Abstract
Microglia play important roles in the process of neuronal injury and recovery. Numeous surface receptors have been described to regulate microglial activation. These receptors tightly mediate normal microglial functions including cell mobility, phagocytosis, and production of inflammatory mediators or trophic factors. In recent years, significant progresses have been achieved for understanding the signaling mechanisms underlying these receptors. Their specific roles in neurological diseases have been documented. This review will focus on the signal regulatory protein (SIRP) and its ligand CD47, two surface receptors expressed on microglia and other cells in the central nervous system (CNS) such as neurons. We will discuss the involvement of SIRP/CD47 signaling in microglial activation and in the interplay between microglia and other CNS cells. Current studies reveal the importance of CD47 and SIRPα in the process of neuroinflammation in the CNS disorders. The dual and contradictory role of CD47 suggests that targeting the SIRPα/CD47 signaling may achieve different effects depending on disease stage.
1. Introduction
About 20 years ago, microglia were first regarded as a distinct cell population in the central nervous system (CNS) with almost the same functions as peripheral macrophages (Barron, 1995). Nowadays, accumulating studies have highlighted the importance of microglia in CNS health and in the short and long-term progress of CNS diseases. On the one hand, microglial activation benefits the injured tissue by cleaning cell debris and reconstructing tissue integrity (Hanisch and Kettenmann, 2007; Lalancette-Hebert et al., 2007; Thored et al., 2009). On the other hand, excessive microglial activation may lead to secondary damage and impair CNS repair by releasing a bunch of harmful substances, including nitric oxide (NO), reactive oxygen species (ROS), and proinflammatory cytokines (Hu et al., 2014a). Due to their janus-like roles, microglia are well-regulated by a variety of mechanisms so that they can be promptly switched on as the first responders to noxious stimuli and rapidly turned off to avoid unwanted effects. Many surface recognition receptors on microglia have been identified to sense the “turning on” or “turning off” signals released from other CNS cells and play critical role in modulating microglial responses in many CNS injuries and neurodegerative diseases (Hu et al., 2014b).
The signal regulatory protein (SIRP, also known as CD172) and its ligand CD47 (also known as integrin-associated protein), are both expressed on the surface of microglia as well as on other types of CNS cells. Accumulating evidence documents that the interaction between SIRP and CD47 is important in mediating the cell-cell communication in the CNS. In this manuscript, we will discuss the functions of SIRP and CD47 in microglial activity and in the interplay between microglia and other CNS cells. The current understanding of the SIRP and CD47 sinaling will also be reviewed.
1. Signal regulatory protein (SIRP) family
The SIRP is a family of surface receptors. The SIRP family consists of SIRPα, SIRPβ, and SIRPγ as well as some other closely related proteins. Among them, SIRPα and SIRPβ1 are mainly expressed on myeloid cells and have been detected on microglia (Gaikwad et al., 2009; Gitik et al., 2011). Interestingly, SIRPα and SIRPβ1 may play opposite roles on regulating microglial activities.
1.1 Functions of SIRPα in microglia and other CNS cells
SIRPα (also known as SHPS-1, p84 and BIT) is expressed on a variety of myeloid cells. The activation of SIRPα has been related to the inhibition of cell activities, including the blunting of cytokine production (Kong et al., 2007; Latour et al., 2001; Smith et al., 2003), reduced monocyte adhesion to the extracellular matrix (Liu et al., 2005), reduced phagocytosis (Ide et al., 2007; Janssen et al., 2008; Latour et al., 2001; Oldenborg et al., 2000), and arrest of maturation of dendritic cells (Latour et al., 2001). In addition to these negative regulatory functions, a few positive effects of SIRPα have also been reported. For instance, engagement of SIRPα on monocytes mediates the transmigration of these cells across the endothelial lining of the blood-brain barrier (BBB) (de Vries et al., 2002). Ligation of SIRPα has also been shown to induce NO production from macrophages (Alblas et al., 2005). The expression of SIRPα on microglia is evident with immunohistochemical staining (Gitik et al., 2011). SIRPα is known to inhibit microglial phagocytic activity because the engulfment of myelin is augmented when microglial SIRPα is blocked with antibodies or knocked down by SIRPα-shRNA (Gitik et al., 2011). This inhibitory property of SIRPα is thought to be critical for the maintainence of myelin integrity under normal conditions or following mild brain damage. However, the inhibitory property of SIRPα may be disadvantageous in massive brain injuries or degeneration when rapid clearance of damaged myelin is essential.
