Abstract
The blood-brain barrier (BBB) is a physical and biochemical barrier that precisely regulates the ability of endogenous and exogenous substances to accumulate within brain tissue. It possesses structural and biochemical features (i.e., tight junction and adherens junction protein complexes, influx and efflux transporters) that work in concert to control solute permeation. Oxidative stress, a critical component of several diseases including cerebral hypoxia/ischemia and peripheral inflammatory pain, can cause considerable injury to the BBB and lead to significant CNS pathology. This suggests a critical need for novel therapeutic approaches that can protect the BBB in diseases with an oxidative stress component. Recent studies have identified molecular targets (i.e., endogenous transporters, intracellular signaling systems) that can be exploited for optimization of endothelial drug delivery or for control of transport of endogenous substrates such as the antioxidant glutathione (GSH). In particular, targeting transporters offers a unique approach to protect BBB integrity by promoting repair of cell-cell interactions at the level of the brain microvascular endothelium. This review summarizes current knowledge in this area and emphasizes those targets that present considerable opportunity for providing BBB protection and/or promoting BBB repair in the setting of oxidative stress.
Keywords: Blood-Brain Barrier, Endothelial Cell, Membrane Transporter, Multidrug Resistance Proteins, Organic Anion Transporting Polypeptides, Oxidative Stress
1. Introduction
The blood-brain barrier (BBB) is an essential physical and biochemical barrier that separates the central nervous system (CNS) from the peripheral circulation. This barrier is primarily formed by cerebral endothelial cells that interact with each other to maintain CNS homeostasis and reduce the probability of cerebral toxicity due to xenobiotic accumulation. Pathologies with an oxidative stress component (i.e., cerebral hypoxia/ischemia, peripheral inflammatory pain) are known to disrupt BBB inter-endothelial cell interactions, which can cause profound pharmacotherapeutic challenges. Therefore, there is a critical need for novel therapeutic strategies that can protect the BBB from pathological damage and, by extension, ensure more effective drug delivery across the endothelial cell plasma membrane. One approach is to target endogenous transporters localized to BBB endothelial cells. Here, we review BBB molecular characteristics (i.e., tight junction and adherens junction protein complexes, influx and efflux drug transporters) and those mechanisms associated with oxidative stress that can cause BBB injury. Additionally, we provide insights on endothelial transporter targets that have great potential to be exploited for promoting BBB repair in the setting of oxidative stress.
2. The Blood-Brain Barrier
The CNS is the most critical and sensitive organ system in the body. Proper function requires precise control of the brain extracellular milieu. Additionally, metabolic demands of brain tissue are considerable with the CNS accounting for approximately 20% of human oxygen consumption (Rolfe and Brown, 1997). Therefore, the interface between CNS and systemic circulation must possess highly effective mechanisms that can facilitate transport of specific nutrients, exactly regulate ion balance, and limit blood-to-brain uptake of toxic substances. The absolute necessity for a tissue that is both a physical and biochemical barrier is emphasized by the sensitivity of brain parenchyma cellular compartments to xenobiotics. That is, brain entry of specific substances must be permitted while flux of other molecules into brain parenchyma must be excluded. This function of the cerebral microvasculature primarily occurs at the level of the endothelial cell. It is essential to note that brain microvessel endothelial cells are not intrinsically capable of forming a fully functional BBB. In fact, formation and maintenance of the BBB phenotype requires interactions with adjacent glial cells as well as neurons, pericytes, and extracellular matrix (Ronaldson and Davis, 2012; Ronaldson and Davis, 2013). This intricate relationship implies existence of a neurovascular unit, a concept that emphasizes the requirement for coordinated cell-cell interactions and signaling that precisely regulates BBB homeostasis.
Anatomically, BBB endothelial cells are characterized by a lack of fenestrations, limited pinocytotic activity, and presence of tight junction protein complexes between apposing endothelial cells (Abbott et al. 2010). Additionally, the cerebral microvascular endothelium is identified by increased mitochondrial content as compared to endothelial cells from other tissues (Oldendorf et al. 1977). This increased content of mitochondria is required for both protection against deleterious effects of oxidative stress and maintenance of brain Ca2+ homeostasis (Sochocka et al. 2013). Additionally, several receptors, ion channels, uptake transporters, and efflux transporters are prominently expressed in brain microvascular endothelial cells. Functionally, these transport systems are similar to well-characterized systems in other tissues (i.e., D-glucose transporter, L-amino acid carrier systems, Na+/K+ ATPase), although transport kinetics can vary. Transporters involved in transendothelial flux of drugs have also been identified and characterized at the BBB and include ATP-dependent efflux transporters such as P-glycoprotein (P-gp) (Roberts et al. 2008; Yousif et al. 2008; McCaffrey et al. 2012; Ohtsuki et al. 2013), Multidrug Resistance Proteins 1–6 (MRP1–6 in humans; Mrp1–6 in rodents) (Dallas et al. 2006; Bauer et al. 2008; Roberts et al. 2008; Cartwright et al. 2013), and Breast Cancer Resistance Protein (BCRP in humans; Bcrp in rodents) (Yousif et al. 2012; Ohtsuki et al. 2013). Transporters that facilitate BBB drug permeation include organic anion transporting polypeptides (OATPs in humans; Oatps in rodents) (Ose et al. 2010; Ronaldson et al. 2011; Thompson et al. 2014), organic anion transporters (Hawkins et al. 2007; Ose et al. 2009; Miyajima et al. 2011), monocarboxylate transporters (Vijay and Morris, 2014), nucleoside transporters (Lepist et al. 2013), and peptide transporters (Dogrukol-Ak et al. 2009).
2.1. Molecular Characteristics of the BBB
2.1.1. Tight Junction Protein Complexes
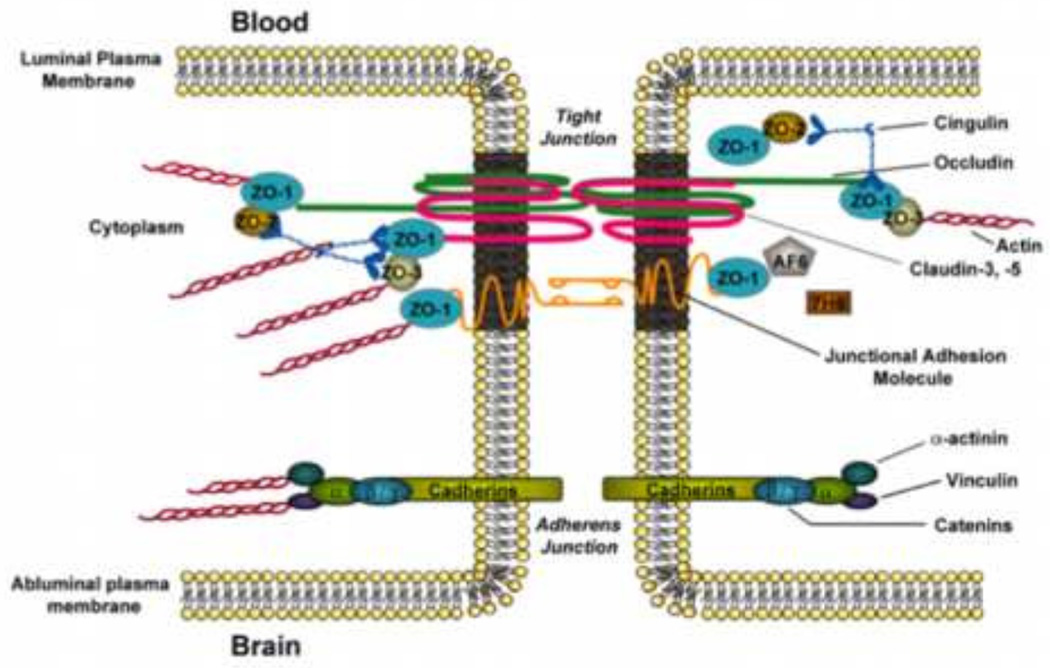
BBB endothelial cells are interconnected by tight junctions (Figure 1), which are large multi-protein complexes maintained by astrocytic trophic factors. Evidence for this role of astrocytes comes from in vivo experiments in which male Fisher F344 rats were injected with 3-chloropropanediol, an astrocyte-selective toxin (Willis et al. 2004a, b). Focal astrocyte loss induced by treatment with 3-chloropropanediol led to disassembly of tight junction protein complexes and increased paracellular dextran leak (Willis et al. 2004a, b), suggesting a central role for astrocytes in maintenance of the BBB phenotype. Physiologically, tight junctions form a continuous, almost impermeable barrier that limits paracellular diffusion of blood-borne substances with the exception of small, lipid soluble molecules (Abbott et al. 2010). The high BBB transendothelial resistance (1,500–2,000 Ωcm2) further restricts free flow of water and solutes (Butt et al. 1990). BBB tight junction formation involves specific transmembrane proteins such as junction adhesion molecules (JAMs), occludin, and claudins (i.e., claudin-1, −3, and −5). These transmembrane proteins are linked to cytoskeletal filaments by interactions with accessory proteins (i.e., zonula occluden (ZO)-1, −2, and −3) (Ronaldson and Davis, 2012). ZO proteins act as a scaffold for multiple intracellular signaling pathways and are involved in regulation of tight junction function (Bauer et al. 2014). Additionally, other protein constituents (i.e., cingulin, AF6, 7H6, EMP-1) have been localized to the tight junction (Ronaldson and Davis, 2012); however, their exact role has yet to be elucidated and these proteins will not be addressed in this review. Characteristics of primary proteins that comprise BBB tight junction protein complexes are highlighted below.
Figure 1.
Basic molecular organization of tight junction protein complexes and adherens junctions at the blood-brain barrier. Adapted from Ronaldson and Davis (2012). Curr Pharm Des. 18: 3624–3644.
Several JAM isoforms have been identified at the mammalian BBB including JAM-1, JAM-2, and JAM-3 (Hawkins and Davis, 2005; Ronaldson and Davis, 2012). JAMs regulate transendothelial migration of neutrophils and monocytes/macrophages (Williams et al. 2013; Sladojevic et al. 2014). JAM-1 is a 40-kDa member of the IgG superfamily and mediates early attachment of adjacent endothelial cells during BBB development (Dejana et al. 2000). Additionally, loss of JAM protein expression is directly correlated with BBB disruption and injury (Yeung et al. 2008; Hoffman et al. 2009; Wang et al. 2014). Additionally, studies in an immortalized human brain endothelial cell line (hCMEC/d3) showed that inflammatory stimulation led to increased paracellular solute diffusion, which correlated with JAM movement away from the tight junction, further suggesting a central role in maintaining BBB integrity (Haarmann et al. 2010).
Monomeric occludin is a 60- to 65 kDa protein that is highly expressed along endothelial cell margins in brain vasculature (Hawkins et al. 2004; McCaffrey et al. 2008). Recent studies by our group have shown that occludin is a critical regulator of BBB permeability in vivo (McCaffrey et al. 2007; McCaffrey et al. 2008; Lochhead et al. 2010). This essential role for occludin occurs via interaction between extracellular loops on occludin monomers and homologous segments on occludin molecules localized to adjacent endothelial cells (Lacaz-Vieira et al. 1999; Feldman et al. 2005). Occludin assembles into dimers and higher order oligomers, a characteristic that is required for restriction of paracellular permeability. Altered occludin expression is associated with BBB dysfunction in several pathologies with an oxidative stress component including hypoxia/aglycemia (Brown and Davis, 2005), hypoxia/reoxygenation stress (Lochhead et al. 2010), and inflammatory pain (Lochhead et al. 2012).
Claudins have similar membrane topography to occludin but no sequence homology (Furuse et al. 1993). Claudins are 20- to 24-kDa proteins that form homophilic and heterophilic interactions between adjacent endothelial cells and contribute to the physiological “seal” of the tight junction (Furuse et al. 1999). In cerebral microvascular endothelial cells, various claudin isoforms have been detected including claudin-1, −3, and −5 (Witt et al. 2003; Hawkins et al. 2004; Forster et al. 2008; Ronaldson et al. 2009; Wang et al. 2011). In experimental stroke models (Gibson et al. 2014; Wang et al. 2014) and inflammatory pain (Huber et al. 2001; Brooks et al. 2006; Ronaldson et al. 2009), altered expression of claudin-5 that correlated with enhanced paracellular permeability has been reported. In contrast, studies in bovine brain microvascular endothelial cells subjected showed that in vitro hypoxia did not alter cellular expression of claudin-1 (Mark and Davis, 2002). More recently, claudin-1 expression was shown to decrease at the in vivo BBB following exposure to human amyloid-β (Aβ40) peptide (Hartz et al. 2012). Aβ deposition at the BBB is characteristic of cerebral amyloid angiopathy, a pathological condition that contributes to microvascular injury including hemorrhages and ischemia.
