaptic plasticity. After stroke, ischemic penumbral tissue releases angiogenic factors that induce proliferation of ECs through migration of endothelial progenitor cells to form new blood vessels. Angiogenesis contributes to the neurorepair processes, including neurogenesis and synaptogenesis.(Brea et al., 2009; Hermann and Zechariah, 2009; Nakagomi et al., 2009; Snapyan et al., 2009; Teng et al., 2008). Furthermore, factors released by ECs trigger neural stem cell proliferation.(Shen et al., 2004) The leading process of migrating neural progenitor cells (NPCs) are closely associated with blood vessels, indicating that this interaction provides directional guidance to the NPCs. These findings suggest that blood vessels play a key role as a scaffold for NPC migration toward the infarct. Angiogenesis requires degradation of the vascular basement membrane and remodeling of the ECM in order to allow ECs to migrate and invade into the surrounding tissue(Rundhaug, 2005). Long-term MMP inhibition reduces neuronal plasticity, impairs new vessel formation, and promotes hemorrhage and tissue damage in peri-infarct cortex. Animal models and clinical studies have established a timeline for MMP-9 expression and corresponding perihematomal edema (PHE) after intracerebral hemorrhage that include an initial peak on days 1-3 and a secondary peak on day 7.(Chang et al., 2014) The temporal profile of MMP-9 after intracerebral hemorrhage is similar with the observation in brains after stroke/reperfusion, suggesting a bimodal pattern of MMP-9 elevation. (Yang and Rosenberg, 2011b; Yang et al., 2013; Zhao et al., 2006b) At 7 and 14 days, increased MMP-9 signals were found in peri-infarct cortex, which co-localized with NeuN-positive and GFAP-positive cells.(Zhao et al., 2006b) MMP-9 expression in astrocytes is correlated with markers of ECs. Consistent with these finding, inhibition of MMP at 7 days after stroke results in reduction in neuronal plasticity and vascular remodeling and additional tissue damage in peri-infarct cortex, (Zhao et al., 2006b; Zhao et al., 2007) suggesting that the second modal pattern of MMP-9 elevation during 7-14 days could be involved in neurovascular remodeling in the peri-infarct areas.
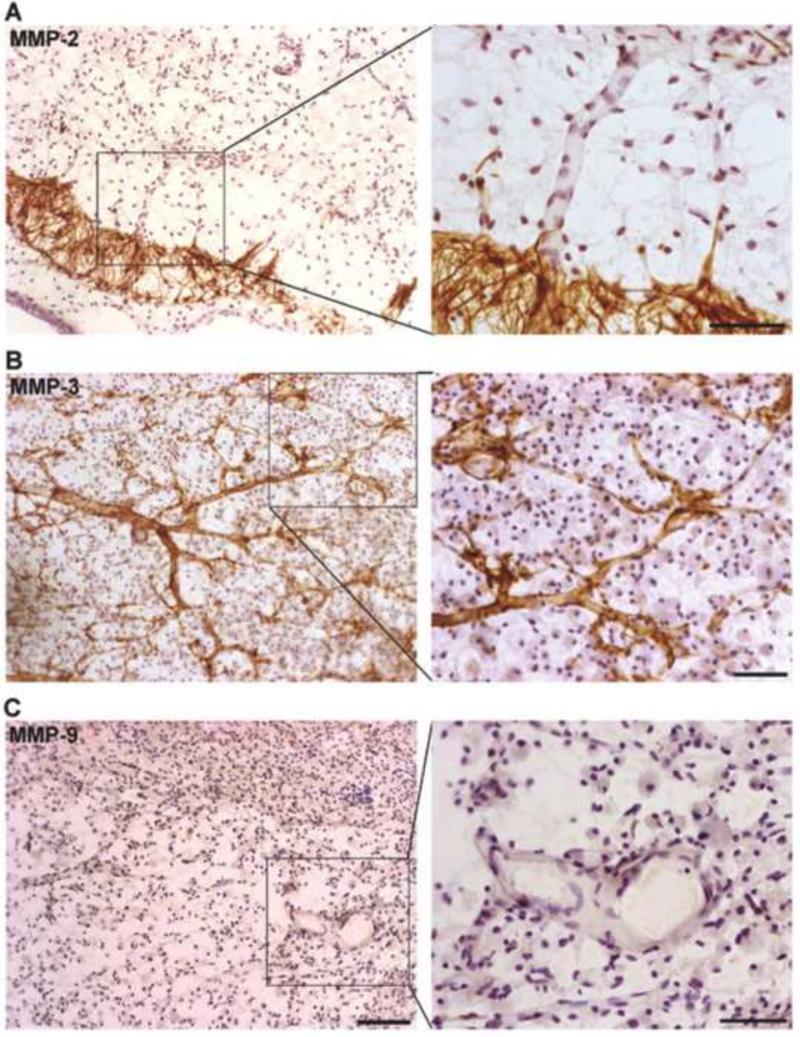
In the brain, MMPs are expressed during development and contribute to morphogenesis of the central nervous system. Specific MMPs have been shown to enhance angiogenesis by helping to detach pericytes from vessels undergoing angiogenesis, by releasing ECM-bound angiogenic growth factors, by exposing cryptic proangiogenic integrin binding sites in the ECM, by generating promigratory ECM component fragments, and by cleaving endothelial cell-cell adhesions.