1.2 Function of SIRPβ 1 in microglial phagocytosis
In contrast to the negative functions of SIRPα discussed above, the activation of SIRPβ1 usually leads to cell activation. SIRPβ1 has been shown to positively regulate macrophage phagocytosis (Hayashi et al., 2004) and neutrophil migration (Liu et al., 2005). The expression of SIRPβ1 on microglia was upregulated in APP transgenic mice and in Alzheimer's disease (AD) patients (Gaikwad et al., 2009). Activation of SIRPβ1 on microglia by cross-linking antibodies induces reorganization of cytoskeletal proteins and increased microglial phagocytosis of microsphere beads, neural debris, and fibrillary Aβ. In contrast, lentiviral knockdown of SIRPβ1 impairs microglial phagocytosis of neural cell debris and Aβ. These findings support the hypothesis that ligation of SIRPβ1 triggers microglial phagocytosis. Interestingly, SIRPβ1 activation suppresses LPS-induced gene transcription of tumor necrosis factor α (TNFα) and nitric oxide synthase-2 in microglia, suggesting that the impact of SIRPβ1 on microglia might not be a simple binary function such as activation or suppression, but rather a subtler directional regulation geared toward maximizing the beneficial effects of microglia.
2. CD47 − the ligand of SIRPα
CD47 is the first documented ligand for SIRPα. Some other soluble factors (surfactant proteins A and D) are also shown to bind to SIRPα in competition with CD47; however, these factors are highly specific to lung tissues (Janssen et al., 2008). CD47 is a cell surface transmembrane glycoprotein that is ubiquitously expressed in most cell types. Both SIRP and CD47 are members of the immunoglobulin superfamily and have one or more immunoglobulin superfamily (IgSF) structural domains.
2.1 SIRPα and CD47 interaction
Structural and mutagenesis studies have provided clues for the structural requirements for SIRPα-CD47 interactions. The extracellular region of SIRPα contains three IgSF domains: one membrane distal IgV domain (N-terminal) and two membrane proximal IgC domains. The N-terminal IgV domain of SIRPα is responsible for binding to CD47 (de Vries et al., 2002; Vernon-Wilson et al., 2000). For CD47, the extracellular IgV-like fold is responsible for its interaction with SIRPα as well as its association with 3 integrin (Gaikwad et al., 2009). In the SIRPα/CD47 complex, four loop structures (BC, C'D, DE, FG loops) at the end of the IgV domain of SIRPα comprise the interface with CD47 structures (BC and FG loops, C' strand surrounding the FG loop, N-terminal pyroglutamic acid) (Hatherley et al., 2008). The CD47/SIRPα interface is highly convoluted, extensive, and well fitted, better resembling that of specific antigen–antibody interactions than that of moderate-affinity IgSF domain-mediated cell-surface interactions. However, this binding model based on domain-domain structures may be oversimplified; the true protein interactions may involve the formation of higher order structures, such as dimerization of SIRPα or CD47. Lee et al. reported that cell surface SIRPα dimerizes in cis mainly through the membrane proximal IgC domains (Lee et al., 2010). Although subsequent studies suggested that dimerization of SIRPα may be independent of CD47 binding, it is likely that SIRPα dimers on the cell surface act as a bivalent receptor that can bind two CD47 molecules. This adds a new dimension to the sophisticated interactions between SIRPα and CD47.
Interestingly, although the extracellular regions of SIRPβ1 and SIRPα are highly homologous, CD47 can only bind to SIRPα (and SIRPγ), but not to SIRPβ1. Such specificity has been attributed to the complementary determining loops of the SIRPs, which allow slight sequence changes between members of the family to produce dramatic changes in binding affinity (Hatherley et al., 2008). SIRPβ1 is expressed on the cell surface as a disulfide-linked homodimer with bond formation mediated by Cys-320 in the membrane-proximal Ig loop (Liu et al., 2005). The ligand of SIRPβ is still unknown.