Proper physiological functioning of the BBB, particularly restriction of paracellular solute transport, requires association of JAMs, occludin, and claudins with accessory proteins localized within the endothelial cell cytoplasm. In brain microvascular endothelial cells, membrane-associated guanylate kinase-like (MAGUK) proteins are involved in clustering of tight junction protein complexes to the cell membrane (Gonzalez-Mariscal et al. 2000). Three MAGUK proteins have been identified at the tight junction: ZO-1, −2, and −3. ZO-1, the first tight junction protein that was identified (Stevenson et al. 1986), links transmembrane tight junction proteins (i.e., JAMs, occludin, claudins) to the actin cytoskeleton (Fanning et al. 1998). Previous studies have shown that dissociation of ZO-1 from the junction complex is associated with increased permeability, suggesting that the ZO-1-transmembrane protein interaction is critical to tight junction stability and function (Abbruscato et al. 2002; Fischer et al. 2002; Mark et al. 2002). ZO-1 may also act as a signaling molecule that communicates information on tight junction integrity to the cellular interior, or vice versa. ZO-1 has been shown to localize to the endothelial cell nucleus under conditions of proliferation or injury (Gottardi et al. 1996), following Ca2+ depletion (Riesen et al. 2002), and in response to nicotine (Hawkins et al. 2004). Similarly, ZO-2 is known to bind tight junction constituents, signaling molecules and transcription factors (Betanzos et al. 2004). In fact, ZO-2 may act redundantly with ZO-1 as it has been shown to facilitate formation of morphologically intact tight junctions in cultured cells lacking ZO-1 (Umeda et al. 2004). ZO-3 is expressed at the BBB but its exact role in formation of tight junctions and/or maintenance of tight junction integrity has not been elucidated (Takenaga et al. 2009). CNS pathologies with an oxidative stress component have been shown to be associated with reduced ZO-1 expression at the BBB (Ronaldson et al. 2009; Zehendner et al. 2013; Li et al. 2014). In fact, Jiao and colleagues reported that movement of ZO-1 away from the tight junction in cerebral microvessels correlated with large-scale BBB disruption as evidenced by increased blood-to-brain leak of Evan’s blue-albumin (Jiao et al. 2011).
2.1.2. Adherens Junctions
Adherens junctions are specialized cell-cell interactions, which are formed by cadherins and associated proteins that are directly linked to actin filaments (Redzic, 2011). Cadherins regulate endothelial function by activation of phosphoinositide 3-kinase signaling, an intracellular pathway that organizes the cytoskeleton and enables complex formation with vascular endothelial growth factor receptor 2. Therefore, cadherin-mediated signaling is essential for endothelial cell layer integrity and for the spatial organization of new microvessels (Lampugnani and Dejana, 2007). Barrier-forming endothelium have been shown to express higher levels of cadherin-10 relative to VE-cadherin (Williams et al. 2005). In contrast, circumventricular organs and choroid plexus capillaries (i.e., brain microvasculature that is devoid of BBB properties) primarily express VE-cadherin (Williams et al. 2005). Optimal function of cadherins requires association of their C terminus with catenins; cadherins bind directly to β-catenin and to p120 catenin, which can bind to α-catenin, a protein that binds to actin (Vestweber, 2008; Redzic, 2011). At least four catenin isoforms (i.e., β, α, x, and p120) are expressed at the BBB, with β-catenin linking cadherin to α-catenin, which binds this protein complex to the actin cytoskeleton (Meng and Takeishi, 2009). Using the murine brain endothelial cell line bEnd3, Steiner and colleagues demonstrated that the heparin sulfate proteoglycan agrin also contributes to barrier properties of adherens junction by promoting localization of VE-cadherin and β-catenin to endothelial cell junctions (Steiner et al. 2014). Paracellular expression of VE-cadherin and/or β-catenin is associated with BBB repair following focal astrocyte loss, an in vivo experimental condition in which endothelial cells display reduced expression of tight junction constituent proteins (Willis et al. 2013). In vitro, hypoxia induced degradation of VE-cadherin in endothelial cells, an effect that contributed to increased paracellular permeability (Hyun and Jung, 2014). VE-cadherin expression was also decreased at the BBB in mice subjected to MCAO, suggesting that adherens junction disruption contributes to BBB dysfunction in diseases with an oxidative stress component (Wacker et al. 2012).
2.1.3. Transporters
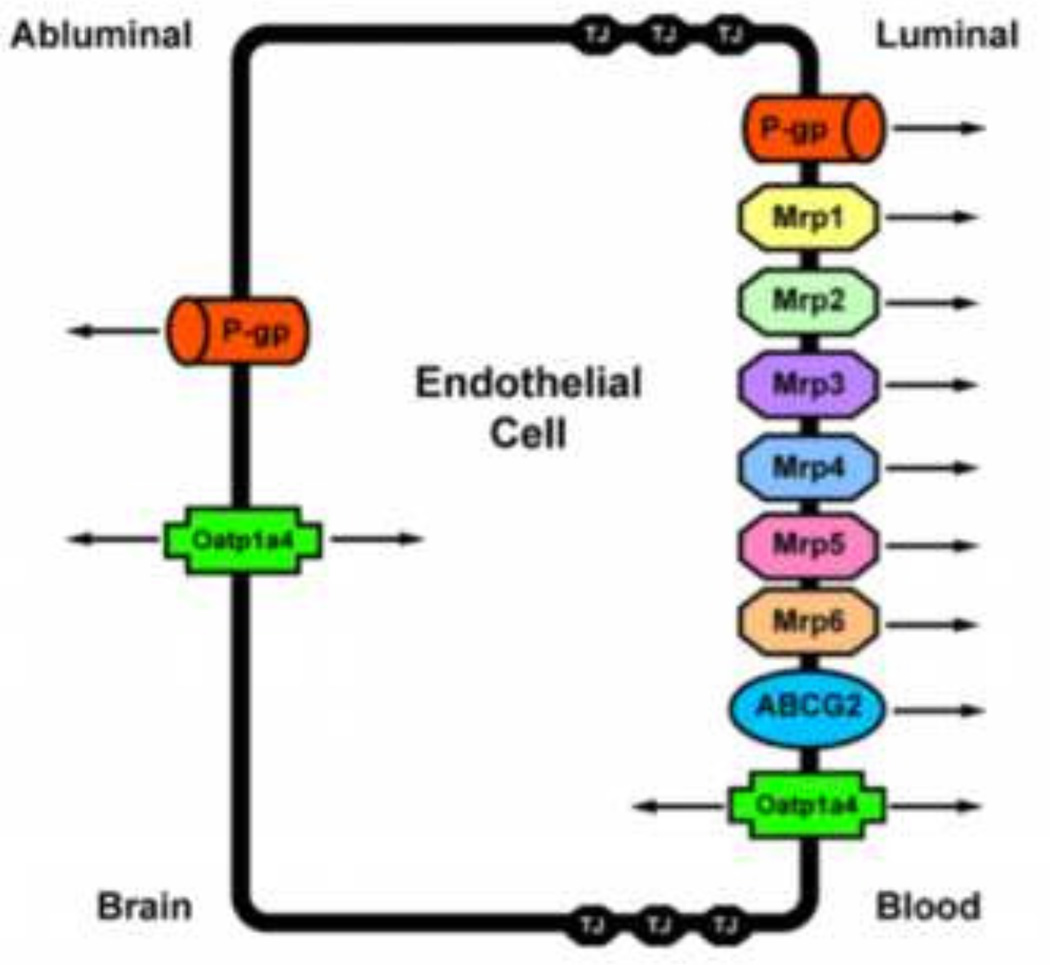
For many endogenous and exogenous substances, proteins endogenously expressed at the BBB govern the ability of these molecules to traverse biological membranes. Such transport proteins include ATP-binding cassette (ABC) transporters and solute carrier (SLC) transporters (Sanchez-Covarrubias et al. 2013). In order to target transporters for optimization of pharmacotherapy, it is critical to understand localization (i.e., luminal versus abluminal) and functional expression of transport proteins at the BBB endothelium. Below, we summarize current knowledge on membrane transporters that are known to determine CNS delivery of therapeutic agents. Localization of those specific transport proteins that are involved in CNS drug delivery is depicted in figure 2.
Figure 2.
Endothelial localization of drug transporters known to be involved in transport of therapeutic agents at the blood-brain barrier. Adapted from Ronaldson and Davis (2012). Curr Pharm Des. 18: 3624–3644.
2.1.3.1. ABC Transporters
The ABC superfamily is among the largest and most ubiquitously expressed protein families. ABC transporters are involved in translocation of drugs and their metabolites against their concentration gradient. The energy to transport xenobiotics is provided by binding and subsequent hydrolysis of ATP. In humans, 48 ABC genes have been identified and are classified according to seven subfamilies (Leslie et al. 2005). ABC drug transporters, specifically P-gp, MRPs/Mrps and BCRP/Bcrp (also known as ABCG2) are involved in cellular extrusion of drugs and thus constitute a considerable barrier to effective therapeutic delivery to the brain. In general, P-gp transports cationic or basic and neutral compounds, whereas MRPs/Mrps are involved in cellular efflux of anionic drugs as well as their glucuronidated, sulfated, and glutathione-conjugated metabolites (Ronaldson et al. 2008; Sanchez-Covarrubias et al. 2013). BCRP/Bcrp has significant overlap in substrate specificity profile with P-gp and has been shown to recognize a vast array of sulfoconjugated organic anions, hydrophobic, and amphiphilic compounds (Robey et al. 2009).
P-gp is a 170-kDa ATP-dependent integral membrane protein that is believed to primarily function as a biological defense mechanism against entry of toxic substances into vital organ systems such as the brain (Gottesman et al. 1995). P-gp orthologues from different species have greater than 70% sequence identity (Gottesman et al. 1995) and are encoded by closely related genes (i.e., multidrug resistance (MDR) genes), which have two isoforms in humans (MDR1, MDR2) and three isoforms in both mice (i.e., mdr1, mdr2, mdr3) and rats (i.e., mdr1a, mdr1b, mdr2). The human MDR2 gene and the murine/rodent mdr2 gene products are exclusively involved in hepatic transport of phosphatidylcholine. In contrast, human MDR1, murine mdr1/mdr3, and rodent mdr1a/mdr1b are involved in transport of drugs in several tissues including BBB endothelium. Specifically, P-gp expression has been identified at both the luminal (Beaulieu et al. 1997; Virgintino et al. 2002; Bendayan et al. 2006) and abluminal membrane (Golden and Pardridge, 1999; Schlachetzki et al. 2003; Bendayan et al. 2006) of brain microvascular endothelial cells. Abluminal localization of P-gp has been identified on perivascular astrocyte foot processes (Golden and Pardridge, 1999; Schlachetzki et al. 2003) and on the abluminal plasma membrane of the endothelial cell itself (Bendayan et al. 2006). Increased expression and/or activity of P-gp has been reported in hippocampal microvessels isolated from stroke-prone spontaneously hypertensive rats (Ueno et al. 2009) and in brain microvessels harvested from Sprague-Dawley rats subjected to peripheral inflammatory pain (Seelbach et al. 2007; McCaffrey et al. 2012), which suggests that alterations in P-gp expression and/or activity may constitute a component of the BBB response to pathological stressors.
The mammalian MRP family belongs to the ABCC group of proteins, which contains 13 members including one ion channel (i.e., CFTR), two surface receptors (i.e., SUR1 and 2) and a truncated protein that does not mediate transport (i.e., ABCC13) (Dallas et al. 2006). Many of the functionally characterized MRP isoforms that are known to be involved in drug transport have been localized to the mammalian BBB. These include MRP1/Mrp1, MRP2/Mrp2, MRP4/Mrp4, Mrp5 and Mrp6 (Miller et al. 2000; Leggas et al. 2004; Zhang et al. 2004; Bandler et al. 2008; Bauer et al. 2008; Uchida et al. 2011; Sanchez-Covarrubias et al. 2013). The presence of multiple MRP homologues at the BBB may be a vital determinant in controlling the delivery of therapeutic agents to the brain. Additionally, the ability of Mrp isoforms to actively efflux the endogenous antioxidant glutathione (GSH) may have significant implications in diseases with an oxidative stress component. GSH is responsible for maintenance of cellular redox balance and antioxidant defense in the brain. It has been previously demonstrated that functional expression of Mrps is upregulated in response to oxidative stress conditions, which leads to increased cellular efflux of GSH (Ronaldson and Bendayan, 2008). Enhanced functional expression of Mrp isoforms at the BBB can cause reduced brain and/or endothelial cell concentrations of GSH, an alteration in cellular redox status, and increased potential for cell injury and death. Therefore, discrete changes in Mrp activity at the BBB in response to oxidative stress warrant further investigation.