(Rundhaug, 2005) At 21 days, we found that increased MMP-2 was expressed by astrocytes around the margin of peri-infarct areas with astrocyte end-feet contacted vessels inside peri-infarct regions (FIGURE 2A). Strong MMP-3 immunostaining (FIGURE 2B) was seen in PDGFR-β- and NG2-positive pericytes closely along with the new vessels (Yang et al., 2013). Very low signal of MMP-9 was detectable at this time point (FIGURE 2C). (Yang et al., 2013) Along with the new vessels, the NG2-positive pericytes expressed ZO-1 and vascular endothelial growth factor (VEGF)-A, astrocytes expressed ZO-1, occludin, and VEGF-A, while ECs expressed claudin-5.(Yang et al., 2013) The specificity of these findings emphasize that pericytes and astrocytes act spatiotemporally, contributing to extra endothelial TJP formation, and that MMPs are involved in BBB restoration during stroke recovery. In angiogenesis, loss of vascular integrity and degradation of the ECM are crucial initiating steps. Most likely this function is performed by the MMPs, particularly by MMP-3 expressed by NG2-positive pericytes that are located next to new vessels, where they can degrade ECM and facilitate vessel migration into the infarcted tissues. This endothelial migration is aided by growth factors, including VEGF.
Figure 2.
Expression of MMPs in ischemic hemispheres at 3 weeks of reperfusion. A. MMP-2 (brown) was expressed by astrocyte-like cells around the peri-infarct region. End-feet of the astrocyte-like cells contact vascular structures (right panel). B. Expression of MMP-3 (brown) was seen along with vessels in peri-infarct region. Small branches of these vessels were wrapped with MMP-3 (right panel). C. Little MMP-9 (brown) was seen in ischemic hemisphere at this time point. Scale bars= 100 in left panels or 50 μm in right panels. Modified from (Yang et al., 2013).
Conclusion
A large number of studies have shown the importance of the MMPs in stroke. The main site of action is the cerebral blood vessel where they attack the extracellular molecules in the basal lamina and disrupt the TJPs. There are constitutively expressed MMPs that initiate the injury cascade early in the acute hypoxic/ischemic phase and inducible MMPs that perpetuate the damage over hours and days. As recovery begins and angiogenesis starts, the MMPs participate in the regrowth of the damaged vessels. Because of this dual role treatment with MMP inhibitors needs to be started early and stopped prior to the onset of the recovery phase. Currently, there are number of MMP inhibitors that are able to block the early injury, and as more information is gained they most likely will enter clinical trials.
Highlights for Review.