2.2 SIRPα and CD47 signaling
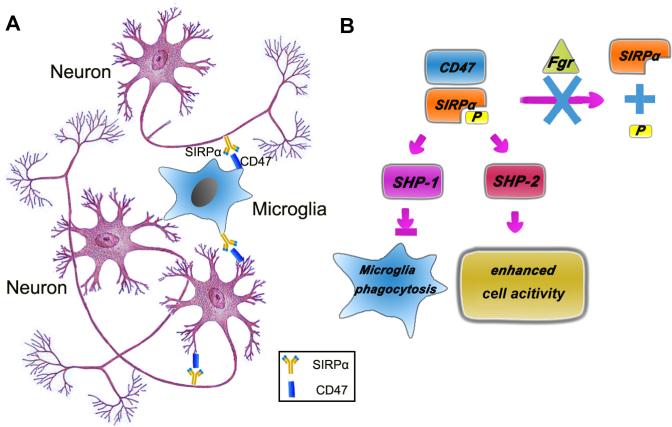
As a general rule in the immunoglobulin superfamily, the receptors react with the “turning off” signal usually keep microglia rest by regulating through a cytoplasmic-domain immunoreceptor tyrosine-based inhibition motif (ITIM); while the receptors activate microglia regulate through a cytoplasmic-domain immunoreceptor tyrosine-based activation motif (ITAM) (Linnartz and Neumann, 2013). As a receptor recognizing the “off” signal, the cytoplasmic region of SIRPα contains two ITIM with four highly conserved tyrosine residues. Ligation of SIRPα by CD47 induces phosphorylation of these tyrosine residues. The two C-terminal tyrosine phosphorylation sites (Y449 and Y473) provide docking sites for inhibitory phosphatases SH2 domain-containing phosphatase (SHP)-1 or SHP-2 (Kharitonenkov et al., 1997). Interestingly, these phosphorylated tyrosine residues could also be the substrate of SHPs and serve as the dephosphorylation site for their catalytic domains. Surprisingly, in spite of their highly homologous structures, SHP-1 and SHP-2 perform opposing biological functions (Neel et al., 2003). (Figure 1) SHP-1 negatively regulates various signaling pathways to inhibit multiple cell functions; SHP-2, in contrast, positively regulates signaling events involved in cellular activities such as growth and migration. For example, SIRPα engagement has been shown to inhibit macrophage phagocytosis through the recruitment of SHP-1 (Ohnishi et al., 2005; Veillette et al., 1998; Yamao et al., 2002). In contrast, IGF-1 stimulation of vascular smooth muscle cells results in the phosphorylation of SIRPα and recruitment of SHP-2 as well as other downstream molecules, leading to enhanced cell survival (Shen et al., 2010). Fcγ receptor (FcγR) activation has been shown to stimulate the association between SHP-1 and SIRPα, which in turn inhibits FcγR- and complementary receptor-mediated phagocytosis (Gresham et al., 2000; Latour et al., 2001). Crosslinking of FcγRs induces the association of Fgr, a member of the Src kinase family, with SIRPα. However, Fgr is not involved in the phosphorylation of SIRPα. Instead, it protects SIRPα from dephosphorylation, allowing the recruitment and docking of SHP-1. Fgr therefore inhibits the phagocytic activity of macrophages. These results demonstrate how functionally opposed signaling pathways are activated in phagocytes to fine-tune their responses to specific external signals.
Figure. Cell-cell crosstalk through signal regulatory protein (SIRP)α-CD47 signaling.