A third ABC superfamily member that may be involved in xenobiotic efflux is BCRP. Several recent studies have demonstrated localization of BCRP at the brain microvasculature, particularly along the luminal side of the BBB (Hori et al. 2004; Lee et al. 2005). In terms of transport activity, data from recent in vitro and in vivo studies are controversial. Although some studies have suggested that BCRP is not functional at the BBB (van Herwaarden et al. 2003; Lee et al. 2005; Lee et al. 2007) or plays a minimal role in xenobiotic efflux from the brain (Zhao et al. 2009), more detailed analyses have confirmed that BCRP is a critical determinant of drug permeation across the BBB (de Vries et al. 2007; Zhou et al. 2009; Kodaira et al. 2010; Agarwal et al. 2011). The effect of oxidative stress on BBB functional expression of BCRP is a critical point for future study.
2.1.3.2. Solute Carrier (SLC) Transporters
The second major group of drug transport proteins at the BBB endothelium is the SLC transporters. In contrast to ABC transporters, membrane transport of SLC family members is governed by either an electrochemical gradient utilizing an inorganic or organic solute as a driving force or the transmembrane concentration gradient of the substance actually being transported. To date, 319 SLC genes (i.e., SLC1 - SLC43 families) have been identified in humans (Sugiura et al. 2006). Of the 43 known families of SLC transporters, members of SLC21A/SLCO and SLC22 are known to be expressed at the BBB and play a critical role in determining xenobiotic permeation across the brain microvascular endothelium (Kusuhara and Sugiyama, 2005).
Of the SLC transporters known to transport drugs at the BBB, perhaps the most viable candidates for transporter targeting are members of the SLC21A/SLCO family that includes organic anion transporting polypeptides (OATPs in humans; Oatps in rodents). OATPs/Oatps have distinct substrate preferences for amphipathic solutes (Hagenbuch and Meier, 2004). OATPs/Oatps are well known to transport 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase inhibitors (i.e., statins), which have been recently shown to exhibit considerable neuroprotective and antioxidant activity in the CNS (Ponce et al. 2008; Wood et al. 2010; Barone et al. 2011; Butterfield et al. 2011). For example, studies in Xenopus laevis oocytes have shown Oatp1a4-mediated uptake of pravastatin (Tokui et al. 1999). Additionally, experimentation in Oatp1a4(−/−) mice demonstrated reduced blood-to-brain transport of pitavastatin and rosuvastatin as compared to wild-type controls, which suggests that Oatp1a4 is involved in statin transport across the BBB (Ose et al. 2010). Although OATP isoforms are expressed in several tissues, not all exist at the BBB. Immunofluorescence staining of human brain frontal cortex demonstrated OATP1A2 localization at the level of the brain microvascular endothelium (Gao et al. 2000). In rodent brain, expression of Oatp1a4, Oatp1 c1, and Oatp2a1 has been reported in capillary enriched fractions, capillary endothelial cells and/or isolated brain microvessels (Sugiyama et al. 2003; Taogoshi et al. 2005; Chu et al. 2008; Westholm et al. 2009a, b; Ronaldson et al. 2011; Thompson et al. 2014). Oatp1 c1 is selectively expressed at the BBB (Chu et al. 2008) and has relatively narrow substrate specificity and primarily transports thyroxine and conjugated sterols (Westholm et al. 2009a, b). Oatp2a1 regulates BBB transport of prostaglandins (Kis et al. 2006). It has proposed that Oatp1a4, a rodent homologue of OATP1A2, is the primary drug transporting Oatp isoform expressed at the rat BBB (Hagenbuch and Meier, 2004). Recently, our laboratory demonstrated that Oatp1a4 is a BBB transporter that can be effectively targeted for facilitation of effective CNS drug delivery (Ronaldson et al. 2011; Ronaldson and Davis, 2013; Thompson et al. 2014; Thompson and Ronaldson, 2014).
3. Effects of Oxidative Stress on the BBB
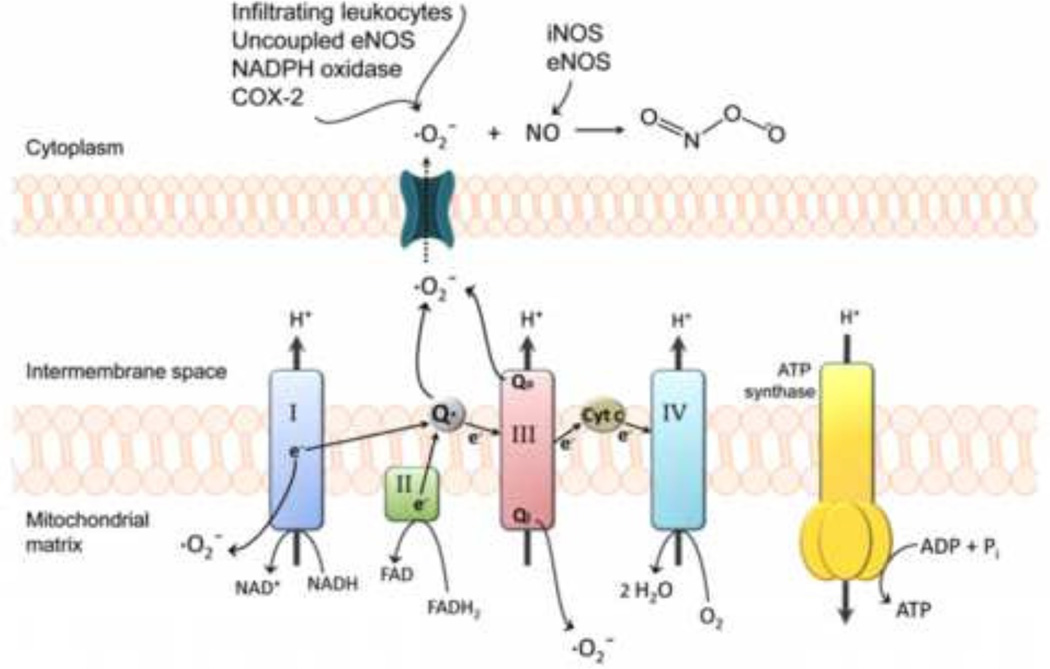
Functional BBB integrity can be disrupted by production of reactive oxygen species (ROS) and subsequent oxidative stress (Figure 3). Production of superoxide anion, a potent ROS generated when molecular oxygen is reduced by only one electron, is a known component of CNS pathologies with an oxidative stress component (Kim et al. 2001; Heo et al. 2005). Additionally, superoxide production has been reported in the chronic constriction injury model of neuropathic pain (Pathak et al. 2014). Superoxide dismutase (SOD) enzymes tightly control biological activity of superoxide anion, a by-product of normal physiological processes. Under oxidative stress conditions, superoxide is produced at high levels that overwhelm the metabolic capacity of SOD. This phenomenon is supported by the observation that infarct size and cerebral edema were markedly reduced in mice engineered to overexpress SOD as compared to wild type controls (Kim et al. 2001). Increased levels of superoxide also contribute to BBB endothelial dysfunction (Strasser et al. 1997; Nito et al. 2008; Lochhead et al. 2012). BBB damage can be intensified by conjugation of superoxide and NO to form peroxynitrite, a cytotoxic and proinflammatory molecule. Peroxynitrite causes significant injury to cerebral microvessels through lipid peroxidation, consumption of endogenous antioxidants (i.e., reduced GSH), and induction of mitochondrial failure (Pacher et al. 2007; Thompson and Ronaldson, 2014). Peroxynitrite is also known to induce endothelial damage by its ability to nitrosylate tyrosine, leading to functional modifications of critical proteins (Salvemini et al. 2006). Peroxynitrite formation in BBB endothelial cells becomes more likely with activation of endothelial NOS (eNOS) and inducible NOS (iNOS) because NO diffuses easily through membranes and readily reacts with superoxide anion (Pacher et al. 2007).
Figure 3.
Generation of reactive oxygen species (ROS) in brain microvascular endothelial cells. During disease, mitochondrial superoxide levels increase via NO inhibition of cytochrome complexes and oxidation of reducing equivalents in the electron transport chain. Complex I as well as both sides of complex III (i.e., Qi and Qo sites) are the most common sources of mitochondrial superoxide. Superoxide generated within the intermembrane space of mitochondria can reach the cytosol through voltage-dependent mitochondrial anion channels. Superoxide levels further increase via cyclooxygenase-2, NADPH oxidase, uncoupled eNOS, and infiltrating leukocytes. The resulting high levels of superoxide coupled with the activation of NO-producing eNOS and iNOS increases the probability of peroxynitrite formation. Peroxynitrite-induced cellular damage includes protein oxidation, tyrosine nitration, DNA damage, poly(ADP-ribose) polymerase activation, lipid peroxidation, and mitochondrial dysfunction. Adapted from Thompson and Ronaldson (2014). Adv Pharmacol. 71: 165–209.
Overall, oxidative stress contributes to disruption of endothelial cell-cell interactions and BBB injury by promoting redistribution and/or downregulation of critical tight junction proteins such as claudin-5, occludin, ZO-1, and JAM-1 (Schreibelt et al. 2007; Lochhead et al. 2010; Yang et al. 2013b; Rochfort et al. 2014). Downregulation of VE-cadherin was also shown to occur at the BBB in response to oxidative stress, an effect that led to increased permeability to FITC-labeled 40-kDa dextran (Rochfort et al. 2014). In the context of focal cerebral ischemia, reorganization of tight junction complexes enables considerable movement of vascular fluid across the microvascular endothelium (i.e., leak) and development of vasogenic edema (Heo et al. 2005; Sandoval and Witt, 2008; Pillai et al. 2009). In vivo, Evan’s blue dye conjugated with plasma proteins to form a large solute protein complex (i.e., in excess of 60,000 Da) that can only traverse the BBB under considerable pathological stress (Ronaldson and Davis, 2012). In other pathologies such as peripheral inflammatory pain, permeability changes at the BBB are more subtle, enabling movement of small vascular markers (i.e., sucrose) and drugs (i.e., codeine) while restricting movement of large substances such as Evan’s blue-albumin (Hau et al. 2004; Ronaldson et al. 2009; Lochhead et al. 2012). Decreased expression of occludin is associated with increased BBB permeability as shown in in vivo models of hypoxia/reoxygenation stress (Witt et al. 2003; Witt et al. 2005) and peripheral inflammatory pain (Huber et al. 2001; Ronaldson et al. 2009). Additionally, pathological stressors cause trafficking of occludin away from BBB tight junction protein complexes (McCaffrey et al. 2007; McCaffrey et al. 2008; McCaffrey et al. 2009; Lochhead et al. 2010; Lochhead et al. 2012). This occludin relocalization can be prevented in vivo by treatment with 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPOL), an antioxidant that readily crosses the BBB (Lochhead et al. 2012). Specifically, TEMPOL prevents breakage of disulfide bonds on occludin thereby blocking disruption of occludin oligomeric assemblies and subsequent blood-to-brain leak of circulating solutes, an effect that suggests efficacy in protecting against pathology-induced BBB injury (Lochhead et al. 2012).
The increase in BBB permeability observed in response to pathologies with an oxidative stress component involves changes to transcellular transport pathways in addition to tight junction modifications. For example, Yeh and colleagues showed that hypoxia upregulates glucose transporters (i.e., GLUT1) (Yeh et al. 2008). Additionally, the sodium-glucose cotransporter-1 (SGLT1) plays a significant role in glucose uptake and edema formation during ischemia (Vemula et al. 2009). Recently, our own laboratory discovered that the endogenous BBB transporter Oatp1a4 is upregulated at the BBB following global cerebral hypoxia (Thompson et al. 2014) or following peripheral inflammatory pain (Ronaldson et al. 2011). This transporter is capable of promoting blood-to-brain transport of therapeutic drugs (Ronaldson et al. 2011; Thompson et al. 2014). Increased functional expression of efflux transport systems such as P-gp has also been reported at the BBB in an in vivo model of peripheral inflammatory pain (Seelbach et al. 2007; McCaffrey et al. 2012; Sanchez-Covarrubias et al. 2013). In addition to putative membrane transporters, an increase in specific vesicular transport and pinocytosis within BBB endothelial cells has been reported (Cipolla et al. 1997).