Matrix metalloproteinases are important in injury and recovery
Blood-brain barrier disruption is an early MMP-mediated process
Angiogenesis is a late event mediated by MMPs
Treatments will need to consider the early and late effects
ACKNOWLEDGMENTS
These studies were supported by grants from the NIH (RO1NS045847) to GR, AHA (0765473Z and 10BGIA4310034) and NIH COBRE project 7 (5P20RR015636-09) to YY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alexander JS, et al. Activated T-lymphocytes express occludin, a component of tight junctions. Inflammation. 1998;22:573–82. doi: 10.1023/a:1022310429868. [DOI] [PubMed] [Google Scholar]
- Ali MA, Schulz R. Activation of MMP-2 as a key event in oxidative stress injury to the heart. Front Biosci. 2009;14:699–716. doi: 10.2741/3274. [DOI] [PubMed] [Google Scholar]
- Amantea D, et al. Brain regional and cellular localization of gelatinase activity in rat that have undergone transient middle cerebral artery occlusion. Neuroscience. 2008;152:8–17. doi: 10.1016/j.neuroscience.2007.12.030. [DOI] [PubMed] [Google Scholar]
- Amantea D, et al. Early reperfusion injury is associated to MMP2 and IL-1beta elevation in cortical neurons of rats subjected to middle cerebral artery occlusion. Neuroscience. 2014;277:755–63. doi: 10.1016/j.neuroscience.2014.07.064. [DOI] [PubMed] [Google Scholar]
- Bang OY, et al. Prediction of hemorrhagic transformation after recanalization therapy using T2*-permeability magnetic resonance imaging. Ann Neurol. 2007;62:170–6. doi: 10.1002/ana.21174. [DOI] [PubMed] [Google Scholar]
- Bauer AT, et al. Matrix metalloproteinase-9 mediates hypoxia-induced vascular leakage in the brain via tight junction rearrangement. J Cereb Blood Flow Metab. 2010;30:837–48. doi: 10.1038/jcbfm.2009.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brea D, et al. [Reorganisation of the cerebral vasculature following ischaemia]. Rev Neurol. 2009;49:645–54. [PubMed] [Google Scholar]
- Brun A. Pathology and pathophysiology of cerebrovascular dementia: pure subgroups of obstructive and hypoperfusive etiology. Dementia. 1994;5:145–7. doi: 10.1159/000106712. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158:983–94. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DI, et al. Activation systems for latent matrix metalloproteinase-2 are upregulated immediately after focal cerebral ischemia. J Cereb Blood Flow Metab. 2003;23:1408–19. doi: 10.1097/01.WCB.0000091765.61714.30. [DOI] [PubMed] [Google Scholar]
- Chang JJ, et al. Matrix metalloproteinase-9: dual role and temporal profile in intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2014;23:2498–505. doi: 10.1016/j.jstrokecerebrovasdis.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Cuadrado E, et al. Matrix metalloproteinase-13 is activated and is found in the nucleus of neural cells after cerebral ischemia. J Cereb Blood Flow Metab. 2009;29:398–410. doi: 10.1038/jcbfm.2008.130. [DOI] [PubMed] [Google Scholar]
- Cui J, et al. Inhibition of MMP-9 by a selective gelatinase inhibitor protects neurovasculature from embolic focal cerebral ischemia. Mol Neurodegener. 2012;7:21. doi: 10.1186/1750-1326-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English JL, et al. Individual Timp deficiencies differentially impact pro-MMP-2 activation. J Biol.Chem. 2006;281:10337–10346. doi: 10.1074/jbc.M512009200. [DOI] [PubMed] [Google Scholar]
- Fagan SC, et al. Minocycline to improve neurologic outcome in stroke (MINOS): a dose-finding study. Stroke. 2010;41:2283–7. doi: 10.1161/STROKEAHA.110.582601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasche Y, et al. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:1393–400. doi: 10.1097/00004647-200112000-00003. [DOI] [PubMed] [Google Scholar]
- Gu Z, et al. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297:1186–90. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- Gu Z, et al. A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J.Neurosci. 2005;25:6401–6408. doi: 10.1523/JNEUROSCI.1563-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haorah J, et al. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. J Neurochem. 2007;101:566–76. doi: 10.1111/j.1471-4159.2006.04393.x. [DOI] [PubMed] [Google Scholar]