A. Bidirectional signaling bwtween SIRPα and CD47. SIRPα and CD47 may be coexpressed on the same cell and their ligation might mediate intercellular signaling in a bidirectional manner. SIRPα-CD47 binding between neighboring neurons enhances neuronal network formation in the hippocampus. Similarly, CD47 and SIRPα are colocalized in microglia. Although SIRPα-CD47 signaling in microglia has not been thoroughly investigated, the individual roles of CD47 and SIRPα have been addressed. Both SIRPα and CD47 participate in the phagocytic functions of microglia. B. SIRP signaling in microglia. The phosphorylation of SIRPα allows the recruitment and docking of SHP-1 and SHP-2. SHP-1 and SHP-2 perform opposing biological functions. SHP-1 negatively regulates various signaling pathways to inhibit multiple cell functions such as phagocytosis; SHP-2, in contrast, positively regulates signaling events involved in cellular activities such as growth and migration. Fgr, a member of the Src kinase family that can be activated by crossling of Fcγ receptor, inhibits the SIRPα dephosphorylation, allowing the recruitment and docking of SHP-1. Fgr therefore inhibits the phagocytic activity of microglia. SHP1, SH2 domain-containing phosphatase-1.
In addition to SHPs, SIRPα was also shown to associate with two adaptor molecules (Srckinase-associated protein of 55 kDa homologue (SKAP55hom/R) and Fyn-binding protein/SLP-76-associated protein of 130 kDa (FYB/SLAP-130), or a tyrosine kinase PYK2 (Timms et al., 1999). It is possible that SIRPα has scaffold-like functions to assemble multi-protein complexes. The function of these complexes is not clear, but might involve the transmission of adhesion-regulated signals or termination of these signals via SHP-1 recruitment.
2.2 Bidirectional signaling between SIRPα and CD47
Unique to SIRPα-CD47 signaling is that these two proteins may be coexpressed on the same cell and their ligation might mediate intercellular signaling in a bidirectional manner (Gaikwad et al., 2009). (Figure 1) This feature is especially important in the CNS where the expression of SIRPα and CD47 is largely overlapping (Gresham et al., 2000; Ohnishi et al., 2005). For instance, both SIRPα and CD47 are expressed on the surface of hippocampal neurons, with SIRPα present on the axon and dendrites and CD47 restricted to dendrites (Ohnishi et al., 2005). The CD47-SIRPα interaction between neighboring neurons plays an important role in neuronal network formation in the hippocampus (Murata et al., 2006). Similarly, CD47 and SIRPα are colocalized in microglia. Although SIRPα-CD47 signaling in microglia has not been thoroughly investigated, the individual roles of CD47 and SIRPα have been addressed. Interestingly, both SIRPα and CD47 participate in the phagocytic functions of microglia. As mentioned above, SIRPα inhibits microglial phagocytosis. CD47, however, works together with other surface receptors (CD36, integrinα6β1, TLR4, TLR2 and scavenger receptor A) as a complex involved in microglial interactions with Aβ fibrils (Bamberger et al., 2003; Koenigsknecht and Landreth, 2004). The subsequent phagocytosis of Aβ fibrils and activation of intracellular signaling pathways leads to a proinflammatory response. Using acutely isolated microglia from mice of different ages, phagocytosis of Aβ fibrils has been shown to be prominent in young microglia (prepared from day 0 pups) in a CD47-dependent manner but is lost in aged microglia prepared from 6-month-old mice (Floden and Combs, 2011). This study suggests that dysfunctional CD47 might be responsible for the impaired phagocytic function of aged microglia. SIRP/CD47 signaling also play an instrumental role in cerebral infiltration of circulating monocytes. The interaction between the SIRP expressed on monocytes and CD47 on endothelial cells (ECs) promotes the transmigration of monocytes across the brain endothelial, which requires G1-protein activation instead of monocytes adhesion.(de Vries et al., 2002).
3. SIRPβ1 signaling through the transmembrane adaptor protein DAP12
Despite the similarity of the extracellular domains of SIRPα and SIRPβ1, the cytoplasmic domains differ greatly, leading to the activation of distinct downstream signaling cascades. SIRP 1 has a very short cytoplasmic region that lacks signaling motifs. It associates with DNAX activation protein 12 (DAP12), a dimeric adaptor protein containing an ITAM, through a basic amino acid residue in the transmembrane region. Engagement of SIRP 1 has been shown to activate the tyrosine kinase Syk, which subsequently activates mitogen-activated protein kinase (MAPK) and enhances microglial phagocytosis (Hayashi et al., 2004).