4. Targeting Endogenous BBB Transporters
The ability of a drug to elicit a pharmacological effect at the level of the BBB requires the achievement of efficacious concentrations within the endothelial cell cytoplasm. This therapeutic objective is dependent upon multiple mechanisms of transport that may include uptake into the cell by an influx transporter and/or extrusion from the endothelium by an efflux transporter. For many drugs, it is this discrete balance between influx and efflux that determines if a therapeutic agent will be able to protect the BBB from oxidative stress-associated injury. The complexity of drug transporter biology at the BBB is further underscored by the observation that functional expression of transporters can be dramatically altered by oxidative stress (Hong et al. 2006; Ronaldson and Bendayan, 2008; Wang et al. 2014). A thorough understanding of the regulation and functional expression of endogenous BBB transporters in both health and disease is essential for the provision of effective pharmacotherapy. Furthermore, such information will enable effective targeting of transporters and/or transporter regulatory mechanisms, thus allowing endogenous BBB transport systems to be exploited for purposes of conferring BBB protection and/or repair.
Considerable research has focused on studying mechanisms that limit endothelial membrane transport by describing the role of P-gp in restricting drug uptake from the systemic circulation (Hartz and Bauer, 2010; Tournier et al. 2011; Slosky et al. 2013; Sanchez-Covarrubias et al. 2014); however, clinical trials targeting P-gp with small molecule inhibitors have been unsuccessful in improving pharmacotherapy due to inhibitor toxicity and/or enhanced global tissue penetration of drugs (Potschka, 2010; Kalvass et al. 2011; Palmeira et al. 2012). An alternative approach for optimizing delivery of drugs across the endothelial plasma membrane is to focus on BBB transporters that are involved in cellular uptake of drugs. One intriguing candidate is Oatp1a4, which is known to transport HMG-CoA reductase inhibitors (i.e., statins). Recent evidence suggests that statins can act as ROS scavengers independent of their well-documented effects on cholesterol biosynthesis (Kassan et al. 2010; Barone et al. 2011). Specifically, studies in dogs demonstrated that atorvastatin reduced expression of oxidative and nitrosative stress markers (i.e., protein carbonyls, 4-hydroxy-2-noneal, 3-nitrotyrosine) and increased brain GSH levels (Kassan et al. 2010; Barone et al. 2011; Butterfield et al. 2011). Interestingly, Cui and colleagues showed, in vivo, that atorvastatin administration during the acute phase of cerebral ischemia prevented the increased BBB permeability that is commonly associated with this disease (Cui et al. 2010). More recently, simvastatin was demonstrated to preserve barrier function following experimental intracerebral hemorrhage in an in vivo study involving MRI measurements of T1sat, a marker of BBB integrity (Yang et al. 2013a). Taken together, these studies suggest that effective delivery of statins to the brain microvascular endothelium may be an effective strategy for reducing deleterious effects of oxidative stress and protecting the BBB. We have shown, in vivo, that Oatp1a4 is a BBB transporter target that can be exploited to optimize delivery of drugs, including statins, across the plasma membrane of the brain microvascular endothelium (Ronaldson et al. 2011; Thompson et al. 2014). Specifically, our data in an in vivo model of peripheral inflammatory pain reported increased influx and efflux rate constants for taurocholate, a known Oatp substrate (Ronaldson et al. 2011). These data strongly indicate that drugs can be delivered to the endothelial cell cytoplasm by targeted Oatp1a4-mediated delivery (Ronaldson et al. 2011).
Although pathophysiological stressors can modulate BBB transporters, such changes must be controlled to provide optimal delivery of drugs. For example, we have demonstrated increased functional expression of Oatp1a4 only between 1 h and 6 h after induction of pain/inflammation (Ronaldson et al. 2011). Similarly, we showed increased Oatp1a4 expression after 1 h hypoxia followed by up to 1 h reoxygenation (Thompson et al. 2014). If Oatp1a4 is to facilitate effective delivery of drugs (i.e., statins), its functional expression must be precisely controlled over a more desirable time course than is possible by relying on pathophysiological changes. This objective can be accomplished by pharmacological targeting of Oatp regulatory pathways such as the transforming growth factor-β (TGF-β) system (Ronaldson et al. 2011; Thompson and Ronaldson, 2014; Thompson et al. 2014). TGF-ps are cytokines that signal by binding to a heterotetrameric complex of type I and type II receptors (Derynck and Zhang, 2003). The type I receptors, also known as activin receptor-like kinases (ALKs) propagate intracellular signals through phosphorylation of receptor-specific Smad proteins (i.e., (R)-Smads). Phosphorylated (R)-Smads complex with Smad4, which enables nuclear translocation and changes in target gene transcription. At the BBB, only two ALK receptors (ALK1, ALK5) have been identified (Ronaldson et al. 2009). We have shown that pharmacological inhibition of TGF-β/ALK5 signaling can increase Oatp1a4 functional expression (Ronaldson et al. 2011; Thompson and Ronaldson, 2014; Thompson et al. 2014). This observation suggests that targeting of the TGF-β/ALK5 pathway may enable control of BBB Oatp1a4 expression and/or activity, thereby providing novel strategies for improved BBB protection in diseases with an oxidative stress component.
Optimization of drug delivery is not the only benefit that can be achieved from targeting transporters. BBB transporters mediate flux of endogenous substrates, many of which are essential to the cellular response to pathological insult. One such substance is the endogenous antioxidant GSH. During oxidative stress, GSH is rapidly oxidized to glutathione disulfide (GSSG). Therefore, the redox state of a cell is represented by the ratio of GSH to GSSG (Park et al. 2000; Namba et al. 2001; Li et al. 2012). In vitro studies using human and rodent brain microvascular endothelial cells have demonstrated that hypoxia reduces intracellular GSH levels and decreases the GSH:GSSG ratio, suggesting significant oxidative stress at the level of the BBB (Plateel et al. 1995; Muruganandam et al. 2000; Namba et al. 2001). Using an in vivo model, oxidative stress was shown to cause BBB disruption characterized by altered expression/assembly of tight junction proteins occludin (Witt et al. 2003; Brown and Davis, 2005; McCaffrey et al. 2009; Lochhead et al. 2010), claudin-5 (Witt et al. 2003; Willis et al. 2010), and ZO-1 (Mark and Davis, 2002; Willis et al. 2010). These tight junction modifications correlated with increased BBB permeability to sucrose, an established vascular marker (Witt et al. 2008; Lochhead et al. 2010), and dextrans (Willis et al. 2010). Such increases in BBB permeability can result in leak of neurotoxic substances from blood into brain and/or contribute to vasogenic edema. BBB protection and/or repair in diseases with an oxidative stress component are paramount to protecting the brain from neurological damage. One approach that can accomplish this therapeutic objective is to prevent cellular loss of GSH from endothelial cells (i.e., preservation of the endothelial antioxidant defense system) by targeting endogenous BBB transporters. BBB transporters that can transport GSH and GSSG include MRPs/Mrps. Both GSH and GSSG are substrates for MRP1/Mrp1 (Hirrlinger et al. 2001; Hirrlinger and Dringen, 2005; Ronaldson and Bendayan, 2008; Tadepalle et al. 2014), MRP2/Mrp2 (Paulusma et al. 1999), and MRP4/Mrp4 (Rius et al. 2006). In contrast, MRP5/Mrp5 is not involved in GSH and/or GSSG transport (Minich et al. 2006). It has well known that increased cellular concentrations of GSH are cytoprotective while processes that promote GSH loss from cells are damaging (Ballatori et al. 2009). Therefore, it stands to reason that pharmacological targeting of Mrps during oxidative stress may have profound therapeutic benefits. Using the known Mrp transport inhibitor MK571, Tadepalle and colleagues showed that inhibition of Mrp1 -mediated GSH transport resulted prevented GSH depletion in primary cultures of rat astrocytes (Tadepalle et al. 2014). Indeed, the effect of Mrp transport inhibition at the BBB and its effect on endothelial redox status and barrier integrity requires further study.
Previous studies have shown that Mrp expression and/or activity can change in response to oxidative stress (Maher et al. 2007; Ronaldson and Bendayan, 2008). Altered BBB expression of Mrps may prevent endothelial cells from retaining effective GSH concentrations. A thorough understanding of signaling pathways involved in Mrp regulation during oxidative stress will enable development of pharmacological approaches to target Mrp-mediated efflux (i.e., GSH transport) for the purpose of preventing BBB dysfunction in diseases with an oxidative stress component. One intriguing pathway is signaling mediated by nuclear factor E2-related factor-2 (Nrf2), a sensor of oxidative stress (Alfieri et al. 2011; Wang et al. 2014). In the presence of ROS, the cytosolic Nrf2 repressor Kelch-like ECH-associated protein 1 (Keap1) undergoes structural alterations that cause dissociation from the Nrf2-Keap1 complex. This enables Nrf2 to translocate to the nucleus and induce transcription of genes that possess an antioxidant response element at their promoter (Hayashi et al. 2003; Ma, 2013). It has been demonstrated that activation of Nrf2 signaling induces expression of Mrp1, Mrp2, and Mrp4 (Hayashi et al. 2003; Vollrath et al. 2006; Maher et al. 2007; Aleksunes et al. 2008; Xu et al. 2010; Wang et al. 2014). An emerging concept is that Nrf2 acts as a double-edged sword (Ma, 2013): on one hand, Nrf2 is required for protecting tissues from oxidative stress; on the other, its activation can lead to deleterious effects. Therefore, an alteration in the balance of Mrp isoforms via activation of Nrf2 signaling may adversely affect redox balance and antioxidant defense at the brain microvascular endothelium. Indeed, this points towards a need for rigorous study of pharmacological approaches (i.e., use of antioxidant drugs such as TEMPOL) that can modulate Nrf2 signaling and control expression of Mrp isoforms and/or GSH transport at the BBB.
5. Conclusions and Future Perspectives
The field of BBB biology, particularly the study of tight junction/adherens junction protein complexes and endogenous transport systems, has rapidly advanced over the past several years. It is now established that tight junctions and adherens junctions are dynamic in nature and can organize and reorganize in response to oxidative stress. These changes can lead to increased BBB permeability to endogenous and exogenous solutes. Transporters (i.e., Oatps, Mrps) provide a considerable opportunity to protect the BBB and/or promote BBB repair by either facilitating endothelial uptake of drugs with cytoprotective/antioxidant properties or by preventing cellular loss of critical endogenous substances (i.e., GSH). Furthermore, molecular mechanisms involved in regulating endogenous BBB transport systems such as TGF-β/ALK5 signaling and the Nrf2 pathway are just now being identified and characterized. These discoveries have identified discrete molecular targets that can be exploited for control of BBB xenobiotic transport. Perhaps targeting of novel drugs to influx transporters such as Oatp1a4 or to efflux systems such as Mrps will lead to significant advances in treatment of diseases such as cerebral hypoxia/ischemia and peripheral inflammatory pain. Characterization of intracellular signaling pathways that can regulate functional expression of BBB transporters provides another approach for pharmacological control of transport systems in an effort to prevent BBB dysfunction. Future work will continue to provide more information on the interplay of cell-cell interactions, transporters, and signaling pathways at the BBB endothelium and how these systems can be effectively targeted. Ultimately, data derived from these studies will enable achievement of more precise and more efficient drug concentrations within brain microvessel endothelial cells and improved BBB protection and/or repair following pathophysiological insult.
Highlights.
We provide an overview of physical and biochemical properties of the blood-brain barrier (BBB).
We review oxidative stress mechanisms that can lead to BBB injury.
We outline transporter systems that can be targeted for BBB protection and/or promotion of BBB repair in disease.
Acknowledgements
This work was supported by grants from the National Institutes of Health to PTR (R01 NS084941) and to TPD (R01 NS42652 and R01 DA1 1271).