- Hermann DM, Zechariah A. Implications of vascular endothelial growth factor for postischemic neurovascular remodeling. J Cereb Blood Flow Metab. 2009;29:1620–43. doi: 10.1038/jcbfm.2009.100. [DOI] [PubMed] [Google Scholar]
- Higashida T, et al. The role of hypoxia-inducible factor-1alpha, aquaporin-4, and matrix metalloproteinase-9 in blood-brain barrier disruption and brain edema after traumatic brain injury. J Neurosurg. 2011;114:92–101. doi: 10.3171/2010.6.JNS10207. [DOI] [PubMed] [Google Scholar]
- Hill JW, et al. Intranuclear matrix metalloproteinases promote DNA damage and apoptosis induced by oxygen-glucose deprivation in neurons. Neuroscience. 2012;220:277–90. doi: 10.1016/j.neuroscience.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjort N, et al. MRI detection of early blood-brain barrier disruption: parenchymal enhancement predicts focal hemorrhagic transformation after thrombolysis. Stroke. 2008;39:1025–8. doi: 10.1161/STROKEAHA.107.497719. [DOI] [PubMed] [Google Scholar]
- Hom J, et al. Blood-brain barrier permeability assessed by perfusion CT predicts symptomatic hemorrhagic transformation and malignant edema in acute ischemic stroke. AJNR Am J Neuroradiol. 2011;32:41–8. doi: 10.3174/ajnr.A2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornig CR, et al. Hemorrhagic transformation in cardioembolic cerebral infarction. Stroke. 1993;24:465–468. doi: 10.1161/01.str.24.3.465. [DOI] [PubMed] [Google Scholar]
- Jian LK, Rosenberg GA. Matrix metalloproteinases and free radicals in cerebral ischemia. Free Radic.Biol.Med. 2005;39:71–80. doi: 10.1016/j.freeradbiomed.2005.03.033. [DOI] [PubMed] [Google Scholar]
- Jin X, et al. Spatiotemporal evolution of blood brain barrier damage and tissue infarction within the first 3h after ischemia onset. Neurobiol Dis. 2012;48:309–16. doi: 10.1016/j.nbd.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Jin X, et al. Normobaric hyperoxia combined with minocycline provides greater neuroprotection than either alone in transient focal cerebral ischemia. Exp Neurol. 2013;240:9–16. doi: 10.1016/j.expneurol.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassner A, et al. Recombinant tissue plasminogen activator increases blood-brain barrier disruption in acute ischemic stroke: an MR imaging permeability study. AJNR Am J Neuroradiol. 2009;30:1864–9. doi: 10.3174/ajnr.A1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PJ, et al. Oxidative stress and matrix metalloproteinase-9 in acute ischemic stroke: the Biomarker Evaluation for Antioxidant Therapies in Stroke (BEAT-Stroke) study. Stroke. 2008;39:100–4. doi: 10.1161/STROKEAHA.107.488189. [DOI] [PubMed] [Google Scholar]
- Kim YS, et al. Matrix metalloproteinase-3: a novel signaling proteinase from apoptotic neuronal cells that activates microglia. J Neurosci. 2005;25:3701–11. doi: 10.1523/JNEUROSCI.4346-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura A, et al. Selective white matter abnormalities in a novel rat model of vascular dementia. Neurobiol Aging. 2012;33:1012, e25–35. doi: 10.1016/j.neurobiolaging.2011.10.033. [DOI] [PubMed] [Google Scholar]
- Kuchiwaki H, et al. An electron microscopic and electron probe study of the microcirculation in cold-induced oedema. Acta Neurochir Suppl (Wien) 1990;51:82–3. doi: 10.1007/978-3-7091-9115-6_28. [DOI] [PubMed] [Google Scholar]
- Kwan JA, et al. Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (ADP-ribose) polymerase (PARP) in vitro. Faseb J. 2004;18:690–2. doi: 10.1096/fj.02-1202fje. [DOI] [PubMed] [Google Scholar]
- Lee HS, et al. Hydrogen peroxide-induced alterations of tight junction proteins in bovine brain microvascular endothelial cells. Microvasc Res. 2004;68:231–8. doi: 10.1016/j.mvr.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Lenglet S, Montecucco F, Mach F. Role of matrix metalloproteinases in animal models of ischemic stroke. Curr Vasc Pharmacol. 2013 doi: 10.2174/15701611113116660161. [DOI] [PubMed] [Google Scholar]