4. CD47 and SIRPα in neuroinflammation and CNS disorders
4.1 CD47 and SIRPa in experimental stroke and CNS injuries
A recent study documented an important role of SIRPα in the pathology of ischemic stroke. The infarct volume, neurological deficits, neuronal apoptosis, and oxidative stress after focal cerebral ischemia were all attenuated in SIRPα deficient mice (Wang et al., 2012). This study focused on the effect of SIRPα on neuronal stressors. Actually, SIRPα, together with its ligand CD47, are also expressed by neurons and involved in neuronal apoptosis, neurite outgrowth, and synaptic activities (Gresham et al., 2000; Jiang et al., 1999; Wang et al., 2003). So far, the contribution of microglial SIRPα to ischemic stroke or other types of neurological disorders has not yet been addressed.
While the effect of SIRP in CNS injuries is relatively few, CD47 has been documented to be important in a variety of CNS injuries including ischemic and hemorrhagic brain injury, and spinal cord injury (SCI). A study using CD47 knockout mice suggests a deteriorate effect of CD47 in ischemic brain (Jin et al., 2009). The absence of the CD47 gene reduces brain infarct and swelling at acute stage after focal ischemic brain damage. Further study revealed that CD47 promotes matrix metalloproteinase-9 (MMP-9) upregulation after stroke and enhances the damage in BBB, resulting in increased inflammatory cell infiltration and aggravated neuroinflammation in the ischemic brain. In vitro studies further demonstrated that the engagement of CD47 upregulated vascular endothelial growth factor (VEGF) and MMP-9, two major neurovascular mediators, in brain endothelial cells and astrocytes, which could in turn contribute to CD47-mediated BBB damage after stroke (Xing et al., 2010).
The involvement of CD47 in hemorrhagic stroke has also been investigated in a pig model of inintracerebral hemorrhage (ICH). (Zhou et al., 2014) The expression of CD47 in the perihematomal white and grey matter is upregulated soon after ICH and such upregulation can last for at least 2 weeks. Increased expression of CD47 is observed in microglia/macrophages, neurons and oliogodendrocytes. However, the exact roles of CD47 in these cells after ICH are still unknown.
Similar to the study in ischemic brain injury, targeted deletion of CD47 leads to protection in a model of SCI, as manefested by white matter sparing, decreased neutrophil extravasation and improved functional recovery (Myers et al., 2011). Further study revealed that the benefitial effects of CD47 ablation could be attributed to reduced endothelial cells-neutrophil interaction through CD47-SIRPα signaling. The kockout of CD47 also inhibits angiogenesis that mediated by CD47 interaction with its angiogenic ligand, thrombospondin-1 (TSP-1) (Benton et al., 2008) and results in improvements in penumbral microvascularity after SCI. This anti-angiogenic effect alone, however, can not lead to functional improvement as there is no significant behavioral improvement between TSP-1 knockout mice and wildtype mice.
Taken together, these studies highlight the importance of CD47-SIRPα signaling in the development of neuroinflammation after CNS injury.
4.2 CD47 and SIRPα in multiple sclerosis
In addition to the CNS injuries, CD47 is also important in CNS autoimmune diseases such as multiple sclerosis (MS). (Han et al., 2012) CD47 localizes in normal myelin and also in foamy macrophages and activated astrocytes around the active MS lesions. The expression of CD47 is downregulated in MS brain lesions. CD47 deficient mice are resistant to experimental autoimmune encephalomyelitis (EAE), a model of MS, together with an ameliorated inflammatory responses in T cells and other immune cells. Surprisingly, inhibition against CD47 with a monoclonal antibody at the peak of paralysis deteriorated EAE severity in spite of ameliorated immune cell activation. In vitro assays demonstrate that blocking CD47 enhance myelin phagocytosis which is largely dependent on CD47/SIRPα binding. Therefore, lack of CD47 may promote demyelination by enhancing SIRPα–dependent phagocytosis of myelin. This study exemplifies the dual and contradictory effects of CD47 during neuroinflammation, which are likely caused by differential expression of CD47 in different cell types, location, and disease phase.