Non-standard abbreviations
- ABC
ATP Binding Cassette
- ALK
activin receptor-like kinase
- BBB
blood-brain barrier
- BCRP
Breast Cancer Resistance Protein
- CNS
central nervous system
- GSH
glutathione
- GSSG
glutathione disulfide
- JAM
junctional adhesion molecule
- MAGUK
membrane-associated guanylate kinase-like
- MCAO
middle cerebral artery occlusion
- MDR
multidrug resistance
- MRP
Multidrug Resistance Protein
- Nrf2
nuclear factor E2-related factor-2
- OATP
organic anion transporting polypeptide
- P-gp
P-glycoprotein
- SLC
solute carrier
- SOD
superoxide dismutase
- TEMPOL
4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl
- TGF
transforming growth factor
- ZO
zonula occluden
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Abbruscato TJ, Lopez SP, Mark KS, Hawkins BT, Davis TP. Nicotine and cotinine modulate cerebral microvascular permeability and protein expression of ZO-1 through nicotinic acetylcholine receptors expressed on brain endothelial cells. J Pharm Sci. 2002;91:2525–2538. doi: 10.1002/jps.10256. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Sane R, Ohlfest JR, Elmquist WF. The role of the breast cancer resistance protein (ABCG2) in the distribution of sorafenib to the brain. J Pharmacol Exp Ther. 2011;336:223–233. doi: 10.1124/jpet.110.175034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksunes LM, Slitt AL, Maher JM, Augustine LM, Goedken MJ, Chan JY, Cherrington NJ, Klaassen CD, Manautou JE. Induction of Mrp3 and Mrp4 transporters during acetaminophen hepatotoxicity is dependent on Nrf2. Toxicol Appl Pharmacol. 2008;226:74–83. doi: 10.1016/j.taap.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfieri A, Srivastava S, Siow RCM, Modo M, Fraser PA, Mann GE. Targeting the Nrf2-Keap1 antioxidant defence pathway for neurovascular protection in stroke. J Physiol. 2011;58:4125–4136. doi: 10.1113/jphysiol.2011.210294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballatori N, Krance SM, Marchan R, Hammond CL. Plasma membrane glutathione transporters and their roles in cell physiology and pathophysiology. Mol Aspects Med. 2009;30:13–28. doi: 10.1016/j.mam.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandler PE, Westlake CJ, Grant CE, Cole SP, Deeley RG. Identification of regions required for apical membrane localization of human multidrug resistance protein 2. Mol Pharmacol. 2008;74:9–19. doi: 10.1124/mol.108.045674. [DOI] [PubMed] [Google Scholar]
- Barone E, Cenini G, Di Domenico F, Martin S, Sultana R, Mancuso C, Murphy MP, Head E, Butterfield DA. Long-term high-dose atorvastatin decreases brain oxidative and nitrosative stress in a preclinical model of Alzheimer disease: a novel mechanism of action. Pharmacol Res. 2011;63:172–180. doi: 10.1016/j.phrs.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer B, Hartz AM, Lucking JR, Yang X, Pollack GM, Miller DS. Coordinated nuclear receptor regulation of the efflux transporter, Mrp2, and the phase-II metabolizing enzyme, GSTpi, at the blood-brain barrier. J Cereb Blood Flow Metab. 2008;28:1222–1234. doi: 10.1038/jcbfm.2008.16. [DOI] [PubMed] [Google Scholar]
- Bauer HC, Krizbal IA, Bauer H, Traweger A. “You shall not pass”-tight junctions of the blood brain barrier. Front Neurosci. 2014;8:392. doi: 10.3389/fnins.2014.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu E, Demeule M, Ghitescu L, Beliveau R. P-glycoprotein is strongly expressed in the luminal membranes of the endothelium of blood vessels in the brain. Biochem J. 1997;326:539–544. doi: 10.1042/bj3260539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendayan R, Ronaldson PT, Gingras D, Bendayan M. In situ localization of P-glycoprotein (ABCB1) in human and rat brain. J Histochem Cytochem. 2006;54:1159–1167. doi: 10.1369/jhc.5A6870.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betanzos A, Huerta M, Lopez-Bayghen E, Azuara E, Amerena J, Gonzalez-Mariscal L. he tight junction protein ZO-2 associates with Jun, Fos and C/EBP transcription factors in epithelial cells. Exp Cell Res. 2004;292:51–66. doi: 10.1016/j.yexcr.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Brooks TA, Ocheltree SM, Seelbach MJ, Charles RA, Nametz N, Egleton RD, Davis TP. Biphasic cytoarchitecture and functional changes in the BBB induced by chronic inflammatory pain. Brain Res. 2006;1120:172–182. doi: 10.1016/j.brainres.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RC, Davis TP. Hypoxia/aglycemia alters expression of occludin and actin in brain endothelial cells. Biochem Biophys Res Commun. 2005;327:1114–1123. doi: 10.1016/j.bbrc.2004.12.123. [DOI] [PubMed] [Google Scholar]
- Butt AM, Jones HC, Abbott NJ. Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J Physiol. 1990;429:47–62. doi: 10.1113/jphysiol.1990.sp018243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Barone E, Mancuso C. Cholesterol-independent neuroprotective and neurotoxic activities of statins: perspectives for statin use in Alzheimer disease and other age-related neurodegenerative disorders. Pharmacol Res. 2011;64:180–186. doi: 10.1016/j.phrs.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright TA, Campos CR, Cannon RE, Miller DS. Mrp1 is essential for sphingolipid signaling to p-glycoprotein in mouse blood-brain and blood-spinal cord barriers. J Cereb Blood Flow Metab. 2013;33:381–388. doi: 10.1038/jcbfm.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C, Li JY, Boado RJ, Pardridge WM. Blood-brain barrier genomics and cloning of a novel organic anion transporter. J Cereb Blood Flow Metab. 2008;28:291–301. doi: 10.1038/sj.jcbfm.9600538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla MJ, Crete R, Vitullo L, Rix RD. Transcellular transport as a mechanism of blood-brain barrier disruption during stroke. Front Biosci. 2004;9:777–785. doi: 10.2741/1282. [DOI] [PubMed] [Google Scholar]
- Cui L, Zhang X, Yang R, Wang L, Liu L, Li M, Du W. Neuroprotection of early and short-time applying atorvastatin in the acute phase of cerebral ischemia: down-regulated 12/15-LOX, p38MAPK and cPLA2 expression, ameliorated BBB permeability. Brain Res. 2010;1325:164–173. doi: 10.1016/j.brainres.2010.02.036. [DOI] [PubMed] [Google Scholar]
- Dallas S, Miller DS, Bendayan R. Multidrug resistance-associated proteins: expression and function in the central nervous system. Pharmacol Rev. 2006;58:140–161. doi: 10.1124/pr.58.2.3. [DOI] [PubMed] [Google Scholar]
- de Vries NA, Zhao J, Kroon E, Buckle T, Beijnen JH, van Tellingen O. P-glycoprotein and breast cancer resistance protein: two dominant transporters working together in limiting the brain penetration of topotecan. Clin Cancer Res. 2007;13:6440–6449. doi: 10.1158/1078-0432.CCR-07-1335. [DOI] [PubMed] [Google Scholar]
- Dejana E, Lampugnani MG, Martinez-Estrada O, Bazzoni G. The molecular organization of endothelial junctions and their functional role in vascular morphogenesis and permability. Int J Dev Biol. 2000;44:743–748. [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signaling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Dogrukol-Ak D, Kumar VB, Ryerse JS, Farr SA, Verma S, Nonaka N, Nakamachi T, Ohtaki H, Niehoff ML, Edwards JC, Shioda S, Morley JE, Banks WA. Isolation of peptide transport system-6 from brain endothelial cells: therapeutic effects with antisense inhibition in Alzheimer and stroke models. J Cereb Blood Flow Metab. 2009;29:411–422. doi: 10.1038/jcbfm.2008.131. [DOI] [PubMed] [Google Scholar]
- Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- Feldman GJ, Mullin JM, Ryan MP. Occludin: structure, function and regulation. Adv Drug Deliv Rev. 2005;57:883–917. doi: 10.1016/j.addr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Fischer S, Wobben M, Marti HH, Renz D, Schaper W. Hypoxia-induced hyperpermeability in brain microvessel endothelial cells involves VEGF-mediated changes in the expression of zonula occludens-1. Microvasc Res. 2002;63:70–80. doi: 10.1006/mvre.2001.2367. [DOI] [PubMed] [Google Scholar]
- Forster C, Burek M, Romero IA, Weksler B, Couraud PO, Drenckhahn D. Differential effects of hydrocortisone and TNFalpha on tight junction proteins in an in vitro model of the human blood-brain barrier. J Physiol. 2008;586:1937–1949. doi: 10.1113/jphysiol.2007.146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol. 1999;147:891–903. doi: 10.1083/jcb.147.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Hagenbuch B, Kullak-Ublick GA, Benke D, Aguzzi A, Meier PJ. Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J Pharmacol Exp Ther. 2000;294:73–79. [PubMed] [Google Scholar]
- Gibson CL, Srivastava K, Sprigg N, Bath PM, Bayraktutan U. Inhibition of Rho-kinase protects cerebral barrier from ischaemia-evoked injury through modulations of endothelial cell oxidative stress and tight junctions. J Neurochem. 2014;129:816–826. doi: 10.1111/jnc.12681. [DOI] [PubMed] [Google Scholar]
- Golden PL, Pardridge WM. P-glycoprotein on astrocyte foot processes of unfized isolated human brain capillaries. Brain Res. 1999;819:143–146. doi: 10.1016/s0006-8993(98)01305-5. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Betanzos A, Avila-Flores A. MAGUK proteins: structure and role in the tight junction. Semin Cell Dev Biol. 2000;11:315–324. doi: 10.1006/scdb.2000.0178. [DOI] [PubMed] [Google Scholar]
- Gottardi CJ, Arpin M, Fanning AS, Louvard D. The junction-associated protein, zonula occludens-1, localizes to the nucleus before the maturation and during the remodeling of cell-cell contacts. Proc Natl Acad Sci U S A. 1996;93:10779–10784. doi: 10.1073/pnas.93.20.10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman MM, Hrycyna CA, Schoenlein PV, Germann UA, Pastan I. Genetic analysis of the multidrug transporter. Annu Rev Genet. 1995;29:607–649. doi: 10.1146/annurev.ge.29.120195.003135. [DOI] [PubMed] [Google Scholar]
- Haarmann A, Deiss A, Prochaska J, Foerch C, Weksler B, Romero I, Couraud PO, Stoll G, Rieckmann P, Buttmann M. Evaluation of soluble junctional adhesion molecule-A as a biomarker of human brain endothelial barrier breakdown. PLoS One. 2010;5:e13568. doi: 10.1371/journal.pone.0013568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447:653–665. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- Hartz AM, Bauer B. Regulation of ABC transportes at the blood-brain barrier: new targets for CNS therapy. Mol Interv. 2010;10:293–304. doi: 10.1124/mi.10.5.6. [DOI] [PubMed] [Google Scholar]
- Hartz AM, Bauer B, Soldner EL, Wolf A, Boy S, Backhaus R, Mihaljevic I, Bogdahn U, Klunemann HH, Schuierer G, Schlachetzki F. Amyloid-β contributes to blood-brain barrier leakage in transgenic human amyloid precursor protein mice and in humans with cerebral amyloid angiopathy. Stroke. 2012;43:514–523. doi: 10.1161/STROKEAHA.111.627562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hau VS, Huber JD, Campos CR, Davis RT, Davis TP. Effect of lambda-carrageenan-induced inflammatory pain on brain uptake of codeine and antinociception. Brain Res. 2004;1018:257–264. doi: 10.1016/j.brainres.2004.05.081. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Abbruscato TJ, Egleton RD, Brown RC, Huber JD, Campos CR, Davis TP. Nicotine increases in vivo blood-brain barrier permeability and alters cerebral microvascular tight junction protein distribution. Brain Res. 2004;1027:48–58. doi: 10.1016/j.brainres.2004.08.043. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Ocheltree SM, Norwood KM, Egleton RD. Decreased blood-brain barrier permeability to fluorescein in streptozotocin-treated rats. Neurosci Lett. 2007;411:1–5. doi: 10.1016/j.neulet.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi A, Suzuki H, Itoh K, Yamamoto M, Sugiyama Y. Transcription factor Nrf2 is required for the constitutive and inducible expression of multidrug resistance-associated protein 1 in mouse embryo fibroblasts. Biochem Biophys Res Commun. 2003;310:824–829. doi: 10.1016/j.bbrc.2003.09.086. [DOI] [PubMed] [Google Scholar]