- Liu J, et al. Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J Neurosci. 2012;32:3044–57. doi: 10.1523/JNEUROSCI.6409-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, et al. Normobaric hyperoxia attenuates early blood-brain barrier disruption by inhibiting MMP-9-mediated occludin degradation in focal cerebral ischemia. J Neurochem. 2009;108:811–20. doi: 10.1111/j.1471-4159.2008.05821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaner J, et al. Matrix metalloproteinase expression is related to hemorrhagic transformation after cardioembolic stroke. Stroke. 2001;32:2762–2767. doi: 10.1161/hs1201.99512. [DOI] [PubMed] [Google Scholar]
- Nakagomi N, et al. Endothelial cells support survival, proliferation, and neuronal differentiation of transplanted adult ischemia-induced neural stem/progenitor cells after cerebral infarction. Stem Cells. 2009;27:2185–95. doi: 10.1002/stem.161. [DOI] [PubMed] [Google Scholar]
- Nakaji K, et al. Matrix metalloproteinase-2 plays a critical role in the pathogenesis of white matter lesions after chronic cerebral hypoperfusion in rodents. Stroke. 2006;37:2816–23. doi: 10.1161/01.STR.0000244808.17972.55. [DOI] [PubMed] [Google Scholar]
- Owen CA, et al. Membrane-bound matrix metalloproteinase-8 on activated polymorphonuclear cells is a potent, tissue inhibitor of metalloproteinase-resistant collagenase and serpinase. J Immunol. 2004;172:7791–803. doi: 10.4049/jimmunol.172.12.7791. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27:1641–1646. doi: 10.1161/01.str.27.9.1641. [DOI] [PubMed] [Google Scholar]
- Power C, et al. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Ann.Neurol. 2003;53:731–742. doi: 10.1002/ana.10553. [DOI] [PubMed] [Google Scholar]
- Pustovrh MC, et al. Oxidative stress promotes the increase of matrix metalloproteinases-2 and -9 activities in the feto-placental unit of diabetic rats. Free Radic Res. 2005;39:1285–93. doi: 10.1080/10715760500188796. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, et al. Collagenase-induced intracerebral hemorrhage in rats. Stroke. 1990;21:801–7. doi: 10.1161/01.str.21.5.801. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, et al. Injury-induced 92-kDa gelatinase and urokinase expression in rat brain. Lab.Invest. 1994;71:417–422. [PubMed] [Google Scholar]
- Rosenberg GA, Navratil M. Metalloproteinase inhibition blocks edema in intracerebral hemorrhage in the rat. Neurology. 1997;48:921–6. doi: 10.1212/wnl.48.4.921. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998;29:2189–95. doi: 10.1161/01.str.29.10.2189. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Yang Y. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg Focus. 2007;22:E4. doi: 10.3171/foc.2007.22.5.5. [DOI] [PubMed] [Google Scholar]
- Roycik MD, et al. Matrix metalloproteinase inhibition in atherosclerosis and stroke. Curr Mol Med. 2013;13:1299–313. doi: 10.2174/15665240113139990067. [DOI] [PubMed] [Google Scholar]
- Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9:267–85. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariahmetoglu M, et al. Regulation of matrix metalloproteinase-2 (MMP-2) activity by phosphorylation. FASEB J. 2007;21:2486–95. doi: 10.1096/fj.06-7938com. [DOI] [PubMed] [Google Scholar]
- Schubert-Unkmeir A, et al. Neisseria meningitidis induces brain microvascular endothelial cell detachment from the matrix and cleavage of occludin: a role for MMP-8. PLoS Pathog. 2010;6:e1000874. doi: 10.1371/journal.ppat.1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–40. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Shi H, Liu KJ. Cerebral tissue oxygenation and oxidative brain injury during ischemia and reperfusion. Front Biosci. 2007;12:1318–28. doi: 10.2741/2150. [DOI] [PubMed] [Google Scholar]
- Si-Tayeb K, et al. Matrix metalloproteinase 3 is present in the cell nucleus and is involved in apoptosis. Am J Pathol. 2006;169:1390–401. doi: 10.2353/ajpath.2006.060005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironi L, et al. Analysis of pathological events at the onset of brain damage in stroke-prone rats: a proteomics and magnetic resonance imaging approach. J Neurosci Res. 2004;78:115–22. doi: 10.1002/jnr.20219. [DOI] [PubMed] [Google Scholar]