5. Future directions
Despite our increased understanding of SIRPα/CD47 interaction bwtween CNS cells and their roles in CNS homeostasis and abnormalties, there are still unsolved questions in this field (Barclay and Van den Berg, 2014). First and foremost, current studies lack of a cell-specific view of SIRPα-CD47 interplay and the mobilization of downstream signaling mediators. In addition, the complexity of SIRPα/CD47 contribution to CNS injuries and disorders need to be further characterized and the underlying mechanisms need to be elicidated. For example, the previous study documented increased expression of CD47 in microglia/macrophage, neurons and oliogodendrocytes after ICH. However, the cell specific function of CD47 is unknown and its downstream signaling is not clear. Secondly, it is still uncertain whether CD47 or SIRPα can be a therapeutic target for the treatment of CNS diseases. Indeed, the therapeutic role of CD47/SIRPα binding has been demonstated in cancer therapy. A promising anti-cancer strategy depending on a unique CD47/SIRPα interaction between tumor cells and macrophages/microglia has been addressed (Weiskopf et al., 2013). However, in the CNS, both CD47 and SIRPα are widely expressed on various cells and actively involve in multipul cell activities. Moreover, CD47 and SIRPα may play janus roles in certain pathological events such as neuroinflammation (Han et al., 2012). It therefore would be important and challenging to decide whether modulating CD47/SIRPα signaling may have different effects depending on disease progress. In this regards, better characterization of cell-specific CD47/SIRPα interaction during different stages of certain disease would be necessary for the development successful traetment strategies targeting these molecules.
Highlights.
SIRP and CD47 regulate microglial activation.
SIRP and CD47 mediate the interplay between microglia and other CNS cells.
CD47 and SIRP are important in neuroinflammation in the CNS disorders.
CD47 and SIRPα may play janus roles in certain pathological events.
Modulating CD47/SIRPα may have different effects depending on disease progress.
Acknowledgments
XIAOMING HU is supported by a Scientist Development Grant (13SDG14570025) from the American Heart Association. JUN CHEN is supported by National Institutes of Health Grants NS36736, NS43802, and NS45048.
Abbreviation
- Aβ
amyloid β
- AD
Alzheimer's disease
- CNS
central nervous system
- IgSF
immunoglobulin superfamily
- ICH
inintracerebral hemorrhage
- DAP12
DNAX activation protein 12
- ITAM
immunoreceptor tyrosine-based activation motif
- ITIM
immunoreceptor tyrosine-based inhibition motif
- MAPK
mitogen-activated protein kinase
- MMP
matrix metalloproteinase
- MS
multiple sclerosis
- NO
nitric oxide
- ROS
reactive oxygen species
- SCI
spinal cord injury
- SHP1
SH2 domain-containing phosphatase-1
- SIRP
Signal regulatory protein
- TNFα
tumor necrosis factor α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure Statement
No competing financial interests exist.
References
- Alblas J, et al. Signal regulatory protein alpha ligation induces macrophage nitric oxide production through JAK/STAT- and phosphatidylinositol 3-kinase/Rac1/NAPDH oxidase/H2O2-dependent pathways. Mol Cell Biol. 2005;25:7181–92. doi: 10.1128/MCB.25.16.7181-7192.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger ME, et al. A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation. J Neurosci. 2003;23:2665–74. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay AN, Van den Berg TK. The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: structure, function, and therapeutic target. Annu Rev Immunol. 2014;32:25–50. doi: 10.1146/annurev-immunol-032713-120142. [DOI] [PubMed] [Google Scholar]
- Barron KD. The microglial cell. A historical review. J Neurol Sci. 1995;134(Suppl):57–68. doi: 10.1016/0022-510x(95)00209-k. [DOI] [PubMed] [Google Scholar]
- Benton RL, et al. Transcriptomic screening of microvascular endothelial cells implicates novel molecular regulators of vascular dysfunction after spinal cord injury. J Cereb Blood Flow Metab. 2008;28:1771–85. doi: 10.1038/jcbfm.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries HE, et al. Signal-regulatory protein alpha-CD47 interactions are required for the transmigration of monocytes across cerebral endothelium. J Immunol. 2002;168:5832–9. doi: 10.4049/jimmunol.168.11.5832. [DOI] [PubMed] [Google Scholar]