- Heo JH, Han SW, Lee SK. Free radicals as triggers of brain edema formation after stroke. Free Radic Biol Med. 2005;39:51–70. doi: 10.1016/j.freeradbiomed.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Hirrlinger J, Dringen R. Multidrug resistance protein 1-mediated export of glutathione and glutathione disulfide from brain astrocytes. Methods Enzymol. 2005;400:395–409. doi: 10.1016/S0076-6879(05)00023-6. [DOI] [PubMed] [Google Scholar]
- Hirrlinger J, Konig J, Keppler D, Lindenau J, Schulz JB, Dringen R. The multidrug resistance protein MRP1 mediates the release of glutathione disulfide from rat astrocytes during oxidative stress. J Neurochem. 2001;76:627–636. doi: 10.1046/j.1471-4159.2001.00101.x. [DOI] [PubMed] [Google Scholar]
- Hoffman WH, Stamatovic SM, Andjelkovic AV. Inflammatory mediators and blood brain barrier disruption in fatal brain edema of diabetic ketoacidosis. Brain Res. 2009;1254:138–148. doi: 10.1016/j.brainres.2008.11.100. [DOI] [PubMed] [Google Scholar]
- Hong H, Lu Y, Ji ZN, Liu GQ. Up-regulation of P-glycoprotein expression by glutathione depletion-induced oxidative stress in rat brain microvessel endothelial cells. J Neurochem. 2008;98:1465–1473. doi: 10.1111/j.1471-4159.2006.03993.x. [DOI] [PubMed] [Google Scholar]
- Hori S, Ohtsuki S, Tachikawa M, Kimura N, Kondo T, Watanabe M, Nakashima E, Terasaki T. Functional expression of rat ABCG2 on the luminal side of brain capillaries and its enhancement by astrocyte-derived soluble factor(s) J Neurochem. 2004;90:526–536. doi: 10.1111/j.1471-4159.2004.02537.x. [DOI] [PubMed] [Google Scholar]
- Huber JD, Witt KA, Hom S, Egleton RD, Mark KS, Davis TP. Inflammatory pain alters blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol. 2001;280:H1241–H1248. doi: 10.1152/ajpheart.2001.280.3.H1241. [DOI] [PubMed] [Google Scholar]
- Hyun SW, Jung YS. Hypoxia induces FoxO3-mediated dysfunction of blood-brain barrier. Biochem Biophys Res Commun. 2014;450:1638–1642. doi: 10.1016/j.bbrc.2014.07.055. [DOI] [PubMed] [Google Scholar]
- Jiao H, Wang Z, Liu Y, Wang P, Xue Y. Specific role of tight junction proteins claudin-5, occludin, and ZO-1 of the blood-brain barrier in a focal cerebral ischemic insult. J Mol Neurosci. 2011;44:130–139. doi: 10.1007/s12031-011-9496-4. [DOI] [PubMed] [Google Scholar]
- Kalvass JC, Polli JW, Bourdet DL, Feng B, Huang SM, Liu X, Smith QR, Zhang LK, Zamek-Gliszczynski MJ. Why clinical modulation of efflux transport at the human blood-brain barrier is unlikely: the ITC evidence-based position. Clin Pharmacol Ther. 2013;94:80–94. doi: 10.1038/clpt.2013.34. [DOI] [PubMed] [Google Scholar]
- Kassan M, Montero MJ, Sevilla MA. In vitro antioxidant activity of pravastatin provides vascular protection. Eur J Pharmacol. 2010;630:107–111. doi: 10.1016/j.ejphar.2009.12.037. [DOI] [PubMed] [Google Scholar]
- Kim MY, Zhang T, Kraus WL. Poly(ADP-ribosyl)ation by PARP-1: ‘PAR-laying’ NAD+ into a nuclear signal. Genes Dev. 2005;19:1951–1967. doi: 10.1101/gad.1331805. [DOI] [PubMed] [Google Scholar]
- Kis B, isse T, Snipes JA, Chen L, Yamashita H, Ueta Y, Busija DW. Effects of LPS stimulation on the expression of prostaglandin carriers in the cells of the blood-brain and blood-cerebrospinal fluid barriers. J Appl Physiol. 2006;100:1392–1399. doi: 10.1152/japplphysiol.01259.2005. [DOI] [PubMed] [Google Scholar]
- Kodaira H, Kusuhara H, Ushiki J, Fuse E, Sugiyama Y. Kinetic analysis of the cooperation of P-glycoprotein (P-gp/Abcb1) and breast cancer resistance protein (Bcrp/Abcg2) in limiting the brain and testis penetration of erlotinib, flavopiridol, and mitoxantrone. J Pharmacol Exp Ther. 2010;333:788–796. doi: 10.1124/jpet.109.162321. [DOI] [PubMed] [Google Scholar]
- Kusuhara H, Sugiyama Y. Active efflux across the blood-brain barrier: role of the solute carrier family. NeuroRx. 2005;2:73–85. doi: 10.1602/neurorx.2.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaz-Vieira F, Jaeger MM, Farshori P, Kachar B. Small synthetic peptides homologous to segments of the first external loop of occludin impair tight junction resealing. J Membr Biol. 1999;168:289–297. doi: 10.1007/s002329900518. [DOI] [PubMed] [Google Scholar]
- Lampugnani MG, Dejana E. The control of endothelial cell functions by adherens junctions. Novartis Found Symp. 2007;283:4–13. doi: 10.1002/9780470319413.ch2. [DOI] [PubMed] [Google Scholar]
- Lee G, Babakhanian K, Ramaswamy M, Prat A, Wosik K, Bendayan R. Expression of the ATP-binding cassette membrane transporter, ABCG2, in human and rodent brain microvessel endothelial and glial cell culture systems. Pharm Res. 2007;24:1262–1274. doi: 10.1007/s11095-007-9244-1. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Kusuhara H, Jonker JW, Schinkel AH, Sugiyama Y. Investigation of efflux transport of dehydroepiandrosterone sulfate and mitoxantrone at the mouse blood-brain barrier: a minor role of breast cancer resistance protein. J Pharmacol Exp Ther. 2005;312:44–52. doi: 10.1124/jpet.104.073320. [DOI] [PubMed] [Google Scholar]
- Leggas M, Adachi M, Scheffer GL, Sun D, Wielinga P, Du G, Mercer KE, Zhuang Y, Panetta JC, Johnston B, Scheper RJ, Stewart CF, Schuetz JD. Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol. 2004;24:7612–7621. doi: 10.1128/MCB.24.17.7612-7621.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepist EI, Damaraju VL, Zhang J, Gati WP, Yao SY, Smith KM, Karpinski E, Young JD, Leung KH, Cass CE. Transport of A1 adenosine receptor agonist tecadenoson by human and mouse nucleoside transporters: evidence for blood-brain barrier transport by murine equilibrative nucleoside transporter 1 mENT1. Drug Metab Dispos. 2013;41:916–922. doi: 10.1124/dmd.112.049858. [DOI] [PubMed] [Google Scholar]
- Leslie EM, Deeley RG, Cole SP. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol. 2005;204:216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Li H, Gao A, Feng D, Wang Y, Zhang L, Cui Y, Li B, Wang Z, Chen G. Evaluation of the protective potential of brain microvascular endothelial cell autophagy on blood-brain barrier integrity during experimental cerebral ischemia-reperfusion injury. Transl Stroke Res. 2014;5:618–626. doi: 10.1007/s12975-014-0354-x. [DOI] [PubMed] [Google Scholar]
- Li W, Busu C, Circu ML, Aw TY. Glutathione in cerebral microvascular endothelial biology and pathobiology: Implications for brain homeostasis. Int J Cell Biol. 2012;2012:434971. doi: 10.1155/2012/434971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead JJ, McCaffrey G, Quigley CE, Finch J, DeMarco KM, Nametz N, Davis TP. Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab. 2010;30:1625–1636. doi: 10.1038/jcbfm.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead JJ, McCaffrey G, Sanchez-Covarrubias L, Finch JD, DeMarco KM, Quigley CE, Davis TP, Ronaldson PT. Tempol modulates changes in xenobiotic permeability and occludin oligomeric assemblies at the blood-brain barrier during inflammatory pain. Am J Physiol Heart Circ Physiol. 2012;302:H582–H593. doi: 10.1152/ajpheart.00889.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. Role of Nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxiciol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher JM, Dieter MZ, Aleksunes LM, Slitt AL, Guo G, Tanaka Y, Scheffer GL, Chan JY, Manautou JE, Chen Y, Dalton TP, Yamamoto M, Klaassen CD. Oxidative and electrophilic stress induces multidrug resistance-associated protein transporters via the nuclear factor-E2-related factor-2 transcriptional pathway. Hepatology. 2007;46:1597–1610. doi: 10.1002/hep.21831. [DOI] [PubMed] [Google Scholar]
- Mark KS, Davis TP. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol. 2002;282:H1485–H1494. doi: 10.1152/ajpheart.00645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey G, Staatz WD, Sanchez-Covarrubias L, Finch JD, DeMarco K, Laracuente ML, Ronaldson PT, Davis TP. P-glycoprotein trafficking at the blood-brain barrier altered by peripheral inflammatory hyperalgesia. J Neurochem. 2012;122:962–975. doi: 10.1111/j.1471-4159.2012.07831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey G, Staatz WD, Quigley CA, Nametz N, Seelbach MJ, Campos CR, Brooks TA, Egleton RD, Davis TP. Tight junctions contain oligomeric protein assembly critical for maintaining blood-brain barrier integrity in vivo. J Neurochem. 2007;103:2540–2555. doi: 10.1111/j.1471-4159.2007.04943.x. [DOI] [PubMed] [Google Scholar]
- McCaffrey G, Seelbach MJ, Staatz WD, Nametz N, Quigley C, Campos CR, Brooks TA, Davis TP. Occludin oligomeric assembly at tight junctions of the blood-brain barrier is disrupted by peripheral inflammatory hyperalgesia. J Neurochem. 2008;106:2395–2409. doi: 10.1111/j.1471-4159.2008.05582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng W, Takeichi M. Adherens junction: molecular architecture and regulation. Cold Spring Harb Perspect Biol. 2009;1:a002899. doi: 10.1101/cshperspect.a002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DS, Nobmann SN, Gutmann H, Toeroek M, Drewe J, Fricker G. Xenobiotic transport across isolated brain microvessels studied by confocal microscopy. Mol Pharmacol. 2000;58:1357–1367. doi: 10.1124/mol.58.6.1357. [DOI] [PubMed] [Google Scholar]
- Minich T, Riemer J, Schulz JB, Wielinga P, Wijnholds J, Dringen R. The multidrug resistance protein 1 (Mrp1), but not Mrp5, mediates export of glutathione and glutathione disulfide from brain astrocytes. J Neurochem. 2005;97:373–384. doi: 10.1111/j.1471-4159.2006.03737.x. [DOI] [PubMed] [Google Scholar]
- Miyajima M, Kusuhara H, Fujishima M, Adachi Y, Sugiyama Y. Organic anion transporter 3 mediates the efflux transport of an amphipathic organic anion, dehydroepiandrosterone sulfate, across the blood-brain barrier in mice. Drug Metab Dispos. 2011;39:814–819. doi: 10.1124/dmd.110.036863. [DOI] [PubMed] [Google Scholar]
- Muruganandam A, Smith C, Ball R, Herring T, Stanimirovic D. Glutathione homeostasis and leukotriene-induced permeability in human blood-brain barrier endothelial cells subjected to in vitro ischemia. Acta Neurochir Suppl. 2000;76:29–34. doi: 10.1007/978-3-7091-6346-7_6. [DOI] [PubMed] [Google Scholar]
- Namba K, Takeda Y, Sunami K, Hirakawa M. Temporal profiles of the levels of endogenous antioxidants after four-vessel occlusion in rats. J Neurosurg Anesthesiol. 2001;13:131–137. doi: 10.1097/00008506-200104000-00010. [DOI] [PubMed] [Google Scholar]
- Nito C, Kamada H, Endo H, Niizuma K, Myer DJ, Chan PH. Role of the p38 mitogen-activated protein kinase/cytosolic phospholipase A2 signaling pathway in blood-brain barrier disruption after focal cerebral ischemia and reperfusion. J Cereb Blood Flow Metab. 2008;28:1686–1696. doi: 10.1038/jcbfm.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki S, Ikeda C, Uchida Y, Sakamoto Y, Miller F, Glacial F, Decleves X, Scherrmann JM, Couraud PO, Kubo Y, Tachikawa M, Terasaki T. Quantitative targeted absolute proteomic analysis of transporters and junction proteins for validation of human cerebral microvascular endothelial cell line hCMEC/D3 as a human blood-brain barrier model. Mol Pharm. 2013;10:289–296. doi: 10.1021/mp3004308. [DOI] [PubMed] [Google Scholar]