- Snapyan M, et al. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci. 2009;29:4172–88. doi: 10.1523/JNEUROSCI.4956-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville RP, Oblander SA, Apte SS. Matrix metalloproteinases: old dogs with new tricks. Genome Biol. 2003;4:216. doi: 10.1186/gb-2003-4-6-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, et al. Oxygen therapy reduces secondary hemorrhage after thrombolysis in thromboembolic cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:1651–60. doi: 10.1038/jcbfm.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng H, et al. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J Cereb Blood Flow Metab. 2008;28:764–71. doi: 10.1038/sj.jcbfm.9600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandooren J, Van Damme J, Opdenakker G. On the structure and functions of gelatinase B/matrix metalloproteinase-9 in neuroinflammation. Prog Brain Res. 2014;214:193–206. doi: 10.1016/B978-0-444-63486-3.00009-8. [DOI] [PubMed] [Google Scholar]
- Wakisaka Y, et al. Spontaneous intracerebral hemorrhage during acute and chronic hypertension in mice. J Cereb Blood Flow Metab. 2010;30:56–69. doi: 10.1038/jcbfm.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Tsirka SE. Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain. 2005;128:1622–33. doi: 10.1093/brain/awh489. [DOI] [PubMed] [Google Scholar]
- Wasserman JK, Schlichter LC. Minocycline protects the blood-brain barrier and reduces edema following intracerebral hemorrhage in the rat. Exp Neurol. 2007;207:227–37. doi: 10.1016/j.expneurol.2007.06.025. [DOI] [PubMed] [Google Scholar]
- Wu S, et al. Relative recirculation: a fast, model-free surrogate for the measurement of blood-brain barrier permeability and the prediction of hemorrhagic transformation in acute ischemic stroke. Invest Radiol. 2009;44:662–8. doi: 10.1097/RLI.0b013e3181ae9c40. [DOI] [PubMed] [Google Scholar]
- Xu L, et al. Low dose intravenous minocycline is neuroprotective after middle cerebral artery occlusion-reperfusion in rats. BMC Neurol. 2004;4:7. doi: 10.1186/1471-2377-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, et al. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- Yang Y, et al. Increased intranuclear matrix metalloproteinase activity in neurons interferes with oxidative DNA repair in focal cerebral ischemia. J Neurochem. 2010;112:134–49. doi: 10.1111/j.1471-4159.2009.06433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Hill JW, Rosenberg GA. Chapter 6--multiple roles of metalloproteinases in neurological disorders. Prog Mol Biol Transl Sci. 2011a;99:241–63. doi: 10.1016/B978-0-12-385504-6.00006-3. [DOI] [PubMed] [Google Scholar]
- Yang Y, Hill JW, Rosenberg GA. Multiple roles of metalloproteinases in neurological disorders. Prog Mol Biol Transl Sci. 2011b;99:241–63. doi: 10.1016/B978-0-12-385504-6.00006-3. [DOI] [PubMed] [Google Scholar]
- Yang Y, et al. Tissue inhibitor of metalloproteinases-3 mediates the death of immature oligodendrocytes via TNF-alpha/TACE in focal cerebral ischemia in mice. J Neuroinflammation. 2011c;8:108. doi: 10.1186/1742-2094-8-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Rosenberg GA. MMP-mediated disruption of claudin-5 in the blood-brain barrier of rat brain after cerebral ischemia. Methods Mol Biol. 2011a;762:333–45. doi: 10.1007/978-1-61779-185-7_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011b;42:3323–8. doi: 10.1161/STROKEAHA.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, et al. Early inhibition of MMP activity in ischemic rat brain promotes expression of tight junction proteins and angiogenesis during recovery. J Cereb Blood Flow Metab. 2013;33:1104–14. doi: 10.1038/jcbfm.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong VW, et al. Metalloproteinases in biology and pathology of the nervous system. Nat.Rev.Neurosci. 2001;2:502–511. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yrjanheikki J, et al. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao BQ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat.Med. 2006a;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- Zhao BQ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006b;12:441–5. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- Zhao BQ, Tejima E, Lo EH. Neurovascular proteases in brain injury, hemorrhage and remodeling after stroke. Stroke. 2007;38:748–52. doi: 10.1161/01.STR.0000253500.32979.d1. [DOI] [PubMed] [Google Scholar]