- Floden AM, Combs CK. Microglia demonstrate age-dependent interaction with amyloid-beta fibrils. J Alzheimers Dis. 2011;25:279–93. doi: 10.3233/JAD-2011-101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaikwad S, et al. Signal regulatory protein-beta1: a microglial modulator of phagocytosis in Alzheimer's disease. Am J Pathol. 2009;175:2528–39. doi: 10.2353/ajpath.2009.090147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitik M, et al. Myelin down-regulates myelin phagocytosis by microglia and macrophages through interactions between CD47 on myelin and SIRPalpha (signal regulatory protein-alpha) on phagocytes. J Neuroinflammation. 2011;8:24. doi: 10.1186/1742-2094-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresham HD, et al. Negative regulation of phagocytosis in murine macrophages by the Src kinase family member, Fgr. J Exp Med. 2000;191:515–28. doi: 10.1084/jem.191.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MH, et al. Janus-like opposing roles of CD47 in autoimmune brain inflammation in humans and mice. J Exp Med. 2012;209:1325–34. doi: 10.1084/jem.20101974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–94. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hatherley D, et al. Paired receptor specificity explained by structures of signal regulatory proteins alone and complexed with CD47. Mol Cell. 2008;31:266–77. doi: 10.1016/j.molcel.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Hayashi A, et al. Positive regulation of phagocytosis by SIRPbeta and its signaling mechanism in macrophages. J Biol Chem. 2004;279:29450–60. doi: 10.1074/jbc.M400950200. [DOI] [PubMed] [Google Scholar]
- Hu X, et al. Microglial and macrophage polarization-new prospects for brain repair. Nat Rev Neurol. 2014a doi: 10.1038/nrneurol.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, et al. Neurobiology of microglial action in CNS injuries: receptor-mediated signaling mechanisms and functional roles. Prog Neurobiol. 2014b;119-120:60–84. doi: 10.1016/j.pneurobio.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide K, et al. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci U S A. 2007;104:5062–6. doi: 10.1073/pnas.0609661104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen WJ, et al. Surfactant proteins A and D suppress alveolar macrophage phagocytosis via interaction with SIRP alpha. Am J Respir Crit Care Med. 2008;178:158–67. doi: 10.1164/rccm.200711-1661OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Lagenaur CF, Narayanan V. Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J Biol Chem. 1999;274:559–62. doi: 10.1074/jbc.274.2.559. [DOI] [PubMed] [Google Scholar]
- Jin G, et al. CD47 gene knockout protects against transient focal cerebral ischemia in mice. Exp Neurol. 2009;217:165–70. doi: 10.1016/j.expneurol.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, et al. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature. 1997;386:181–6. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- Koenigsknecht J, Landreth G. Microglial phagocytosis of fibrillar beta-amyloid through a beta1 integrin-dependent mechanism. J Neurosci. 2004;24:9838–46. doi: 10.1523/JNEUROSCI.2557-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong XN, et al. LPS-induced down-regulation of signal regulatory protein {alpha} contributes to innate immune activation in macrophages. J Exp Med. 2007;204:2719–31. doi: 10.1084/jem.20062611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalancette-Hebert M, et al. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latour S, et al. Bidirectional negative regulation of human T and dendritic cells by CD47 and its cognate receptor signal-regulator protein-alpha: down-regulation of IL-12 responsiveness and inhibition of dendritic cell activation. J Immunol. 2001;167:2547–54. doi: 10.4049/jimmunol.167.5.2547. [DOI] [PubMed] [Google Scholar]