- Oldendorf WH, Cornford ME, Brown WJ. The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol. 1977;1:409–417. doi: 10.1002/ana.410010502. [DOI] [PubMed] [Google Scholar]
- Ose A, Ito M, Kusuhara H, Yamatsugu K, Kanai M, Shibasaki M, Hosokawa M, Schuetz JD, Sugiyama Y. Limited brain distribution of [3R,4R,5S]-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate phosphate (Ro 64–0802), a pharmacologically active form of oseltamivir, by active efflux across the blood-brain barrier mediated by organic anion transporter 3 (Oat3/Slc22a8) and multidrug resistance-associated protein 4 (Mrp4/Abcc4) Drug Metab Dispos. 2009;37:315–321. doi: 10.1124/dmd.108.024018. [DOI] [PubMed] [Google Scholar]
- Ose A, Kusuhara H, Endo C, Tohyama K, Miyajima M, Kitamura S, Sugiyama Y. Functional characterization of mouse organic anion transporting peptide 1a4 in the uptake and efflux of drugs across the blood-brain barrier. Drug Metab Dispos. 2010;38:168–176. doi: 10.1124/dmd.109.029454. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmeira A, Sousa E, Vasconcelos MH, Pinto MM. Three decades of Pgp inhibitors: skimming through several generations and scaffolds. Curr Med Chem. 2012;19:1946–2025. doi: 10.2174/092986712800167392. [DOI] [PubMed] [Google Scholar]
- Park EM, Choi JH, Park JS, han MY, Park YM. Measurement of glutathione oxidation and 8-hydroxy-2’-deoxyguanosine accumulation in the gerbil hippocampus following global ischemia. Brain Res Brain Res Protoc. 2000;6:25–32. doi: 10.1016/s1385-299x(00)00033-7. [DOI] [PubMed] [Google Scholar]
- Pathak NN, Balaganur V, Lingaraju MC, Kant V, Latief N, More AS, Kumar D, Kumar D, Tandan SK. Atorvastatin attenuates neuropathic pain in rat neuropathy model by down-regulating oxidative damage at peripheral, spinal and supraspinal levels. Neurochem Int. 2014;68:1–9. doi: 10.1016/j.neuint.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Paulusma CC, van Geer MA, Evers R, Heijn M, Ottenhoff R, Borst P, Oude Elferink RP. Canalicular multispecific organic transporter/multidrug resistance protein 2 mediates low-affinity transport of reduced glutathione. Biochem J. 1999;338:393–401. [PMC free article] [PubMed] [Google Scholar]
- Pillai DR, Dittmar MS, Baldaranov D, Heidemann RM, Henning EC, Schulerer G, Bogdahn U, Schlachetzki F. Cerebral ischemia-reperfusion injury in rats--a 3 T MRI study on biphasic blood-brain barrier opening and the dynamics of edema formation. J Cereb Blood Flow Metab. 2009;29:1846–1855. doi: 10.1038/jcbfm.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plateel M, Dehouck MP, Torpier G, Cecchelli R, Tessier E. Hypoxia increases the susceptibility to oxidant stress and the permeability of the blood-brain barrier endothelial cell monolayer. J Neurochem. 1995;65:2138–2145. doi: 10.1046/j.1471-4159.1995.65052138.x. [DOI] [PubMed] [Google Scholar]
- Ponce J, de la Ossa NP, Hurtado O, Millan M, Arenillas JF, Davalos A, Gasull T. Simvastatin reduces the association of NMDA receptors to lipid rafts: a cholesterol-mediated effect in neuroprotection. Stroke. 2008;39:1269–1275. doi: 10.1161/STROKEAHA.107.498923. [DOI] [PubMed] [Google Scholar]
- Potschka H. Modulating P-glycoprotein regulation: future perspectives for pharmacoresistant epilepsies? Epilepsia. 2010;51:1333–1347. doi: 10.1111/j.1528-1167.2010.02585.x. [DOI] [PubMed] [Google Scholar]
- Redzic Z. Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: similarities and differences. Fluids Barriers CNS. 2011;8:3. doi: 10.1186/2045-8118-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesen FK, Rothen-Rutishauser B, Wunderli-Allenspach H. A ZO1-GFP fusion protein to study the dynamics of tight junctions in living cells. Histochem Cell Biol. 2002;117:307–315. doi: 10.1007/s00418-002-0398-y. [DOI] [PubMed] [Google Scholar]
- Rius M, Hummel-Eisenbeiss J, Hofmann AF, Keppler D. Substrate specificity of human ABCC4 (MRP4)-mediated cotransport of bile acids and reduced glutathione. Am J Physiol Gastrointest Liver Physiol. 2006;290:G640–G649. doi: 10.1152/ajpgi.00354.2005. [DOI] [PubMed] [Google Scholar]
- Roberts LM, Black DS, Raman C, Woodford K, Zhou M, Haggerty JE, Yan AT, Cwirla SE, Grindstaff KK. Subcellular localization of transporters along the rat blood-brain barrier and blood-cerebral-spinal fluid barrier by in vivo biotinylation. Neuroscience. 2008;155:423–438. doi: 10.1016/j.neuroscience.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Robey RW, To KK, Polgar O, Dohse M, Fetsch P, Dean M, Bates SE. ABCG2: a perspective. Adv Drug Deliv Rev. 2009;61:3–13. doi: 10.1016/j.addr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochfort KD, Collins LE, Murphy RP, Cummins PM. Downregulation of blood-brain barrier phenotype by proinflammatory cytokines involves NADPH oxidase-dependent ROS generation: consequences for interendothelial adherens and tight junctions. PLoS One. 2014;9:e101815. doi: 10.1371/journal.pone.0101815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Bendayan R. HIV-1 viral envelope glycoprotein gp120 produces oxidative stress and regulates the functional expression of multidrug resistance protein-1 (Mrp1) in glial cells. J Neurochem. 2008;106:1298–1313. doi: 10.1111/j.1471-4159.2008.05479.x. [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Davis TP. Blood-brain barrier integrity and glial support: mechanisms that can be targeted for novel therapeutic approaches in stroke. Curr Pharm Des. 2012;18:3624–3644. doi: 10.2174/138161212802002625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson PT, Davis TP. Targeted drug delivery to treat pain and cerebral hypoxia. Pharmacol Rev. 2013;65:291–314. doi: 10.1124/pr.112.005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson PT, DeMarco KM, Sanchez-Covarrubias L, Solinsky CM, Davis TP. Transforming growth factor-beta signaling alters substrate permeability and tight junction protein expression at the blood-brain barrier during inflammatory pain. J Cereb Blood Flow Metab. 2009;29:1084–1098. doi: 10.1038/jcbfm.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson PT, Finch JD, DeMarco KM, Quigley CE, Davis TP. Inflammatory pain signals an increase in functional expression of organic anion transporting polypeptide 1a4 at the blood-brain barrier. J Pharmacol Exp Ther. 2011;336:827–839. doi: 10.1124/jpet.110.174151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson PT, Persidsky Y, Bendayan R. Regulation of ABC membrane transporters in glial cells: relevance to the pharmacotherapy of brain HIV-1 infection. Glia. 2008;56:1711–1735. doi: 10.1002/glia.20725. [DOI] [PubMed] [Google Scholar]
- Salvemini D, Doyle TM, Cuzzocrea S. Superoxide, peroxynitrite and oxidative/nitrative stress in inflammation. Biochem Soc Trans. 2006;34:965–970. doi: 10.1042/BST0340965. [DOI] [PubMed] [Google Scholar]
- Sanchez-Covarrubias L, Slosky LM, Thompson BJ, Davis TP, Ronaldson PT. Transporters at CNS barrier sites: obstacles or opportunities for drug delivery? Curr Pharm Des. 2014;20:1422–1449. doi: 10.2174/13816128113199990463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Covarrubias L, Slosky LM, Thompson BJ, Zhang Y, Laracuente ML, DeMarco KM, Ronaldson PT, Davis TP. P-glycoprotein modulates morphine uptake into the CNS: a role for the non-steroidal anti-inflammatory drug diclofenac. PLoS One. 2014;9:e88516. doi: 10.1371/journal.pone.0088516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Schlachetzki F, Pardridge WM. P-glycoprotein and caveolin-1 alpha in endothelium and astrocytes of primate brain. Neuroreport. 2003;14:2041–2046. doi: 10.1097/00001756-200311140-00007. [DOI] [PubMed] [Google Scholar]
- Schreibelt G, Kooij G, Reijerkerk A, van Doorn R, Gringhuis SI, van der Pol S, Weksler BB, Rmero IA, Couraud PO, Piontek J, Blasig IE, Dijkstra CD, Ronken E, de Vries HE. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J. 2007;21:3666–3676. doi: 10.1096/fj.07-8329com. [DOI] [PubMed] [Google Scholar]
- Seelbach MJ, Brooks TA, Egleton RD, Davis TP. Peripheral inflammatory hyperalgesia modulates morphine delivery to the brain: a role for P-glycoprotein. J Neurochem. 2007;102:1677–1690. doi: 10.1111/j.1471-4159.2007.04644.x. [DOI] [PubMed] [Google Scholar]
- Sladojevic N, Stamatovic SM, Keep RF, Grailer JJ, Sarma JV, Ward PA, Andjelkovic AV. Inhibition of junctional adhesion molecule-A/LFA interaction attenuates leukocyte trafficking and inflammation in brain ischemia/reperfusion injury. Neurobiol Dis. 2014;67:57–70. doi: 10.1016/j.nbd.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slosky LM, Thompson BJ, Sanchez-Covarrubias L, Zhang Y, Laracuente ML, Vanderah TW, Ronaldson PT, Davis TP. Acetaminophen modulates P-glycoprotein functional expression at the blood-brain barrier by a constitutive androstane receptor-dependent mechanism. Mol Pharmacol. 2013;84:774–786. doi: 10.1124/mol.113.086298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sochocka M, lKoutsouraki ES, Gasiorowski K, Leszek J. Vascular oxidative stress and mitochondrial failure in the pathobiology of Alzheimer’s disease: a new approach to therapy. CNS Neurol Disord Drug Targets. 2013;12:870–881. doi: 10.2174/18715273113129990072. [DOI] [PubMed] [Google Scholar]
- Steiner E, Enzmann GU, Lyck R, Lin S, Ruegg MA, Kroger S, Engelhardt B. The heparan sulfate proteoglycan agrin contributes to barrier properties of mouse brain endothelial cells by stabilizing adherens junctions. Cell Tissue Res. 2014;358:465–479. doi: 10.1007/s00441-014-1969-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A, Stanimirovic D, Kawai N, McCarron RM, Spatz M. Hypoxia modulates free radical formation in brain microvascular endothelium. Acta Neurochir Suppl. 1997;70:8–11. doi: 10.1007/978-3-7091-6837-0_3. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kato Y, Tsuji A. Role of SLC xenobiotic transporters and their regulatory mechanisms PDZ proteins in drug delivery and disposition. J Control Release. 2006;116:238–246. doi: 10.1016/j.jconrel.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Sugiyama D, Kusuhara H, Taniguchi H, Ishikawa S, Nozaki Y, Aburatani H, Sugiyama Y. Functional characterization of rat brain-specific organic anion transporter (Oatp14) at the blood-brain barrier: high affinity transporter for thyroxine. J Biol Chem. 2003;278:43489–43495. doi: 10.1074/jbc.M306933200. [DOI] [PubMed] [Google Scholar]
- Tadepalle N, Koehler Y, Brandmann M, Meyer N, Dringen R. Arsenite stimulates glutathione export and glycolytic flux in viable primary rat brain astrocytes. Neurochem Int. 2014;76:1–11. doi: 10.1016/j.neuint.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Takenaga Y, Takagi N, Murotomi K, Tanonaka K, Takeo S. Inhibition of Src activity decreases tyrosine phosphorylation of occludin in brain capillaries and attenuates increase in permeability of the blood-brain barrier after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2009;29:1099–1108. doi: 10.1038/jcbfm.2009.30. [DOI] [PubMed] [Google Scholar]
- Taogoshi T, Nomura A, Murakami T, Nagai J, Takano M. Transport of prostaglandin E1 across the blood-brain barrier in rats. J Pharm Pharmacol. 2005;57:61–66. doi: 10.1211/0022357055173. [DOI] [PubMed] [Google Scholar]