- Lee WY, et al. The role of cis dimerization of signal regulatory protein alpha (SIRPalpha) in binding to CD47. J Biol Chem. 2010;285:37953–63. doi: 10.1074/jbc.M110.180018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnartz B, Neumann H. Microglial activatory (immunoreceptor tyrosine-based activation motif)- and inhibitory (immunoreceptor tyrosine-based inhibition motif)-signaling receptors for recognition of the neuronal glycocalyx. Glia. 2013;61:37–46. doi: 10.1002/glia.22359. [DOI] [PubMed] [Google Scholar]
- Liu SQ, et al. Negative regulation of monocyte adhesion to arterial elastic laminae by signal regulatory protein alpha and Src homology 2 domain-containing protein-tyrosine phosphatase-1. J Biol Chem. 2005;280:39294–301. doi: 10.1074/jbc.M503866200. [DOI] [PubMed] [Google Scholar]
- Murata T, et al. CD47 promotes neuronal development through Src- and FRG/Vav2-mediated activation of Rac and Cdc42. J Neurosci. 2006;26:12397–407. doi: 10.1523/JNEUROSCI.3981-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers SA, et al. CD47 knockout mice exhibit improved recovery from spinal cord injury. Neurobiol Dis. 2011;42:21–34. doi: 10.1016/j.nbd.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel BG, Gu H, Pao L. The 'Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284–93. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- Ohnishi H, et al. Differential localization of Src homology 2 domain-containing protein tyrosine phosphatase substrate-1 and CD47 and its molecular mechanisms in cultured hippocampal neurons. J Neurosci. 2005;25:2702–11. doi: 10.1523/JNEUROSCI.5173-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenborg PA, et al. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–4. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- Shen X, et al. Recruitment of Pyk2 to SHPS-1 signaling complex is required for IGF-I-dependent mitogenic signaling in vascular smooth muscle cells. Cell Mol Life Sci. 2010;67:3893–903. doi: 10.1007/s00018-010-0411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RE, et al. A novel MyD-1 (SIRP-1alpha) signaling pathway that inhibits LPS-induced TNFalpha production by monocytes. Blood. 2003;102:2532–40. doi: 10.1182/blood-2002-11-3596. [DOI] [PubMed] [Google Scholar]
- Thored P, et al. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57:835–49. doi: 10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- Timms JF, et al. SHPS-1 is a scaffold for assembling distinct adhesion-regulated multi-protein complexes in macrophages. Curr Biol. 1999;9:927–30. doi: 10.1016/s0960-9822(99)80401-1. [DOI] [PubMed] [Google Scholar]
- Veillette A, Thibaudeau E, Latour S. High expression of inhibitory receptor SHPS-1 and its association with protein-tyrosine phosphatase SHP-1 in macrophages. J Biol Chem. 1998;273:22719–28. doi: 10.1074/jbc.273.35.22719. [DOI] [PubMed] [Google Scholar]
- Vernon-Wilson EF, et al. CD47 is a ligand for rat macrophage membrane signal regulatory protein SIRP (OX41) and human SIRPalpha 1. Eur J Immunol. 2000;30:2130–7. doi: 10.1002/1521-4141(2000)30:8<2130::AID-IMMU2130>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Wang L, et al. SHPS-1 deficiency induces robust neuroprotection against experimental stroke by attenuating oxidative stress. J Neurochem. 2012;122:834–43. doi: 10.1111/j.1471-4159.2012.07818.x. [DOI] [PubMed] [Google Scholar]
- Wang XX, Dangott LJ, Pfenninger KH. The heterogeneous growth cone glycoprotein gp93 is identical to the signal regulatory protein SIRPalpha/SHPS-1/BIT. J Neurochem. 2003;86:55–60. doi: 10.1046/j.1471-4159.2003.01810.x. [DOI] [PubMed] [Google Scholar]
- Weiskopf K, et al. Engineered SIRPalpha variants as immunotherapeutic adjuvants to anticancer antibodies. Science. 2013;341:88–91. doi: 10.1126/science.1238856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing C, et al. Induction of vascular endothelial growth factor and matrix metalloproteinase-9 via CD47 signaling in neurovascular cells. Neurochem Res. 2010;35:1092–7. doi: 10.1007/s11064-010-0159-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamao T, et al. Negative regulation of platelet clearance and of the macrophage phagocytic response by the transmembrane glycoprotein SHPS-1. J Biol Chem. 2002;277:39833–9. doi: 10.1074/jbc.M203287200. [DOI] [PubMed] [Google Scholar]
- Zhou X, et al. Brain CD47 expression in a swine model of intracerebral hemorrhage. Brain Res. 2014;1574:70–6. doi: 10.1016/j.brainres.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]