- Thompson BJ, Ronaldson PT. Drug delivery to the ischemic brain. Adv Pharmacol. 2014;71:165–202. doi: 10.1016/bs.apha.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BJ, Sanchez-Covarrubias L, Slosky LM, Zhang Y, Laracuente ML, Ronaldson PT. Hypoxia/reoxygenation stress signals an increase in organic anion transporting polypeptide 1a4 (Oatp1a4) at the blood-brain barrier: relevance to CNS drug delivery. J Cereb Blood Flow Metab. 2014;34:699–707. doi: 10.1038/jcbfm.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokui T, Nakai D, Nakagomi R, Yawo H, Abe T, Sugiyama Y. Pravastatin, an HMG-CoA reductase inhibitor, is transported by rat organic anion transporting polypeptide, oatp2. Pharm Res. 1999;16:904–908. doi: 10.1023/a:1018838405987. [DOI] [PubMed] [Google Scholar]
- Tournier N, Decleves X, Saubamea B, Scherrmann JM, Cisternino S. Opioid transport by ATP-binding cassette transporters at the blood-brain barrier: implications for neuropsychopharmacology. Curr Pharm Des. 2011;17:2829–2842. doi: 10.2174/138161211797440203. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C, Suzuki T, Kamiie J, Terasaki T. Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J Neurochem. 2011;117:333–345. doi: 10.1111/j.1471-4159.2011.07208.x. [DOI] [PubMed] [Google Scholar]
- Ueno M, Nakagawa T, Huang CL, Ueki M, Kusaka T, Hosomi N, Kanenishi K, Onodera M, Wu B, Sakamoto H. The expression of P-glycoprotein is increased in vessels with blood-brain barrier impairment in a stroke-prone hypertensive model. Neuropathol Appl Neurobiol. 2009;35:147–155. doi: 10.1111/j.1365-2990.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- Umeda K, Matsui T, Nakayama M, Furuse K, Sasaki H, Furuse M, Tsukita S. Establishment and characterization of cultured epithelial cells lacking expression of ZO-1. J Biol Chem. 2004;279:44785–44794. doi: 10.1074/jbc.M406563200. [DOI] [PubMed] [Google Scholar]
- van Herwaarden AE, Jonker JW, Wagenaar E, Brinkhuis RF, Schellens JH, Beijnen JH, Schinkel AH. The breast cancer resistance protein (Bcrp1/Abcg2) restricts exposure to the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Cancer Res. 2003;63:6447–6452. [PubMed] [Google Scholar]
- Vemula S, Roder KE, Yang T, Bhat GJ, Thekkumkara TJ, Abbruscato TJ. A functional role for sodium-dependent glucose transport across the blood-brain barrier during oxygen glucose deprivation. J Pharmacol Exp Ther. 2009;328:487–495. doi: 10.1124/jpet.108.146589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestweber D. VE-cadherin: the major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler Thromb Vasc Biol. 2008;28:223–232. doi: 10.1161/ATVBAHA.107.158014. [DOI] [PubMed] [Google Scholar]
- Vijay N, Morris ME. Role of monocarboxylate transporters in drug delivery to the brain. Curr Pharm Des. 2014;20:1487–1498. doi: 10.2174/13816128113199990462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgintino D, Robertson D, Errede M, Benagiano V, Girolamo F, Maiorano E, Roncali L, Bertossi M. Expression of P-glycoprotein in human cerebral cortex microvessels. J Histochem Cytochem. 2002;50:1671–1676. doi: 10.1177/002215540205001212. [DOI] [PubMed] [Google Scholar]
- Vollrath V, Wielandt AM, Iruretagoyena M, Chianale J. Role of Nrf2 in the regulation of the Mrp2 (ABCC2) gene. Biochem J. 2006;395:599–609. doi: 10.1042/BJ20051518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker BK, Freie AB, Perfater JL, Gidday JM. Junctional protein regulation by sphingosine kinase 2 contributes to blood-brain barrier protection in hypoxic preconditioning-induced cerebral ischemic tolerance. J Cereb Blood Flow Metab. 2012;32:1014–1023. doi: 10.1038/jcbfm.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Campos CR, Peart JC, Smith LK, Boni JL, Cannon RE, Miller DS. Nrf2 upregulates ATP binding cassette transporter expression and activity at the blood-brain and blood-spinal cord barriers. J Neurosci. 34:8585–8593. doi: 10.1523/JNEUROSCI.2935-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XS, Fang HL, Chen Y, Liang SS, Zhu ZG, Zeng QY, Li J, Xu HG, Shao B, He JC, Hou ST, Zheng RY. Idazoxan reduces blood-brain barrier damage during experimental autoimmune encephalomyelitis in mouse. Eur J Pharmacol. 2014;736:70–76. doi: 10.1016/j.ejphar.2014.04.034. [DOI] [PubMed] [Google Scholar]
- Wang P, Liu Y, Shang X, Xue Y. CRM197-induced blood-brain barrier permeability increase is mediated by upregulation of caveolin-1 protein. J Mol Neurosci. 2011;43:485–492. doi: 10.1007/s12031-010-9471-5. [DOI] [PubMed] [Google Scholar]
- Westholm DE, Salo DR, Viken KJ, Rumbley JN, Anderson GW. The blood-brain barrier thyroxine transporter organic anion-transporting polypeptide 1c1 displays atypical transport kinetics. Endocrinology. 2009a;150:5153–5162. doi: 10.1210/en.2009-0769. [DOI] [PubMed] [Google Scholar]
- Westholm DE, Stenehjem DD, Rumbley JN, Drewes LR, Anderson GW. Competitive inhibition of organic anion transporting polypeptide 1c1-mediated thyroxine transport by the fenamate class of nonsteroidal antiinflammatory drugs. Endocrinology. 2009b;150:1025–1032. doi: 10.1210/en.2008-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DW, Calderon TM, Lopez L, Carvallo-Torres L, Gaskill PJ, Eugenin EA, Morgello S, Berman JW. Mechanisms of HIV entry into the CNS: increased sensitivity of HIV infected CD14+CD16+ monocytes to CCL2 and key roles of CCR2, JAM-A, and ALCAM in diapedesis. PLoS One. 2013;8:e69270. doi: 10.1371/journal.pone.0069270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MJ, Lowrie MB, Bennett JP, Firth JA, Clark P. Cadherin-10 is a novel blood-brain barrier adhesion molecule in human and mouse. Brain Res. 2005;1058:62–72. doi: 10.1016/j.brainres.2005.07.078. [DOI] [PubMed] [Google Scholar]
- Willis CL, Camire RB, Brule SA, Ray DE. Partial recovery of the damaged rat blood-brain barrier is mediated by adherens junction complexes, extracellular matrix remodeling and macrophage infiltration following focal astrocyte loss. Neuroscience. 2013;250:773–785. doi: 10.1016/j.neuroscience.2013.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis CL, Leach L, Clarke GJ, Nolan CC, Ray DE. Reversible disruption of tight junction complexes in the rat blood-brain barrier following transitory focal astrocyte loss. Glia. 2004a;48:1–13. doi: 10.1002/glia.20049. [DOI] [PubMed] [Google Scholar]
- Willis CL, Meske DS, Davis TP. Protein kinase C activation modulates reversible increase in cortical blood-brain barrier permeability and tight junction protein expression during hypoxia and posthypoxic reoxygenation. J Cereb Blood Flow Metab. 2010;30:1847–1859. doi: 10.1038/jcbfm.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis CL, Nolan CC, Reith SN, Lister T, Prior MJ, Guerin CJ, Mavroudis G, Ray DE. Focal astrocyte loss is followed by microvascular damage, with subsequent repair of the blood-brain barrier in the apparent absence of direct astrocytic contact. Glia. 2004b;45:325–337. doi: 10.1002/glia.10333. [DOI] [PubMed] [Google Scholar]
- Witt KA, Mark KS, Hom S, Davis TP. Effects of hypoxia-reoxygenation on rat blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol. 2003;285:H2820–H2831. doi: 10.1152/ajpheart.00589.2003. [DOI] [PubMed] [Google Scholar]
- Witt KA, Mark KS, Huber J, Davis TP. Hypoxia-inducible factor and nuclear factor kappa-B activation in blood-brain barrier endothelium under hypoxic/reoxygenation stress. J Neurochem. 2005;92:203–214. doi: 10.1111/j.1471-4159.2004.02871.x. [DOI] [PubMed] [Google Scholar]
- Witt KA, Mark KS, Sandoval KE, Davis TP. Reoxygenation stresson blood-brain barrier paracellular permeability and edema in the rat. Microvasc Res. 2008;75:91–96. doi: 10.1016/j.mvr.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood WG, Eckert GP, Igbavboa U, Muller WE. Statins and neuroprotection: a prescription to move the field forward. Ann N Y Acad Sci. 2010;1199:69–76. doi: 10.1111/j.1749-6632.2009.05359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Weerachayaphorn J, Cai SY, Soroka CJ, Boyer JL. Aryl hydrocarbon receptor and NF-E2-related factor 2 are key regulators of human MRP4 expression. Am J Physiol Gastrointest Liver Physiol. 2010;299:G126–G135. doi: 10.1152/ajpgi.00522.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Knight RA, Han Y, Karki K, Zhang J, Chopp M, Seyfried DM. Statins protect the blood-brain barrier acutely after experimental intracerebral hemorrhage. J Behav Brain Sci. 2013a;3:100–106. doi: 10.4236/jbbs.2013.31010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Thompson JF, Taheri S, Salayandia VM, McAvoy TA, Hill JW, Yang Y, Estrada EY, Rosenberg GA. Early inhibition of MMP activity in ischemic rat brain promotes expression of tight junction proteins and angiogenesis during recovery. J Cereb Blood Flow Metab. 2013b;33:1104–1114. doi: 10.1038/jcbfm.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh WL, Lin CJ, Fu WM. Enhancement of glucose transporter expression of brain endothelial cells by vascular endothelial growth factor derived from glioma exposed to hypoxia. Mol Pharmacol. 2008;73:170–177. doi: 10.1124/mol.107.038851. [DOI] [PubMed] [Google Scholar]
- Yeung D, Manias JL, Stewart DJ, Nag S. Decreased junctional adhesion molecule-A expression during blood-brain barrier breakdown. Acta Neuropathol. 2008;115:635–642. doi: 10.1007/s00401-008-0364-4. [DOI] [PubMed] [Google Scholar]
- Yousif S, Chaves C, Potin S, Margalli I, Scherrmann JM, Decleves X. Induction of P-glcyoprotein and Bcrp at the rat blood-brain barrier following a subchronic morphine treatment is mediated through NMDA/COX-2 acitivation. J Neurochem. 2012;123:491–503. doi: 10.1111/j.1471-4159.2012.07890.x. [DOI] [PubMed] [Google Scholar]
- Yousif S, Saubamea B, Cisternino S, Marie-Claire C, Dauchy S, Scherrmann JM, Decleves X. Effect of chronic exposure to morphine on the rat blood-brain barrier: focus on the P-glycoprotein. J Neurochem. 2008;107:647–657. doi: 10.1111/j.1471-4159.2008.05647.x. [DOI] [PubMed] [Google Scholar]
- Zehendner CM, Librizzi L, Hedrich J, Bauer NM, Angamo EA, de Curtis M, Luhmann HJ. Moderate hypoxia followed by reoxygenation results in blood-brain barrier breakdown via oxidative stress-dependent tight-junction protein disruption. PLoS One. 2013;8:e82823. doi: 10.1371/journal.pone.0082823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Schuetz JD, Elmquist WF, Miller DW. Plasma membrane localization of multidrug resistance-associated protein homologs in brain capillary endothelial cells. J Pharmacol Exp Ther. 2004;311:449–455. doi: 10.1124/jpet.104.068528. [DOI] [PubMed] [Google Scholar]
- Zhao R, Raub TJ, Sawada GA, Kasper SC, Bacon JA, Bridges AS, Pollack GM. Breast cancer resistance protein interacts with various compounds in vitro, but plays a minor role in substrate efflux at the blood-brain barrier. Drug Metab Dispos. 2009;37:1251–1258. doi: 10.1124/dmd.108.025064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Schmidt K, Nelson FR, Zelesky V, Troutman MD, Feng B. The effect of breast cancer resistance protein and P-glycoprotein on the brain penetration of flavopiridol, imatinib mesylate (Gleevec), prazosin, and 2-methoxy-3-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)phenyl)propanoic acid (PF-407288) in mice. Drug Metab Dispos. 2009;37:946–955. doi: 10.1124/dmd.108.024489. [DOI] [PubMed] [Google Scholar]